Abstract
A simple and reliable method that could be used in developing countries to pasteurize milk and water with solar energy is described. A cardboard reflector directs sunshine onto a black jar, heating water to pasteurizing temperatures in several hours. A reusable water pasteurization indicator verifies that pasteurization temperatures have been reached.
Exposing water in clear plastic or glass jars to sunshine has been shown to inactivate bacteria. However, much variability–from no inactivation to an approximately 3-log decrease in 1.5 h–has been reported (1, 2, 10–12, 14, 15, 17, 18, 20). Reasons for this variability include the transparency of the container, water turbidity, water temperature reached, altitude, aerobic or anaerobic conditions, and the amount of solar radiation received (2, 10–12, 15, 17). In addition, only a few studies have included viruses or protozoan cysts, which might not be as sensitive to sunshine as bacteria.
A major limitation of exposing clear containers of water to sunshine is that there is no simple test to perform which would ensure that contaminated water has been pasteurized by direct sunshine. Another limitation of this procedure is its restriction to clear liquids; exposure to sunshine cannot pasteurize raw milk, another source of disease in developing countries.
The objective of this study was to develop a simple and reliable method to pasteurize milk and water with solar energy which could be used primarily in developing countries but also in emergency situations elsewhere.
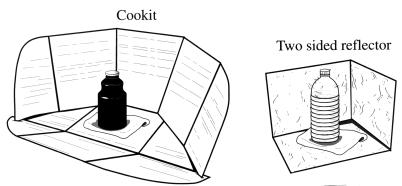
We previously reported that water heated to 65°C in a solar box cooker would pasteurize water and make it safe to drink (5). However, solar box cookers were designed for cooking and are awkward to use for water pasteurization. In 1995, a simple reflective solar cooker, the Cookit, was developed by Solar Cookers International (SCI; Sacramento, Calif.) and subsequently introduced as a cooking device to three refugee camps in Kenya and Ethiopia (Fig. 1). For cooking, the foiled reflective panels of the Cookit direct sunshine onto a dark pot which is enclosed in a clear polypropylene bag. The dark pot converts sunshine into heat, which is trapped within the bag and cooks the food. We discovered that when using the Cookit on sunny days, a plastic bag was not needed to enclose a black jar for heating water to pasteurization temperatures.
FIG. 1.
Solar reflectors used in this study.
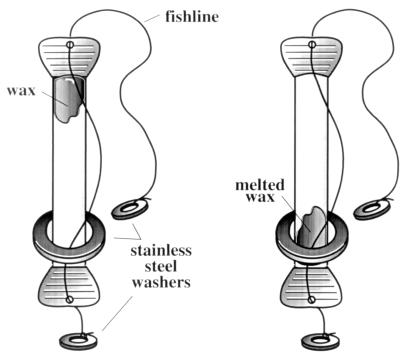
In order to verify that sufficient water temperatures (at least 65°C) were obtained, we included a reusable water pasteurization indicator (WAPI) which was developed for SCI (Fig. 2). The WAPI is a clear polycarbonate tube partially filled with a soybean wax which melts at about 70°C. The WAPI tube is placed at the bottom of a black jar of water which is solar heated. If the WAPI wax melts and falls to the bottom of the tube, it indicates that pasteurization conditions have been reached.
FIG. 2.
Water pasteurization indicator. Wax at the top of the tube before heating (left) falls to the bottom when water temperatures reach 70°C (right).
The bacteriophage T2 was included in these studies. Although T2 is not a human pathogen, its inclusion tests the validity of extrapolating to nonbacterial microbes Escherichia coli’s response.
Cultures and test conditions.
E. coli ATCC 11775 (American Type Culture Collection, Rockville, Md.) was maintained in brain heart infusion broth (BHI; Acumedia, Baltimore, Md.). A stock of T2 phage with 4 × 109 PFU/ml was obtained by seeding 100 ml of BHI broth with E. coli B and a drop of T2 and incubating at 35°C for 12 h when complete lysis was observed.
Two reflectors were used (Fig. 1). The smaller, two-sided reflector was made from a cardboard box and foiled on the inside and was 25 cm high with sides 25 and 35 cm wide. This reflector was included in the study to compare the effect of enhanced solar reflection on clear containers with that on unreflected clear bottles, the type used in previous studies. The Cookit was purchased from SCI. It has aluminum foil laminated onto cardboard which is folded at three locations to create the vertical reflective section (121 by 34 cm), the middle section, which holds the black jar (84 by 28 cm), and the front section, which is adjusted to reflect the maximum amount of sunshine onto the jar (86 by 28 cm). The edges of the vertical piece fit through slits on each side of the front piece and are held in place by clothespins clamped to the edge under the front flap. Bricks on the back of the flat middle section anchor the Cookit against strong winds.
To simulate heavily contaminated water, an 18-h E. coli culture in BHI broth and the T2 stock were diluted and added to autoclaved American River water to obtain about 104 E. coli/ml and 104 T2/0.1 ml. The inoculated river water was mixed in a large container, and 20 ml was removed for use as an inside control. The remaining water was poured into 1-liter polyethylene terephthalate clear plastic bottles and 1.4-liter glass jars which had been spray painted black. The inoculated containers were placed in full sun for 3 h (usually from 11 a.m. to 2 p.m. Pacific Daylight Time). The reflectors were repositioned approximately hourly during the experiments to maximize incident sunshine.
Water temperatures were monitored with a Trendicator 400 thermometer (Doric Scientific, San Diego, Calif.) with thermocouple probes. To keep water in clear bottles at temperatures not lethal to E. coli and T2, when these bottles reached 45 to 50°C, they were swirled in ice water for a few minutes to cool the water to about 35°C.
At sampling times, 5 ml of water was withdrawn from the bottom of the container, placed into 5-in. sterile tubes, and taken to the laboratory for plating. For E. coli counts, 1 ml of undiluted or diluted (in saline) sample was added to a sterile petri dish, to which melted and cooled BHI agar was added, mixed, and allowed to solidify. To enumerate T2 bacteriophage, 3 ml of melted and cooled (to 45°C) BHI soft agar (0.75% agar), 2 drops of a 24-h culture of E. coli B, and 0.1 ml of the undiluted or diluted water sample were added, mixed, and poured over a BHI agar plate. All plates were incubated for 24 h at 35°C. Average counts were obtained from duplicate or triplicate plates. Incubation for an additional 1 to 3 days did not produce an increase in microbial counts. Water samples were exposed to Sacramento sunshine nine times on cloudless days from July through October 1997. Although the maximum daily sun angle varied from 73° in July to 41° in October, results were comparable over this time.
Effect of sunshine on E. coli and T2.
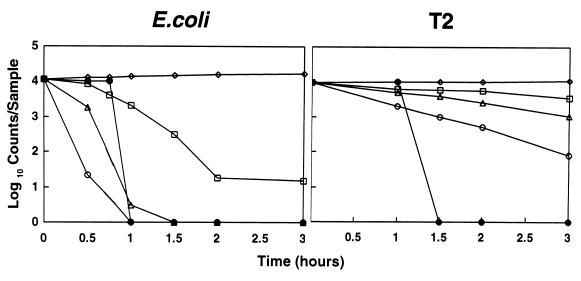
The results from one experiment performed on a cloudless day are presented in Fig. 3. In clear containers, the rate of E. coli inactivation increased threefold with the smaller, two-sided reflector and 4.5-fold with the Cookit reflector, compared to that of an unreflected bottle. The rate of T2 inactivation with the small and Cookit reflectors was enhanced by 2.5 and 5.3 times, respectively, compared with that of the unreflected sample. However, T2 was much more resistant to solar inactivation than E. coli. In 3 h, there was less than a 0.5-log/ml decrease in T2 viability in the unreflected sample, a 1-log decrease with the two-sided reflector, and a 2-log decrease with the Cookit.
FIG. 3.
Solar inactivation of E. coli and T2. E. coli counts/sample are shown as CFU per milliliter. T2 counts/sample are shown as PFU per 0.1 ml. Open symbols represent data from clear plastic bottles. ◊, inside control; □, flat (no reflector); ▵, two-sided reflector; ○, Cookit; •, black glass in Cookit.
When sunshine was converted to heat with a black jar in a Cookit, there was no microbial inactivation until lethal temperatures were reached (Fig. 3). E. coli was still fully viable at 52.4°C, but was completely inactivated when the water temperature reached 60°C in 1 h. The T2 phage was still viable at 62°C, partially inactivated at 65°C, and completely inactivated at 70°C in 1.5 h. In order to determine when pasteurizing conditions had been reached in the black jar, we used a WAPI whose wax melts at about 70°C. In most experiments, it took 1.5 to 2 h for water in the 1.4-liter jar to melt the WAPI wax. In separate studies using a 1-gal (3.7-liter) black glass jar in a Cookit, 3 to 4 h was needed to heat water to 70°C on sunny days from July through October. The smaller, two-sided reflector heated water in 1.4-liter black jars only to between 50 to 60°C in 3 h.
Discussion and conclusions.
Worldwide, unsafe water is a major health problem. An estimated 1 billion people do not have access to safe water. The World Health Organization estimates that diarrheal diseases that result from contaminated water kill about 2 million children and cause about 900 million episodes of illness each year (21). A major study in Cebu, Philippines, estimated that if families using moderately contaminated water (100 fecal coliforms/100 ml) were able to use a high-quality water source, diarrhea among their children would be reduced by over 30% (19). As people in sun-rich areas of developing countries often cannot afford modern fuels and suffer from ever-increasing fuelwood shortages, the use of sunshine to cook and pasteurize contaminated water and milk could provide a new approach to these debilitating problems.
Most efforts using solar energy to kill pathogenic microbes in contaminated water have used transparent containers and direct sunshine. We report that the use of reflectors substantially increases the solar and heat inactivation of E. coli and bacteriophage T2 in clear bottles. However, solar inactivation of microbes is variable. In our studies, T2 was 7.8 to 10.8 times as resistant to sunshine inactivation than E. coli. Wegelin et al. (20) tested the solar inactivation of three viruses suspended in phosphate buffer and held in quartz tubes. They reported that a bovine rotavirus and the E. coli RNA bacteriophage f2 had inactivation rates similar to that of E. coli, but that encephalomyocarditis virus, a picornavirus, was more than twice as resistant as E. coli. Acra et al. (2) reported that compared to solar inactivation of E. coli, it took 2.4 times as much sunshine to inactivate Candida sp., Geotrichum sp., and spores of Aspergillus flavus and 6.4 times as much sunshine to inactivate Penicillium spores.
A major field study in Kenya compared the incidence of diarrhea in children 5 to 16 years old who drank water from plastic bottles exposed to sunshine for most of the day with that in children who drank from bottles kept inside (6). Although there was a statistically significant difference between the groups, if the incidence of diarrhea during the 12-week trail was projected over 1 year, the group with solar-treated water would still have an average of 17.8 diarrhea incidents per child, only 9% fewer than the control group with 19.5 diarrhea incidents per child. A possible factor contributing to the small benefit of sunlight-exposed water would be the greater resistance to solar inactivation which pathogenic viruses and protozoan cysts may have compared to E. coli. Although simple reflectors could increase microbial inactivation in clear bottles severalfold, this procedure may be inadequate to inactivate all possible pathogens.
We found that if there is sufficient sunshine, a more effective and reliable method to pasteurize water is to heat water in dark containers placed in a reflector like the Cookit and to use a WAPI indicator. As temperatures reach 50°C or greater, pathogenic microbes are inactivated. The temperatures which cause approximately a 1-log decrease in viability within 1 min are 55°C for protozoan cysts; 60°C for E. coli, enteric bacteria, and rotavirus; and 65°C for hepatitis A virus (3, 5, 7–9, 16). When the WAPI wax melts at about 70°C, the cumulative lethal effect of heat will be greater than the heat ordinarily used to pasteurize milk, 15 s at 71.7°C or 62.7°C for 30 min. This is because of the gradual increase in temperature, often at least 30 min from 50 to 70°C, as well as the gradual cooling once the water is removed. In addition, the water at the bottom of the black jar is often 5 to 10°C cooler than the water at the top of the jar. Microbes in the upper portion will have been inactivated before those in the bottom portion, where the WAPI is located.
In a small trial, the WAPI was successfully used by Somali refugee women in the Dadaab, Kenya, refugee camp to pasteurize camel’s milk which contained >200 E. coli/ml (13). A WAPI could also be used in nonsolar conditions, saving much energy when people are told to boil their water, heat far in excess of what is needed to pasteurize either water or milk.
The limitations of this procedure include the quantities of water which can be treated daily with a Cookit (6 to 7 liters) and the need for good sunshine. Although clear plastic bags enclose dark pots for cooking with a Cookit, we found that dark jars in a Cookit heated well to pasteurization temperatures without a plastic bag on sunny days over a wide range of solar angles, from high sun angles in July to low sun angles in October. On partially sunny days, the addition of a clear plastic bag over the black jar could assist in heating water to pasteurization temperatures. On cloudy days it will not work, but this still enables solar pasteurization on 200 to 300 days/year in many developing countries. Effective solar thermal water pasteurization units capable of producing many liters of pasteurized water on sunny days are available (4). However, these units require materials and financial means which are unlikely to be available in the areas of greatest need.
The effective method of pasteurizing contaminated water and milk we have described, using a dark jar with a WAPI in a Cookit reflector, could be copied at low cost by individuals and groups in many sun-rich areas around the world and possibly contribute to improved health among the world’s poorest citizens.
Acknowledgments
We thank Cynthia Maravich, Richard Abdallah, Debra Hanson, Gigi Montemayer, Taraneh Sharif, and Michelle Puzdrakiewicz for their preliminary studies of solar water pasteurization and Madeline Ewing for her technical assistance.
REFERENCES
- 1.Acra A, Karahagopian Y, Raffoul Z, Dajani R. Disinfection of oral rehydration solutions by sunlight. Lancet. 1980;ii:1257–1258. doi: 10.1016/s0140-6736(80)92530-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Acra A, Raffoul Z, Karahagopian Y. Solar disinfection of drinking water and oral rehydration solutions. Guidelines for household applications in developing countries. Beirut, Lebanon: UNICEF Illustrated Publications; 1984. [Google Scholar]
- 3.Anderson B C. Moist heat inactivation of Cryptosporidium sp. Am J Public Health. 1985;75:1433–1434. doi: 10.2105/ajph.75.12.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burch J, Thomas K E. An overview of water disinfection in developing countries and the potential for solar thermal water pasteurization. Golden, Colo: National Renewable Energy Laboratory; 1998. [Google Scholar]
- 5.Ciochetti D A, Metcalf R H. Pasteurization of naturally contaminated water with solar energy. Appl Environ Microbiol. 1984;47:223–228. doi: 10.1128/aem.47.2.223-228.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy R M, Elmore-Meegan M, Joyce T, McGuigan K G, Barnes J. Solar disinfection of drinking water and diarrhoea in Maasai children: a controlled field trial. Lancet. 1996;348:1695–1697. doi: 10.1016/S0140-6736(96)02309-4. [DOI] [PubMed] [Google Scholar]
- 7.Faechem R G, Bradley D J, Garelick H, Mara D D. Sanitation and disease; health aspects of excreta and wastewater management. New York, N.Y: John Wiley & Sons; 1983. [Google Scholar]
- 8.Fayer R. Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol. 1994;60:2732–2735. doi: 10.1128/aem.60.8.2732-2735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harp J A, Fayer R, Pesch B A, Jackson G J. Effect of pasteurization on infectivity of Cryptosporidium parvum oocysts in water and milk. Appl Environ Microbiol. 1996;62:2866–2868. doi: 10.1128/aem.62.8.2866-2868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce T, Kenny V, McGuigan K, Barnes J. Disinfection of water by sunlight. Lancet. 1992;340:921. doi: 10.1016/0140-6736(92)93340-s. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 11.Joyce T M, McGuigan K G, Elmore-Meegan M, Conroy R M. Inactivation of fecal bacteria in drinking water by solar heating. Appl Environ Microbiol. 1996;62:399–402. doi: 10.1128/aem.62.2.399-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKenzie T D, Ellison III R T, Mostow S R. Sunlight and cholera. Lancet. 1992;340:367. doi: 10.1016/0140-6736(92)91439-f. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 13.Metcalf, R. H. 1995. Unpublished data.
- 14.Morley D. Sunlight and drinking water. Lancet. 1988;ii:686. doi: 10.1016/s0140-6736(88)90499-0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 15.Odeyemi O, Lawand T, Alward R, Collett R. Microbial aspects of solar water disinfection. In: Lawand T A, Alward R, Odeyemi O, Hahn J, Kandpal T C, Ayoub J, editors. Proceedings, Solar Water Disinfection Workshop. Montreal, Quebec, Canada: Brace Research Institute; 1988. pp. 155–171. [Google Scholar]
- 16.Parry J V, Mortimer P P. The heat sensitivity of hepatitis A virus determined by simple tissue culture method. J Med Virol. 1984;14:277–283. doi: 10.1002/jmv.1890140312. [DOI] [PubMed] [Google Scholar]
- 17.Reed R H. Solar inactivation of faecal bacteria in water: the critical role of oxygen. Lett Appl Microbiol. 1997;24:276–280. doi: 10.1046/j.1472-765x.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 18.Sommer B, Mariño A, Solarte Y, Salas M L, Dierolf C, Valiente C, Mora D, Rechsteiner R, Setter P, Wirojanagud W, Ajarmeh H, Al-Hassan A, Wegelin M. SODIS - an emerging water treatment process. J Water Supply Res Tech - Aqua. 1997;46:127–137. [Google Scholar]
- 19.VanDerslice J, Briscoe J. All coliforms are not created equal: a comparison of the effects of water source and in-house water contamination on infantile diarrheal disease. Water Resour Res. 1993;29:1983–1995. [Google Scholar]
- 20.Wegelin M, Canonica S, Meschner K, Tleischmann T, Pasaro F, Metzler A. Solar water disinfection: scope of the process and analysis of radiation experiments. J Water Supply Res Tech - Aqua. 1994;43:154–169. [Google Scholar]
- 21.World Bank. World Development Report 1992. Development and the environment. New York, N.Y: Oxford University Press; 1992. p. 44. [Google Scholar]