Abstract
Introduction
Anakinra is being empirically considered for the treatment of COVID‐19 patients. The aim is to assess the efficacy of anakinra treatment on inflammatory marker reduction, including c‐reactive protein (CRP) concentrations, serum ferritin, and serum d‐dimer levels.
Methods
Adhering to PRISMA 2020 statement guidelines, a systematic search was conducted across the following databases from December 2019 until January 10, 2022: PubMed/MEDLINE, Cochrane Central, Web of Science, Scopus, and EMBASE. The following keywords were employed: Anakinra, COVID*, SARS‐CoV‐2, inflammatory, CRP, D‐dimer, Ferritin, hematological, laboratory, clinical, trials. The findings were collated and presented in a tabulated manner, and statistically analyzed using Review Manger 5.4 (Cochrane).
Results
In total, 2032 patients were included (881 in the anakinra and 1151 in the control/standard care group); 69.1% of them were males. Overall, the mean difference from admission until last follow‐up in CRP values was −9.66, where notable reductions were seen in the anakinra group (SMD = −0.46, p < 0.00001, N = 655). Serum ferritin mean values were reduced by 1467.16 in the anakinra group (SMD = −0.31, p = 0.004, N = 537). D‐dimer mean values were largely reduced by 4.04 in the anakinra group (SMD = −0.38, p = 0.0004, N = 375).
Conclusion
This study finds that anakinra is potentially a strong candidate as an anti‐inflammatory agent to reduce mortality in COVID‐19 patients, specifically in patients with elevated inflammatory biomarkers.
Keywords: acute‐phase reactant, anakinra, c‐reactive protein, d‐dimer, interleukin‐1, serum ferritin
Anakinra is a drug being considered for treating COVID‐19 patients. In this paper, we collate novel and pertinent findings of the mean difference in CRP values (−9.66), serum ferritin (−1467 17), and D‐dimer values (−4.04). Anakinra serves as a strong candidate particularly for its anti‐inflammatory properties in reducing mortality among COVID‐19 patients and curbing elevated inflammatory biomarkers.

1. INTRODUCTION
Inflammation is a key driver of the severity of COVID‐19 infection among patients. 1 Preliminary reports since the initial stages of the pandemic have demonstrated that elevated concentrations of pro‐inflammatory markers are more pronounced in severe or critically ill COVID‐19 patients than milder cases. 2 Anakinra is a recombinant interleukin‐1 receptor antagonist (IL‐1Ra), a regulatory molecule with activity against both IL‐1α and IL‐1β, that emerged as a candidate to reduce hyper‐inflammation and concomitant mortality in COVID‐19 patients. 3 The pro‐inflammatory cascade is similar to the macrophage activation syndrome (MAS), and COVID‐19 hyper‐inflammation may benefit from cytokine‐blocking agents such as Anakinra. 4 , 5 The severe hyper‐inflammation in a subset of COVID‐19 patients resembles MAS and is associated with hyper‐ferritinemia, fever, and diffuse intravascular coagulation (DIC). 4 , 6 Anakinra has been used empirically across several clinical settings in COVID‐19 and has had positive effects, including mortality reduction. 7 , 8 , 9 As the potential effect of Anakinra is due to inhibition of the pro‐inflammatory cascades, it is likely to lead to suppression of systematic inflammation, control of fever, and inhibition of host inflammatory responses to viral replication in COVID‐19. 7 , 10 Although systematic inflammation may not be significant in all patients, there is evidence of localized hyper‐inflammation in the lungs, making Anakinra an attractive candidate for moderate‐to‐severe COVID‐19 infections as concomitant therapy. 7 , 11 , 12 , 13
So far, Anakinra has been administered as an off‐label medication for patients with clinical or laboratory signs of hyper‐inflammation in COVID‐19 patients to mitigate IL‐1‐mediated pro‐inflammatory cascades. 14 The severe hyper‐inflammation in these patients is characterized by laboratory markers including c‐reactive protein (CRP), serum ferritin, and d‐dimer levels. 15 It remains unclear whether Anakinra confers an additional advantage to COVID‐19 patients to mitigate hyper‐inflammation beyond the current standard of care. Reports of COVID‐19 increasingly recognize two types of distinct, yet overlapping phenotypic and pathological subsets of pneumonia—these include viral pneumonitis and virus‐triggered overreacting immune response. 16 , 17 The later phenotype is a more severe form of disease and is typified by rapid progression to acute respiratory failure, often necessitating invasive ventilatory support. With severe COVID‐19 pneumonia on invasive ventilatory support, excessive mortality is documented. 16 , 17 The excessively high rates of death are attributed to severe hyper‐inflammation. 4 , 8 , 16 The aim of this meta‐analysis is to document whether anakinra in patients with COVID‐19 pneumonia is beneficial for improvement of inflammatory biomarkers (i.e., CRP, serum ferritin, and d‐dimer).
2. MATERIALS AND METHODS
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement 2020, randomized controlled trials and observational studies employing an intervention group (anakinra) compared to the control/standard of care (SoC) group were included. The studies reported inflammatory biomarkers at baseline (on admission or enrollment) and on endline (completion of study or last follow‐up). These laboratory‐based outcomes were reported in anakinra and control/SoC groups. The data were systematically reviewed and meta‐analyzed to quantify the standardized changes of inflammatory laboratory markers (CRP, serum ferritin, and d‐dimer).
Randomized clinical trials, retrospective or prospective cohorts, were considered. Case series, case reports, systematic reviews, meta‐analyses, and letters were omitted due to the high risk of biases associated with the study types and lack of control groups for comparison purposes. The studies were required to include adult participants, aged 18 or above, with no restrictions to genders of enrolled individuals. The follow‐up period was not predetermined due to the limitations of existent data. Instead, the maximum follow‐up of the reported outcomes was enlisted as the endline laboratory value. The target condition was COVID‐19, and all the studies were in‐hospital based with patients of any severity of disease (mild, moderate, or severe) as per the NIH classification. 18
Databases including PubMed/MEDLINE, Cochrane Central, Web of Science, Scopus, and EMBASE were systematically searched from December 2019 until January 10, 2022. Other sources were manually searched including Science Direct, SAGE, Elsevier, and Google Scholar to ensure that no studies were omitted. The search terms across the databases and additional sources were a combination of the following: Anakinra, COVID*, SARS‐CoV‐2, inflammatory, CRP, D‐dimer, Ferritin, hematological, laboratory, clinical, trials. No language restrictions were applied, and in case a non‐English study was identified, google translate was to be used.
The titles and abstracts of all screened studies from the databases and additional sources were screened together by two authors (AS, ZS). On shortlisting studies, all authors conducted a full‐text review of the studies. All disagreements were actively resolved through consensus and with the final reviewer (ZS). The Cohen's Coefficient of Agreement was calculated to compute the inter‐reviewer agreement. The search process with PRISMA flowchart is depicted in Figure 1.
FIGURE 1.

PRISMA flowchart
All the identified studies were de‐duplicated by entering bibliographic details into EndNote X9 (Clarivate Analytics). The methodology was both quantitative and analytical where the mean difference (MD) on admission/enrollment and on last follow‐up was analyzed. The mean difference is a common meta‐analytical measure to note the difference in means from the baseline to endline, which may be both negative and positive. These laboratory values were continuous in nature and were aligned to a single scale across CRP, serum ferritin, and d‐dimer. The standardized mean difference (SMD), which is an effect measure, was computed applying 95% confidence intervals (CI). A fixed‐effects model was applied for the laboratory outcomes since they were converted to a single unit of measure, as recommended by Cochrane's handbook. The additional outcome for mortality was conducted using a random‐effects model, with outcomes reported as Risk Ratio (RR) using 95% CI. Forest plots were generated for all the outcomes that represented the effect size (SMD and RR), heterogeneity, and overall test results. Funnel plots accompanied the forest plots for all outcomes. At least 2 or more studies were required to report the same outcome measure to be meta‐analyzed. Heterogeneity was testing using the χ²‐based Q test and the I 2 index. All statistical tests were conducted using Review Manger 5.4 (Cochrane). The protocol is attached in the supplementary materials.
3. RESULTS
The overall Kappa score computed for the inter‐reviewer agreement was 0.91, suggesting good agreement. The characteristics of included studies are enlisted in Table 1. In total, 2032 patients were included, of which 881 were intervened with anakinra, whereas 1151 were treated with standard care of were controls. A total of 12 observational studies (N = 1444) and 4 randomized controlled trials (N = 588) were meta‐analyzed. The countries represented in this analysis include Belgium (1), France (3), Greece (2), Iran (1), Italy (4), the Netherlands (1), Oman (1), and Spain (3). Anakinra dosage and route of administration parameters are described in Table 1.
TABLE 1.
Characteristics of included studies
| ID | Author, year | Study type | Country | Sample size (Anakinra vs. Control/SoC) | Gender (Male) (Anakinra vs. Control/SoC) | Anakinra dosage and route of administration |
|---|---|---|---|---|---|---|
| 1 | Cauchois et al., 2020 46 | Observational | France | 22 (12 vs. 10) | 6 (50%) vs. 6 (60%) | Infused IV over 2 h as a single daily dose of 300 mg for 5 days, then tapered to 200 mg for two days, and then 100 mg for 1 day |
| 2 | Huet et al., 2020 32 | Observational | France | 96 (52 vs. 44) | 36 (69%) 25 (57%) | Subcutaneously at a dose of 100 mg twice daily for 72 h, followed by 100 mg daily for 7 days |
| 3 | Cavalli et al., 2020 33 | Observational | Italy | 52 (36 vs. 16) | 29 (80.5%) vs. 14 (88%) | Low‐dose anakinra was administered subcutaneously at a dose of 100 mg twice daily; high‐dose anakinra was administered IV at 10 mg/kg per day (5 mg/kg twice daily, infused over 1 hour) |
| 4 | Bozzi et al., 2021 47 | Observational | Italy | 120 (65 vs. 55) | 52 (80%) vs. 44 (80%) | Anakinra was administered subcutaneously at 200 mg every 8 h for 3 days, then 100 mg every 8 hours up to day 14; IV approach was used if the patient was on invasive mechanical ventilation |
| 5 | Kooistra et al., 2020 14 | Observational | The Netherlands | 60 (21 vs. 39) | 14 (67%) vs. 33 (85%) | 300 mg anakinra IV followed by 100 mg IV every six hours |
| 6 | Balkhair et al., 2021 48 | Observational | Oman | 69 (45 vs. 24) | 35 (78%) vs 17 (71%) | 100 mg twice daily for 3 days, followed by 100 mg daily for a maximum of 7 days, subcutaneous |
| 7 | CORIMUNO−19, 2021 49 | Randomized controlled trial | France | 114 (59 vs. 55) | 43 (73%) vs. 37 (67%) | 200 mg IV twice a day (total 400 mg) on days 1–3, then at 100 mg IV twice a day (total 200 mg) on day 4, and 100 mg IV once on day 5; 3 supplementary treatment at 400 mg IV per day on days 4–6, followed by 200 mg IV per day on day 7 and 100 mg IV per day on day 8 |
| 8 | Kyriazopoulou et al., 2021 50 | Observational | Greece | 260 (130 vs. 130) | 81 (62.3%) vs. 84 (64.6%) | Subcutaneous anakinra 100 mg once daily for 10 days |
| 9 | Pontali et al., 2021 51 | Observational | Italy | 128 (63 vs. 65) | 42 (66.7%) vs. 45 (69.2%) | Anakinra 100 mg IV every 8 h for 3 days, followed by tapering (100 mg every 12 h for 1–3 days, followed by 100 mg every 24 h for 1–3 days) |
| 10 | Aomar‐Millán et al., 2021 52 | Observational | Spain | 143 (10 vs. 133) | 10 (100%) vs. 79 (59.4%) | Patients weighing 50–60 kg received 100 mg/12 h, patients weighing 60–75 kg received 100 mg/8 h, and patients weighing >75 kg received 100 mg/6 h. On the second day, all patients received 100 mg/12 h from day 2 to day 6; subcutaneous |
| 11 | Kharazmi et al., 2021 53 | Randomized controlled trial | Iran | 30 (15 vs. 15) | 8 (53.3%) vs. 11 (73.3%) | Anakinra 100 mg IV once daily |
| 12 | Garcia et al., 2021 54 | Observational | Spain | 342 (125 vs. 217) | 70 (56%) vs. 127 (58%) | Anakinra administrated subcutaneously at a standard dose of 200 mg twice on day 1, followed by 100 mg twice daily until a course of 10 days was completed |
| 13 | Franzetti et al., 2021 55 | Observational | Italy | 112 (56 vs. 56) | 41 (73.2%) vs. 46 (82.1%) | Subcutaneously for 7 days at 100 mg, four times a day in a regular ward or 200 mg three times daily IV if in intensive care |
| 14 | Calle et al., 2021 56 | Observational | Spain | 40 (20 vs. 20) | 14 (70%) vs 12 (60%) | Subcutaneously at 100 mg/12 h on day 0, then at 100 mg/24 h from day 1 to day 5; re‐evaluated on day 6 based on clinical progress |
| 15 | Karakike et al., 2021 57 | Randomized controlled trial | Greece | 102 (60 vs. 42) | 45 (75%) vs. 34 (81%) | Anakinra was administered at 200 mg IV every 8 hours for 7 days |
| 16 | Declercq et al., 2021 58 | Randomized controlled trial | Belgium | 342 (112 vs. 230) | 87 (78%) vs. 178 (77%) | 100 mg once daily subcutaneously for 28 days or until hospital discharge on top of standard of care |
The laboratory values for d‐dimer (mg/L), CRP (mg/L), and ferritin (ng/ml) are reported in Table S1. The values were reported at baseline, that is, on admission and on the last day of follow‐up or peak where applicable, were presented as mean (SD) unless stated otherwise (Table S1).
Of 2032 patients included in this study, a total of 613 (69.6%) were males in the anakinra group (N = 881), and 792 (68.8%) were males in the control/SoC group (N = 1151). The MD for CRP was computed for all included studies based on the differences in admission (baseline) and endline. The overall MD was −9.66, 95% −15.47, −3.84 (p = 0.001), suggesting that the anakinra group had notable reductions in CRP laboratory values compared to control/SoC. CRP values were reported among 286 patients in the anakinra group and 369 patients in the control/SOC group. The SMD was yielded as follows: −0.46, 95% CI = −0.63, −0.28 (p < 0.001) (Figure 2).
FIGURE 2.

Forest plot and funnel plot of CRP (mg/L) (mean difference) in anakinra versus control/SoC groups. Heterogeneity: Chi² = 76.15, df = 8 (p < 0.00001); I² = 89%. Test for overall effect: Z = 5.10 (p < 0.00001)
Ferritin values were reported for 222 and 315 patients in the anakinra and control/SoC groups. The overall MD was calculated as −1467.16, 95% CI = −1583.81, −1350.5 in favor of anakinra (p < 0.001). The SMD was yielded as follows: −0.31, 95% CI = −0.52, −0.1 (p = 0.004) (Figure 3).
FIGURE 3.
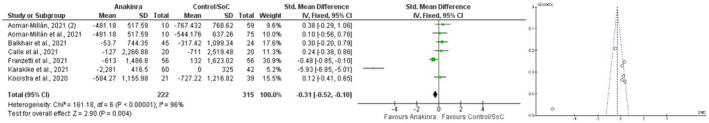
Forest plot and funnel plot of ferritin (ng/ml) (mean difference) in anakinra versus control/SoC groups. Heterogeneity: Chi² = 161.18, df = 6 (p < 0.00001); I² = 96%. Test for overall effect: Z = 2.90 (p = 0.004)
D‐dimer laboratory values were reported at endline and baseline, both in a total of 375 patients (141 in the anakinra group and 234 in the anakinra/SoC group). The MD was computed as −4.04, 95% CI = −4.43, −3.64, favoring the anakinra group (p < 0.001). The SMD was yielded as follows: −0.38, 95% CI = −0.63, −0.12 (p = 0.004) (Figure 4).
FIGURE 4.

Forest plot and funnel plot of D‐dimer (mg/L) (mean difference) in anakinra versus control/SoC groups. Heterogeneity: Chi² = 94.71, df = 4 (p < 0.00001); I² = 96%. Test for overall effect: Z = 2.88 (p = 0.004)
The death (mortality) trends were reported as a common endpoint across all studies with a patient population of 917 participants in the anakinra group and 1390 in the control/SoC group. The results were yielded as follows: RR = 0.64, 95% CI = 0.49–0.83 (p < 0.001). The results indicate that the risk of mortality was reduced in the anakinra group by 36% (I 2 = 48%) (Figure 5).
FIGURE 5.

Forest plot for mortality (endpoint) in anakinra versus control/SoC groups. Heterogeneity: Tau² = 0.13; Chi² = 31.05, df = 16 (p = 0.01); I² = 48%. Test for overall effect: Z = 3.38 (p = 0.0007)
To note any sources of bias, a funnel plot was generated and studied. While we expected for there to be large sources of heterogeneity due to the differing nature of study types included, the funnel plot shows an otherwise inverted funnel shape with only 3 studies deviating from the total of 16. It may be stated that minimal‐moderate sources of bias were present in this meta‐analysis (Figure 6).
FIGURE 6.

Funnel plot to assess for publication bias
4. DISCUSSION
Our results indicate that, in patients admitted with COVID‐19, anakinra reduces hyper‐inflammatory markers compared to the standard of care. This effect is significant for CRP concentrations, serum ferritin, and d‐dimer levels. Of these biomarkers, CRP is the most commonly tested to document the extent of inflammation in COVID‐19 patients. Our results demonstrate a profound reduction in the hyper‐inflammatory phenotype of COVID‐19, known as cytokine storm syndrome (CSS), which is associated with a high mortality rate. The underlying etiologies that are currently undergoing investigation include systemic inflammation, hyper‐ferritinemia, hemodynamic instability, and multiorgan failure, for which we have evaluated the most important biomarkers.
CRP is clinically used as a plasma inflammatory signature, and it serves as an important biomarker to document pathological inflammatory responses. 19 CRP is the prototype of acute‐phase reactants (APPs) produced primarily by hepatocytes in response to various inflammatory cytokines, including IL‐1β and IL‐6. 20 Up to 86% of severe COVID‐19 patients have elevated CRP, which is considered a relevant downstream biomarker. 21 The clinical utility of CRP as a prognostic test for COVID‐19 severity has previously been established, and it remains broadly relevant to correlate with severity and treatment responses. 22 The IL‐1 signaling pathway is a major target for immune modulation and serves as the apical pro‐inflammatory mediator, especially in innate immunity. 23 Anakinra is a short‐acting IL‐1Ra leading to dual IL‐1α and IL‐1β inhibition, which is associated with lower CRP concentration. 24 IL‐β is the primary form of circulating IL‐1, whereas IL‐1α remains largely membrane‐bound. 25 IL‐1α and IL‐1β bind to the universally expressed cell surface receptor, IL‐1R, to activate the cascade of IL‐1‐mediated inflammatory responses downstream. 23 Anakinra antagonizes the IL‐1R to control the activity of the NLRP3 inflammasome through caspase‐1‐mediated activation of IL‐1β and production of a variety of innate inflammatory responses downstream, including IL‐6, which is closely linked to the severity of COVID‐19 infection. 26 , 27 Overall, anakinra is mechanistically and clinically emerging as a strong candidate to target the pro‐inflammatory syndrome observed in COVID‐19 patients with a significant reduction in CRP levels reported in our findings as well as other studies. 28
Anakinra has previously shown efficacy in patients with sepsis and features of MAS. 29 MAS is associated with an excessive inflammatory response, specifically high ferritin concentrations. 30 The cutoff established for ferritin levels in MAS is higher than 2000 ng/ml. 31 While the hyper‐inflammation in COVID‐19 and MAS cannot be equated, ferritin levels are being assessed as a biomarker to detect clinical improvement in COVID‐19 patients following high‐dose anakinra treatment across clinical trials. 10 , 32 , 33 Ferritin is an APP that seems to augment immediately in COVID‐19 patients. 34 The H‐chain of ferritin‐activating macrophages and increased iron metabolism required for controlling viral infections is a potential mechanism for increased pro‐inflammatory cytokines in COVID‐19. 35 Also, ferritin may be related to inflammatory parameters and suggestive of cellular damage when its value is over 600 ng/ml. 36 The high ferritin levels observed in COVID‐19 patients also result in ferroptosis, one of the underlying mechanisms in acute respiratory distress syndrome (ARDS), similar to COVID‐19 pneumonia. 37 Iron parameters including ferritin have also been correlated with improvement in the sequential organ failure assessment (SOFA) score, which provides further rationale for the utility of Anakinra in COVID‐19 patients. 38 While the role of ferritin as an inflammatory biomarker in COVID‐19 remains unclear, Anakinra has a potential role in the hyper‐ferritinemia associated with COVID‐19 progression and severity. 39 We demonstrate the reduction in serum ferritin levels with anakinra use which is encouraging.
D‐dimer is a fibrin degradation product and a well‐recognized biomarker for thrombotic disorders. 40 Its utility as a prognostic marker has already been established in community‐acquired pneumonia and more recently in COVID‐19 patients. 41 , 42 While there is variation in the cutoff for morbidity and mortality prediction, a value less than 0.5 ug/ml is widely considered normal. 43 Anakinra treatment has been shown to reduce d‐dimer levels in COVID‐19 patients in clinical trials, suggesting a positive effect on underlying cardiovascular and thrombotic pathologies. 44 , 45 Our results similarly demonstrate beneficial outcomes with anakinra treatment and d‐dimer reduction in COVID‐19 patients.
On noting the mortality trends and outcomes across all studies, we find favorable results among patients that have elevated inflammatory markers with markedly low risk ratio (RR = 0.64). The analysis highlights the requirement for antithrombotic therapy in patients with COVID‐19 that are hospitalized. In hospitalized patients with COVID‐19, coagulation and hematologic parameters are typically measured. Patients who are receiving anticoagulant therapy for underlying chronic conditions ought to continue medications once diagnosed with COVID‐19. However, current guidelines for nonhospitalized patients with COVID‐19 state that anticoagulant therapy ought not to be initiated for the prevention of VTE or arterial thrombosis unless the patient has other indications for therapy. On the whole, our laboratory analysis and mortality outcomes suggest that there is a requirement for better antithrombotic management and guidelines for hospitalized patients because thrombosis may be more driven with inflammation at this stage.
To the best of our knowledge, this is the first patient‐level meta‐analysis that evaluates the effect of anakinra treatment in COVID‐19 patients and relevant hyper‐inflammatory biomarkers to advance the current understanding of this treatment. Our findings suggest a positive impact on the hyper‐inflammatory biomarkers noted in COVID‐19 patients. However, anakinra is an immunosuppressive drug that may theoretically cause more harm than benefit when targeting “beneficial” inflammation. 28 Anakinra is noted to have positive effects on hyper‐inflammation, which needs to be evaluated further. The current criteria for anakinra administration in clinical trials consider CRP, serum ferritin, and d‐dimer levels; there is no consensus on the standard cutoff in COVID‐19 patients. Another important consideration is the dose and route of administration of anakinra due to its short half‐life. This study's main limitation is the observational design of many studies included in the meta‐analysis. These studies may be biased and have possible confounders such as concomitant use or lack of dexamethasone as the standard of care. Anakinra, regardless, remains a strong potential candidate, as demonstrated by our findings, and it is recommended to conduct randomized clinical trials with caution. These trials may focus on homogenous eligibility criteria and dosing regimens to provide meaningful insight into the efficacy of anakinra in COVID‐19 patients with hyper‐inflammation.
5. CONCLUSION
This meta‐analysis reports the favorable effects of anakinra treatment in patients with COVID‐19 in reducing hyper‐inflammatory markers characteristic of the severity of the disease, specifically CRP concentrations, serum ferritin, and d‐dimer levels. Large randomized control trials are required to confirm the benefits of anakinra treatment in COVID‐19 patients. As the COVID‐19 pandemic is challenged with new variants, it is of utmost importance to consider the evaluation of anakinra in the anti‐COVID‐19 armamentarium.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Material
Table S1
Naveed Z, Sarwar M, Ali Z, et al. Anakinra treatment efficacy in reduction of inflammatory biomarkers in COVID‐19 patients: A meta‐analysis. J Clin Lab Anal. 2022;36:e24434. doi: 10.1002/jcla.24434
Contributor Information
Zouina Sarfraz, Email: zouinasarfraz@gmail.com.
Ivan Cherrez‐Ojeda, Email: Ivancherrez@gmail.com.
DATA AVAILABILITY STATEMENT
All data obtained for the purpose of this meta‐analysis are freely available online.
REFERENCES
- 1. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID‐19 patients. Int J Hematol. 2020;112(4):553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iglesias‐Julián E, López‐Veloso M, de‐la‐Torre‐Ferrera N, et al. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID‐19 patients. J Autoimmun. 2020;115:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine‐directed therapies. Annu Rev Med. 2015;66:145‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasin L, Cavalli G, Navalesi P, et al. Anakinra for patients with COVID‐19: a meta‐analysis of non‐randomized cohort studies. Eur J Intern Med. 2021;86:34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khamees A, Bani‐Issa J, Al Zoubi MS, et al. SARS‐CoV‐2 and coronavirus disease mitigation: treatment options, vaccinations and variants. Pathogens. 2022;11(2):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NICE . COVID‐19 rapid guideline: Managing COVID‐19. 2022. https://www.nice.org.uk/guidance/ng191/resources/covid19‐rapid‐guideline‐managing‐covid19‐pdf‐51035553326. Accessed April 3, 2022 [PubMed]
- 10. Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe Covid‐19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28(1):117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jouan Y, Baranek T, Si‐Tahar M, Paget C, Guillon A. Lung compartmentalization of inflammatory biomarkers in COVID‐19‐related ARDS. Crit Care. 2021;25(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bendib I, Beldi‐Ferchiou A, Schlemmer F, et al. Alveolar compartmentalization of inflammatory and immune cell biomarkers in pneumonia‐related ARDS. Crit Care. 2021;25(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarfraz A, Sarfraz Z, Sarfraz M, Aftab H, Pervaiz Z. Tocilizumab and COVID‐19: a meta‐analysis of 2120 patients with severe disease and implications for clinical trial methodologies. Turkish J Med Sci. 2021;51(3):890. doi: 10.3906/SAG-2010-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kooistra EJ, Waalders NJB, Grondman I, et al. Anakinra treatment in critically ill COVID‐19 patients: a prospective cohort study. Crit Care. 2020;24(1):1‐12. doi: 10.1186/s13054-020-03364-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melo AKG, Milby KM, Caparroz ALMA, et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID‐19 cases: A living systematic review and meta‐analysis. PLoS One. 2021;16(6):e0253894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juanes‐Velasco P, Landeira Viñuela A, García‐Vaquero L, et al. SARS‐CoV‐2 infection triggers auto‐immune response in ARDS. Front Immunol. 2022;13:732197. doi: 10.3389/fimmu.2022.732197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Hear Lung Transplant. 2020;39(5):405‐407. doi: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NIH . Clinical Spectrum of SARS‐CoV‐2 Infection. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/ Accessed April 3, 2022.
- 19. Sproston NR, Ashworth JJ. Role of C‐reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL‐1β/IL‐6/CRP and IL‐18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12(12):e1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali N. Elevated level of C‐reactive protein may be an early marker to predict risk for severity of COVID‐19. J Med Virol. 2020;92(11):2409‐2411. doi: 10.1002/jmv.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharifpour M, Rangaraju S, Liu M, et al. C‐Reactive protein as a prognostic indicator in hospitalized patients with COVID‐19. PLoS One. 2020;15(11):e0242400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinarello CA. Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridker PM. From C‐reactive protein to interleukin‐6 to interleukin‐1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanakaraj P, Schafer PH, Cavender DE, et al. Interleukin (IL)‐1 receptor–associated kinase (IRAK) requirement for optimal induction of multiple IL‐1 signaling pathways and IL‐6 production. J Exp Med. 1998;187(12):2073‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis. J Med Virol. 2020;92(11):2283‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. King A, Vail A, O’Leary C, et al. Anakinra in COVID‐19: important considerations for clinical trials. Lancet Rheumatol. 2020;2(7):e379‐e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin‐1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial∗. Crit Care Med. 2016;44(2):275‐281. doi: 10.1097/CCM.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol. 2020;2(7):e437‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cavalli G, Dagna L. Effect of anakinra in COVID‐19–Authors’ reply. Lancet Rheumatol. 2020;2(9):e524‐e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2(7):e393‐e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325‐e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kappert K, Jahić A, Tauber R. Assessment of serum ferritin as a biomarker in COVID‐19: bystander or participant? Insights by comparison with other infectious and non‐infectious diseases. Biomarkers. 2020;25(8):616‐625. [DOI] [PubMed] [Google Scholar]
- 35. Bozkurt FT, Tercan M, Patmano G, Tanrıverdi TB, Demir HA, Yurekli UF. Can ferritin levels predict the severity of illness in patients with COVID‐19? Cureus. 2021;13(1):e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748‐773. [DOI] [PubMed] [Google Scholar]
- 37. Banchini F, Cattaneo GM, Capelli P. Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID‐19 and emergency surgical non‐COVID‐19 patients. World J Emerg Surg. 2021;16(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brandtner A, Tymoszuk P, Nairz M, et al. Linkage of alterations in systemic iron homeostasis to patients’ outcome in sepsis: a prospective study. J Intensive Care. 2020;8(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID‐19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double‐blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adam SS, Key NS, Greenberg CS. D‐dimer antigen: current concepts and future prospects. Blood J Am Soc Hematol. 2009;113(13):2878‐2887. [DOI] [PubMed] [Google Scholar]
- 41. Arslan S, Ugurlu S, Bulut G, Akkurt I. The association between plasma D‐dimer levels and community‐acquired pneumonia. Clinics. 2010;65:593‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chalmers JD, Singanayagam A, Scally C, Hill AT. Admission D‐dimer can identify low‐risk patients with community‐acquired pneumonia. Ann Emerg Med. 2009;53(5):633‐638. [DOI] [PubMed] [Google Scholar]
- 43. Poudel A, Poudel Y, Adhikari A, et al. D‐dimer as a biomarker for assessment of COVID‐19 prognosis: D‐dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID‐19. PLoS One. 2021;16(8):e0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Otsuka R, Seino K. Macrophage activation syndrome and COVID‐19. Inflamm Regen. 2020;40(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ombrello MJ, Schulert GS. COVID‐19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Trans Res. 2021;232:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cauchois R, Koubi M, Delarbre D, et al. Early IL‐1 receptor blockade in severe inflammatory respiratory failure complicating COVID‐19. Proc Natl Acad Sci. 2020;117(32):18951‐18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bozzi G, Mangioni D, Minoia F, et al. Anakinra combined with methylprednisolone in patients with severe COVID‐19 pneumonia and hyperinflammation: An observational cohort study. J Allergy Clin Immunol. 2021;147(2):561‐566.e4. doi: 10.1016/J.JACI.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Balkhair A, Al‐Zakwani I, Al Busaidi M, et al. Anakinra in hospitalized patients with severe COVID‐19 pneumonia requiring oxygen therapy: results of a prospective, open‐label, interventional study. Int J Infect Dis. 2021;103:288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tharaux P‐L, Pialoux G, Pavot A, et al. Effect of anakinra versus usual care in adults in hospital with COVID‐19 and mild‐to‐moderate pneumonia (CORIMUNO‐ANA‐1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID‐19. Elife. 2021;10:e66125. doi: 10.7554/ELIFE.66125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pontali E, Volpi S, Signori A, et al. Efficacy of early anti‐inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID‐19 pneumonia. J Allergy Clin Immunol. 2021;147(4):1217‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aomar‐Millán IF, Salvatierra J, Torres‐Parejo Ú, et al. Anakinra after treatment with corticosteroids alone or with tocilizumab in patients with severe COVID‐19 pneumonia and moderate hyperinflammation. A retrospective cohort study. Intern Emerg Med. 2021;16(4):843‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kharazmi AB, Moradi O, Haghighi M, et al. A randomized controlled clinical trial on efficacy and safety of anakinra in patients with severe COVID‐19. Imm Inflam Dis. 2022;10(2):201‐208. doi: 10.1002/iid3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García‐García JA, Pérez‐Quintana M, Ramos‐Giráldez C, et al. Anakinra versus Baricitinib: different strategies for patients hospitalized with COVID‐19. J Clin Med. 2021;10(17):4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Franzetti M, Forastieri A, Borsa N, et al. IL‐1 receptor antagonist anakinra in the treatment of COVID‐19 acute respiratory distress syndrome: a retrospective. Observational Study. J Immunol. 2021;206(7):1569‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de la Calle C, López‐Medrano F, Pablos JL, et al. Effectiveness of anakinra for tocilizumab‐refractory severe COVID‐19: A single‐centre retrospective comparative study. Int J Infect Dis. 2021;105:319‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karakike E, Dalekos GN, Koutsodimitropoulos I, et al. ESCAPE: an open‐label trial of personalized immunotherapy in critically Ill COVID‐19 patients. MedRxiv. Published online 2021. [DOI] [PMC free article] [PubMed]
- 58. Declercq J, Van Damme KFA, De Leeuw E, et al. Effect of anti‐interleukin drugs in patients with COVID‐19 and signs of cytokine release syndrome (COV‐AID): a factorial, randomised, controlled trial. Lancet Respir Med. 2021;9(12):1427‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1
Data Availability Statement
All data obtained for the purpose of this meta‐analysis are freely available online.


