Abstract
Respiratory viruses pose a significant threat to global health. They initially infect the naso‐ and oropharyngeal regions, where they amplify, cause symptoms, and may also be transmitted to new hosts. Preventing initial infection or reducing viral loads upon infection might soothe symptoms, prevent dissemination into the lower airways, or transmission to the next individual. Several natural products have well‐described direct antiviral activity or may ameliorate symptoms of respiratory infections. We thus analyzed the potential of plant‐derived products to inactivate respiratory viral pathogens and determined the antiviral activity of black chokeberry (Aronia melanocarpae [Michx.] Elliott), elderberry (Sambucus nigra L.), and pomegranate (Punica granatum L.) juice, as well as green tea (Camellia sinensis [L.] Kuntze) on the infectivity of the surrogate‐modified vaccinia virus Ankara, and the respiratory viruses severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), influenza A virus (IAV), and adenovirus Type 5. Black chokeberry and pomegranate juice, and green tea reduced SARS‐CoV‐2 and IAV titers by ≥80% or ≥99%. This suggests that oral rinsing with these products may reduce viral loads in the oral cavity which might prevent viral transmission.
Keywords: black chokeberry (Aronia melanocarpae), COVID‐19, elderberry (Sambucus nigra), oral rinses, pomegranate (Punica granatum), virus transmission
1. INTRODUCTION
Respiratory viruses such as influenza viruses and coronaviruses (CoVs) pose a significant threat to global health and are a substantial social, economic, and healthcare burden, as exemplified by the current COVID‐19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Bar‐On, Flamholz, Phillips, & Milo, 2020). For SARS‐CoV‐2, the long incubation period of up to 14 days, subclinical course, and high transmissibility before the onset of symptoms have led to unprecedented spread around the globe (Bar‐On et al., 2020; Gandhi, Yokoe, & Havlir, 2020). Respiratory viruses initially infect the upper airways, both the naso‐ and oropharyngeal areas, where they amplify, may cause respiratory symptoms (Hou et al., 2020) and spread to new hosts. Recent studies suggest gargling with commercial oral rinses may reduce virus spread and potentially infection (Ather, Parolia, & Ruparel, 2021; Carrouel et al., 2021; Meister et al., 2020; O'Donnell et al., 2020; Schürmann, Aljubeh, Tiemann, & Sudhoff, 2021; Seneviratne et al., 2021). Several natural products also have direct antiviral activity or may ameliorate symptoms of respiratory infections. Pomegranate (Punica granatum L.) (Haidari, Ali, Ward Casscells, & Madjid, 2009) and black chokeberry (Aronia melanocarpa) (Park et al., 2013) extracts have been shown to be antivirally active against influenza viruses in vitro, elderberry syrup (Sambucus nigra L.) showed improved symptom relief in influenza patients (Harnett et al., 2020; Zakay‐Rones, Thom, Wollan, & Wadstein, 2004; Zakay‐Rones et al., 1995), and a meta‐analysis showed that gargling green tea (Camellia sinensis) lowered incidences of influenza infections (Ide, Yamada, & Kawasaki, 2016). Natural products with a broad‐spectrum antiviral activity could therefore be highly useful to reduce the spread of respiratory viruses in the population, as they are inexpensive and rapidly deployable.
Here, we evaluated the in vitro virucidal activity of green tea and herbal juices with prospective use as oral rinses against relevant respiratory viruses, SARS‐CoV‐2, and influenza A virus (IAV). Additionally, we investigated activity against model viruses representing enveloped (modified vaccinia virus Ankara, MVA) and naked (adenovirus type 5, AdV5) viruses in general.
2. METHODS
2.1. Herbal substances
Green tea (Bio‐Grüntee Japan Sencha Tee‐Gschwendner Nr. 700; pH 4.46) was prepared by infusing 3 g of leaves with 0.1 g ascorbic acid (Sigma Aldrich) for 4 min in 300 ml freshly boiled water under gentle movement, followed by filtration and directly used for experiments. Black chokeberry juice (Bio‐Aronia Direktsaft Fa. Aronia original L2719; pH: 3.69), pomegranate juice (Satower Granatapfelsaft Direktsaft klar; pH: 2.99), and elderberry juice (Satower Fliederbeersaft; pH: 4.13) with valid best before date were kept refrigerated until use. Juices were purchased directly from the manufacturer (on‐line store). Juices production: Fresh fruit crushed and pressed, centrifuged and filtered. The juice thus separated (without the addition of water or other substances) was pasteurized (85Grd.C). Storage in tank and filled into glass bottles with pasteurization.
2.2. Cell culture
Vero E6 (Cercopithecus aethiops derived epithelial kidney) cells were grown in Dulbecco's modified Eagle's medium (DMEM, Gibco) which was supplemented with 2.5% heat‐inactivated fetal calf serum (FCS), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L glutamine, 1 mM sodium pyruvate, and 1× non‐essential amino acids. Madin Darby canine kidney cells (MDCK) and A549 (adenocarcinomic human alveolar basal epithelial) cells were grown in minimal essential medium with Earle's salts (EMEM; Biochrom AG, Berlin, Germany) supplemented with 1% non‐essential aminoacids (Biochrom AG, Berlin, Germany) and 10% FCS. BHK‐21 (Mesocricetus auratus kidney) cells were grown in DMEM (CCPro) with 10% FCS. For experiments, cells were seeded in a medium containing 2% FCS. Cells were incubated at 37°C in a 5% CO2 humidified incubator.
2.3. Virus test strains and cultivation
Viruses were propagated by inoculation of respective target cells and culturing until strong cytopathic effect was visible. The supernatant was then harvested, centrifuged to deplete cellular debris, aliquoted, and stored at −80°C as virus stocks. MVA (provided from the Institute of Animal Hygiene and Veterinary Public Health of the University Leipzig) was passaged on BHK‐21 cells (provided by Friedrich Löffler institute), IAV A/H1N1/Brisbane/59/2007 (Novartis Vaccines and Diagnostics GmbH & Co. KG) on MDCK cells (ATCC), adenovirus Type 5, strain adenoid 75 (kindly provided by Prof. Sauerbrei, University of Jena, Jena, Germany) on A549 cells (ATCC) and SARS‐CoV‐2 BetaCoV/France/IDF0372/2020 (obtained through European Virus Archive global) on Vero E6 cells (ATCC).
2.4. Quantitative suspension tests according to EN 14476
To determine the virucidal activity of the herbal substances, quantitative suspensions tests were carried out as described in EN 14476 (EN 14476: 2019‐10). Briefly, efficacy against the respective test viruses MVA as a European surrogate for all enveloped viruses (Eggers et al., 2021) and adenovirus Type 5 (AdV 5) as well as influenza virus A and SARS‐CoV‐2 was studied using four herbal substances (chokeberry, elderberry, pomegranate juice, and green tea). Concentrations and contact times used throughout this study are indicated. In brief, an amount of 100 μl of virus was mixed with 800 μl of the test product, in the presence of 100 μl tripartite soil load ([5% (w/v) BSA Fraction V (Sigma Aldrich), 0.4% (w/v) Mucin bovine Glandula submandibularis Type I‐S (Sigma Aldrich), 5% w/v] yeast extract [Sigma Aldrich]) mimicking body fluids as an interfering substance. Due to the addition of test virus suspension and interfering substance an 80.0% solution resulted. Virus controls with 800 μl medium in place of the test products were included. SARS‐CoV‐2 was analyzed in 90% product. After the intended contact time, the reaction was stopped by transfer of 100‐μl aliquots to 900 μl cell culture medium with 2% FBS. Immediately, tenfold serial dilutions were performed and 100 μl of each dilution was used to inoculate cells (in sextuplicate) in a 96‐well microtitre plate. Due to the immediate titration, no after‐effect of the test product could occur.
After the specified contact time, the test mixture was serially diluted 10‐fold and titrated onto a 96 microtiter plate containing a confluent monolayer of the respective target cells in sextuplicates and the cells cultured until strong cytopathic effect was visible. IAV‐infected cells were additionally immunostained as described previously (Eggers et al., 2011). Cells were then examined by light microscopy, the infected wells counted. Viral titres were calculated as median tissue culture infectious dose (TCID50/ml) using the Spearman–Kärber method (Kärber, 1931; Spearman, 1908). If the cytotoxicity of the compound succeeded the lower limit of quantification (LLOQ), we rated the cytotoxicity as the viral infectious titer (marked with an #) to indicate that this titer might be masked by the cytotoxicity but could be even lower. The virucidal activity was determined by the difference of the logarithmic titer of the virus control minus the logarithmic titer of the virus incubated with the substance to test.
3. RESULTS
To assess the virucidal potential of four plant‐derived products, we performed a quantitative suspension test using MVA (EN 14476) which is a resilient surrogate virus that is used for the validation of virucidal disinfectants against all enveloped viruses according to the European Guidance on the Biocidal Products Regulation (European Chemicals Agency, 2018). Vaccinia virus (MVA or vaccinia virus strain Elstree, respectively) was introduced as a surrogate virus for the European claim “active against enveloped viruses” in 2015 in the European standardization. In vitro studies comparing MVA with EBOV, SARS‐CoV‐1 MERS‐CoV and SARS‐CoV‐2 showed that vaccinia virus was the most resilient virus (Eggers et al., 2021). While no reduction in viral titer was observed upon incubation with control buffer, 5‐min incubation with chokeberry juice, elderberry juice, pomegranate juice, or green tea yielded a 3.17, 0.67, 1.0, or 1.0 log10 decrease in infectivity, respectively (Figure 1, Table 1), indicating that the tested products are generally active against enveloped viruses. An incubation time of 20 min was only marginally more potent, suggesting a rapid‐acting antiviral effect. We then analyzed the two respiratory enveloped viruses responsible for the “swine flu” in 2009/2010 and the ongoing COVID‐19 pandemic, IAV and SARS‐CoV‐2, respectively, as well as AdV5 as a naked control virus. A 5‐min incubation with chokeberry juice yielded most potent antiviral activities and inactivated IAV, SARS‐CoV‐2 and also AdV5 by 99.99, 96.98, and 93.23%, respectively (Figure 1, Table 1). IAV was most susceptible to all products and infectivity reduced >99% by elderberry juice, pomegranate juice, and green tea. SARS‐CoV‐2 titers were reduced approximately 80% by pomegranate juice and green tea already after 1‐min incubation, however, unaffected by elderberry juice, corresponding to the results obtained with the more resistant surrogate MVA. The naked AdV5 was resistant to three out of four products, however, susceptible to chokeberry juice (Figure 1, Table 1). In summary, IAV is highly susceptible to all analyzed products, whereas SARS‐CoV‐2 can be efficiently inactivated by chokeberry juice and is to a lower level affected by pomegranate juice or green tea.
FIGURE 1.
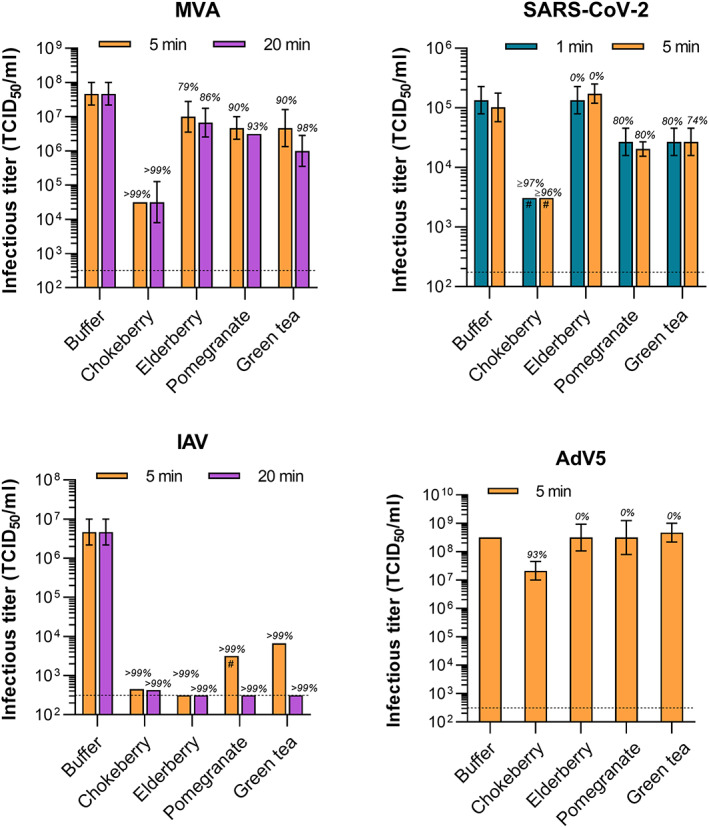
Virucidal activity of the products against MVA, IAV, SARS‐CoV‐2, and AdV5. MVA, SARS‐CoV‐2, IAV, or AdV5 were incubated with green tea, black chokeberry juice, pomegranate juice, or elderberry juice for indicated contact times before serial titration and inoculation of BHK‐21, Vero E6, MDCK, or A549 cells, respectively. Viral titers were determined by monitoring cytopathic effect and calculated as tissue culture infectious dose 50 (TCID50) according to Spearman‐Kaerber (Kärber, 1931). The lower limit of quantification (LLOQ) is defined by the limit of titration (dotted line) or cytotoxicity of the compound (#). Error bars indicate standard deviation and italics above corresponding bars the decrease of titers compared to control
TABLE 1.
Antiviral activity of natural products against MVA, IAV, SARS‐CoV‐2, and AdV5
| Virus | Contact time (min) | Control | Chokeberry | Elderberry | Pomegranate | Green tea | |
|---|---|---|---|---|---|---|---|
| MVA | 5 | Titer | 7.67 ± 0.33 | 4.50 ± 0.00 | 7.00 ± 0.45 | 6.67 ± 0.33 | 6.67 ± 0.54 |
| Log10 reduction factor | 3.17 | 0.67 | 1.00 | 1.00 | |||
| Infectivity reduction (%) | 99.93 | 78.62 | 90.00 | 90.00 | |||
| 20 | Titer | 7.67 ± 0.33 | 4.50 ± 0.60 | 6.83 ± 0.42 | 6.50 ± 0.00 | 6.00 ± 0.45 | |
| Log10 reduction factor | 3.17 | 0.84 | 1.17 | 1.67 | |||
| Infectivity reduction (%) | 99.93 | 85.55 | 93.24 | 97.86 | |||
| IAV | 5 | Titer | 6.67 ± 0.33 | 2.66 ± 0.0 | ≤2.5 ± 0.0 | ≤3.5 ± 0.0 | 3.83 ± 0.0 |
| Log10 reduction factor | 4.01 | ≥4.17 | ≥3.17 | 2.84 | |||
| Infectivity reduction (%) | 99.99 | ≥99.99 | ≥99.93 | 99.86 | |||
| 20 | Titer | 6.67 ± 0.33 | 2.63 ± 0.0 | ≤2.5 ± 0.0 | ≤2.5 ± 0.0 | ≤2.5 ± 0.0 | |
| Log10 reduction factor | 4.04 | ≥4.17 | ≥4.17 | ≥4.17 | |||
| Infectivity reduction (%) | 99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |||
| SARS‐CoV‐2 | 1 | Titer | 5.13 ± 0.23 | ≤3.49 ± 0.00 | 5.13 ± 0.23 | 4.43 ± 0.23 | 4.43 ± 0.23 |
| Log10 reduction factor | ≥1.64 | 0 | 0.70 | 0.70 | |||
| Infectivity reduction (%) | ≥97.71 | 0 | 80.05 | 80.05 | |||
| 5 | Titer | 5.01 ± 0.24 | ≤3.49 ± 0 | 5.24 ± 0.16 | 4.31 ± 0.12 | 4.43 ± 0.23 | |
| Log10 reduction factor | ≥1.52 | 0 | 0.70 | 0.58 | |||
| Infectivity reduction (%) | ≥96.98 | 0 | 80.05 | 73.70 | |||
| AdV5 | 5 | Titer | 8.50 ± 0.00 | 7.33 ± 0.33 | 8.50 ± 0.47 | 8.50 ± 0.60 | 8.67 ± 0.33 |
| Log10 reduction factor | 1.17 | 0 | 0 | 0 | |||
| Infectivity reduction (%) | 93.24 | 0 | 0 | 0 |
Notes: Log10 reduction factor and antiviral activity of chokeberry, elderberry, pomegranate juice, and green tea against MVA, IAV, SARS‐CoV‐2, and AdV5 after indicated contact times.
Abbreviations: AdV5 adenovirus type 5 (adenoid 75); IAV, influenza A virus (A/H1N1/Brisbane/59/2007); MVA, modified vaccinia virus Ankara; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2 (BetaCoV/France/IDF0372/2020).
4. DISCUSSION
We analyzed the virucidal activities of four natural beverages on surrogate and respiratory viruses and found that chokeberry juice, green tea, and pomegranate juice reduced infectious titers of enveloped viruses with chokeberry juice being most efficient. The tested food products showed the highest antiviral efficacy against IAV (H1N1), with ≥4 log10 reduction, corresponding to the required effectiveness of antiseptics (EN 14476). The high susceptibility of IAV, which is also a representative of influenza B and other influenza strains with regard to chemical stability, indicates low resilience of this virus family. SARS‐CoV‐2 behaved similar to MVA (a standard model for enveloped viruses) and proved to be more resilient. Nevertheless, chokeberry juice inactivated SARS‐CoV‐2 by more than 96%, while pomegranate juice and green tea were less active. Notably, activity against MVA is prerequisite for validation of general disinfectant properties of chemicals according to the European Chemicals Agency (European Chemicals Agency, 2018) and suggestive of broad activity against all enveloped viruses. As expected, the non‐enveloped adenovirus, was less susceptible to the tested products, however, was also affected by the very potent chokeberry juice. Generally, differences in contact times did not have a strong influence on the efficiency of inactivation suggesting a rapid‐acting antiviral effect.
The antiviral activities of the plant products can be based on an acidic pH that may directly inactivate virus particles or by (poly‐)phenols such as catechins, tannins, or flavonoids that can act on viral and cellular proteins (Hensel et al., 2020; Mhatre, Srivastava, Naik, & Patravale, 2020). For example, pomegranate polyphenols were shown to inhibit influenza viruses by acting on virion surface glycoproteins and causing structural damage to the virion (Sundararajan et al., 2010) or SARS‐CoV‐2 by preventing spike protein binding to its angiotensin‐converting enzyme 2 (ACE2) receptor, where Punicalagin and Ellagic acid derivatives are the essential ingredients of pomegranate that determine this activity. (Tito et al., 2021). Similarly, elderberry extracts might interfere with the spike receptor interaction (Boroduske et al., 2021) and green tea catechins have been shown to destroy the virion structure and its main catechin epigallocatechin gallate (EGCG) aggregates virus particles to prevent their interaction with target cells (Nishimura, Okamoto, Dapat, Katsumi, & Oshitani, 2021; Xu, Xu, & Zheng, 2017). Specifically for SARS‐CoV‐2, EGCG has been shown to inhibit the activity of the 3CL protease, prevent attachment to ACE2 and directly inactivate viral particles (Jang et al., 2020; Liu et al., 2021; Ohgitani et al., 2021). Catechins not only act on the virus particles but have additionally been shown to prevent fusion by interfering with endosome acidification and viral enzymes (Xu et al., 2017). For theaflavin‐3,3′‐digallat (from black tea) computer modeling suggested that it might prevent SARS‐CoV‐2 infection by interacting with its cellular receptor ACE2 (Mhatre et al., 2020; Xu et al., 2017). Therefore, pilot studies using green tea extracts in SARS‐CoV‐2 infected individuals have already been performed (Bettuzzi, Gabba, & Cataldo, 2021).
Of note, the composition of natural food products may vary between batches, which might affect their antiviral efficiency. We thus performed phytochemical analysis of all tested products (Table 2), however, found no clear correlation of pH, total polyphenols, or anthocyanins with antiviral activity. This result is not surprising, because tannins can in principle strongly change the functions of proteins in different ways, which depends on both the structure of the proteins under consideration and the structure of the tannins. In plants, there are always very complex mixtures of tannins, which are often characteristic for the respective plant but only rarely can a certain effect be attributed to a single one of the tannins. It follows that the overall effect of a mixture of tannins in terms of their strength against a particular protein is not simply determined by the amount alone, but additionally by their specific individual binding properties. It is therefore not surprising that the polyphenol concentrations of different classes of tannins in the different juices and green teas do not correlate by content with anti‐infective efficacy and also not the ph value as an additional effect. However, we have made this quantification of the typical and characteristic ingredient groups for the respective products, so that one has an orientation on the basis of these data if one wants to repeat the tests or carry out other tests with similar products.
TABLE 2.
Phytochemical characterization of used products
| Product | pH | Total polyphenols a (mg/100 ml) | Anthocyanins b (mg/100 ml) | Caffeine c (mg/100 ml) | Ellagic acid d mg/100 ml) | Punicalagin A d (mg/100 ml) | Punicalagin B d (mg/100 ml) | Total catechines e (mg/100 ml) |
|---|---|---|---|---|---|---|---|---|
| Green tea | 4.46 | N/D | N/D | 24 | N/D | N/D | N/D | 95 |
| Black chokeberry juice | 3.69 | 913 | 27.3 | N/D | N/D | N/D | N/D | N/D |
| Pomegranate juice | 2.99 | 347 | 3.34 | N/D | 6.27 | 1.04 | 3.12 | N/D |
| Elderberry juice | 4.13 | 778 | 182 | N/D | N/D | N/D | N/D | N/D |
Notes: Single catechine analysis: (+)‐Gallocatechin: 0.33%/2.7 mg/100 ml; (–)‐Epigallocatechin: 5.02%/35 mg/100 ml; (+)‐Catechin: 0.05%/0.4 mg/100 ml; (–)‐Epigallocatechin‐3‐O‐gallat: 7.41%/39 mg/100 ml; (–)‐Epicatechin: 1.12%/9.4 mg/100 ml; (–)‐Gallocatechin‐3‐O‐gallat: 0.07%/0.6 mg/100 ml; (–)‐Epicatechin‐3‐O‐gallat: 1.50%/8 mg/100 ml.
Analysis method Folin‐Ciocalteu (Wern, Haron, & Keng, 2016): In short: Photometric assay: substance + Folin‐Ciocalteu reagent and Na2CO3 solution measurement of absorption at 765 nm. Reference and calculation as: gallic acid.
Analysis method in analogy to Pharmacopoea Europaea Ed. 10.0 mon. 1602 Myrtilli fructus recens. In short: Photometric assay: dillution in hydrochloric—methanolic and measurement of absorption at 528 nm. Calculation as Cyanidin‐3‐O‐glucosid‐chlorid by specific absorption of 718.
Analysis according to Pharmacopoea Europaea Ed. 10.0 HPLC with Coffein CRS calculation with external standard.
Analysis according to Li, Chen, Jia, Liu, and Peng (2016) HPLC calculation via external standard and calibration curve.
Calculated as EGCG, analysis according to Pharmacopoea Europaea Ed. 10.0. HPLC with external reference mixture HRS for peak identity and (−)‐Epigallocatechin‐3‐O‐gallat CRS calculation with external standard as (−)‐Epigallocatechin‐3‐O‐gallat.
These substances, however, together with others or mixtures thereof likely contribute to the (specific) antiviral effects observed. Nevertheless, the composition of various antivirally active components, acting by different mechanisms, represents a potent mix interfering with virus infection.
Since respiratory virus infection and replication primarily occur in the naso‐ and oropharyngeal area, reducing viral titers as early as possible might represent a proactive strategy to prevent ongoing replication, dissemination, and transmission. The herbal products are common and available food preparations that could be applied as convenient “oral rinses.” Antiseptic oral rinses containing membrane‐damaging agents (i.e., ethanol, chlorhexidine, cetylpyridinium chloride, hydrogen peroxide, and povidone‐iodine) are used in various private or clinical situations for prophylactic and therapeutic purposes and have further been applied in the context of viral infections (Ather et al., 2021; Carrouel et al., 2021; Meister et al., 2020; O'Donnell et al., 2020; Schürmann et al., 2021; Seneviratne et al., 2021). In contrast to these chemical preparations, green tea and herbal juices can be applied more frequently and may be simply swallowed. Gargling tea, tea extracts or plant juice followed by drinking has already been shown to lower the incidence of influenza virus infections, viral loads, and symptoms (Harnett et al., 2020; Ide et al., 2016; Z. Zakay‐Rones et al., 2004; Zichria Zakay‐Rones et al., 1995). Similarly, antivirally active plant products such as chokeberry (Park et al., 2013) or pomegranate (Haidari et al., 2009) juice might be translated into “clinical” use against influenza viruses and SARS‐CoV‐2.
In the case of SARS‐CoV‐2, the virus may be passed before symptom onset, which is particularly treacherous. Oral rinsing and gargling with the tested juices and tea are largely unproblematic in long‐term use and might be a suitable pre‐ and postexposure prophylaxis against SARS‐CoV‐2 during the current COVID‐19 pandemic for any individual but especially those with high risk of infection or severe disease including healthcare workers, elderly, or immunocompromised. Additionally, the possibility of swallowing the “oral rinse” is practical in many situations such as during a flight, train, or car ride, in day‐care centers or schools, and can even be part of a healthy diet. Furthermore, it would be helpful to reduce the risk of transmission in school classes and children using nutritionally valuable and healthy food products instead of aggressive disinfectants or obstructive measures. Thus, the administration of virucidal plant products such as chokeberry juice could minimize the spread of enveloped respiratory viruses, ease symptoms, and potentially contribute to disease prevention and clinical investigation of their benefits is warranted.
CONFLICT OF INTEREST
Bruno Frank and Uwe Kessler are partners of CogniVerde GmbH Groß‐Umstadt, Germany.
ACKNOWLEDGEMENTS
We thank Carina Conzelmann, Tatjana Weil, Rüdiger Groß, Janis Müller, and Jan Münch for experimental assistance.
Eggers, M. , Jungke, P. , Wolkinger, V. , Bauer, R. , Kessler, U. , & Frank, B. (2022). Antiviral activity of plant juices and green tea against SARS‐CoV‐2 and influenza virus. Phytotherapy Research, 36(5), 2109–2115. 10.1002/ptr.7431
Funding information German Cancer Aid (Deutsche Krebshilfe)
DATA AVAILABILITY STATEMENT
Raw data are available upon request.
REFERENCES
- Ather, A. , Parolia, A. , & Ruparel, N. B. (2021). Efficacy of mouth rinses against SARS‐CoV‐2: A scoping review. Frontiers in Dental Medicine, 2. 10.3389/fdmed.2021.648547 [DOI] [Google Scholar]
- Bar‐On, Y. M. , Flamholz, A. , Phillips, R. , & Milo, R. (2020). SARS‐CoV‐2 (COVID‐19) by the numbers. eLife, 9(10), 697–698. 10.7554/eLife.57309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettuzzi, S. , Gabba, L. , & Cataldo, S. (2021). Efficacy of a polyphenolic, standardized green tea extract for the treatment of COVID‐19 syndrome: A proof‐of‐principle study. COVID, 1(1), 2–12. 10.3390/covid1010002 [DOI] [Google Scholar]
- Boroduske, A. , Jekabsons, K. , Riekstina, U. , Muceniece, R. , Rostoks, N. , & Nakurte, I. (2021). Wild Sambucus nigra L. from north‐east edge of the species range: A valuable germplasm with inhibitory capacity against SARS‐CoV2 S‐protein RBD and hACE2 binding in vitro. Industrial Crops and Products, 165, 113438. 10.1016/j.indcrop.2021.113438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel, F. , Gonçalves, L. S. , Conte, M. P. , Campus, G. , Fisher, J. , Fraticelli, L. , … Bourgeois, D. (2021). Antiviral activity of reagents in mouth rinses against SARS‐CoV‐2. Journal of Dental Research, 100(2), 124–132. 10.1177/0022034520967933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, M. , Roth, B. , Schweiger, B. , Schmid, M. , Gregersen, J.‐P. , & Enders, M. (2011). Comparison of the novel ResPlex III assay and existing techniques for the detection and subtyping of influenza virus during the influenza season 2006–2007. European Journal of Clinical Microbiology & Infectious Diseases, 31(6), 1257–1265. 10.1007/s10096-011-1437-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, M. , Schwebke, I. , Suchomel, M. , Fotheringham, V. , Gebel, J. , Meyer, B. , … Steinhauer, K. (2021). The European tiered approach for virucidal efficacy testing ‐ rationale for rapidly selecting disinfectants against emerging and re‐emerging viral diseases. Euro Surveillance Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin, 26(3), 2000708. 10.2807/1560-7917.ES.2021.26.3.2000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Chemicals Agency (2018). Guidance on the biocidal products regulation: Volume II efficacy – Assessment and evaluation (parts B + C) (Vol. V3, Issue April).
- Gandhi, M. , Yokoe, D. S. , & Havlir, D. V. (2020). Asymptomatic transmission, the Achilles' heel of current strategies to control Covid‐19. New England Journal of Medicine, 382(22), 2158–2160. 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidari, M. , Ali, M. , Ward Casscells, S. , & Madjid, M. (2009). Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine, 16(12), 1127–1136. 10.1016/j.phymed.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Harnett, J. , Oakes, K. , Carè, J. , Leach, M. , Brown, D. , Cramer, H. , … Anheyer, D. (2020). The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Advances in Integrative Medicine, 7(4), 240–246. 10.1016/j.aimed.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel, A. , Bauer, R. , Heinrich, M. , Spiegler, V. , Kayser, O. , Hempel, G. , & Kraft, K. (2020). Challenges at the time of COVID‐19: Opportunities and innovations in antivirals from nature. Planta Medica, 86(10), 659–664. 10.1055/a-1177-4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Y. J. , Okuda, K. , Edwards, C. E. , Martinez, D. R. , Asakura, T. , Dinnon, K. H. , … Baric, R. S. (2020). SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell, 429–446, 429–446.e14. 10.1016/j.cell.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, K. , Yamada, H. , & Kawasaki, Y. (2016). Effect of gargling with tea and ingredients of tea on the prevention of influenza infection: A meta‐analysis. BMC Public Health, 16(1), 1–7. 10.1186/s12889-016-3083-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, M. , Park, Y.‐I. , Cha, Y.‐E. , Park, R. , Namkoong, S. , Lee, J. I. , & Park, J. (2020). Tea polyphenols EGCG and theaflavin inhibit the activity of SARS‐CoV‐2 3CL‐protease in vitro. Evidence‐Based Complementary and Alternative Medicine, 2020, 5630838. 10.1155/2020/5630838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärber, G. (1931). Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn‐Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie, 162(4), 480–483. [Google Scholar]
- Li, R. , Chen, X. G. , Jia, K. , Liu, Z. P. , & Peng, H. Y. (2016). A systematic determination of polyphenols constituents and cytotoxic ability in fruit parts of pomegranates derived from five Chinese cultivars. Springerplus, 5(914), 1–9. 10.1186/s40064-016-2639-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Bodnar, B. H. , Meng, F. , Khan, A. I. , Wang, X. , Saribas, S. , … Ho, W. (2021). Epigallocatechin gallate from green tea effectively blocks infection of SARS‐CoV‐2 and new variants by inhibiting spike binding to ACE2 receptor. Cell & Bioscience, 11(1), 168. 10.1186/s13578-021-00680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, T. L. , Brüggemann, Y. , Todt, D. , Conzelmann, C. , Müller, J. A. , Groß, R. , … Steinmann, E. (2020). Virucidal efficacy of different oral rinses against SARS‐CoV‐2. The Journal of Infectious Diseases, 222, 1289–1292. 10.1093/infdis/jiaa471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre, S. , Srivastava, T. , Naik, S. , & Patravale, V. (2020). Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID‐19: A review. Phytomedicine, 85, 153286. 10.1016/j.phymed.2020.153286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, H. , Okamoto, M. , Dapat, I. , Katsumi, M. , & Oshitani, H. (2021). Inactivation of SARS‐CoV‐2 by catechins from green tea. Japanese Journal of Infectious Diseases, 74(5), 421–423. 10.7883/yoken.JJID.2020.902 [DOI] [PubMed] [Google Scholar]
- O'Donnell, V. B. , Thomas, D. , Stanton, R. , Maillard, J.‐Y. , Murphy, R. C. , Jones, S. A. , … Sattar, S. A. (2020). Potential role of oral rinses targeting the viral lipid envelope in SARS‐CoV‐2 infection. Function, 1(1), zqaa002. 10.1093/function/zqaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgitani, E. , Shin‐Ya, M. , Ichitani, M. , Kobayashi, M. , Takihara, T. , Kawamoto, M. , … Mazda, O. (2021). Significant inactivation of SARS‐CoV‐2 in vitro by a green tea catechin, a catechin‐derivative, and black tea galloylated theaflavins. Molecules, 26(12), 3572. 10.3390/molecules26123572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. , Kim, J. I. , Lee, I. , Lee, S. , Hwang, M. W. , Bae, J. Y. , … Park, M. S. (2013). Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochemical and Biophysical Research Communications, 440(1), 14–19. 10.1016/j.bbrc.2013.08.090 [DOI] [PubMed] [Google Scholar]
- Schürmann, M. , Aljubeh, M. , Tiemann, C. , & Sudhoff, H. (2021). Mouthrinses against SARS‐CoV‐2: Anti‐inflammatory effectivity and a clinical pilot study. European Archives of Oto‐Rhino‐Laryngology, 278(12), 5059–5067. 10.1007/s00405-021-06873-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne, C. J. , Balan, P. , Ko, K. K. K. , Udawatte, N. S. , Lai, D. , Ng, D. H. L. , … Sim, X. Y. J. (2021). Efficacy of commercial mouth‐rinses on SARS‐CoV‐2 viral load in saliva: Randomized control trial in Singapore. Infection, 49(2), 305–311. 10.1007/s15010-020-01563-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman, C. (1908). The method of right and wrong cases (constant stimuli) without Gauss's formulae. British Journal of Psychology, 2(3), 227. 10.1111/j.2044-8295.1908.tb00176.x [DOI] [Google Scholar]
- Sundararajan, A. , Ganapathy, R. , Huan, L. , Dunlap, J. R. , Webby, R. J. , Kotwal, G. J. , & Sangster, M. Y. (2010). Influenza virus variation in susceptibility to inactivation by pomegranate polyphenols is determined by envelope glycoproteins. Antiviral Research, 88(1), 1–9. 10.1016/j.antiviral.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tito, A. , Colantuono, A. , Pirone, L. , Pedone, E. , Intartaglia, D. , Giamundo, G. , … Apone, F. (2021). Pomegranate peel extract as an inhibitor of SARS‐CoV‐2 spike binding to human ACE2 receptor (in vitro): A promising source of novel antiviral drugs. Frontiers in Chemistry, 9, 638187. 10.3389/fchem.2021.638187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wern, K. H. , Haron, H. , & Keng, C. B. (2016). Comparison of total phenolic contents (TPC) and antioxidant activities of fresh fruit juices, commercial 100% fruit juices and fruit drinks. Sains Malaysiana, 45(9), 1319–1327. https://www.ukm.my/jsm/pdf_files/SM-PDF-45-9-2016/04%20%20Khaw%20Hui%20Wern.pdf [Google Scholar]
- Xu, J. , Xu, Z. , & Zheng, W. (2017). A review of the antiviral role of green tea catechins. Molecules, 22(8), 1337. 10.3390/molecules22081337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakay‐Rones, Z. , Thom, E. , Wollan, T. , & Wadstein, J. (2004). Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. Journal of International Medical Research, 32(2), 132–140. 10.1177/147323000403200205 [DOI] [PubMed] [Google Scholar]
- Zakay‐Rones, Z. , Varsano, N. , Zlotnik, M. , Manor, O. , Regev, L. , Schlesinger, M. , & Mumcuoglu, M. (1995). Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. Journal of Alternative and Complementary Medicine, 1(4), 361–369. 10.1089/acm.1995.1.361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available upon request.


