Abstract
Phthalates are one of the most commonly used endocrine disruptors and have been considered a risk factor for respiratory disease including asthma. However, it is not yet known how they are related to urticaria. We investigated the association between phthalate exposure and urticaria in 10 healthy controls and 20 adult patients with active urticaria. The urinary levels of mono-n-butyl phthalate (MnBP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-(2-ethyl-5-oxohexyl) phthalate, and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) were measured by using high performance liquid chromatography tandem mass spectrometry, and mast cell releasability was determined after phthalate treatment. The levels of phthalate metabolites, especially di-ethylhexyl phthalate (DEHP), are significantly increased in the urine of patients with urticaria compared to the healthy controls. The release of β-hexosaminidase in human mast cells is more significantly increased by MnBP, mono-benzyl phthalate, MEHHP, and MECPP compared to the negative controls; interestingly, the highest secretion of β-hexosaminidase is observed after the lowest stimulation of MECPP. Phthalates, including DEHP, may act as aggravating factors for chronic spontaneous urticaria and can be used as potential therapeutic targets in future studies.
Keywords: Urticaria, DEHP, asthma, chromatography
INTRODUCTION
Urticaria is a common skin disease that can be experienced by approximately 10%–20% of people in their lifetime,1 and its prevalence has been continuously rising worldwide.2 Chronic urticaria is defined as a recurrent condition lasting more than 6 weeks. Food, medication, and infection can cause acute urticaria, but no specific etiologies can be identified in most cases of chronic urticaria.3 Approximately 30% of patients with chronic urticaria are caused by physical stimuli and diagnosed as having chronic inducible urticaria, while the remaining 70% develop without any specific causes and are diagnosed with chronic spontaneous urticaria. Although some cases of chronic spontaneous urticaria have been suggested to be related to autoimmune mechanisms, recent studies have found that lifestyle as well as indoor and environmental pollutants may aggravate this uriticaria.4,5
Phthalates are common endocrine-disrupting chemicals and used as a plasticizer contained in various products in our daily life, including cosmetics, medical devices, food packaging, children’s toys, and polyvinyl chloride plastics.6 Therefore, humans are exposed to phthalates through several routes of exposure such as water, breathing air, dermal contact, and food. The levels of phthalate metabolites are positively associated with allergy symptoms and sensitization in the National Health and Nutrition Examination Survey in the US7, and phthalate exposure can increase atopic dermatitis symptoms in children.8 However, it is not yet known how phthalates contribute to the worsening of urticaria.
MATERIALS AND METHODS
We prospectively enrolled 10 healthy controls and 20 adult patients with active urticaria. All patients had chronic spontaneous urticaria for at least 6 weeks; those with inducible urticaria caused by drugs, foods, or physical factors were excluded from the study. Urine samples were obtained from all participants and stored at −70°C until analyzed. The urinary metabolites of phthalates, including mono-n-butyl phthalate (MnBP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and creatinine, were measured using high-performance liquid chromatography tandem mass spectrometry (HPLC-MS) at low parts-per-billion levels. The urinary concentration of each compound is expressed as a fraction of urinary creatinine concentration. For an in vitro study, the human mast cell line, laboratory of allergic diseases 2 (LAD2) cells was cultured in StemPro-34 medium supplemented with StemPro nutrient supplement (10 mL/mL), L-glutamine (2 µM), human stem cell factor (100 ng/mL), and penicillin-streptomycin (HyClone, 1:100, UT) in a 37°C, 5% CO2 humidified incubator. The culture medium was replaced every 7 days, and the cells were maintained at a density of 2 × 106 cells/mL. LAD2 cells were treated with different concentrations of phthalates (0.5, 1, 50, and 100 μM) for 1 and 4 hours, and the cell viability and proliferation were determined using Cell counting kit-8 (Dojindo, Rockville, MD, USA) according to the manufacturer’s instructions. Phthalate-induced β-hexosaminidase release from LAD2 cells was accomplished by the following protocol. Briefly, LAD2 cells were sensitized with dinitrophenol (DNP)-immunoglobulin E (IgE; 1 μg/mL) overnight. LAD2 cells (1 × 105 cells/well in a 96-well plate) were suspended in Tyrode’s buffer (10 nmol/L HEPES, 137 mmol/L NaCl, 2.7 mmol/L KCl, 0.4 mmol/L Na2HPO4, 5.6 mmol/L D-glucose, 1.8 mmol/L CaCl2, and 1.3 mmol/L MgSO4) containing 0.025% bovine serum albumin. Cells were treated with MnBP, MECPP, mono-benzyl phthalate (MBzP), and MEHHP (0.5, 1, 50, and 100 μM) in Tyrode’s solution for 1 hour and then stimulated with 100 μL of DNP-HSA (1 μg/mL, in TM buffer) for another 30 minutes. The release of β-hexosaminidase in the supernatants and the cell pellets (solubilized with 0.5% Triton X-100 in Tyrode’s buffer) were measured using p-nitrophenyl N-acetyl-b-D-glucosaminide in 0.1 M sodium citrate (pH = 4.5) for 30 minutes at 37°C. The reaction was stopped using 0.4 mol/L glycine (pH = 10.7), and the absorbance was determined at 405 nm. The percentage of β-hexosaminidase released was calculated as follows: [(β-hexosaminidase in supernatant × 2)/(β-hexosaminidase in supernatant + {total β-hexosaminidase in cell pellet × 4 })] × 100%. The study protocol was approved by the Institutional Review Board, and written informed consent was obtained from each patient (AJIRB-MED-SUR-18-246).
Statistical analysis
The sample size was calculated by setting the mean differences of urinary phthalate levels, SD, 5% level of significance, and 95% statistical power. Data are shown as mean ± SEs. The differences between the urticaria patients and the healthy controls were assessed using the Wilcoxon signed-rank test. All statistical analyses were performed by using SPSS software version 23.0 (SPSS, Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
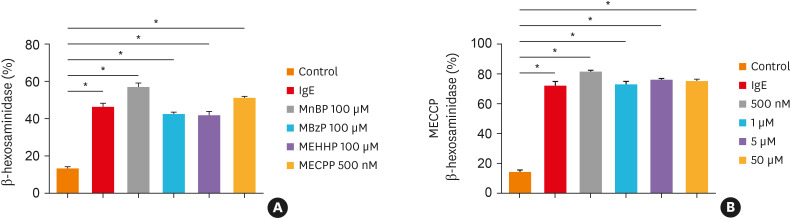
First, the viability of LAD2 cells was measured after treatment with different concentrations of phthalates. There was no significant decrease in the viability of LAD2 cells even when the maximum concentrations of each phthalate were used at 1 and 4 hours (data not shown). MnBP, MBzP, MECPP, or MEHHP did not have any effect on cell viability. Next, the stimulatory effects of each phthalate were evaluated on LAD2 cells. LAD2 cells were incubated with MnBP, MBzP, MECPP, or MEHHP for 1 hour. The releases of β-hexosaminidase were increased by 57.80% in MnBP, 43.21% in MBzP, 42.55% in MEHHP and 51.96% in MECPP compared to the negative controls (Figure A). Interestingly, in the case of MECPP, the strongest mast cell activation was shown at the lowest treatment concentration of 500 nM (Figure B). Finally, we measured major phthalates in urine to determine whether chronic spontaneous urticaria patients excrete more metabolites of phthalate compared to the healthy controls by HPLC-MS. There was no difference between the 2 groups in general characteristics, such as age, sex, or allergic status (data not shown). MnBP is representative of short-branched phthalates, whereas MECPP, MEOHP, and MEHHP are the metabolites of DEHP which are widely used as long-branched phthalates. All the phthalate values measured in urine using the non-parametric method were significantly higher in the patients with chronic spontaneous urticaria than in the healthy controls (Table).
Figure. Stimulatory effects of phthalates on β-hexosaminidase release in LAD2 cells. (A) LAD2 cells were incubated with MnBP, MBzP, MEHHP, and MECPP for 1 hour. The levels of β-hexosaminidase were determined in cell culture supernatants. (B) LAD2 cells were treated with different concentrations of MECPP. Data are represented as absolute intensity and the means ± SE.
IgE, immunoglobulin E; LAD2, laboratory of allergic diseases 2; MnBP, mono-n-butyl phthalate; MBzP, mono-benzyl phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate.
*P < 0.001 versus the negative controls.
Table. The comparisons between concentrations of phthalate metabolites in urine.
| Phthalates | Chronic spontaneous urticaria (n = 20) | Healthy controls (n = 10) | P value |
|---|---|---|---|
| MnBP | 27.1 ± 15.6 (29.1)* | 18.8 ± 8.2 (10.8) | 0.009 |
| MEHHP | 15.2 ± 15.9 (16.9)* | 7.9 ± 3.8 (4.6) | 0.038 |
| MEOHP | 8.7 ± 8.0 (9.6)* | 4.8 ± 1.9 (2.7) | 0.015 |
| MECPP | 17.0 ± 11.2 (20.6)* | 10.8 ± 2.1 (7.1) | 0.008 |
The levels of phthalate metabolites were compared between the healthy controls and the chronic spontaneous urticaria patients by the high-performance liquid chromatography tandem mass spectrometry method. The unit of the measured values is µg/g.
MnBP, mono-n-butyl phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate.
*P < 0.05 versus the negative controls.
DISCUSSION
It has recently been reported that phthalate exposure is a risk factor for allergic diseases such as asthma and atopic dermatitis.7,8 However, there have been few studies on the role of phthalates as potential risk factors for urticaria. The secretion of β-hexosaminidase and histamine was increased in human mast cells by the stimulation of DEHP in vitro.9 Yon et al.10 have demonstrated that urinary concentrations of phthalate metabolites correlate with the development of acute urticaria in children. Their study was the first to prove the role of phthalates as potential risk factors for adult chronic spontaneous urticaria.
In this study, we found that phthalates had different stimulatory effects in human mast cells. A short-branched phthalate, MnBP, as well as long-branched phthalates, MECPP and MEHHP, had similar stimulatory effects on IgE in mast cells. In particular, the highest secretion of β-hexosaminidase was observed after the lowest stimulation of MECPP. DEHP is a long-branched phthalate widely used as a plasticizer, and some of the soft plastic materials contain up to 40% DEHP. Because of the known toxicities of DEHP, the European Union has issued regulations on DEHP and set it at less than 0.1% of total product, but there are no standards for the concentration of DEHP in the human body.6 DEHP is a nonpersistent chemical that is rapidly metabolized and excreted in the urine. DEHP is rapidly hydrolyzed to mono (2-ethylhexyl) phthalate by unspecific lipases and then further metabolized to a wide range of secondary metabolites such as MECPP, MEHHP, and MEOHP. In a previous human study, the major metabolite of DEHP was MECPP, followed by MEHHP and MEOHP.11 In this study, we confirmed that DEHP metabolites were significantly higher in the urine of chronic spontaneous urticaria patients than of the healthy controls, which is evidence that DEHP exposure could be an aggravating factor for chronic spontaneous urticaria. To the best of our knowledge, this is the first study to demonstrate that DEHP acts as an exacerbating factor for chronic spontaneous urticaria. Further studies are needed to confirm the role of DEHP as an exacerbating factor for urticaria patients.
In conclusion, phthalates, including DEHP, may act as aggravating factors for chronic spontaneous urticaria and be used as potential therapeutic targets.
ACKNOWLEDGMENTS
The manuscript has been corrected for English grammar by Seoul Medical Paper Consulting.
This research was supported by the National Research Foundation of Korea (NRF) grant, funded by a grant from the Ministry and Health & Welfare, Republic of Korea (HI16C0992).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 2.Altrichter S, Fok JS, Jiao Q, Kolkhir P, Pyatilova P, Romero SM, et al. Total IgE as a marker for chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2021;13:206–218. doi: 10.4168/aair.2021.13.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novembre E, Cianferoni A, Mori F, Barni S, Calogero C, Bernardini R, et al. Urticaria and urticaria related skin condition/disease in children. Eur Ann Allergy Clin Immunol. 2008;40:5–13. [PubMed] [Google Scholar]
- 4.Wang W, Zhang W, Zhao J, Li H, Wu J, Deng F, et al. Short-term exposure to ambient air pollution and increased emergency room visits for skin diseases in Beijing, China. Toxics. 2021;9:108. doi: 10.3390/toxics9050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo KJL, Ho AFW, Gui H, Tay PJM, Lee HY, Koh MS, et al. Relationship between local weather, air pollution and hospital attendances for urticaria in children: time stratified analysis of 12,002 cases. Clin Exp Allergy. 2022;52:180–182. doi: 10.1111/cea.14015. [DOI] [PubMed] [Google Scholar]
- 6.Schierow LJ, Lee MM. Phthalates in plastics and possible human health effect. Washington, D.C.: Congressional Research Service; 2008. [Google Scholar]
- 7.Hoppin JA, Jaramillo R, London SJ, Bertelsen RJ, Salo PM, Sandler DP, et al. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005–2006. Environ Health Perspect. 2013;121:1129–1134. doi: 10.1289/ehp.1206211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EH, Jeon BH, Kim J, Kim YM, Han Y, Ahn K, et al. Exposure to phthalates and bisphenol A are associated with atopic dermatitis symptoms in children: a time-series analysis. Environ Health. 2017;16:24. doi: 10.1186/s12940-017-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Oh PS, Lim KT. Allergy-related cytokines (IL-4 and TNF-α) are induced by Di(2-ethylhexyl) phthalate and attenuated by plant-originated glycoprotein (75 kDa) in HMC-1 cells. Environ Toxicol. 2011;26:364–372. doi: 10.1002/tox.20563. [DOI] [PubMed] [Google Scholar]
- 10.Yon DK, Cho YS, Ha EK, Jee HM, Song JY, Jung YH, et al. Exposure to phthalates is associated with acute urticaria in children. Pediatr Allergy Immunol. 2018;29:657–660. doi: 10.1111/pai.12932. [DOI] [PubMed] [Google Scholar]
- 11.Silva MJ, Reidy JA, Preau JL, Jr, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006;11:1–13. doi: 10.1080/13547500500382868. [DOI] [PubMed] [Google Scholar]