Abstract
The high incidence of post‐covid symptoms in humans confirms the need for effective treatment. Due to long‐term complications across several disciplines, special treatment programs emerge for affected patients, emphasizing multidisciplinary care. For these reasons, we decided to look at current knowledge about possible long‐term complications of COVID‐19 disease and then present the effect of flavonoids, which could help alleviate or eliminate complications in humans after overcoming the COVID‐19 infection. Based on articles published from 2003 to 2021, we summarize the flavonoids‐based molecular mechanisms associated with the post‐COVID‐19 syndrome and simultaneously provide a complex view regarding their prophylactic and therapeutic potential. Review clearly sorts out the outcome of post‐COVID‐19 syndrome according particular body systems. The conclusion is that flavonoids play an important role in prevention of many diseases. We suggest that flavonoids as critical nutritional supplements, are suitable for the alleviation and shortening of the period associated with the post‐COVID‐19 syndrome. The most promising flavonoid with noteworthy therapeutic and prophylactic effect appears to be quercetin.
Keywords: antiinflammatory, flavonoids, post‐COVID‐19 syndrome, SARS‐CoV‐19
1. INTRODUCTION
At present, physicians and scientists are investigating why COVID‐19 disease (COVID‐19) causes such long‐term health problems or permanent damage, even to systems other than the respiratory system. It is probably the key question to address.
Studies show that COVID‐19 is induced by a SARS‐CoV‐2 virus, which attaches to its S protein's angiotensin‐converting enzyme 2 (ACE2) receptor. Present on the surface of host respiratory cells (Jia et al., 2005) such as alveolar cells type II in the lung (Hamming et al., 2004), cells in tissues such as heart, kidney, testis (Tipnis et al., 2000), brain, liver, intestine (Doobay et al., 2007). The binding of the SARS‐CoV‐2 virus to the ACE2 receptor triggers a cascade of reactions, including inhibition of the ACE2 angiotensin II receptor, which does not change angiotensin II to angiotensin 1–7. Angiotensin II levels rise in patients' blood. Angiotensin II interacts with the AT1 receptor (angiotensin receptor) of the kidneys or blood vessels, further manifested by vasoconstriction, increased blood pressure, water retention, inflammatory reactions, and subsequent apoptosis. In the lung interstitium, angiotensin II causes apoptosis, which initiates the inflammatory process and produces inflammatory cytokines. The first aid for a patient in this condition is increased oxygen uptake by pulmonary ventilation, which is, however, insufficient in the case of so‐called cytokine storm (an exaggerated autoimmune response) that causes lung damage (Rothlin, Vetulli, Duarte, & Pelorosso, 2020) (Figure 1).
FIGURE 1.
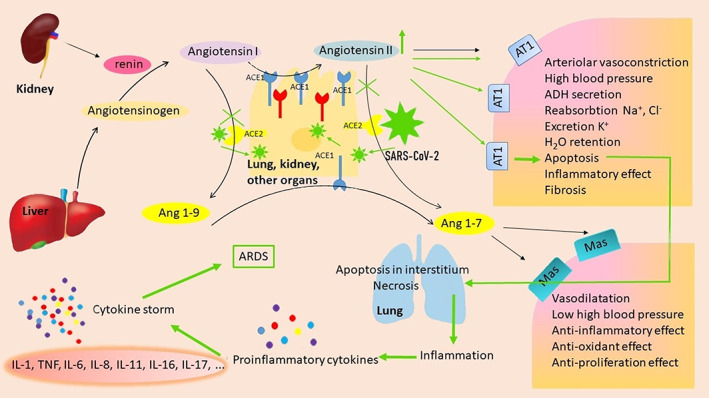
Mechanism of action of angiotensin II under physiological conditions (black arrows) and its negative effect induced by inhibition of ACE2 receptors by SARS‐CoV‐2 virus (green arrows). Inhibition of ACE2 receptors by SARS‐CoV‐2 virus binding results in a fourfold increase in angiotensin II levels, resulting in cell apoptosis followed by inflammation. As a result of the cytokine storm, acute respiratory distress syndrome development occurs. Original figure made for this review using the Zoner Photo Studio X
Since ACE2 receptors are expressed in many human tissues, including kidney epithelial cells, heart, small intestine, blood vessels, liver, endothelial cells (Hamming et al., 2004), hematopoietic stem, and progenitor cells (Jarajapu, 2021), they also damaged by cytokine storms. Thus, the complications associated with COVID‐19 are not surprising.
COVID‐19 therapy is currently mesmerizing and approached very carefully. We believe that COVID‐19 treatment could be potentially more effective by understanding the interaction of SARS‐CoV‐2 with ACE2 receptors. Specifically, how SARS‐CoV‐2 inhibits its binding to the cell surface receptor. Nevertheless, the inhibition of ACE2 receptors could lead to an undesirable angiotensin II increase. Another approach to facilitating the course and outcome of COVID‐19 would be using the ACE1 inhibitors, which would reduce angiotensin II level and inhibit the conversion of nonapeptide angiotensin 1–9 to heptapeptide angiotensin 1–7. This could lead hypothetically to the undesirable upregulation of ACE2 receptors and increases the possibility of SARS‐CoV‐2 virus binding to the cell surface. On the other hand, stimulation of ACE2 activity toward angiotensin 1–7 production could lead to an antiinflammatory effect, which would have a beneficial protective effect on the lungs and other organs possibly.
Hypothetically, the effective COVID‐19 therapy, in addition to ACE2 inhibitors, should include the use of the ACE1 inhibitors, which reduce the level of angiotensin II by blocking the formation of angiotensin II from angiotensin I. Moreover, the ACE1 inhibitors converts angiotensin I to angiotensin II, which increases blood pressure. Thus, increased ACE1 activity and angiotensin II levels higher than basal levels cause hypertension and damage (Benigni, Cassis, & Remuzzi, 2010). On the other side, these inhibitors would block both the site of interaction between SARS‐CoV‐2 virus and cell, as well as the renin‐angiotensin signaling pathway, causing acute lung injury (Imai, Kuba, & Penninger, 2008) associated with high levels of angiotensin II.
The severity of COVID‐19 is also associated with high levels of phospholipase A2. Müller et al. revealed that inhibition of cPLA2 impairs an early step of coronavirus replication in the cell culture (Müller et al., 2018). sPLA2 group IIA (sPLA2‐IIA) plays a central role in severe and fatal COVID‐19 disease outcomes (Snider et al., 2021). Snider et al. demonstrated that a high level of circulating enzymatically active secreted PLA2‐IIA is an essential indicator of the COVID‐19 severity. He introduced the PLA‐BUN index with his team, characterized by PLA2 and blood urea nitrogen (BUN) concentration in plasma. The PLA‐BUN index is a factor for mitochondrial dysfunction, sustained inflammatory injury, and lethal COVID‐19. A high level of circulating catalytically active PLA2‐IIA (≥10 ng/ml) is an indicator for severe COVID‐19 course associated with hyperglycemia, kidney dysfunction, hypoxia, anemia, and multiple organ dysfunctions (Snider et al., 2021). Early administration of PLA2 inhibitors represents an option to reduce COVID‐19 mortality and treat the chronic inflammatory conditions in the post‐COVID syndrome.
Theoretically, the drug used in COVID‐19 therapy should interact with ACE2 and ACE1 receptors and have a higher affinity for ACE2 than virus affinity (SARS‐CoV‐2) for the ACE2. Treatment should stimulate ACE2 enzymatic activity in the active conversion of angiotensin II to angiotensin 1–7 and inhibit the ACE 1 to reduce the angiotensin II levels, and inhibits phospholipase A2 (PLA2‐group IIA) activity. At the same time, it is necessary to consider that these concepts are hypotheses. Only clinical trials will show us the real potentials of COVID‐19 treatment.
2. METHODS
The information was collected from articles published from 2003 to 2021 from international and national scientific databases such PubMed, Medline, Scopus, ScienceDirect using the following key words “flavonoids” and “COVID‐19,” or “antiinflammatory.”
3. FLAVONOIDS
Currently, great attention is paid to research focused on bioactive substances of natural origin. Many studies point to a significant effect of plant polyphenols—chalcones and their flavonoid derivatives, which have been shown to modulate cell pathophysiological processes. Flavonoids possess many antioxidant (Bhuiyan, Mitsuhashi, Sigetomi, & Ubukata, 2017; Brunetti, Di Ferdinando, Fini, Pollastri, & Tattini, 2013; Losada‐Barreiro & Bravo‐Diaz, 2017), anticancer (Gorlach, Fichna, & Lewandowska, 2015; Kello, Kuliková, Vašková, Nagyová, & Mojžiš, 2017), antiinflammatory (Alissa & Ferns, 2017; Maleki, Crespo, & Cabanillas, 2019), antimutagenic (Makhafola, Elgorashi, McGaw, Verschaeve, & Eloff, 2016; Miyazawa & Hisama, 2003), antimicrobial (Xie, Yang, Tang, Chen, & Ren, 2015), and antiviral (Kaul, Middleton, & Ogra, 1985) properties, and their primary sources are fruits, vegetables, herbs, and other plants (Babu, Liu, & Gilbert, 2013).
The antiviral properties of flavonoids concerning human viruses were already mentioned in 1985 by Kaul et al. (1985). Where Schwarz et al. 2014 found that kaempferol derivatives have antiviral activity. Later, Dai et al. 2019 demonstrated the antiviral efficacy of flavonoids against Enterovirus 71 infection.
Flavonoids are generally considered safe. However, repeated application of high doses of flavonoids may lead to side effect/toxicity including hematotoxicity, hepatotoxicity, nephrotoxicity, reproductive toxicity or allergy (Galati & O'Brien, 2004). Due to the possible side effects of flavonoids in terms of overdose, their administration should be performed under the supervision of a doctor or pharmacist.
3.1. Flavonoids and COVID‐19 infection
Researchers began intensively addressing the interactions of flavonoids with host cell ACE receptors during the massive spread of the SARS‐CoV virus in 2002–2003. Many scientific studies have come from these and later periods, with the result that flavonoids interact with ACE1 and ACE2 receptors, inhibit their activity and have an antiviral effect (Baez‐Santos, St John, & Mesecar, 2015; Deng, Aluko, Jin, Zhang, & Yuan, 2012; Gong et al., 2008; L. Guerrero et al., 2012; D. P. Han, Penn‐Nicholson, & Cho, 2006; Hettihewa, Hemar, & Rupasinghe, 2018; Ho, Wu, Chen, Li, & Hsiang, 2007; W. Li et al., 2003; Loizzo et al., 2007; Nguyen et al., 2012; Ojeda et al., 2010; Sui et al., 2010; Takahashi, Yoshiya, Yoshizawa‐Kumagaye, & Sugiyama, 2015; M. S. Yu et al., 2012).
Recent research teams characterized the interaction site of the S protein of the SARS‐CoV‐2 virus and revealed that flavonoids can bind ACE2, regulate the ACE1 and ACE2 levels, and maintain their basal levels (Bhowmik, Nandi, Prakash, & Kumar, 2021; Derosa, Maffioli, D'Angelo, & Di Pierro, 2021; El‐Missiry, Fekri, Kesar, & Othman, 2021; J. Gao et al., 2021; Gour, Manhas, Bag, Gorain, & Nandi, 2021; H. Chen & Du, 2020; Cherrak, Merzouk, & Mokhtari‐Soulimane, 2020; Khan et al., 2021; Mendonca & Soliman, 2020; Muchtaridi, Fauzi, Ikram, Gazzali, & Wahab, 2020; Omotuyi et al., 2021; Russo, Moccia, Spagnuolo, Tedesco, & Russo, 2020; Tutunchi, Naeini, Ostadrahimi, & Hosseinzadeh‐Attar, 2020; J. W. Yu, Wang, & Bao, 2020). In addition, disruption of the interaction between ACE2 and the SARS‐CoV‐2 spike protein is the main effect of flavonoids. Thus, SARS‐CoV‐2 cannot fuse with ACE2 (Abderrazak et al., 2015; Muchtaridi et al., 2020; J. W. Yu et al., 2020; Zhai et al., 2020). Baicalin, scutellarin, hesperetin, nicotianamine, glycyrrhizin (H. Chen & Du, 2020), epigallocatechin‐3‐gallate, theaflavin gallate (Maiti & Banerjee, 2021), naringenin, quercetin, luteolin (Maurya, Kumar, Prasad, Bhatt, & Saxena, 2020), fisetin, kaempferol (Pandey et al., 2020) were due to anti‐SARS‐CoV‐2 effect and to the low toxicity selected as potential candidates for COVID‐19 treatment.
Because of multisystemic disease COVID‐19, many studies have been published confirming the anti‐SARS‐CoV‐19 effect of flavonoids (Palit, Mukhopadhyay, & Chattopadhyay, 2021; Santana et al., 2021).
The use of flavonoids is hypothetically a prerequisite for successful respiratory viral infections (Brendler et al., 2021) and COVID‐19 supportive therapy. However, caution should be exercised in the conclusions, whether inhibition of ACE2 in patients with severe COVID‐19 would be desirable.
As mentioned, the severity of COVID‐19 is also associated with high levels of phospholipase A2. Flavonoids specifically affect the activity of inflammatory enzymes, which include phospholipase A2, cyclooxygenases, lipooxygenases, tyrosine kinase, serine‐threonine protein kinase, phospholipase C, thus reducing the concentrations of crucial mediators of inflammation such as arachidonic acid, prostaglandins, leukotrienes, and NO. Phospholipase A2 (PLA2) is an enzyme that catalyzes the hydrolysis of membrane phospholipids, releasing free fatty acids and lysophospholipids. There are six main groups of phospholipases A2: secretory (sPLA2), cytosolic (cPLA2), calcium‐independent (iPLA2), platelet‐activating factor acetyl hydrolases (PAF‐AH), lysosomal PLA2 (LyPLA2), and adipose‐specific PLA2 (AdPLA2), including their different isoforms (Murakami et al., 2014). Phospholipase A2 classification and pathologies associated with secretory PLA2 are reported by Quach, Arnold, and Cummings (2014).
The best‐known flavonoid that inhibits sPLA2‐II is quercetin (Lattig et al., 2007; Lesjak et al., 2018; Valerio et al., 2009). Myricetin and kaempferol also have a significant inhibitory effect on cPLA2 and COX‐2 (Kang, Belal, Choe, Shin, & Shim, 2018; Lindahl & Tagesson, 1997). However, a weaker inhibition is observed with hesperidin, naringenin, apigenin, amentoflavone (Jin et al., 2020; Lee, Son, Chang, Kang, & Kim, 1997; Welton et al., 1986). Known flavonoids inhibiting sPLA2‐IIA activity include a group of biflavonoids such as ochnaflavone, amentoflavone which inhibit the enzymatic and biological activities of sPLA2‐IIA (Chang et al., 1994; Moon et al., 2002). Ginkgetin reduces a pro‐inflammatory gene expression such as inducible nitric oxide synthase (iNOS), cyclooxygenase‐2 (COX‐2) (100 mg/kg) (Q. Li, Ye, Long, & Peng, 2019), and inhibits sPLA2‐IIA. Flavonoids have been shown to affect inflammatory responses. Quercetin and kaempferol cause inhibition of iNOS, cyclooxygenase‐2, and C‐ reactive protein (CRP) (Garcia‐Mediavilla et al., 2007).
An essential function of flavonoids is the reduction of inflammatory markers, cytokine expression, modulation of transcription factors, and the effect on transduction signals. Silymarin inhibition of the NF‐κB pathway (Hrčková, Mačák Kubašková, Mudroňová, & Bardelčíková, 2020) has a potential therapeutic role in alleviating the severe form of COVID‐19. Ginkgetin, a biflavonoid from Ginkgo biloba, effectively decreased the expression of inflammation‐related protein prostaglandin E2 (PGE2), tumor necrosis factor‐alpha (TNF‐α), interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and interleukin‐8 (IL‐8) (Q. Li et al., 2019). At the same time, these inflammatory markers are associated with chronic inflammation.
Theoharides and Cont described the significant effect of luteolin due to its antiviral and antiinflammatory properties. It could be administered to patients in the acute phase of COVID‐19 together with dexamethasone. Luteolin can inhibit mast cells, a significant source of cytokines in the lung (Theoharides & Conti, 2020). Pawar et al. points out the possible use of quercetin in COVID‐19 therapy because of structural resemblance with dexamethasone (Pawar & Pal, 2020).
4. POST‐COVID‐19 SYNDROME
The COVID‐19 infection is associated with the development of inflammatory syndromes known as post‐COVID‐19 inflammation syndrome with related clinical manifestations (Chandrashekara et al., 2020).
Post‐COVID‐19 symptoms include long‐term health problems with lungs, heart, brain, kidney, and digestion. In some patients, there is a presumption of post‐COVID‐19 chronic diseases associated with damaged lungs, heart, brain, and persistent problems with blood clotting, impaired immune system. All of this is related to clinical manifestations, especially myalgia, intense fatigue, the sensation of fever, shortness of breath, chest tightness, tachycardia, headaches, and anxiety (Davido, Seang, Tubiana, & de Truchis, 2020; Oronsky et al., 2021), which could persist for several years, as it was the case in the 2003 SARS epidemic (P. Zhang et al., 2020).
Not only the elderly and people with many serious health problems but also younger people may develop persistent post‐COVID‐19 symptoms. Even for weeks or months after infection. These people are referred to as “long haulers.” At present, it is not yet possible to say what long‐term consequences it may have or whether they are temporary or permanent. This condition is also called “Long covid” or post‐COVID‐19 syndrome.
The most common complications of patients seeking medical attention after COVID‐19 include muscle pain, severe fatigue, chronic fatigue, sleep disturbances, persistent fever, chronic cough, chest pain, palpitations, joint pain, headache and anxiety, mood swings, cognitive dysfunction (memory disorders), taste and smell disorders, diarrhea, hair loss (Carfi, Bernabei, Landi, & Gemelli Against, 2020; Fu et al., 2020; C. Huang et al., 2021; Mo et al., 2020; Rai, Sharma, & Kumar, 2021; Stavem, Ghanima, Olsen, Gilboe, & Einvik, 2020; Zhou et al., 2020). The most severe complications are difficulty in breathing, persistent shortness of breath, pulmonary fibrosis (Rai et al., 2021), decreased total lung vital capacity (Torres‐Castro et al., 2020), impaired liver function (An et al., 2021; Cai et al., 2020) and kidneys (D. H. Jiang & McCoy, 2020; Oyelade, Alqahtani, & Canciani, 2020), mass muscle reduction (Mo et al., 2020; Rai et al., 2021; Stavem et al., 2020). Other complications include hematological complications (Debuc & Smadja, 2021), such as increased platelet activity and the formation of small blood clots (Vechi, Maia, & Alves, 2020). All these findings indicate the need for ongoing investigation regarding the long‐term consequences of COVID‐19 where long‐term respiratory, cardiovascular, neuropsychological, gastrointestinal, nephrological, hematological, dermatological, musculoskeletal problems may persist for more than 4 weeks after the coronavirus infection.
4.1. Flavonoids and their therapeutic potential in post‐COVID‐19 syndrome
Improper treatment and association of bacterial infection, an inflammatory mediators increase might lead to several chronic diseases where the recovery period may be prolonged. The inflammatory cytokines present after the acute phase of COVID‐19 are therapeutic targets to treat inflammatory disease. Flavonoids could be used to alleviate or even eliminate the post‐COVID‐19 symptoms associated with post‐infection inflammation.
Their antiinflammatory effect is one of the most important activities and probably depends on their chemical structure (Figure 2).
FIGURE 2.
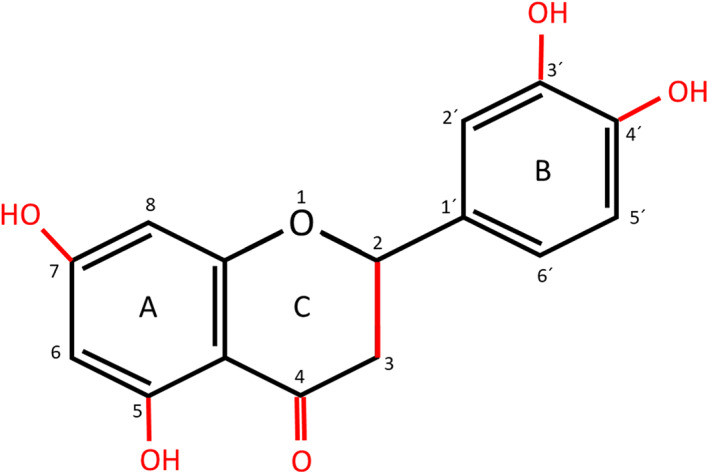
Flavonoids chemical structure of flavonoids with red marked sites showing their effect. Original figure made for this review using the Zoner Photo Studio X
The essential features modulating the antiinflammatory effect of flavonoids include (a) number, and position of hydroxyl groups at the A and B rings, namely at C5 and C7 in the A ring, at C3' and C4' in the B ring, (b) presence of keto group at C4 in C ring, (c) glycosylation/non‐glycosylation of the molecule, (d) presence/absence of double bond between C2 and C3 in the C ring (Kim, Son, Chang, & Kang, 2004; Lago et al., 2014; Ribeiro et al., 2014).
Numerous experimental studies have tried to analyze the effect of flavonoids depending on their structure and present the hydroxyl groups. The 3′, 4′‐dihydroxylated flavonoids—quercetin and luteolin—have a proven higher ability to inhibit one of the most important cytokines, tumor necrosis factor (TNF‐α, in a concentration‐dependent manner, however. The 4′‐hydroxylated flavonoids as kaempferol and apigenin reduced TNF‐α,β secretion approximately 40–50% at 50 μM (Comalada et al., 2006). The inhibitory capacity is virtually abolished if the flavonoid does not have an OH group on its B‐ring.
The effect of flavonoids is monitored at several levels: (a) at the cell level, (b) at the level of enzyme activity, (c) at the level of modulation of gene expression. Regarding flavonoids with anti‐SARS‐CoV‐19 effect such as baicalin, scutellarin, hesperetin, nicotianamine, glycyrrhizin (H. Chen & Du, 2020), epigallocatechin‐3‐gallate, theaflavin gallate (Maiti & Banerjee, 2021), naringenin, quercetin, luteolin (Maurya et al., 2020), fisetin, kaempferol (Pandey et al., 2020), the info about their therapeutic in post‐COVID‐19 syndrome or preventive effects are vastly missing due to minimal data availability. Flavonoids with great antiinflammatory potential possess an influence on the function of different immune cell types; B cells—chrysin (C. C. Lin et al., 2012), rutin (Roseghini et al., 2009), T cells—genistein (Abron et al., 2018), icariin (Tao et al., 2013), NK cells—quercetin (Middleton Jr., Kandaswami, & Theoharides, 2000), tangeritin (Vanhoecke et al., 2005), basophils—quercetin (Chirumbolo et al., 2010), fisetin, apigenin (Middleton Jr. & Drzewiecki, 1984), neutrophils—quercetin, kaempferol, and isorhamnetin (Zielinska, Kostrzewa, & Ignatowicz, 2000), cirsimaritin (J. P. Wang, Chang, Hsu, Chen, & Kuo, 2002), eosinophils—quercetin (X. J. Weng, Chen, & Zhang, 2008), baicalein (Nakajima, Imanishi, Yamamoto, Cyong, & Hirai, 2001), macrophages—luteolin (Xagorari, Roussos, & Papapetropoulos, 2002), apigenin (X. Zhang, Wang, Gurley, & Zhou, 2014), mast cells—chrysin, kaempferol, pinocembrin, galangin (Nakamura et al., 2010), and platelets—quercetin, catechin (Pignatelli et al., 2000).
The flavonoid's mechanism of action on cells is mainly through apoptosis induction, proliferation inhibition, cell cycle arrest induction, differentiation induction, cytokine inhibition, chemokines, or antibodies production.
We should mention that untreated inflammation caused by inflammatory cytokine overproduction might lead to chronic disease. The cytokines involved in chronic inflammation and mediating humoral responses are IL‐4, IL‐5, IL‐6, IL‐7, and IL‐17 (Feghali & Wright, 1997; Leyva‐Lopez, Gutierrez‐Grijalva, Ambriz‐Perez, & Heredia, 2016).
In chronic diseases, studies such as cancer, obesity, liver, and Alzheimer's disease associated with inflammation significant progress was made. This is primarily done by investigating inflammasome NLRP3 (nucleotide‐binding domain, leucine‐rich‐repeat‐containing receptor, pyrin domain‐containing‐3) who may inhibit or activate pro‐inflammatory cytokine release. In the case of COVID‐19, the overproduction of inflammatory markers induced by NLRP3 inflammation may lead to a post‐COVID‐19 syndrome as a chronic disease. This might explain many of the symptoms in humans after overcoming COVID‐19 infection. Inflammasome NLRP3 is considered a target site for the treatment of chronic diseases (119, 120). This piece of evidence could be used to eliminate or alleviate the symptoms of the post‐COVID‐19 syndrome, which is accompanied by many complications leading to chronic diseases. Flavonoids affect the release of NLRP3‐mediated cytokines (Lim, Min, Park, & Kim, 2018). The NLRP3 inflammasome activation inhibitors are myricetin (H. Chen et al., 2019), rutin (Aruna, Geetha, & Suguna, 2014), murine (Tianzhu, Shihai, & Juan, 2014), quercetin, luteolin, and apigenin (Marquez‐Flores, Villegas, Cardeno, Rosillo, & Alarcon‐de‐la‐Lastra, 2016). The effect of flavonoids on the release of inflammatory markers in chronic diseases is apparent. The mechanism of inhibiting NLRP3 by flavonoids is likely to inhibit cytokine release in post‐COVID‐19 patients.
The following sections describe particular body systems with their post‐COVID 19 symptoms and review available data where the assessment and potential use of flavonoids were suggested or studied.
4.2. Pulmonary post‐COVID‐19 syndrome
The severity of the lung injury caused by the SARS‐CoV‐2 virus has been primarily described. A severe complication in individuals after COVID‐19 infection is pulmonary fibrosis (Rai et al., 2021), difficulty breathing, persistent shortness of breath, and reduced overall vital capacity of the lungs (Torres‐Castro et al., 2020).
Weerahandi has found that even a month after patients were discharged, more than 70% of post‐COVID‐19 patients reported shortness of breath, and 13.5% were still using oxygen at home (Weerahandi et al., 2021). Other reports state that 43% of patients in Italy suffered from persistent dyspnea an average of 60 days after symptom onset (Carfi et al., 2020). In France, 42% of COVID19 survivors had dyspnea an average of 111 days after hospital admission (Garrigues et al., 2020). The highest risk to develop lung fibrosis is in an elderly patient who requires ICU care and mechanical ventilation. Currently, no fully proven options for post‐inflammatory COVID‐19 pulmonary fibrosis (Rai et al., 2021). Pulmonary fibrosis is preceded by cytokine storm, and dysregulated release of matrix metalloproteinases is a known consequence of acute respiratory distress syndrome (ARDS). It has been found that 40% of patients with COVID‐19 develop ARDS, and 20% of ARDS cases were severe (Rai et al., 2021; Wu et al., 2020). Although SARS‐CoV‐2 induced pulmonary fibrosis differs from idiopathic pulmonary fibrosis, treatment includes antifibrotic therapy. Antifibrotic and antiinflammatory drugs, such as pirfenidone and nintedanib, should be used even in the acute phase of COVID‐19 pneumonia (Collins & Raghu, 2019).
Flavonoids can also be used to support respiratory disease treatment. Several studies confirm the antifibrotic potential of flavonoids (Cordoba et al., 2015). Apigenin (L. Chen & Zhao, 2016), epigallocatechin (EGCC) (Sriram, Kalayarasan, & Sudhandiran, 2009) and quercetin (Baowen et al., 2010) are flavonoids that reduce bleomycin‐induced pulmonary fibrosis. Grape seed extract recovers lung fibrosis by inhibiting of inflammatory cytokine TGF‐β1 and metalloproteinase MMP‐9 expression (Liu et al., 2017).
Leaf extract of flavonoid‐rich antioxidant Ginkgo biloba improves blood flow, protects from free radicals, blocks platelet aggregation and blood clotting (Dubey, Shankar, Upadhyaya, & Deshpande, 2004; Ernst, 2002; Mahady, 2002). It also has been examined for antifibrotic effect in the bleomycin model (Daba et al., 2002; Moeller, Ask, Warburton, Gauldie, & Kolb, 2008). The most studied natural ingredients with antifibrotic properties are those used in Traditional Chinese Medicine (L. C. Li & Kan, 2017). The most well‐known and influential agents tested in rat models are baicalein, tectorigenin, quercetin, and luteolin (Baowen et al., 2010; Y. Gao et al., 2013; C. Y. Chen, Peng, Wu, Wu, & Hsu, 2010; L. C. Li & Kan, 2017; H. Zhang et al., 2010). A noteworthy flavonoid associated with pulmonary fibrosis treatment is naringenin which reversed Mycoplasma pneumoniae‐induced lung inflammation and fibrosis by inhibiting autophagy (Y. Lin, Tan, Kan, Xiao, & Jiang, 2018).
4.3. Cardiovascular post‐COVID‐19 syndrome
A German study on 100 patients overcoming COVID‐19 revealed that by MRI exams, heart abnormalities in 78 of them, and 60 showed viral myocarditis. Even in people who experienced only mild COVID‐19 symptoms. Acute myocarditis may develop due to the virus's direct effect or the inflammation on the heart itself. This may increase the risk of heart failure or other heart complications (Puntmann et al., 2020). Altogether, the increased metabolic rate, a severe inflammatory storm can lead to cardiac depression and either to new‐onset heart failure or acute decompensation of chronic heart failure (Bader, Manla, Atallah, & Starling, 2021).
Patients who overcame severe heart failure due to COVID‐19 require special care. It includes both—change in diet and a change in food composition. In terms of flavonoids effectiveness, the diet should be enriched by a higher intake of fruits, vegetables, and other natural resources. The flavonols quercetin and keampferol in apples, broccoli, blueberries, red wine, tea, and onion have a significant cardioprotective effect, which according to Knekt et al., are associated with a decrease in ischemic heart disease mortality (Hertog, Feskens, Hollman, Katan, & Kromhout, 1993; Knekt et al., 2002). The other sources of flavonoids associated with cardiovascular disease are parsley and celery containing luteolin and apigenin (Erdman et al., 2007), honey, propolis with chrysin (Testai, Martelli, Cristofaro, Breschi, & Calderone, 2013), and citrus fruits with naringenin and hesperetin (Sanchez‐Recillas et al., 2019). In addition to the necessary physician‐led therapy, additional supportive care, which may well include flavonoids and become part of innovative therapeutic management, seems appropriate.
The myocarditis, accompanied by significant elevation of creatine kinase and creatine kinase MB in COVID patients (Y. Zhang et al., 2020), is probably related to the action of the pro‐inflammatory enzymes PLA2, COX‐2, and the secretion of pro‐inflammatory cytokines, in conjunction with the production of oxygen radicals. In addition to the antiinflammatory effect, flavonoids also have an antioxidant effect (Sanchez et al., 2019). Effective flavonoids with antioxidant properties include quercetin being the most potent antioxidant (Mirossay, Onderková, Mirossay, Šarišský, & Mojžiš, 2001; Rice‐Evans, Miller, & Paganga, 1996), than kaempferol, myricetin, hesperidin, naringenin (Knekt et al., 2002), catechin (Grzesik, Naparlo, Bartosz, & Sadowska‐Bartosz, 2018), and others.
Significant flavonoids effects concerning cardiovascular diseases were described in the past. Their function is both cardioprotective and regenerative. The German study describes significant heart damage in patients after overcoming COVID‐19. Among others, patients recently recovered from COVID‐19 had lower left ventricular ejection fraction and higher left ventricle volumes (Puntmann et al., 2020). A longer recovery time is expected in these patients. In association with the effect of flavonoids and their products in regeneration processes, it is appropriate to report the positive impact of flavonoids in rats in which flavonoid‐rich diets have been shown to reduce myocardial post‐ischemic damage (Facino et al., 1999). Their diet contained procyanidins—health‐promoting flavonoids from Viti's vinifera seeds. The result of the study was that all rats fed by procyanidins significantly regained left ventricular function at the end of reperfusion. Procyanidins are derived from proanthocyanidins naturally occurring in the plant kingdom. Procyanidins are usually found in fruits, vegetables, legumes, grains, seeds, and nuts. Widely consumed foods containing procyanidins are listed in Rue et al.'s publication (Rue, Rush, & van Breemen, 2018).
Post‐COVID‐19 patients often come to the outpatient clinics due to increased blood pressure. The flavonoids are a great source of functional antihypertensive products (L. Guerrero et al., 2012). Data showed that daily consumption of 150 mg quercetin for 6 weeks reduced systolic blood pressure and simultaneously decreased plasma concentrations of atherogenic oxidized LDL (Egert et al., 2009).
Flavonoids in propolis such as isosakuranetin, dihydrokaempferide, and betuletol are antihypertensive flavonoids affecting vasodilation (Maruyama, Sumitou, Sakamoto, Araki, & Hara, 2009). Their effect depends on the additional structures on the flavonoid skeleton. As mentioned above, flavonoids relative to ACE2, a type I transmembrane metallocarboxypeptidase, have a significant impact on high blood pressure. ACE1 converts angiotensin I to angiotensin II, which increases blood pressure. Flavonoids as ACE inhibitors control the renin‐angiotensin‐aldosterone system by inhibition of the ACE1 activity. Flavonoids are considered cardioprotective drugs. Significant structural features enhancing ACE1 activity include a catechol group in the B‐ring, the double bond between C2, C3 at the C‐ring, and the ketone group in C4 at the C‐ring (L. Guerrero et al., 2012). A study from 1993 showed that dietary intake of flavonoids leads to reduced incidence and mortality of cardiovascular disease (Hertog et al., 1993). Flavonoids with antihypertensive activity such as quercetin, hesperidin, naringenin, epicatechin, epigallocatcheningallate, procyanidin, delphinidin‐3‐O‐glucoside, daidzein, genistein, luteolin, kaempferol (Hugel, Jackson, May, Zhang, & Xue, 2016) were reported by Hugel et al. (2016). Important sources of these flavonoids are blueberries, cranberries, strawberries, blackcurrants, blood orange juice, black tea (Cassidy et al., 2011).
A significant complication of the post‐COVID‐19 syndrome is the persistent presence of blood clots. Flavonoids have a place in this area too. The effect of flavonoids is antithrombotic, anti‐platelet (J. A. Guerrero et al., 2005), and anticoagulant (Correia‐da‐Silva et al., 2011; Kumar, Narwal, Kumar, & Prakash, 2011. Flavonoids can modulate platelet function through many pathways. The mechanism of flavonoids action is through binding of flavonoids to TXA2 (Muñoz, Garrido, & Valladares, 2009 #145), which decreases the levels of TXA2 related to the inhibition of COX‐1—genistein, daidzein, equol (Corvazier & Maclouf, 1985), and kaempferol (H. Wang et al., 2000). Another way is the induction of conformational changes of GPIIb/IIIa receptors with epicatechin (Pearson et al., 2005), tangeritin, naringin, and naringenin (Holt, Actis‐Goretta, Momma, & Keen, 2006), and occupation of GPIIb/IIIa receptors. The most significant antiplatelet activity was shown by flavonoids naringin, naringenin, and coumarins such as franxetin and esculetin. It has been revealed that naringin showed a considerably high ability to interact with GPIIb/IIIa receptors (Zaragoza et al., 2016). The effective antithrombotic agents seem to be quercetin and rutin found in apples (Gryglewski, Korbut, Robak, & Swies, 1987; Shafi et al., 2019), kaempferol, and tiliroside from raspberry leaves (N. Han et al., 2012) myricetin, fisetin, and morin from white mulberry (Tzeng, Ko, Ko, & Teng, 1991).
Because of increased bleeding, which could be a severe adverse effect, only a clinical study will show whether it is appropriate to deliver flavonoids with anticoagulant ability to post‐COVID‐19 patients. In most COVID‐19 patients, the coagulation pathways are usually activated. Some patients may uncommonly develop subarachnoid hemorrhage due to COVID‐19 (Batcik et al., 2021). Therefore, flavonoids should be used with caution in patients with COVID‐19 or post‐COVID‐19.
4.4. Neurological post‐COVID‐19 syndrome
The development of mental disorders such as depression and anxiety are predisposed by genetic background, biological factors, and the population's epidemiologic, social, and economic situation. Headaches and anxiety, depression, mood swings, cognitive dysfunction—memory disorders, chronic fatigue, and sleep disorders (Fu et al., 2020; Pandharipande et al., 2013; Zhou et al., 2020) associated with neurotransmitter imbalance and elevation of pro‐inflammatory cytokines are also common symptoms in people with the post‐COVID‐19 syndrome. Neuropsychiatric disorders, including major depression, are also related to oxidative changes in nucleotides and polymorphisms in several genes associated with the metabolism of reactive oxygen species. The pathophysiology of depression likely lies in mitochondrial dysfunction, which results in free radicals, nonradical molecules, reactive oxygen and nitrogen species, oxidative stress of the cell modulation (Vaváková, Ďuračková, & Trebatická, 2015).
Flavonoids and their derivatives—flavonoid glycosides as antiinflammatory agents can affect the CNS‐mediated activities associated with neurochemical changes. Particularly as either sedative‐hypnotics, analgesics, or both. It was revealed that the flavonoid glycosides such as hesperidin, linarin, naringin, diosmin, neohesperidin, and gossypin induce CNS depressant action in mice (Fernandez et al., 2006; Fernandez et al., 2009). The maximal anxiolytic effect of flavones was reached at the 10 mg/kg concentration without sedation, myorelaxation, or significant reduction in locomotor activity (Fernandez et al., 2006; Marder & Paladini, 2002). Low doses of gossypin and naringin induced a strong anxiolytic effect comparable to diazepam 2 mg/kg. At high doses, both flavonoids caused sedative effects. Moreover, naringin also possesses myorelaxant activity (Fernandez et al., 2006). Another study has shown that baicalein, directly injected into the mice CNS, promoted anxiolytic‐like and sedative effects (de Carvalho, Duarte, & de Lima, 2011). Flavonoids as natural products are classified as new effective antidepressant drugs. A significant antidepressant‐like mechanism of hesperidin has been revealed in mice exposed to chronic mild stress (C. F. Li et al., 2016). Furthermore, the antidepressant effect was described in chrysin from propolis and honey (Borges et al., 2016), astilbin from Hypericum perforatum (Aruna et al., 2014; Lv et al., 2014), icariin from Herba Epidemii (Wei et al., 2016), kaempferitrin from beans, apples, spinach, onions, strawberries (Cassani, Dorantes‐Barron, Novales, Real, & Estrada‐Reyes, 2014), luteolin from Mexico oregano (de la Pena et al., 2014; L. Z. Lin, Mukhopadhyay, Robbins, & Harnly, 2007), vitexin from hawthorn (Can, Demir Ozkay, & Ucel, 2013), and nobiletin from citrus fruit (H. Huang et al., 2016).
Post‐COVID‐19 syndrome also includes chronic fatigue syndrome associated with tiredness, malaise, headaches, sleep disturbance, difficulty in concentration and memory, muscular and skeletal pain. Sathyapalan, Beckett, Rigby, Mellor, and Atkin (2010) stated that high cocoa polyphenol‐rich chocolate might reduce the burden of symptoms associated with chronic fatigue syndrome (Sathyapalan et al., 2010). Many flavonoids are reported to alleviate this syndrome. Still, the most important is epigallocatechin, an essential component of green tea (Sachdeva, Kuhad, & Chopra, 2011), and naringin and curcumin (Gupta et al., 2009; Vij, Gupta, & Chopra, 2009).
4.5. Kidney failure at post‐COVID‐19 syndrome
The new coronavirus SARS‐CoV‐19 also attacks the cells of the excretory system. This observation explains the number of renal damage cases reported worldwide (Legrand et al., 2021), where the virus can infect various organs and exacerbate previous kidney health problems.
Kidney damage can result from several circumstances. The putative mechanism of renal injury is induced by viral cytokine damage of podocytes and renal tubular epithelial cells via ACE2 receptor proteins expressed at high levels in the kidney (Zou et al., 2020). The cells damage can also be caused by abnormally low levels of blood oxygen or by the presence of tiny clots in the bloodstream.
Not surprisingly, many COVID‐19 patients required kidney replacement therapy (F. Jiang, Zhang, Xie, Zhang, & Wang, 2019), and post‐COVID regeneration in such patients took much longer. Kidney involvement in SARS‐CoV‐2 infection is associated with kidney tubular injury, impaired glomerular filtration, and manifests as proteinuria, hematuria with increased serum creatinine and urea nitrogen level (X. Han & Ye, 2021; N. Chen et al., 2020; Naicker et al., 2020). In a study by Richardson et al., 454 of 5,700 hospitalized COVID‐19 patients had underlying kidney disease, of which 81 patients were treated by renal replacement therapy (Richardson et al., 2020).
Regarding renoprotective effectiveness, flavonoids are well‐known antihypertensive substances that promote diuresis and natriuresis. Rutin can be found in onion, apples, red wine, tea, citrus fruit, and figs. It may decrease elevated blood pressure, cardiac fibrosis, left ventricular stiffness, hypertrophy, normalize plasma uric acid, ameliorate renal fibrosis and proteinuria. Thus, improve creatinine clearance and normalize plasma creatinine concentrations (Diwan, Brown, & Gobe, 2017; Y. Han, Lu, Xu, Zhang, & Hong, 2015; Panchal, Poudyal, Arumugam, & Brown, 2011). Oral administration of pectolinaringenin from the aerial parts of Cirsium chanroenicum remarkably improved kidney tubular injury after unilateral ureteral obstruction via inhibition of TGFβ/SMAD3 and JAK2/STAT3 signaling (Y. Li, Guo, Huang, Ma, & Fu, 2021). Astragali radix (AR) root extract from Astragalus membranaceus (Fisch.) is a well‐known source of flavonoids used for the anti‐nephrotic syndrome (L. P. Chen, Zhou, & Yang, 2004). In addition to its antiinflammatory, anticancer, and diuretic action, AR increased kidney blood perfusion and glomerular filtration rate and reduced podocyte injury and urinary protein. The treatment with quercetin improved renal function in a rat model of adenine‐induced chronic kidney disease. Also, it decreased the urine protein‐to‐creatinine ratios, urinary uric acid, creatinine, BUN levels. Reduction in the abnormal histopathological renal changes, including the chronic interstitial inflammation, renal inflammation, and renal tubular damage (H. Yang, Song, Liang, & Li, 2018) was also observed. The grape seed proanthocyanidins show an anti‐nephrotic effect via attenuation of renal JNK and p38 kinase activities resulting in decreased proteinuria, renal hypertrophy, renal fibrosis, and oxidative stress markers (Lan et al., 2015).
Since flavonoids have adverse effects, only clinical studies can confirm or refute the use of flavonoids in the treatment of renal failure. For instance, rutin via mast cell activation can increase the TNF‐α (S. S. Chen, Gong, Liu, & Mohammed, 2000), augment kidney injury, and decrease kidney function. Rutin, together with naringenin and catechin, cannot alleviate a chronic kidney disease (Peng et al., 2012).
As mentioned earlier, flavonoids with their anticoagulant and antithrombotic effect also have a renoprotective impact in preventing kidney damage by blood clots.
4.6. Gastrointestinal post‐COVID‐19 syndrome
Viral disease COVID‐19 also affects the digestive tract what is accompanied by gastrointestinal symptoms such as abdominal pain, diarrhea, vomiting, or loss of smell and taste. Gastrointestinal symptoms have also been observed in similar viral respiratory diseases, such as SARS, which occurred in 2003, and respiratory syndrome in the Middle East, which happened in 2012 (Assiri et al., 2013; Leung et al., 2003). While the presence of SARS‐CoV‐19 in the gastrointestinal tract is demonstrated in COVID‐19 patients, despite multiple SARS‐CoV‐2 RT‐PCR tests performed on oropharyngeal and nasopharyngeal swab samples, were COVID‐19 negative (Brogna et al., 2021) and without respiratory syndrome (Jin et al., 2020). A virus was detected in the feces, thus confirming its feco‐oral transmission (Y. Chen et al., 2020). In some patients' endoscopic examination confirmed the ulcers and bleeding in gastrointestinal tissues associated with COVID‐19. The most severe complication of COVID‐19 related to the ACE2 receptors on pancreatic islets and cytokine storm is pancreatic injury related, resulting in a higher incidence of anorexia and diarrhea (F. Wang et al., 2020).
Due to the highly expressed ACE2 receptors in the colonic tissue, it is expected that patients will be at risk of further GI complications after overcoming COVID‐19 infection. Gastrointestinal sequelae due to COVID‐19 involved loss of appetite, nausea, acid reflux, and diarrhea. They were common in patients 3 months after discharge from hospitalization (J. Weng et al., 2021).
Pancreatic cells express the ACE receptor of SARS‐CoV‐2 on their surface. The risk of viral damage to the pancreas is high. The association between COVID‐19 and acute pancreatitis or pancreas injury is based on evidence provided by many authors (Aloysius et al., 2020; Anand, Major, Pickering, & Nelson, 2020; Lattig et al., 2007; Samies, Yarbrough, & Boppana, 2021; F. Wang et al., 2020). An important issue regarding the consequences of pancreas injury or acute pancreatitis caused by COVID‐19 is the possibility of developing diabetes mellitus type I (Sathish, Kapoor, Cao, Tapp, & Zimmet, 2021; Unsworth et al., 2020; J. K. Yang, Lin, Ji, & Guo, 2010).
The consequences of COVID‐19 on the pancreas related to the origin and development of diabetes mellitus type I (or type II) will probably be known in a few years. Until then, it is necessary to manage both the patients and healthcare resources properly. The role of flavonoids in the prevention of diabetic complications was discovered in animal models. Concerning diabetes mellitus, flavonoids have a significant antidiabetic effect. They can modulate carbohydrate and lipid metabolism, improve adipose tissue metabolism, inhibit α‐glucosidase, attenuate hyperglycemia, insulin resistance, and dyslipidemia (A. Y. Chen & Chen, 2013; Kumar et al., 2011; Sarian et al., 2017). Significant preventive antidiabetic effect of flavonoids was observed in quercetin and chrysin (Lukačínová et al., 2008), apigenin (Panda & Kar, 2007), baicalein (Keshari et al., 2016), hesperidin, and naringin (Ahmed, Mahmoud, Abdel‐Moneim, & Ashour, 2012). Luteolin attenuated insulin resistance and hepatic steatosis (Kwon, Jung, Park, Yun, & Choi, 2015).
The abnormal liver function associated with COVID‐19 is one of the nonpulmonary manifestations. Liver injury is defined as any liver damage that occurs during disease progression related to COVID‐19 treatment in patients with or without a history of previous liver disease (Lozano‐Sepulveda, Galan‐Huerta, Martinez‐Acuna, Arellanos‐Soto, & Rivas‐Estilla, 2020). Elevated hepatic enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are clinical markers of liver dysfunction in blood serum. Their elevated levels are associated with the highest mortality risk (de la Rica et al., 2020). Liver infection in patients with COVID‐19 directly contributes to hepatic impairment and is manifested by microvesicular steatosis and mild lobular and portal activity (Xu et al., 2020). The most liver damage is of hepatocellular type (Cai et al., 2020). The other significant liver damage is antiviral drug‐induced and is potentially hepatotoxic (Piszczatoski & Powell, 2020). After a morin administration, a remarkable effect was observed in rats with IFOS‐induced liver injury. Morin, found in white berry and cranberry branches, decreases the levels of hepatic enzymes AST, ALP, and ALT, reduces the lipid peroxidation product—malondialdehyde, and decreases the levels of inflammatory markers (Ozdemir, Kucukler, Comakli, & Kandemir, 2020). Ternatin, silymarin, quercetin, glycone (rutin), gossypin, and hydroxyetilrutinoside display a vigorous anti‐hepatotoxic activity (Kvasnička, Bíba, Ševčík, VoldŘich, & Krátká, 2003; Rao et al., 1994; Sikder, Kesh, Das, Manna, & Dey, 2014; Vijayaraghavan, Sugendran, Pant, Husain, & Malhotra, 1991).
5. CONCLUSION
This review addresses the systemic complications in patients with the post‐COVID‐19 syndrome and emphasizes the modulatory potential of flavonoids as critical nutritional supplements after the SARS‐CoV‐2 infection. At the same time, we think that potent ACE1 inhibitory and ACE2 stimulatory effects, as well as inhibitory effects on PLA2‐IIA the flavonoids, should be considered in both: prevention and supportive care in asymptomatic patients tested positive for the SARS‐CoV‐2. This study is aimed to instigate a more rigorous interest in flavonoids research and support the hypothesis that flavonoids could represent an excellent supportive care strategy specifically for patients suffering from the post‐COVID‐19 syndrome. The list flavonoids holding therapeutic and prophylactic properties, which may potentially modulate the post post‐COVID‐19 syndrome outcome, is presented in Table 1.
TABLE 1.
Flavonoids list used in modulation of individual clinical manifestations during post‐COVID‐19 syndrome
| Clinical manifestation | Mechanism of action | Flavonoid | References | |
|---|---|---|---|---|
| Pulmonary post‐COVID‐19 syndrome | Pulmonary fibrosis | Inhibition of TGF‐β1 and MMP‐9 expression |
Apigenin Epigallocatechin Quercetin Naringenin Grape seed extract |
L. Chen and Zhao (2016) Sriram et al. (2009) Baowen et al. (2010) Y. Lin et al. (2018) Liu et al. (2017) |
| Cardiovascular post‐COVID‐19 syndrome | Myocarditis | Inhibition of pro‐inflammatory cytokine and inflammatory enzymes PLA2, COX‐2, antioxidant effect |
Quercetin Kaempferol Myricetin Hesperidin Naringenin Catechin |
Mirossay et al. (2001) Rice‐Evans et al. (1996) Knekt et al. (2002) Grzesik et al. (2018) |
| Heart damage | Restrain the release of tissue damaging proteases, increase of PGI2 release, reduction in the coronary perfusion pressure induced by angiotensin II | Procyanidins | Facino et al. (1999) | |
| Increased blood pressure | ACE inhibitors |
Quercetin Hesperidin Naringenin Epicatechin Epigallocatcheningallate Procyanidin Delphinidin‐3‐O‐glucoside Daidzein Genistein Luteolin Kaempferol Isosakuranetin Dihydrokaempferide Betuletol |
Egert et al. (2009) Maruyama et al. (2009) |
|
| Blood clots | Decrease of TXA2, inhibition of COX‐1, interaction with GPIIb/IIIa receptors |
Quercetin Rutin Genistein Daidzein Equol Kaempferol Epicatechin Tangeritin Naringin Naringenin Myricetin Fisetin Morin |
Gryglewski et al. (1987) Shafi et al. (2019) Corvazier and Maclouf (1985) H. Wang et al. (2000) Pearson et al. (2005) Holt et al. (2006) Tzeng et al. (1991) |
|
| Neurological post‐COVID‐19 syndrome |
Anxiety Depression |
Positive modulators of GABAA, modulators of ion channels GIRK and hERG |
Hesperidin Linarin Naringin Diosmin Neohesperidin Gossypin Baicalein Chrysin Astilbin Kaempferitrin Luteolin Vitexin Nobiletin |
Fernandez et al. (2009) Fernandez et al. (2006) de Carvalho et al. (2011) Borges et al. (2016) Aruna et al. (2014) Cassani et al. (2014) de la Pena et al. (2014) Can et al. (2013) H. Huang et al. (2016) |
| Cognitive dysfunction—memory disorders, chronic fatigue syndrome, sleep disorders | Attenuation of oxidative stress, decrease of TNF‐α levels |
Epigallocatechin Naringin Curcumin |
Sachdeva et al. (2011) Vij et al. (2009) Gupta et al. (2009) |
|
| Nephrological post‐COVID‐19 syndrome/kidney failure |
Kidney tubular injury Proteinuria Hematuria Renal fibrosis |
Inhibition of TGFβ/SMAD3 and JAK2/STAT3 |
Quercetin Pectolinaringenin Rutin |
Y. Li et al. (2021) H. Yang et al. (2018) Diwan et al. (2017) Y. Han et al. (2015) Panchal et al. (2011) |
| Gastrointestinal post‐COVID‐19 syndrome | Liver injury elevated ALT, AST |
Decrease of ALT, AST, ALP, MDA Increase of SOD, CAT, GPx, GSH Reduction of NF‐κB and TNF‐α |
Morin Ternatin Silymarin Quercetin Glycone (rutin) Gossypin Hydroxyetilrutinoside |
Ozdemir et al. (2020) Rao et al. (1994) Kvasnička et al. (2003) Sikder et al. (2014) Vijayaraghavan et al. (1991) |
|
Acute pancreatitis Pancreas injury Diabetes mellitus type I |
Increase in SOD and CAT activity and GSH content, decrease of glucose level, serum insulin level, hepatic and muscle glycogen content, AST, LDH and CK‐MB |
Quercetin Chrysin Apigenin Baicalein Hesperidin Naringin |
Lukačínová et al. (2008) Panda and Kar (2007) Keshari et al. (2016) Ahmed et al. (2012) |
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
This publication results from the project implementation: “Open scientific community for modern interdisciplinary research in medicine (OPENMED),” ITMS2014+: 313011V455 supported by the Operational Programme Integrated Infrastructure, funded by the ERDF.
Bardelčíková, A. , Miroššay, A. , Šoltýs, J. , & Mojžiš, J. (2022). Therapeutic and prophylactic effect of flavonoids in post‐COVID‐19 therapy. Phytotherapy Research, 36(5), 2042–2060. 10.1002/ptr.7436
Funding information ERDF
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abderrazak, A. , Syrovets, T. , Couchie, D. , El Hadri, K. , Friguet, B. , Simmet, T. , & Rouis, M. (2015). NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biology, 4, 296–307. 10.1016/j.redox.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abron, J. D. , Singh, N. P. , Price, R. L. , Nagarkatti, M. , Nagarkatti, P. S. , & Singh, U. P. (2018). Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One, 13(7), e0199631. 10.1371/journal.pone.0199631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, O. M. , Mahmoud, A. M. , Abdel‐Moneim, A. , & Ashour, M. B. (2012). Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetologia Croatica, 41(2), 53–67. https://www.researchgate.net/publication/232242638_Antidiabetic_effects_of_hesperidin_and_naringin_in_type_2_diabetic_rats [Google Scholar]
- Alissa, E. M. , & Ferns, G. A. (2017). Dietary fruits and vegetables and cardiovascular diseases risk. Critical Reviews in Food Science and Nutrition, 57(9), 1950–1962. 10.1080/10408398.2015.1040487 [DOI] [PubMed] [Google Scholar]
- Aloysius, M. M. , Thatti, A. , Gupta, A. , Sharma, N. , Bansal, P. , & Goyal, H. (2020). COVID‐19 presenting as acute pancreatitis. Pancreatology, 20(5), 1026–1027. 10.1016/j.pan.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y. W. , Song, S. , Li, W. X. , Chen, Y. X. , Hu, X. P. , Zhao, J. , … Liu, H. Q. (2021). Liver function recovery of COVID‐19 patients after discharge, a follow‐up study. International Journal of Medical Sciences, 18(1), 176–186. 10.7150/ijms.50691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, E. R. , Major, C. , Pickering, O. , & Nelson, M. (2020). Acute pancreatitis in a COVID‐19 patient. The British Journal of Surgery, 107(7), 1–1. 10.1002/bjs.11657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruna, R. , Geetha, A. , & Suguna, P. (2014). Rutin modulates ASC expression in NLRP3 inflammasome: A study in alcohol and cerulein‐induced rat model of pancreatitis. Molecular and Cellular Biochemistry, 396(1–2), 269–280. 10.1007/s11010-014-2162-8 [DOI] [PubMed] [Google Scholar]
- Assiri, A. , Al‐Tawfiq, J. A. , Al‐Rabeeah, A. A. , Al‐Rabiah, F. A. , Al‐Hajjar, S. , Al‐Barrak, A. , … Memish, Z. A. (2013). Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infectious Diseases, 13(9), 752–761. 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, P. V. , Liu, D. , & Gilbert, E. R. (2013). Recent advances in understanding the anti‐diabetic actions of dietary flavonoids. The Journal of Nutritional Biochemistry, 24(11), 1777–1789. 10.1016/j.jnutbio.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader, F. , Manla, Y. , Atallah, B. , & Starling, R. C. (2021). Heart failure and COVID‐19. Heart Failure Reviews, 26(1), 1–10. 10.1007/s10741-020-10008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez‐Santos, Y. M. , St John, S. E. , & Mesecar, A. D. (2015). The SARS‐coronavirus papain‐like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Research, 115, 21–38. 10.1016/j.antiviral.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baowen, Q. , Yulin, Z. , Xin, W. , Wenjing, X. , Hao, Z. , Zhizhi, C. , … Lijuan, C. (2010). A further investigation concerning correlation between anti‐fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. European Journal of Pharmacology, 642(1–3), 134–139. 10.1016/j.ejphar.2010.05.019 [DOI] [PubMed] [Google Scholar]
- Batcik, O. E. , Kanat, A. , Cankay, T. U. , Ozturk, G. , Kazancioglu, L. , Kazdal, H. , … Sevilgen, G. (2021). COVID‐19 infection produces subarachnoid hemorrhage; acting now to understand its cause: A short communication. Clinical Neurology and Neurosurgery, 202, 106495. 10.1016/j.clineuro.2021.106495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni, A. , Cassis, P. , & Remuzzi, G. (2010). Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Molecular Medicine, 2(7), 247–257. 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmik, D. , Nandi, R. , Prakash, A. , & Kumar, D. (2021). Evaluation of flavonoids as 2019‐nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon, 7(3), e06515. 10.1016/j.heliyon.2021.e06515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan, M. N. , Mitsuhashi, S. , Sigetomi, K. , & Ubukata, M. (2017). Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Bioscience, Biotechnology, and Biochemistry, 81(5), 882–890. 10.1080/09168451.2017.1282805 [DOI] [PubMed] [Google Scholar]
- Borges, C. , Jesse, C. R. , Donato, F. , Del Fabbro, L. , de Gomes, M. G. , Goes, A. T. R. , … Boeira, S. P. (2016). Chrysin promotes attenuation of depressive‐like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chemico‐Biological Interactions, 260, 154–162. 10.1016/j.cbi.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Brendler, T. , Al‐Harrasi, A. , Bauer, R. , Gafner, S. , Hardy, M. L. , Heinrich, M. , … Williamson, E. M. (2021). Botanical drugs and supplements affecting the immune response in the time of COVID‐19: Implications for research and clinical practice. Phytotherapy Research, 35(6), 3013–3031. 10.1002/ptr.7008 [DOI] [PubMed] [Google Scholar]
- Brogna, B. , Brogna, C. , Petrillo, M. , Conte, A. M. , Benincasa, G. , Montano, L. , & Piscopo, M. (2021). SARS‐CoV‐2 detection in fecal sample from a patient with typical findings of COVID‐19 pneumonia on CT but negative to multiple SARS‐CoV‐2 RT‐PCR tests on oropharyngeal and nasopharyngeal swab samples. Medicina‐Lithuania, 57(3), 1–7. 10.3390/medicina57030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti, C. , Di Ferdinando, M. , Fini, A. , Pollastri, S. , & Tattini, M. (2013). Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. International Journal of Molecular Sciences, 14(2), 3540–3555. 10.3390/ijms14023540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. , Huang, D. , Yu, H. , Zhu, Z. , Xia, Z. , Su, Y. , … Xu, L. (2020). COVID‐19: Abnormal liver function tests. Journal of Hepatology, 73(3), 566–574. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can, O. D. , Demir Ozkay, U. , & Ucel, U. I. (2013). Anti‐depressant‐like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. European Journal of Pharmacology, 699(1–3), 250–257. 10.1016/j.ejphar.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Carfi, A. , Bernabei, R. , & Landi, F. (2020). Persistent symptoms in patients after acute COVID‐19. JAMA, 324(6), 603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani, J. , Dorantes‐Barron, A. M. , Novales, L. M. , Real, G. A. , & Estrada‐Reyes, R. (2014). Anti‐depressant‐like effect of kaempferitrin isolated from Justicia spicigera Schltdl (Acanthaceae) in two behavior models in mice: Evidence for the involvement of the serotonergic system. Molecules, 19(12), 21442–21461. 10.3390/molecules191221442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy, A. , O'Reilly, E. J. , Kay, C. , Sampson, L. , Franz, M. , Forman, J. P. , … Rimm, E. B. (2011). Habitual intake of flavonoid subclasses and incident hypertension in adults. American Journal of Clinical Nutrition, 93(2), 338–347. 10.3945/ajcn.110.006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekara, S. , Jaladhar, P. , Paramshetti, S. , Ramachandran, V. , Nizar, S. F. , & Kori, D. (2020). Post COVID inflammation syndrome: Different manifestations caused by the virus. The Journal of the Association of Physicians of India, 68(12), 33–34. [PubMed] [Google Scholar]
- Chang, H. W. , Baek, S. H. , Chung, K. W. , Son, K. H. , Kim, H. P. , & Kang, S. S. (1994). Inactivation of phospholipase A2 by naturally occurring biflavonoid, ochnaflavone. Biochemical and Biophysical Research Communications, 205(1), 843–849. 10.1006/bbrc.1994.2741 [DOI] [PubMed] [Google Scholar]
- Chen, A. Y. , & Chen, Y. C. (2013). A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry, 138(4), 2099–2107. 10.1016/j.foodchem.2012.11.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. Y. , Peng, W. H. , Wu, L. C. , Wu, C. C. , & Hsu, S. L. (2010). Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: Implications for therapy of lung fibrosis. Journal of Agricultural and Food Chemistry, 58(22), 11653–11661. 10.1021/jf1031668 [DOI] [PubMed] [Google Scholar]
- Chen, H. , & Du, Q. (2020). Potential natural compounds for preventing 2019‐nCoV infection. Preprint, 1–17. 10.20944/preprints202001.0358.v3 [DOI] [Google Scholar]
- Chen, H. , Lin, H. , Xie, S. , Huang, B. , Qian, Y. , Chen, K. , … Wu, Y. (2019). Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS‐dependent ubiquitination of ASC and promotion of ROS‐independent NLRP3 ubiquitination. Toxicology and Applied Pharmacology, 365, 19–29. 10.1016/j.taap.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Chen, L. , & Zhao, W. (2016). Apigenin protects against bleomycin‐induced lung fibrosis in rats. Experimental and Therapeutic Medicine, 11(1), 230–234. 10.3892/etm.2015.2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. P. , Zhou, Q. L. , & Yang, J. H. (2004). Protective effects of astragali injection on tubular in patients with primary nephrotic syndrome. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 29(2), 152–153. https://pubmed.ncbi.nlm.nih.gov/16145898/ [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , … Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. S. , Gong, J. , Liu, F. T. , & Mohammed, U. (2000). Naturally occurring polyphenolic antioxidants modulate IgE‐mediated mast cell activation. Immunology, 100(4), 471–480. 10.1046/j.1365-2567.2000.00045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Chen, L. , Deng, Q. , Zhang, G. , Wu, K. , Ni, L. , … Cheng, Z. (2020). The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. Journal of Medical Virology, 92(7), 833–840. 10.1002/jmv.25825 [DOI] [PubMed] [Google Scholar]
- Cherrak, S. A. , Merzouk, H. , & Mokhtari‐Soulimane, N. (2020). Potential bioactive glycosylated flavonoids as SARS‐CoV‐2 main protease inhibitors: A molecular docking and simulation studies. PLoS One, 15(10), e0240653. 10.1371/journal.pone.0240653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo, S. , Marzotto, M. , Conforti, A. , Vella, A. , Ortolani, R. , & Bellavite, P. (2010). Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin Mol Allergy, 8, 13. 10.1186/1476-7961-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, B. F. , & Raghu, G. (2019). Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. European Respiratory Review, 28(153), 190022. 10.1183/16000617.0022-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada, M. , Ballester, I. , Bailon, E. , Xaus, J. , Galvez, J. , de Medina, F. S. , & Zarzuelo, A. (2006). Inhibition of pro‐inflammatory markers in primary bone marrow‐derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure‐activity relationship. Biochemical Pharmacology, 72(8), 1010–1021. 10.1016/j.bcp.2006.07.016 [DOI] [PubMed] [Google Scholar]
- Cordoba, A. , Satue, M. , Gomez‐Florit, M. , Hierro‐Oliva, M. , Petzold, C. , Lyngstadaas, S. P. , … Ramis, J. M. (2015). Flavonoid‐modified surfaces: Multifunctional bioactive biomaterials with osteopromotive, anti‐inflammatory, and anti‐fibrotic potential. Advanced Healthcare Materials, 4(4), 540–549. 10.1002/adhm.201400587 [DOI] [PubMed] [Google Scholar]
- Correia‐da‐Silva, M. , Sousa, E. , Duarte, B. , Marques, F. , Carvalho, F. , Cunha‐Ribeiro, L. M. , & Pinto, M. M. (2011). Flavonoids with an oligopolysulfated moiety: A new class of anticoagulant agents. Journal of Medicinal Chemistry, 54(1), 95–106. 10.1021/jm1013117 [DOI] [PubMed] [Google Scholar]
- Corvazier, E. , & Maclouf, J. (1985). Interference of some flavonoids and non‐steroidal anti‐inflammatory drugs with oxidative‐metabolism of Arachidonic‐acid by human‐platelets and neutrophils. Biochimica et Biophysica Acta, 835(2), 315–321. 10.1016/0005-2760(85)90287-5 [DOI] [PubMed] [Google Scholar]
- Daba, M. H. , Abdel‐Aziz, A. A. , Moustafa, A. M. , Al‐Majed, A. A. , Al‐Shabanah, O. A. , & El‐Kashef, H. A. (2002). Effects of L‐carnitine and ginkgo biloba extract (EG b 761) in experimental bleomycin‐induced lung fibrosis. Pharmacological Research, 45(6), 461–467. 10.1006/phrs.2002.0985 [DOI] [PubMed] [Google Scholar]
- Dai, W. , Bi, J. , Li, F. , Wang, S. , Huang, X. , Meng, X. , … Su, W. (2019). Antiviral efficacy of flavonoids against Enterovirus 71 infection in vitro and in newborn mice. Viruses, 11(7), 1–14. 10.3390/v11070625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido, B. , Seang, S. , Tubiana, R. , & de Truchis, P. (2020). Post‐COVID‐19 chronic symptoms: A postinfectious entity? Clinical Microbiology and Infection, 26(11), 1448–1449. 10.1016/j.cmi.2020.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho, R. S. , Duarte, F. S. , & de Lima, T. C. (2011). Involvement of GABAergic non‐benzodiazepine sites in the anxiolytic‐like and sedative effects of the flavonoid baicalein in mice. Behavioural Brain Research, 221(1), 75–82. 10.1016/j.bbr.2011.02.038 [DOI] [PubMed] [Google Scholar]
- de la Pena, J. B. , Kim, C. A. , Lee, H. L. , Yoon, S. Y. , Kim, H. J. , Hong, E. Y. , … Cheong, J. H. (2014). Luteolin mediates the antidepressant‐like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Archives of Pharmacal Research, 37(2), 263–269. 10.1007/s12272-013-0229-9 [DOI] [PubMed] [Google Scholar]
- de la Rica, R. , Borges, M. , Aranda, M. , Del Castillo, A. , Socias, A. , Payeras, A. , … Gonzalez‐Freire, M. (2020). Low albumin levels are associated with poorer outcomes in a case series of COVID‐19 patients in Spain: A retrospective cohort study. Microorganisms, 8(8), 1–13. 10.3390/microorganisms8081106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuc, B. , & Smadja, D. M. (2021). Is COVID‐19 a new hematologic disease? Stem Cell Reviews and Reports, 17(1), 4–8. 10.1007/s12015-020-09987-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. F. , Aluko, R. E. , Jin, Q. , Zhang, Y. , & Yuan, L. J. (2012). Inhibitory activities of baicalin against renin and angiotensin‐converting enzyme. Pharmaceutical Biology, 50(4), 401–406. 10.3109/13880209.2011.608076 [DOI] [PubMed] [Google Scholar]
- Derosa, G. , Maffioli, P. , D'Angelo, A. , & Di Pierro, F. (2021). A role for quercetin in coronavirus disease 2019 (COVID‐19). Phytotherapy Research, 35(3), 1230–1236. 10.1002/ptr.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan, V. , Brown, L. , & Gobe, G. C. (2017). The flavonoid rutin improves kidney and heart structure and function in an adenine‐induced rat model of chronic kidney disease. Journal of Functional Foods, 33, 85–93. 10.1016/j.jff.2017.03.012 [DOI] [Google Scholar]
- Doobay, M. F. , Talman, L. S. , Obr, T. D. , Tian, X. , Davisson, R. L. , & Lazartigues, E. (2007). Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin‐angiotensin system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 292(1), R373–R381. 10.1152/ajpregu.00292.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, A. K. , Shankar, P. R. , Upadhyaya, D. , & Deshpande, V. Y. (2004). Ginkgo biloba‐‐an appraisal. Kathmandu Univ Med J (KUMJ), 2(3), 225–229. PMID: 16400219 [PubMed] [Google Scholar]
- Egert, S. , Bosy‐Westphal, A. , Seiberl, J. , Kurbitz, C. , Settler, U. , Plachta‐Danielzik, S. , … Muller, M. J. (2009). Quercetin reduces systolic blood pressure and plasma oxidised low‐density lipoprotein concentrations in overweight subjects with a high‐cardiovascular disease risk phenotype: A double‐blinded, placebo‐controlled cross‐over study. The British Journal of Nutrition, 102(7), 1065–1074. 10.1017/S0007114509359127 [DOI] [PubMed] [Google Scholar]
- El‐Missiry, M. A. , Fekri, A. , Kesar, L. A. , & Othman, A. I. (2021). Polyphenols are potential nutritional adjuvants for targeting COVID‐19. Phytotherapy Research, 35(6), 2879–2889. 10.1002/ptr.6992 [DOI] [PubMed] [Google Scholar]
- Erdman, J. W. , Balentine, D. , Arab, L. , Beecher, G. , Dwyer, J. T. , Folts, J. , … Burrowes, J. (2007). Flavonoids and heart health: Proceedings of the ILSI North America flavonoids workshop, May 31‐June 1, 2005, Washington, DC. Journal of Nutrition, 137(3), 718s–737s. [DOI] [PubMed] [Google Scholar]
- Ernst, E. (2002). The risk‐benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, ginseng, Echinacea, saw palmetto, and kava. Annals of Internal Medicine, 136(1), 42–53. 10.7326/0003-4819-136-1-200201010-00010 [DOI] [PubMed] [Google Scholar]
- Facino, R. M. , Carini, M. , Aldini, G. , Berti, F. , Rossoni, G. , Bombardelli, E. , & Morazzoni, P. (1999). Diet enriched with procyanidins enhances antioxidant activity and reduces myocardial post‐ischaemic damage in rats. Life Sciences, 64(8), 627–642. 10.1016/S0024-3205(98)00605-5 [DOI] [PubMed] [Google Scholar]
- Feghali, C. A. , & Wright, T. M. (1997). Cytokines in acute and chronic inflammation. Frontiers in Bioscience, 2, d12–d26. 10.2741/a171 [DOI] [PubMed] [Google Scholar]
- Fernandez, S. P. , Nguyen, M. , Yow, T. T. , Chu, C. , Johnston, G. A. , Hanrahan, J. R. , & Chebib, M. (2009). The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochemical Research, 34(10), 1867–1875. 10.1007/s11064-009-9969-9 [DOI] [PubMed] [Google Scholar]
- Fernandez, S. P. , Wasowski, C. , Loscalzo, L. M. , Granger, R. E. , Johnston, G. A. , Paladini, A. C. , & Marder, M. (2006). Central nervous system depressant action of flavonoid glycosides. European Journal of Pharmacology, 539(3), 168–176. 10.1016/j.ejphar.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Fu, W. , Wang, C. , Zou, L. , Guo, Y. , Lu, Z. , Yan, S. , & Mao, J. (2020). Psychological health, sleep quality, and coping styles to stress facing the COVID‐19 in Wuhan, China. Translational Psychiatry, 10(1), 225. 10.1038/s41398-020-00913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati, G. , & O'Brien, P. J. (2004). Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radical Biology & Medicine, 37(3), 287–303. 10.1016/j.freeradbiomed.2004.04.034 [DOI] [PubMed] [Google Scholar]
- Gao, J. , Ding, Y. , Wang, Y. , Liang, P. , Zhang, L. , & Liu, R. (2021). Oroxylin a is a severe acute respiratory syndrome coronavirus 2‐spiked pseudotyped virus blocker obtained from radix Scutellariae using angiotensin‐converting enzyme II/cell membrane chromatography. Phytotherapy Research, 35(6), 3194–3204. 10.1002/ptr.7030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Lu, J. , Zhang, Y. , Chen, Y. , Gu, Z. , & Jiang, X. (2013). Baicalein attenuates bleomycin‐induced pulmonary fibrosis in rats through inhibition of miR‐21. Pulmonary Pharmacology & Therapeutics, 26(6), 649–654. 10.1016/j.pupt.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Garcia‐Mediavilla, V. , Crespo, I. , Collado, P. S. , Esteller, A. , Sanchez‐Campos, S. , Tunon, M. J. , & Gonzalez‐Gallego, J. (2007). The anti‐inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase‐2 and reactive C‐protein, and down‐regulation of the nuclear factor kappaB pathway in Chang liver cells. European Journal of Pharmacology, 557(2–3), 221–229. 10.1016/j.ejphar.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Garrigues, E. , Janvier, P. , Kherabi, Y. , Le Bot, A. , Hamon, A. , Gouze, H. , … Nguyen, Y. (2020). Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. Journal of Infection, 81(6), E4–E6. 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, S. J. , Su, X. J. , Yu, H. P. , Li, J. , Qin, Y. J. , Xu, Q. , & Luo, W. (2008). A study on anti‐SARS‐CoV 3CL protein of flavonoids from litchi chinensis sonn core. Pharmacological Bulletin, 24, 699–700. [Google Scholar]
- Gorlach, S. , Fichna, J. , & Lewandowska, U. (2015). Polyphenols as mitochondria‐targeted anticancer drugs. Cancer Letters, 366(2), 141–149. 10.1016/j.canlet.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Gour, A. , Manhas, D. , Bag, S. , Gorain, B. , & Nandi, U. (2021). Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS‐CoV‐2. Phytotherapy Research, 35(8), 4258–4283. 10.1002/ptr.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski, R. J. , Korbut, R. , Robak, J. , & Swies, J. (1987). On the mechanism of antithrombotic action of flavonoids. Biochemical Pharmacology, 36(3), 317–322. 10.1016/0006-2952(87)90288-7 [DOI] [PubMed] [Google Scholar]
- Grzesik, M. , Naparlo, K. , Bartosz, G. , & Sadowska‐Bartosz, I. (2018). Antioxidant properties of catechins: Comparison with other antioxidants. Food Chemistry, 241, 480–492. 10.1016/j.foodchem.2017.08.117 [DOI] [PubMed] [Google Scholar]
- Guerrero, J. A. , Lozano, M. L. , Castillo, J. , Benavente‐Garcia, O. , Vicente, V. , & Rivera, A. (2005). Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. Journal of Thrombosis and Haemostasis, 3(2), 369–376. 10.1111/j.1538-7836.2004.01099.x [DOI] [PubMed] [Google Scholar]
- Guerrero, L. , Castillo, J. , Quinones, M. , Garcia‐Vallve, S. , Arola, L. , Pujadas, G. , & Muguerza, B. (2012). Inhibition of angiotensin‐converting enzyme activity by flavonoids: Structure‐activity relationship studies. PLoS One, 7(11), e49493. 10.1371/journal.pone.0049493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Vij, G. , Sharma, S. , Tirkey, N. , Rishi, P. , & Chopra, K. (2009). Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology, 214(1), 33–39. 10.1016/j.imbio.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D. P. , Penn‐Nicholson, A. , & Cho, M. W. (2006). Identification of critical determinants on ACE2 for SARS‐CoV entry and development of a potent entry inhibitor. Virology, 350(1), 15–25. 10.1016/j.virol.2006.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, N. , Gu, Y. , Ye, C. , Cao, Y. , Liu, Z. , & Yin, J. (2012). Antithrombotic activity of fractions and components obtained from raspberry leaves (Rubus chingii). Food Chemistry, 132(1), 181–185. 10.1016/j.foodchem.2011.10.051 [DOI] [PubMed] [Google Scholar]
- Han, X. , & Ye, Q. (2021). Kidney involvement in COVID‐19 and its treatments. Journal of Medical Virology, 93(3), 1387–1395. 10.1002/jmv.26653 [DOI] [PubMed] [Google Scholar]
- Han, Y. , Lu, J. S. , Xu, Y. , Zhang, L. , & Hong, B. F. (2015). Rutin ameliorates renal fibrosis and proteinuria in 5/6‐nephrectomized rats by anti‐oxidation and inhibiting activation of TGF β1‐smad signaling. International Journal of Clinical and Experimental Pathology, 8(5), 4725–4734. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4503034/pdf/ijcep0008-4725.pdf [PMC free article] [PubMed] [Google Scholar]
- Hertog, M. G. L. , Feskens, E. J. M. , Hollman, P. C. H. , Katan, M. B. , & Kromhout, D. (1993). Dietary antioxidant flavonoids and risk of coronary heart‐disease ‐ the Zutphen elderly study. Lancet, 342(8878), 1007–1011. 10.1016/0140-6736(93)92876-U [DOI] [PubMed] [Google Scholar]
- Hettihewa, S. K. , Hemar, Y. , & Rupasinghe, H. P. V. (2018). Flavonoid‐rich extract of Actinidia macrosperma (A Wild Kiwifruit) inhibits angiotensin‐converting enzyme in vitro. Food, 7(9), 1–8. 10.3390/foods7090146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, T. Y. , Wu, S. L. , Chen, J. C. , Li, C. C. , & Hsiang, C. Y. (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin‐converting enzyme 2 interaction. Antiviral Research, 74(2), 92–101. 10.1016/j.antiviral.2006.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, R. R. , Actis‐Goretta, L. , Momma, T. Y. , & Keen, C. L. (2006). Dietary flavanols and platelet reactivity. Journal of Cardiovascular Pharmacology, 47, 187–196. 10.1097/00005344-200606001-00014 [DOI] [PubMed] [Google Scholar]
- Hrčková, G. , Mačák Kubašková, T. , Mudroňová, D. , & Bardelčíková, A. (2020). Concentration‐dependent effect of silymarin on concanavalin A‐stimulated mouse spleen cells in vitro. European Pharmaceutical Journal, 67(1), 17–26. 10.2478/afpuc-2020-0003 [DOI] [Google Scholar]
- Huang, C. , Huang, L. , Wang, Y. , Li, X. , Ren, L. , Gu, X. , … Cao, B. (2021). 6‐month consequences of COVID‐19 in patients discharged from hospital: A cohort study. Lancet, 397(10270), 220–232. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Li, L. , Shi, W. , Liu, H. , Yang, J. , Yuan, X. , & Wu, L. (2016). The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evidence‐Based Complementary and Alternative Medicine, 2016, 2918796. 10.1155/2016/2918796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügel, H. M. , Jackson, N. , May, B. , Zhang, A. L. , & Xue, C. C. (2016). Polyphenol protection and treatment of hypertension. Phytomedicine, 23(2), 220–231. 10.1016/j.phymed.2015.12.012 [DOI] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , & Penninger, J. M. (2008). The discovery of angiotensin‐converting enzyme 2 and its role in acute lung injury in mice. Experimental Physiology, 93(5), 543–548. 10.1113/expphysiol.2007.040048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarajapu, Y. P. R. (2021). Targeting angiotensin‐converting enzyme‐2/angiotensin‐(1‐7)/Mas receptor axis in the vascular progenitor cells for cardiovascular diseases. Molecular Pharmacology, 99(1), 29–38. 10.1124/mol.119.117580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, H. P. , Look, D. C. , Shi, L. , Hickey, M. , Pewe, L. , Netland, J. , … McCray, P. B. (2005). ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. Journal of Virology, 79(23), 14614–14621. 10.1128/Jvi.79.23.14614-14621.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, D. H. , & McCoy, R. G. (2020). Planning for the post‐COVID syndrome: How payers can mitigate long‐term complications of the pandemic. Journal of General Internal Medicine, 35(10), 3036–3039. 10.1007/s11606-020-06042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, F. , Zhang, L. , Xie, H. , Zhang, J. , & Wang, M. J. (2019). The effect and clinical significance of continuous renal replacement therapy on sepsis and liver and kidney functions. International Journal of Clinical and Experimental Medicine, 12(10), 12303–12312. shorturl.at/ltuLT [Google Scholar]
- Jin, X. , Lian, J. S. , Hu, J. H. , Gao, J. B. , Zheng, L. , Zhang, Y. M. , … Yang, Y. (2020). Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut, 69(6), 1002–1009. 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, D. R. , Belal, S. A. , Choe, H. S. , Shin, D. K. , & Shim, K. S. (2018). Effect of kaempferol on cyclooxygenase 2 (Cox2) and cytosolic phospholipase A2 (cPLA2) protein expression in BALB/c mice. Iranian Journal of Allergy, Asthma, and Immunology, 17(5), 428–435. 10.18502/ijaai.v17i5.301 [DOI] [PubMed] [Google Scholar]
- Kaul, T. N. , Middleton, E. , & Ogra, P. L. (1985). Antiviral effect of flavonoids on human viruses. Journal of Medical Virology, 15(1), 71–79. 10.1002/jmv.1890150110 [DOI] [PubMed] [Google Scholar]
- Kello, M. , Kuliková, L. , Vašková, J. , Nagyová, A. , & Mojžiš, J. (2017). Fruit peel polyphenolic extract‐induced apoptosis in human breast cancer cells is associated with ROS production and modulation of p38MAPK/Erk1/2 and the Akt signaling pathway. Nutrition and Cancer, 69(6), 920–931. 10.1080/01635581.2017.1339819 [DOI] [PubMed] [Google Scholar]
- Keshari, A. K. , Kumar, G. , Kushwaha, P. S. , Bhardwaj, M. , Kumar, P. , Rawat, A. , … Saha, S. (2016). Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. Journal of Ethnopharmacology, 181, 252–262. 10.1016/j.jep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Khan, A. , Heng, W. , Wang, Y. , Qiu, J. , Wei, X. , Peng, S. , … Wei, D. Q. (2021). In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS‐CoV‐2 main protease (3CLpro). Phytotherapy Research, 35(6), 2841–2845. 10.1002/ptr.6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. P. , Son, K. H. , Chang, H. W. , & Kang, S. S. (2004). Anti‐inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences, 96(3), 229–245. 10.1254/jphs.crj04003x [DOI] [PubMed] [Google Scholar]
- Knekt, P. , Kumpulainen, J. , Jarvinen, R. , Rissanen, H. , Heliovaara, M. , Reunanen, A. , … Aromaa, A. (2002). Flavonoid intake and risk of chronic diseases. American Journal of Clinical Nutrition, 76(3), 560–568. 10.1093/ajcn/76.3.560 [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Narwal, S. , Kumar, V. , & Prakash, O. (2011). Alpha‐glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacognosy Reviews, 5(9), 19–29. 10.4103/0973-7847.79096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvasnička, F. , Bíba, B. , Ševčík, R. , Voldřich, M. , & Krátká, J. (2003). Analysis of the active components of silymarin. Journal of Chromatography A, 990(1–2), 239–245. 10.1016/S0021-9673(02)01971-4 [DOI] [PubMed] [Google Scholar]
- Kwon, E. Y. , Jung, U. J. , Park, T. , Yun, J. W. , & Choi, M. S. (2015). Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet‐induced obesity. Diabetes, 64(5), 1658–1669. 10.2337/db14-0631 [DOI] [PubMed] [Google Scholar]
- Lago, J. H. , Toledo‐Arruda, A. C. , Mernak, M. , Barrosa, K. H. , Martins, M. A. , Tiberio, I. F. , & Prado, C. M. (2014). Structure‐activity association of flavonoids in lung diseases. Molecules, 19(3), 3570–3595. 10.3390/molecules19033570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, C. Z. , Ding, L. , Su, Y. L. , Guo, K. , Wang, L. , Kan, H. W. , … Gao, S. (2015). Grape seed proanthocyanidins prevent DOCA‐salt hypertension‐induced renal injury and its mechanisms in rats. Food & Function, 6(7), 2179–2186. 10.1039/c5fo00253b [DOI] [PubMed] [Google Scholar]
- Lattig, J. , Bohl, M. , Fischer, P. , Tischer, S. , Tietbohl, C. , Menschikowski, M. , … Pisabarro, M. T. (2007). Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: Rationale for lead design. Journal of Computer‐Aided Molecular Design, 21(8), 473–483. 10.1007/s10822-007-9129-8 [DOI] [PubMed] [Google Scholar]
- Lee, S. J. , Son, K. H. , Chang, H. W. , Kang, S. S. , & Kim, H. P. (1997). Inhibition of arachidonate release from rat peritoneal macrophage by biflavonoids. Archives of Pharmacal Research, 20(6), 533–538. 10.1007/BF02975207 [DOI] [PubMed] [Google Scholar]
- Legrand, M. , Bell, S. , Forni, L. , Joannidis, M. , Koyner, J. L. , Liu, K. , & Cantaluppi, V. (2021). Pathophysiology of COVID‐19‐associated acute kidney injury. Nature Reviews Nephrology, 17, 751–764. 10.1038/s41581-021-00452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesjak, M. , Beara, I. , Simin, N. , Pintać, D. , Majkić, T. , Bekvalac, K. , Orčić, D. , & Mimica‐Dukić, N. (2018). Antioxidant and anti‐inflammatory activities of quercetin and its derivatives. Journal of Functional Foods, 40, 68–75. 10.1016/j.jff.2017.10.047 [DOI] [Google Scholar]
- Leung, W. K. , To, K. F. , Chan, P. K. S. , Chan, H. L. Y. , Wu, A. K. L. , Lee, N. , … Sung, J. J. Y. (2003). Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology, 125(4), 1011–1017. 10.1053/S0016-5085(03)01215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva‐López, N. , Gutierrez‐Grijalva, E. P. , Ambriz‐Perez, D. L. , & Heredia, J. B. (2016). Flavonoids as cytokine modulators: A possible therapy for inflammation‐related diseases. International Journal of Molecular Sciences, 17(6), 1–15. 10.3390/ijms17060921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. F. , Chen, S. M. , Chen, X. M. , Mu, R. H. , Wang, S. S. , Geng, D. , … Yi, L. T. (2016). ERK‐dependent brain‐derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Research Bulletin, 124, 40–47. 10.1016/j.brainresbull.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Li, L. C. , & Kan, L. D. (2017). Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. Journal of Ethnopharmacology, 198, 45–63. 10.1016/j.jep.2016.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Ye, T. , Long, T. , & Peng, X. M. (2019). Ginkgetin exerts anti‐inflammatory effects on cerebral ischemia/reperfusion‐induced injury in a rat model via the TLR4/NF‐kB signaling pathway. Bioscience Biotechnology and Biochemistry, 83(4), 675–683. 10.1080/09168451.2018.1553608 [DOI] [PubMed] [Google Scholar]
- Li, W. , Moore, M. J. , Vasilieva, N. , Sui, J. , Wong, S. K. , Berne, M. A. , … Farzan, M. (2003). Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426(6965), 450–454. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Guo, F. , Huang, R. , Ma, L. , & Fu, P. (2021). Natural flavonoid pectolinarigenin alleviated kidney fibrosis via inhibiting the activation of TGFbeta/SMAD3 and JAK2/STAT3 signaling. International Immunopharmacology, 91, 107279. 10.1016/j.intimp.2020.107279 [DOI] [PubMed] [Google Scholar]
- Lim, H. , Min, D. S. , Park, H. , & Kim, H. P. (2018). Flavonoids interfere with NLRP3 inflammasome activation. Toxicology and Applied Pharmacology, 355, 93–102. 10.1016/j.taap.2018.06.022 [DOI] [PubMed] [Google Scholar]
- Lin, C. C. , Yu, C. S. , Yang, J. S. , Lu, C. C. , Chiang, J. H. , Lin, J. P. , … Chung, J. G. (2012). Chrysin, a natural and biologically active flavonoid, influences a murine leukemia model in vivo through enhancing populations of T‐and B‐cells, and promoting macrophage phagocytosis and NK cell cytotoxicity. In Vivo, 26(4), 665–670. [PubMed] [Google Scholar]
- Lin, L. Z. , Mukhopadhyay, S. , Robbins, R. J. , & Harnly, J. M. (2007). Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC‐DAD‐ESI/MS analysis. Journal of Food Composition and Analysis, 20(5), 361–369. 10.1016/j.jfca.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , Tan, D. , Kan, Q. , Xiao, Z. , & Jiang, Z. (2018). The protective effect of naringenin on airway remodeling after Mycoplasma pneumoniae infection by inhibiting autophagy‐mediated lung inflammation and fibrosis. Mediators of Inflammation, 2018, 8753894. 10.1155/2018/8753894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, M. , & Tagesson, C. (1997). Flavonoids as phospholipase A2 inhibitors: Importance of their structure for selective inhibition of group II phospholipase A2. Inflammation, 21(3), 347–356. 10.1023/a:1027306118026 [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Jiang, J. X. , Liu, Y. N. , Ge, L. T. , Guan, Y. , Zhao, W. , … Xie, Q. M. (2017). Grape seed extract ameliorates bleomycin‐induced mouse pulmonary fibrosis. Toxicology Letters, 273, 1–9. 10.1016/j.toxlet.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Loizzo, M. R. , Said, A. , Tundis, R. , Rashed, K. , Statti, G. A. , Hufner, A. , & Menichini, F. (2007). Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytotherapy Research, 21(1), 32–36. 10.1002/ptr.2008 [DOI] [PubMed] [Google Scholar]
- Losada‐Barreiro, S. , & Bravo‐Diaz, C. (2017). Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. European Journal of Medicinal Chemistry, 133, 379–402. 10.1016/j.ejmech.2017.03.061 [DOI] [PubMed] [Google Scholar]
- Lozano‐Sepulveda, S. A. , Galan‐Huerta, K. , Martinez‐Acuna, N. , Arellanos‐Soto, D. , & Rivas‐Estilla, A. M. (2020). SARS‐CoV‐2 another kind of liver aggressor, how does it do that? Annals of Hepatology, 19(6), 592–596. 10.1016/j.aohep.2020.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukačínová, A. , Mojžiš, J. , Beňačka, R. , Keller, J. , Maguth, T. , Kurila, P. , Vaško, L. , Rázz, O. , & Ništiar, F. (2008). Preventive effects of flavonoids on alloxan‐induced diabetes mellitus in rats. Acta Veterinaria Brno, 77(2), 175–182. 10.2754/avb200877020175 [DOI] [Google Scholar]
- Lv, Q. Q. , Wu, W. J. , Guo, X. L. , Liu, R. L. , Yang, Y. P. , Zhou, D. S. , … Liu, J. Y. (2014). Antidepressant activity of astilbin: Involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biological & Pharmaceutical Bulletin, 37(6), 987–995. 10.1248/bpb.b13-00968 [DOI] [PubMed] [Google Scholar]
- Mahady, G. B. (2002). Ginkgo biloba for the prevention and treatment of cardiovascular disease: A review of the literature. The Journal of Cardiovascular Nursing, 16(4), 21–32. 10.1097/00005082-200207000-00004 [DOI] [PubMed] [Google Scholar]
- Maiti, S. , & Banerjee, A. (2021). Epigallocatechin gallate and theaflavin gallate interaction in SARS‐CoV‐2 spike‐protein central channel with reference to the hydroxychloroquine interaction: Bioinformatics and molecular docking study. Drug Development Research, 82(1), 86–96. 10.1002/ddr.21730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhafola, T. J. , Elgorashi, E. E. , McGaw, L. J. , Verschaeve, L. , & Eloff, J. N. (2016). The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complementary and Alternative Medicine, 16(1), 490. 10.1186/s12906-016-1437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki, S. J. , Crespo, J. F. , & Cabanillas, B. (2019). Anti‐inflammatory effects of flavonoids. Food Chemistry, 299, 125124. 10.1016/j.foodchem.2019.125124 [DOI] [PubMed] [Google Scholar]
- Marder, M. , & Paladini, A. C. (2002). GABA(A)‐receptor ligands of flavonoid structure. Current Topics in Medicinal Chemistry, 2(8), 853–867. 10.2174/1568026023393462 [DOI] [PubMed] [Google Scholar]
- Marquez‐Flores, Y. K. , Villegas, I. , Cardeno, A. , Rosillo, M. A. , & Alarcon‐de‐la‐Lastra, C. (2016). Apigenin supplementation protects the development of dextran sulfate sodium‐induced murine experimental colitis by inhibiting canonical and non‐canonical inflammasome signaling pathways. The Journal of Nutritional Biochemistry, 30, 143–152. 10.1016/j.jnutbio.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Maruyama, H. , Sumitou, Y. , Sakamoto, T. , Araki, Y. , & Hara, H. (2009). Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Biological & Pharmaceutical Bulletin, 32(7), 1244–1250. 10.1248/bpb.32.1244 [DOI] [PubMed] [Google Scholar]
- Maurya, V. K. , Kumar, S. , Prasad, A. K. , Bhatt, M. L. B. , & Saxena, S. K. (2020). Structure‐based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS‐CoV‐2 spike glycoprotein and its cellular receptor. Virus, 31(2), 179–193. 10.1007/s13337-020-00598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca, P. , & Soliman, K. F. A. (2020). Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS‐CoV‐2 infection severity. Antioxidants (Basel), 9(8), 1–28. 10.3390/antiox9080659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, E., Jr. , & Drzewiecki, G. (1984). Flavonoid inhibition of human basophil histamine release stimulated by various agents. Biochemical Pharmacology, 33(21), 3333–3338. 10.1016/0006-2952(84)90102-3 [DOI] [PubMed] [Google Scholar]
- Middleton, E., Jr. , Kandaswami, C. , & Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews, 52(4), 673–751. [PubMed] [Google Scholar]
- Mirossay, A. , Onderková, H. , Mirossay, L. , Šarišský, M. , & Mojžiš, J. (2001). The effect of quercetin on light‐induced cytotoxicity of hypericin. Physiological Research, 50(6), 635–637. http://www.biomed.cas.cz/physiolres/pdf/50/50_635.pdf [PubMed] [Google Scholar]
- Miyazawa, M. , & Hisama, M. (2003). Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Bioscience Biotechnology and Biochemistry, 67(10), 2091–2099. 10.1271/bbb.67.2091 [DOI] [PubMed] [Google Scholar]
- Mo, X. , Jian, W. , Su, Z. , Chen, M. , Peng, H. , Peng, P. , … Li, S. (2020). Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. The European Respiratory Journal, 55(6), 2001217. 10.1183/13993003.01217-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, A. , Ask, K. , Warburton, D. , Gauldie, J. , & Kolb, M. (2008). The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? The International Journal of Biochemistry & Cell Biology, 40(3), 362–382. 10.1016/j.biocel.2007.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, T. C. , Lee, E. K. , Lee, S. H. , Son, K. H. , Kim, H. P. , Kang, S. S. , & Chang, H. W. (2002). Inhibitory activity of amentoflavone on arachidonic acid releasing enzyme, Phopholipase A2 and inhibition of histamine release from mast cells. Korean Journal of Pharmacognosy, 33(1), 49–52. [Google Scholar]
- Muchtaridi, M. , Fauzi, M. , Ikram, N. K. K. , Gazzali, A. M. , & Wahab, H. A. (2020). Natural flavonoids as potential angiotensin‐converting enzyme 2 inhibitors for anti‐SARS‐CoV‐2. Molecules, 25(17), 1–20. 10.3390/molecules25173980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, C. , Hardt, M. , Schwudke, D. , Neuman, B. W. , Pleschka, S. , & Ziebuhr, J. (2018). Inhibition of cytosolic phospholipase A2 alpha impairs an early step of coronavirus replication in cell culture. Journal of Virology, 92(4), e01463‐17. 10.1128/JVI.01463-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, Y. , Garrido, A. , & Valladares, L. (2009). Equol is more active than soy isoflavone itself to compete for binding to thromboxane A2 receptor in human platelets. Thrombosis Research, 123(5), 740–744. 10.1016/j.thromres.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Murakami, M. , Taketomi, Y. , Miki, Y. , Sato, H. , Yamamoto, K. , & Lambeau, G. (2014). Emerging roles of secreted phospholipase A(2) enzymes: The 3rd edition. Biochimie, 107, 105–113. 10.1016/j.biochi.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Naicker, S. , Yang, C. W. , Hwang, S. J. , Liu, B. C. , Chen, J. H. , & Jha, V. (2020). The novel coronavirus 2019 epidemic and kidneys. Kidney International, 97(5), 824–828. 10.1016/j.kint.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, T. , Imanishi, M. , Yamamoto, K. , Cyong, J. C. , & Hirai, K. (2001). Inhibitory effect of baicalein, a flavonoid in Scutellaria root, on eotaxin production by human dermal fibroblasts. Planta Medica, 67(2), 132–135. 10.1055/s-2001-11532 [DOI] [PubMed] [Google Scholar]
- Nakamura, R. , Nakamura, R. , Watanabe, K. , Oka, K. , Ohta, S. , Mishima, S. , & Teshima, R. (2010). Effects of propolis from different areas on mast cell degranulation and identification of the effective components in propolis. International Immunopharmacology, 10(9), 1107–1112. 10.1016/j.intimp.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. T. , Woo, H. J. , Kang, H. K. , Nguyen, V. D. , Kim, Y. M. , Kim, D. W. , … Kim, D. (2012). Flavonoid‐mediated inhibition of SARS coronavirus 3C‐like protease expressed in Pichia pastoris. Biotechnology Letters, 34(5), 831–838. 10.1007/s10529-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda, D. , Jimenez‐Ferrer, E. , Zamilpa, A. , Herrera‐Arellano, A. , Tortoriello, J. , & Alvarez, L. (2010). Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin‐ and cyanidin‐3‐O‐sambubiosides from Hibiscus sabdariffa. Journal of Ethnopharmacology, 127(1), 7–10. 10.1016/j.jep.2009.09.059 [DOI] [PubMed] [Google Scholar]
- Omotuyi, I. O. , Nash, O. , Ajiboye, B. O. , Olumekun, V. O. , Oyinloye, B. E. , Osuntokun, O. T. , … Adelakun, N. (2021). Aframomum melegueta secondary metabolites exhibit polypharmacology against SARS‐CoV‐2 drug targets: In vitro validation of furin inhibition. Phytotherapy Research, 35(2), 908–919. 10.1002/ptr.6843 [DOI] [PubMed] [Google Scholar]
- Oronsky, B. , Larson, C. , Hammond, T. C. , Oronsky, A. , Kesari, S. , Lybeck, M. , & Reid, T. R. (2021). A review of persistent post‐COVID syndrome (PPCS). Clinical Reviews in Allergy and Immunology, 1–9. 10.1007/s12016-021-08848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelade, T. , Alqahtani, J. , & Canciani, G. (2020). Prognosis of COVID‐19 in patients with liver and kidney diseases: An early systematic review and meta‐analysis. Tropical Medicine Infectious Diseases, 5(2), 1–14. 10.3390/tropicalmed5020080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir, S. , Kucukler, S. , Comakli, S. , & Kandemir, F. M. (2020). The protective effect of Morin against ifosfamide‐induced acute liver injury in rats associated with the inhibition of DNA damage and apoptosis. Drug and Chemical Toxicology, 1–10. 10.1080/01480545.2020.1822390 [DOI] [PubMed] [Google Scholar]
- Palit, P. , Mukhopadhyay, A. , & Chattopadhyay, D. (2021). Phyto‐pharmacological perspective of Silymarin: A potential prophylactic or therapeutic agent for COVID‐19, based on its promising immunomodulatory, anti‐coagulant and anti‐viral property. Phytotherapy Research, 35(8), 4246–4257. 10.1002/ptr.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal, S. K. , Poudyal, H. , Arumugam, T. V. , & Brown, L. (2011). Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high‐carbohydrate high‐fat diet‐fed rats. Journal of Nutrition, 141(6), 1062–1069. 10.3945/jn.111.137877 [DOI] [PubMed] [Google Scholar]
- Panda, S. , & Kar, A. (2007). Apigenin (4′,5,7‐trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan‐induced diabetic mice. Journal of Pharmacy and Pharmacology, 59(11), 1543–1548. 10.1211/jpp.59.11.0012 [DOI] [PubMed] [Google Scholar]
- Pandey, P. , Rane, J. S. , Chatterjee, A. , Kumar, A. , Khan, R. , Prakash, A. , & Ray, S. (2020). Targeting SARS‐CoV‐2 spike protein of COVID‐19 with naturally occurring phytochemicals: An in silico study for drug development. Journal of Biomolecular Structure & Dynamics, 1‐11, 6306–6316. 10.1080/07391102.2020.1796811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandharipande, P. P. , Girard, T. D. , Jackson, J. C. , Morandi, A. , Thompson, J. L. , Pun, B. T. , … Investigators, B.‐I. S. (2013). Long‐term cognitive impairment after critical illness. The New England Journal of Medicine, 369(14), 1306–1316. 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar, A. , & Pal, A. (2020). Molecular and functional resemblance of dexamethasone and quercetin: A paradigm worth exploring in dexamethasone‐nonresponsive COVID‐19 patients. Phytotherapy Research, 34(12), 3085–3088. 10.1002/ptr.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, D. A. , Holt, R. R. , Rein, D. , Paglieroni, T. , Schmitz, H. H. , & Keen, C. L. (2005). Flavanols and platelet reactivity. Clinical & Developmental Immunology, 12(1), 1–9. 10.1080/10446670410001722140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. C. , Hsieh, C. L. , Ker, Y. B. , Wang, H. Y. , Chen, K. C. , & Peng, R. Y. (2012). Selected nutraceutic screening by therapeutic effects on doxorubicin‐induced chronic kidney disease. Molecular Nutrition & Food Research, 56(10), 1541–1558. 10.1002/mnfr.201200178 [DOI] [PubMed] [Google Scholar]
- Pignatelli, P. , Pulcinelli, F. M. , Celestini, A. , Lenti, L. , Ghiselli, A. , Gazzaniga, P. P. , & Violi, F. (2000). The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. American Journal of Clinical Nutrition, 72(5), 1150–1155. 10.1093/ajcn/72.5.1150 [DOI] [PubMed] [Google Scholar]
- Piszczatoski, C. R. , & Powell, J. (2020). Emergency authorization of chloroquine and hydroxychloroquine for treatment of COVID‐19. The Annals of Pharmacotherapy, 54(8), 827–831. 10.1177/1060028020925558 [DOI] [PubMed] [Google Scholar]
- Puntmann, V. O. , Carerj, M. L. , Wieters, I. , Fahim, M. , Arendt, C. , Hoffmann, J. , … Nagel, E. (2020). Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiology, 5(11), 1265–1273. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach, N. D. , Arnold, R. D. , & Cummings, B. S. (2014). Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochemical Pharmacology, 90(4), 338–348. 10.1016/j.bcp.2014.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai, D. K. , Sharma, P. , & Kumar, R. (2021). Post covid 19 pulmonary fibrosis. Is it real threat? The Indian Journal of Tuberculosis, 68(3), 330–333. 10.1016/j.ijtb.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, V. S. N. , Figueiredo, E. G. , Melo, C. L. , Viana, G. S. B. , Menezes, D. B. , Matos, M. S. F. , & Silveira, E. R. (1994). Protective effect of ternatin, a flavonoid isolated from Egletes viscosa less, in experimental liver‐injury. Pharmacology, 48(6), 392–397. 10.1159/000139206 [DOI] [PubMed] [Google Scholar]
- Ribeiro, D. , Freitas, M. , Tome, S. M. , Silva, A. M. , Porto, G. , Cabrita, E. J. , … Fernandes, E. (2014). Inhibition of LOX by flavonoids: A structure‐activity relationship study. European Journal of Medicinal Chemistry, 72, 137–145. 10.1016/j.ejmech.2013.11.030 [DOI] [PubMed] [Google Scholar]
- Rice‐Evans, C. A. , Miller, N. J. , & Paganga, G. (1996). Structure‐antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine, 20(7), 933–956. 10.1016/0891-5849(95)02227-9 [DOI] [PubMed] [Google Scholar]
- Richardson, S. , Hirsch, J. S. , Narasimhan, M. , Crawford, J. M. , McGinn, T. , Davidson, K. W. , … Zanos, T. P. (2020). Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA, 323(20), 2052–2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseghini, R. , Falcao, G. M. , Costa, J. F. O. , Clarencio, J. , Nascimento, I. , Velozo, E. , … Freire, S. M. (2009). The flavonoid rutin but not the alkaloid arborinine induces apoptosis in a B‐cell hybridoma cell line. Planta Medica, 75(5), 488–493. 10.1055/s-0029-1185316 [DOI] [PubMed] [Google Scholar]
- Rothlin, R. P. , Vetulli, H. M. , Duarte, M. , & Pelorosso, F. G. (2020). Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID‐19. Drug Development Research, 81(7), 768–770. 10.1002/ddr.21679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rue, E. A. , Rush, M. D. , & van Breemen, R. B. (2018). Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochemistry Reviews, 17(1), 1–16. 10.1007/s11101-017-9507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, M. , Moccia, S. , Spagnuolo, C. , Tedesco, I. , & Russo, G. L. (2020). Roles of flavonoids against coronavirus infection. Chemico‐Biological Interactions, 328, 109211. 10.1016/j.cbi.2020.109211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva, A. K. , Kuhad, A. , & Chopra, K. (2011). Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load‐induced chronic fatigue syndrome. Brain Research Bulletin, 86(3–4), 165–172. 10.1016/j.brainresbull.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Samies, N. L. , Yarbrough, A. , & Boppana, S. (2021). Pancreatitis in pediatric patients with COVID‐19. Journal of the Pediatric Infectious Diseases Society, 10(1), 57–59. 10.1093/jpids/piaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchéz, M. , Romero, M. , Goméz‐Guzmán, M. , Tamargo, J. , Pérez‐Vizcaino, F. , & Duarte, J. (2019). Cardiovascular effects of flavonoids. Current Medicinal Chemistry, 26(39), 6991–7034. 10.2174/0929867326666181220094721 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Recillas, A. , Gonzalez‐Rivero, N. A. , Barrera‐Canto, V. , Ibarra‐Barajas, M. , Estrada‐Soto, S. , & Ortiz‐Andrade, R. (2019). Vasorelaxant and antihypertensive activities of citroflavonoids (Hesperidin/Naringenin mixture): Potential prophylactic of cardiovascular endothelial dysfunction. Pharmacognosy Magazine, 15(62), 84–91. 10.4103/pm.pm_489_18 [DOI] [Google Scholar]
- Santana, F. P. R. , Thevenard, F. , Gomes, K. S. , Taguchi, L. , Camara, N. O. S. , Stilhano, R. S. , … Lago, J. H. G. (2021). New perspectives on natural flavonoids on COVID‐19‐induced lung injuries. Phytotherapy Research, 35(9), 4988–5006. 10.1002/ptr.7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarian, M. N. , Ahmed, Q. U. , So'ad, S. Z. M. , Alhassan, A. M. , Murugesu, S. , Perumal, V. , … Latip, J. (2017). Antioxidant and antidiabetic effects of flavonoids: A structure‐activity relationship based study. BioMed Research International, 2017. 8386065. doi: 10.1155/2017/8386065, 1, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish, T. , Kapoor, N. , Cao, Y. T. , Tapp, R. J. , & Zimmet, P. (2021). Proportion of newly diagnosed diabetes in COVID‐19 patients: A systematic review and meta‐analysis. Diabetes Obesity & Metabolism, 23(3), 870–874. 10.1111/dom.14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapalan, T. , Beckett, S. , Rigby, A. S. , Mellor, D. D. , & Atkin, S. L. (2010). High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutrition Journal, 9, 55. 10.1186/1475-2891-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, S. , Sauter, D. , Wang, K. , Zhang, R. , Sun, B. , Karioti, A. , … Schwarz, W. (2014). Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Medica, 80(2–3), 177–182. 10.1055/s-0033-1360277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi, W. , Mansoor, S. , Jan, S. , Singh, D. B. , Kazi, M. , Raish, M. , Alwadei, M. , Mir, J.I. , & Ahmad, P. (2019). Variability in catechin and rutin contents and their antioxidant potential in diverse apple genotypes. Molecules, 24(5), 1–12. 10.3390/molecules24050943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder, K. , Kesh, S. B. , Das, N. , Manna, K. , & Dey, S. (2014). The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food & Function, 5(6), 1294–1303. 10.1039/c3fo60526d [DOI] [PubMed] [Google Scholar]
- Snider, J. M. , You, J. K. , Wang, X. , Snider, A. J. , Hallmark, B. , Seeds, M. C. , … Chilton, F.H. (2021). Group IIA secreted phospholipase A2 plays a central role in the pathobiology of COVID‐19. medRxiv. 10.1101/2021.02.22.21252237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram, N. , Kalayarasan, S. , & Sudhandiran, G. (2009). Epigallocatechin‐3‐gallate exhibits anti‐fibrotic effect by attenuating bleomycin‐induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chemico‐Biological Interactions, 180(2), 271–280. 10.1016/j.cbi.2009.02.017 [DOI] [PubMed] [Google Scholar]
- Stavem, K. , Ghanima, W. , Olsen, M. K. , Gilboe, H. M. , & Einvik, G. (2020). Persistent symptoms 1.5‐6 months after COVID‐19 in non‐hospitalised subjects: A population‐based cohort study. Thorax, 76, 405–407. 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, H. , Yu, Q. , Zhi, Y. , Geng, G. , Liu, H. , & Xu, H. (2010). Effects of apigenin on the expression of angiotensin‐converting enzyme 2 in kidney in spontaneously hypertensive rats. Wei Sheng Yan Jiu, 39(6), 693, 700–696. [PubMed] [Google Scholar]
- Takahashi, S. , Yoshiya, T. , Yoshizawa‐Kumagaye, K. , & Sugiyama, T. (2015). Nicotianamine is a novel angiotensin‐converting enzyme 2 inhibitor in soybean. Biomedical Research, 36(3), 219–224. 10.2220/biomedres.36.219 [DOI] [PubMed] [Google Scholar]
- Tao, F. F. , Qian, C. , Guo, W. J. , Luo, Q. , Xu, Q. , & Sun, Y. (2013). Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochemical Pharmacology, 85(6), 798–807. 10.1016/j.bcp.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Testai, L. , Martelli, A. , Cristofaro, M. , Breschi, M. C. , & Calderone, V. (2013). Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff‐perfused rat hearts. Journal of Pharmacy and Pharmacology, 65(5), 750–756. 10.1111/jphp.12032 [DOI] [PubMed] [Google Scholar]
- Theoharides, T. C. , & Conti, P. (2020). Dexamethasone for COVID‐19? Not so fast. Journal of Biological Regulators and Homeostatic Agents, 34(4), 1241–1243. 10.23812/20-EDITORIAL_1-5 [DOI] [PubMed] [Google Scholar]
- Tianzhu, Z. , Shihai, Y. , & Juan, D. (2014). The effects of morin on lipopolysaccharide‐induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation, 37(6), 1976–1983. 10.1007/s10753-014-9930-1 [DOI] [PubMed] [Google Scholar]
- Tipnis, S. R. , Hooper, N. M. , Hyde, R. , Karran, E. , Christie, G. , & Turner, A. J. (2000). A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. The Journal of Biological Chemistry, 275(43), 33238–33243. 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- Torres‐Castro, R. , Vasconcello‐Castillo, L. , Alsina‐Restoy, X. , Solis‐Navarro, L. , Burgos, F. , Puppo, H. , & Vilaro, J. (2020). Respiratory function in patients post‐infection by COVID‐19: A systematic review and meta‐analysis. Pulmonology., 27, 328–337. 10.1016/j.pulmoe.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunchi, H. , Naeini, F. , Ostadrahimi, A. , & Hosseinzadeh‐Attar, M. J. (2020). Naringenin, a flavanone with antiviral and anti‐inflammatory effects: A promising treatment strategy against COVID‐19. Phytotherapy Research, 34(12), 3137–3147. 10.1002/ptr.6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, S. H. , Ko, W. C. , Ko, F. N. , & Teng, C. M. (1991). Inhibition of platelet aggregation by some flavonoids. Thrombosis Research, 64(1), 91–100. 10.1016/0049-3848(91)90208-e [DOI] [PubMed] [Google Scholar]
- Unsworth, R. , Wallace, S. , Oliver, N. S. , Yeung, S. , Kshirsagar, A. , Naidu, H. , … Logan, K. M. (2020). New‐onset type 1 diabetes in children during COVID‐19: Multicenter regional findings in the U.K. Diabetes Care, 43(11), e170–e171. 10.2337/dc20-1551 [DOI] [PubMed] [Google Scholar]
- Valerio, D. A. , Georgetti, S. R. , Magro, D. A. , Casagrande, R. , Cunha, T. M. , Vicentini, F. T. M. C. , … Verri, W. A. (2009). Quercetin reduces inflammatory pain: Inhibition of oxidative stress and cytokine production. Journal of Natural Products, 72(11), 1975–1979. 10.1021/np900259y [DOI] [PubMed] [Google Scholar]
- Vanhoecke, B. W. , Delporte, F. , van Braeckel, E. , Heyerick, A. , Depypere, H. T. , Nuytinck, M. , … Bracke, M. E. (2005). A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo, 19(1), 103–107. https://iv.iiarjournals.org/content/invivo/19/1/103.full.pdf [PubMed] [Google Scholar]
- Vaváková, M. , Ďuračková, Z. , & Trebatická, J. (2015). Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Medicine and Cellular Longevity, 2015. 898393. doi: 10.1155/2015/898393, 1, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vechi, H. T. , Maia, L. R. , & Alves, M. D. M. (2020). Late acute pulmonary embolism after mild coronavirus disease 2019 (COVID‐19): A case series. Revista Do Instituto de Medicina Tropical de São Paulo, 62, e63. 10.1590/S1678-9946202062063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij, G. , Gupta, A. , & Chopra, K. (2009). Modulation of antigen‐induced chronic fatigue in mouse model of water immersion stress by naringin, a polyphenolic antioxidant. Fundamental & Clinical Pharmacology, 23(3), 331–337. 10.1111/j.1472-8206.2009.00675.x [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan, R. , Sugendran, K. , Pant, S. C. , Husain, K. , & Malhotra, R. C. (1991). Dermal intoxication of mice with bis(2‐chloroethyl)sulphide and the protective effect of flavonoids. Toxicology, 69(1), 35–42. 10.1016/0300-483x(91)90151-p [DOI] [PubMed] [Google Scholar]
- Wang, F. , Wang, H. , Fan, J. , Zhang, Y. , Wang, H. , & Zhao, Q. (2020). Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology, 159(1), 367–370. 10.1053/j.gastro.2020.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Nair, M. G. , Strasburg, G. M. , Booren, A. M. , Gray, I. , & Dewitt, D. L. (2000). Cyclooxygenase active bioflavonoids from Balaton tart cherry and their structure activity relationships. Phytomedicine, 7(1), 15–19. 10.1016/S0944-7113(00)80016-1 [DOI] [PubMed] [Google Scholar]
- Wang, J. P. , Chang, L. C. , Hsu, M. F. , Chen, S. C. , & Kuo, S. C. (2002). Inhibition of formyl‐methionyl‐leucyl‐phenylalanine‐stimulated respiratory burst by cirsimaritin involves inhibition of phospholipase D signaling in rat neutrophils. Naunyn‐Schmiedebergs Archives of Pharmacology, 366(4), 307–314. 10.1007/s00210-002-0631-1 [DOI] [PubMed] [Google Scholar]
- Weerahandi, H. , Hochman, K. A. , Simon, E. , Blaum, C. , Chodosh, J. , Duan, E. , … Horwitz, L. I. (2021). Post‐discharge health status and symptoms in patients with severe COVID‐19. Journal of General Internal Medicine, 36(3), 738–745. 10.1007/s11606-020-06338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, K. , Xu, Y. Z. , Zhao, Z. X. , Wu, X. , Du, Y. J. , Sun, J. , … Liu, B. J. (2016). Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. International Journal of Molecular Medicine, 38(1), 337–344. 10.3892/ijmm.2016.2591 [DOI] [PubMed] [Google Scholar]
- Welton, A. F. , Tobias, L. D. , Fiedler‐Nagy, C. , Anderson, W. , Hope, W. , Meyers, K. , & Coffey, J. W. (1986). Effect of flavonoids on arachidonic acid metabolism. Progress in Clinical and Biological Research, 213, 231–242. [PubMed] [Google Scholar]
- Weng, J. , Li, Y. , Li, J. , Shen, L. , Zhu, L. , Liang, Y. , … Lan, P. (2021). Gastrointestinal sequelae 90 days after discharge for COVID‐19. The Lancet Gastroenterology & Hepatology, 6(5), 344–346. 10.1016/S2468-1253(21)00076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, X. J. , Chen, L. L. , & Zhang, H. Q. (2008). Effect of total flavonoid in leaves of Ginkgo biloba on the apoptosis of eosinophil in broncho alveloar lavage fluid. Yao Xue Xue Bao, 43(5), 480–483. [PubMed] [Google Scholar]
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934–943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagorari, A. , Roussos, C. , & Papapetropoulos, A. (2002). Inhibition of LPS‐stimulated pathways in macrophages by the flavonoid luteolin. British Journal of Pharmacology, 136(7), 1058–1064. 10.1038/sj.bjp.0704803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y. X. , Yang, W. J. , Tang, F. , Chen, X. Q. , & Ren, L. C. (2015). Antibacterial activities of flavonoids: Structure‐activity relationship and mechanism. Current Medicinal Chemistry, 22(1), 132–149. 10.2174/0929867321666140916113443 [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , … Wang, F. S. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Song, Y. , Liang, Y. N. , & Li, R. (2018). Quercetin treatment improves renal function and protects the kidney in a rat model of adenine‐induced chronic kidney disease. Medical Science Monitor, 24, 4760–4766. 10.12659/Msm.909259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. K. , Lin, S. S. , Ji, X. J. , & Guo, L. M. (2010). Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetologica, 47(3), 193–199. 10.1007/s00592-009-0109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. W. , Wang, L. , & Bao, L. D. (2020). Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID‐19) based on molecular docking method. Journal of Functional Foods, 71, 104016. 10.1016/j.jff.2020.104016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M. S. , Lee, J. , Lee, J. M. , Kim, Y. , Chin, Y. W. , Jee, J. G. , … Jeong, Y. J. (2012). Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic & Medicinal Chemistry Letters, 22(12), 4049–4054. 10.1016/j.bmcl.2012.04.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza, C. , Monserrat, J. , Mantecon, C. , Villaescusa, L. , Zaragoza, F. , & Alvarez‐Mon, M. (2016). Antiplatelet activity of flavonoid and coumarin drugs. Vascular Pharmacology, 87, 139–149. 10.1016/j.vph.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Zhai, X. , Sun, J. , Yan, Z. , Zhang, J. , Zhao, J. , Zhao, Z. , … Su, S. (2020). Comparison of severe acute respiratory syndrome coronavirus 2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. Journal of Virology, 94(15), 1–16. 10.1128/JVI.00831-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Liu, X. , Chen, S. , Wu, J. , Ye, X. , Xu, L. , … Wang, Y. (2010). Tectorigenin inhibits the in vitro proliferation and enhances miR‐338* expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis. Journal of Ethnopharmacology, 131(1), 165–173. 10.1016/j.jep.2010.06.022 [DOI] [PubMed] [Google Scholar]
- Zhang, P. , Li, J. , Liu, H. , Han, N. , Ju, J. , Kou, Y. , … Jiang, B. (2020). Long‐term bone and lung consequences associated with hospital‐acquired severe acute respiratory syndrome: A 15‐year follow‐up from a prospective cohort study. Bone Res, 8, 8. 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Wang, G. , Gurley, E. C. , & Zhou, H. (2014). Flavonoid apigenin inhibits lipopolysaccharide‐induced inflammatory response through multiple mechanisms in macrophages. PLoS One, 9(9), e107072. 10.1371/journal.pone.0107072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Geng, X. , Tan, Y. , Li, Q. , Xu, C. , Xu, J. , … Wang, H. (2020). New understanding of the damage of SARS‐CoV‐2 infection outside the respiratory system. Biomedicine & Pharmacotherapy, 127, 110195. 10.1016/j.biopha.2020.110195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Lu, S. , Chen, J. , Wei, N. , Wang, D. , Lyu, H. , … Hu, S. (2020). The landscape of cognitive function in recovered COVID‐19 patients. Journal of Psychiatric Research, 129, 98–102. 10.1016/j.jpsychires.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska, M. , Kostrzewa, A. , & Ignatowicz, E. (2000). Antioxidative activity of flavonoids in stimulated human neutrophils. Folia Histochemica et Cytobiologica, 38(1), 25–30. https://pubmed.ncbi.nlm.nih.gov/10763121/ [PubMed] [Google Scholar]
- Zou, X. , Chen, K. , Zou, J. W. , Han, P. Y. , Hao, J. , & Han, Z. G. (2020). Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Frontiers of Medicine, 14(2), 185–192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


