Abstract
Background
Acute basilar artery occlusion (ABAO) is known to have a poor outcome with a high rate of morbidity and mortality despite endovascular treatment (EVT), highlighting the necessities of exploring factors to limit the efficacy of EVT in these patients. Cerebellar infarctions in ABAO might progress to malignant cerebellar edema (MCE), a life-threatening complication after reperfusion, posing a secondary injury to the brainstem by mass effects. Therefore, the present research aimed to explore the impacts of MCE on a long-term outcome and investigate the prognostic factors for MCE among ABAO after EVT.
Methods
In the national BASILAR registry, a total of 329 ABO patients with cerebellar infarctions treated by EVT met the inclusion criteria. The presence of MCE defined by the Jauss scale ≥4 points, was evaluated on the computed tomography performed 72 h after EVT. The adjusted odds ratio and 95% CI were obtained by logistic regression models. A favorable outcome was defined as a 90-day modified Rankin Scale score of 0–3.
Results
MCE was statistically associated with the decreased incidence of a favorable outcome [adjusted odds ratio, 0.35(95% CI, 0.18-0.68), P=0.002]. The baseline National Institutes of Health Stroke Scale score, collateral circulation, neutrophil count at admission, and recanalization status were predictors for MCE and a favorable functional status at 90 days (all P<0.05). Among all inflammatory factors, the neutrophil count achieved the highest accuracy, sensitivity, and specificity for MCE. Adding the neutrophil count status into the baseline model obviously enhanced its prediction ability for MCE and favorable outcome by increasing the area under curve and achieving both net reclassification and integrated discrimination improvement (all P<0.05). Mediation analysis indicated that MCE mediated the association between the increased neutrophil count and worse functional outcome (P=0.026).
Discussion
MCE acted essential roles in worsening prognosis for ABAO after EVT. A high neutrophil count at admission was linked to MCE and a poor outcome among ABAO patients, which could be further incorporated into the clinical decision-making system and guide immunomodulation therapy.
Keywords: acute basilar artery occlusion, endovascular treatment, malignant cerebellar edema, neutrophil count, outcome
Introduction
Acute basilar artery occlusion (ABAO) is a rare and devastating neurological disorder with high mortality that leaves a substantial part of survivors severely disabled (1). Even if recanalization by endovascular treatment (EVT) is achieved, less than 35% of patients remained functionally independent at follow-up as illustrated by the latest Basilar Artery International Cooperation Study (BASICS) and Basilar Artery Occlusion EndovascularIntervention versus Standard Medical Treatment trail (BEST) trails (2, 3). Increasing focus was required to promote an understanding for the reasons of poor outcome and factors that limit the efficacy of EVT.
Brainstem impairment after the occluding basilar artery was regarded as the major contributor for a poor outcome; nevertheless, the phenomena of cerebellar infarctions were frequently observed in ABAO patients that might progress to malignant cerebellar edema (MCE), which could result in obstructive hydrocephalus and pose a secondary injury to the brainstem by mass effects (4). Previous studies have reported that the mortality rate of MCE was more than three times higher than that of non-MCE among pure acute cerebellar infarction patients (5, 6). However, these related previous study focused on anterior circulation stroke, while the basilar artery supplied blood to the infratenttorial structure , ABAO was excluded in these literatures on MCE, and thus, the evidence regarding the relationship between MCE and the outcome and predictive factors for the MCE in ABAO after EVT were scarce (5, 6).
According to findings from animal studies and cross-sectional observation studies, inflammatory cells could act as critically pathogenic but intervenable factors for brain tissue edema, compared to other unchangeable predictors such as the collateral circulation status and stroke severity degrees (7, 8). However, the patients recruited in previous literatures that investigated the associations of inflammation with brain edema, were anterior circulation infarction, while the basilar artery supplied blood to the infratentorial structure (7–9). The obvious histological and anatomical differences of the cerebellum from the cerebrum, including cellular components, feeding arteries, and vessel autoregulation methods, might imply the distinct roles of inflammatory factors in promoting cerebellar edema from cerebral tissue edema (10). The limited subtentorial residual space also suggested the decreased tolerance ability for mass effects among ABAO patients than an anterior circulation stroke. Uncertainties remained about the relationship between inflammation markers and MCE in ABAO after EVT.
Therefore, based on our preceding national registry of BASILAR (11), we ought to evaluate the impacts of MCE on the clinical outcome in ABAO patients after EVT and explore the predictors for MCE, especially the roles of inflammation factors in determining MCE and the functional outcome among these individuals.
Material and Methods
Study Design and Participants
The BASILAR study was a nationwide, prospective registry of EVT plus medical management vs. medical management alone for patients confirmed with an acute symptomatic and radiological ABAO from 47 comprehensive stroke centers in China between January 2014 and May 2019. The details of the study protocol have been previously published (11). We included the ABAO subjects 1) with confirmed cerebellar infarction based on magnetic resonance imaging (MRI) or computed tomography (CT) perfusion at admission, or the corresponding follow-up imaging scanning (5), and 2) treated by EVT. To reduce the influence of drugs and related medication disease history on inflammatory cells, the main exclusion criteria were 1) taking antibiotics, 2) with an infection within 7 days before admission, 3) with major trauma or surgery events within 28 days before admission, 4) with chronic inflammatory diseases, and 5) accepting corticosteroid treatment. From 829 patients in the BASILAR registry, a total of 329 patients were enrolled in the present study.
Standard Protocol Approvals, Registrations, and Patient Consent
The BASILAR was registered on the Chinese Clinical Trial Registry (ChiCTR1800014759). The study was approved by the research board at each participating center, and informed consent was obtained from all patients or their authorized representatives.
Procedures and Data Collection
We retrospectively collected data on baseline characteristics, including age, sex, vascular risk factors (diabetes mellitus, hypertension, atrial fibrillation, and dyslipidemia), National Institutes of Health Stroke Scale (NIHSS) at admission, and posterior circulation-Alberta Stroke Program Early scores (PC-ASEPCTS). The collateral circulation status was assessed by the posterior circulation collateral score (PC-CS) based on the presence of potential collateral pathways on CT angiography (12). Successful reperfusion was defined as a modified thrombolysis-in-cerebral-infarction (mTICI) score higher than 2a at the end of the intervention. Malignant cerebellar edema (MCE) was quantified by the score of Jauss higher than 3 (≥4) from non-contrast CT within 72 h after EVT (13). Each imaging scan for Jauss score was separately interpreted by 2 trained neuroradiologists (KZ and SL). In the cases of discrepancies, a third neuroradiologist (KZ) confirmed the MCE status. All neuroradiologists were blinded to clinical outcomes when performing imaging data analysis.
Whole blood samples were collected from patients at admission. Five key potential blood-based biomarkers including the neutrophil count, platelet count, lymphocyte count, and their combination ratios [neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)] were obtained. Patients were categorized into high or low neutrophil count groups based on the median value of the neutrophil count in enrolled patients.
The primary functional outcomes at follow-up were assessed using the 90-day modified Rankin Scale (mRS). A score on the 90-day mRS of 0-3 was defined as a favorable outcome. Intracerebral hemorrhages were evaluated according to the Heidelberg Bleeding Classification (14). Symptomatic intracranial hemorrhage (SICH) was defined as a newly observed intracranial hemorrhage, leading to an increase of 4 points on the NIHSS before worsening or an increase of 2 points in one category (2, 11).
Statistical Analysis
The proportion tests for categorical variables were performed using the chi-square test. Depending on the normality of the distribution as assessed by the Kolmogorov–Smirnov test, continuous variables were compared using Student’s t-test for independent samples, or the Mann–Whitney U test or Kruskal–Wallis test for non-normal data. Data are presented as mean ± standard deviation, median [interquartile range(IQR)], or a number (percentage), where appropriate. The interobserver agreement for MCE status assignment was evaluated using the weighted κ statistic.
To determine the independent prognostic factors for favorable outcomes, binary and multivariable logistic regression analyses were performed and these results were summarized as odds ratios (ORs) with 95% confidence intervals (CIs). An ordinal regression model was performed to calculate the common OR for a shift toward a 1-point improvement of mRS. We also analyzed the heterogeneity of the effects of the neutrophil count status within subgroups based on the sex, age (≤60 or >60 years), mTICI (0–2a or 2b–3), NIHSS score (<25 or ≥25), and PC-CS (≤6 or >6). The area under the receiver operating characteristic (ROC) curves (AUCs) were calculated and compared using DeLong’s test. The incremental effects of the neutrophil count status for outcome prediction were examined using the net reclassification improvement (NRI) algorithm and the integrated discrimination improvement (IDI) algorithm, with the baseline model as a reference. We performed a sensitivity analysis to explore the impacts of the neutrophil count on the outcomes in cohorts without any hemorrhage, sICH, and poor reperfusion level (mTICI ≤ 2a). The predictive roles of neutrophils for the long-term outcome were also explored in all ABAO patients after EVT in the BASILAR registry based on logistics analysis. A 2-tailed P<0.05 was considered statistically significant. All statistical analyses were performed using the R software version 4.0.2 (https://www.r-project.org).
Results
Baseline Characteristics
The baseline clinical manifestations of enrolled patients were shown in Supplemental Table 1 . The average age was 63.86 years, and 78.12% of them were men. Among enrolled patients, a total of 89 (27.05%) achieved favorable long-term outcomes (90-day mRS ≤ 3).
With a weighted κ value of 0.77 (95% CI, 0.73–0.80), the interobserver agreement for the grading of MCE status on follow-up non-contrast computed tomography (NCCT) images was good. Patients were stratified into two groups according to their MCE level ( Table 1 and Supplemental Figure 1 ). The proportion of MCE among ABAO patients with cerebellar infarctions treated by EVT was 36.17% (N=119). Significant differences in the proportions of satisfied mTICI level, PC-CS score, onset to recanalization time, and neutrophil count were detected between these MCE and non-MCE groups (all P<0.05).
Table 1.
Clinical features for enrolled patients stratified by different MCE levels.
| All patients (N=329) | Non-MCE (N=210) | MCE (N=119) | P | |
|---|---|---|---|---|
| Age, years, mean ± SD | 63.86 ± 10.83 | 64.21 ± 11.24 | 63.24 ± 10.09 | 0.436 |
| Men, (n%) | 257 (78.12) | 158 (75.24) | 99 (83.19) | 0.094 |
| Baseline NIHSS, median (IQR) | 27.00 (17.00,33.00) | 24.00 (14.00,32.00) | 30.00 (23.00,34.00) | <0.001 |
| Initial PC-ASPECTS, median (IQR) | 8.00 (6.00, 9.00) | 8.00 (7.00, 9.00) | 7.00 (6.00, 9.00) | 0.017 |
| Admission SBP, mmHg, mean ± SD | 150.97 ± 25.79 | 149.55 ± 24.84 | 153.46 ± 27.31 | 0.187 |
| Admission DBP, mmHg, mean ± SD | 86.74 ± 15.67 | 86.43 ± 14.83 | 87.28 ± 17.11 | 0.638 |
| 24 h NIHSS after EVT, median (IQR) | 29.00 (15.00, 35.00) | 21.50 (11.00, 32.00) | 34.00 (28.00, 36.00) | <0.001 |
| 7 d NIHSS after EVT, median (IQR) | 24.00 (9.00, 36.00) | 16.00 (6.00, 31.00) | 35.00 (28.00, 36.00) | <0.001 |
| Intravenous thrombolysis, (n%) | 71 (21.58) | 46 (21.90) | 25 (21.01) | 0.849 |
| Pre-onset mRS, (n%) | ||||
| 0 | 284 (86.32) | 178 (84.76) | 106 (89.08) | 0.448 |
| 1 | 31 (9.42) | 23 (10.95) | 8 (6.72) | |
| 2 | 14 (4.26) | 9 (4.29) | 5 (4.20) | |
| Decompressive craniectomy, (n%) | 11 (3.34) | 0 (0.00) | 11 (9.24) | <0.001 |
| History of risk factors, n (%) | ||||
| Hypertension | 231 (70.21) | 146 (69.52) | 85 (71.43) | 0.717 |
| Diabetes mellitus | 75 (22.80) | 50 (23.81) | 25 (21.01) | 0.516 |
| Dyslipidemia | 109 (33.13) | 68 (32.38) | 41 (34.45) | 0.701 |
| Atrial fibrillation | 59 (17.93) | 39 (18.57) | 20 (16.81) | 0.688 |
| TIA | 3 (0.91) | 1 (0.48) | 2 (1.68) | 0.297 |
| Laboratory results, median (IQR) | ||||
| Neutrophil, 109/L | 9.87 (7.28, 12.40) | 9.15 (6.60, 11.29] | 11.85 (8.88,14.26) | <0.001 |
| Lymphocyte, 109/L | 1.09 (0.80, 1.60) | 1.10 (0.80, 1.61) | 1.06 (0.85,1.56) | 0.826 |
| NLR | 8.83 (5.35, 13.66) | 7.80 (4.64, 12.88) | 10.75 (6.62,15.24) | 0.001 |
| Platelet count, 109/L | 216.00 (177.00,250.00) | 213.00 (178.00, 245.00) | 221.00 (174.50,266.25) | 0.109 |
| PLR | 180.83 (131.09, 277.61) | 178.46 (127.93, 275.63) | 186.92 (137.68, 284.66) | 0.272 |
| TOAST classification, n (%) | 0.392 | |||
| LAA | 227 (69.00) | 138 (65.71) | 89 (74.79) | |
| CE | 73 (22.19) | 52 (24.76) | 21 (17.65) | |
| SOE | 7 (2.13) | 5 (2.38) | 2 (1.68) | |
| SUE | 22 (6.69) | 15 (7.14) | 7 (5.88) | |
| Imaging parameters | ||||
| Occlusion site, n (%) | 0.105 | |||
| BA distal | 96 (29.18) | 68 (32.38) | 28 (23.53) | |
| BA middle | 108 (32.83) | 70 (33.33) | 38 (31.93) | |
| BA proximal | 56 (17.02) | 36 (17.14) | 20 (16.81) | |
| V4 | 69 (20.97) | 36 (17.14) | 33 (27.73) | |
| PC-CS score, median (IQR) | 4.00 (3.00, 6.00) | 5.00 (3.25, 6.00) | 4.00 (2.00, 5.00) | <0.001 |
| Reperfusion status, n (%) | ||||
| mTICI | <0.001 | |||
| 0-2a | 60 (18.24) | 29 (13.81) | 31 (26.05) | |
| 2b-3 a | 269 (81.76) | 181 (86.19) | 88 (73.95) | |
| Treatment delay, min, median (IQR) | ||||
| Onset to puncture | 334.00 (219.75, 497.00) | 315.00 (203.00, 475.00) | 368.00 (262.50, 557.00) | 0.004 |
| Puncture to recanalization | 107.00 (72.00, 152.25) | 103.00 (69.00, 144.00) | 112.00 (86.00, 164.00) | 0.032 |
| Onset to recanalization | 456.00 (327.75, 633.00) | 435.00 (302.75, 585.00) | 504.00 (362.00, 725.50) | 0.001 |
mTICI score of 2b or 3 indicates complete recanalization.
PC-CS, posterior circulation collateral system score; BA, basilar artery; mTICI, modified thrombolysis in cerebral infarction; PCA, posterior cerebral artery; V4, V4 segment of vertebral artery; CE, cardioembolism; NIHSS, National Institutes of Health Stroke Scale; PC-ASPECTS, posterior circulation Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; DBP, diastolic blood pressure; SOE, stroke of other determined cause; SUE, stroke of undetermined cause; TIA, transient ischemic attack; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
The Impacts of Malignant Cerebellar Edema on Clinical Outcome for Acute Basilar Artery Occlusion With Cerebellar Infarctions
As shown in Table 2 , favorable outcomes were least likely among patients with MCE, with much lower percentages of 90-day mRS 0-3 and a decreased median value of 90 day-mRS than the non-MCE group (non-MCE vs. MCE: 90-day mRS ≤ 3, 36.67% vs. 10.08%, P<0.001; mRS, 5(2-6) vs. 6(5-6), P<0.001). In both the unadjusted model and multivariate analysis with adjustment for confounders, MCE was found to be significantly associated with the 90-day mRS value [adjusted common OR, 2.86[95% CI, 1.64–4.92), P<0.001], long-term favorable outcome [adjusted OR, 0.35(95% CI, 0.18–0.68), P=0.002], and mortality at 90 days [adjusted OR, 3.24(95% CI, 1.79–5.95), P<0.001].
Table 2.
The impacts of MCE on clinical outcome.
| MCE | No./No. (%) | P-value | OR (95%CI) | P | Adjusted OR (95%CI) | P | |
|---|---|---|---|---|---|---|---|
| 90-day mRS 0-3, n (%) | Non-MCE | 77 (36.67) | <0.001 c | 0.19 (0.10-0.36) | <0.001 | 0.35 (0.18,0.68) g | 0.002 |
| MCE | 12 (10.08) | ||||||
| mRS, median (IQR) | Non-MCE | 5 (2-6) | <0.001 d | 5.37 (3.35,8.82) e | <0.001 | 2.86 (1.69,4.92) h | <0.001 |
| MCE | 6 (5-6) | ||||||
| Mortality in hospital, n (%) | Non-MCE | 34 (16.19) | 0.005 c | 2.16 (1.26,3.71) | 0.005 | 1.19 (0.63,2.24) g | 0.588 |
| MCE | 35 (29.41) | ||||||
| Mortality at 90 d, n (%) | Non-MCE | 72 (34.29) | <0.001 c | 5.69 (3.47,9.51) | <0.001 | 3.24 (1.79,5.95) g | <0.001 |
| MCE | 89 (74.79) | ||||||
| ΔNIHSS at 24 h
a
, median (IQR) |
Non-MCE | -5.00 (-11.00, 2.00) | <0.001 d | 4.26 (2.17,6.35) f | <0.001 | 3.38 (1.31,5.44) i | 0.001 |
| MCE | 2.00 (0.00, 6.00) | ||||||
| ΔNIHSS at 5-7 d
b
, median (IQR) |
Non-MCE | 0.00 (-5.00 to 2.00) | <0.001 d | 7.29 (4.72,9.86) f | <0.001 | 5.96 (3.46,8.46) i | <0.001 |
| MCE | 2.00 (0.00 to 6.00) |
Change from NIHSS at admission from NIHSS at 24 h after EVT.
Change from NIHSS at admission from NIHSS at 5-7 days after EVT.
Chi-square test.
Wilcoxon test.
Common OR.
ß-values were estimated from a univariate linear regression model.
Adjusted OR; adjusted estimates of outcome were calculated using multiple regression, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, neutrophil count, mTICI, PC-CS score, occlusion sites, and onset to recanalization time.
Adjusted common OR; adjusted estimates of outcome were calculated using multiple regression, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, neutrophil count, mTICI, PC-CS score, occlusion sites, and onset to recanalization time.
ß-values were estimated from a multivariable linear regression model; adjusted estimates of outcome were calculated using multiple regression, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, neutrophil count, mTICI, PC-CS score, occlusion sites, and onset to recanalization time.
NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale score at 90 days; MCE, malignant cerebellar edema; PC-CS, posterior circulation collateral system score; mTICI, modified thrombolysis in cerebral infarction; PC-ASPECTS, posterior circulation Alberta Stroke Program Early CT Score.
Predictors for Malignant Cerebellar Edema and Clinical Outcome
As shown in Table 3 , univariate logistic regression showed that the neutrophil count, NIHSS score at baseline, PC-ASPECTS, PC-CS, mTICI and onset to recanalization time were related with the incidences of MCE (all P<0.05). Multivariable logistic regression that included predictors identified by the univariate analysis (at P<0.05) found the following independent predictors of MCE: NIHSS at admission [adjusted OR, 1.04(5% CI, 1.01-1.07), P = 0.013], neutrophil count [adjusted OR 1.1(95% CI, 1.09-1.25), P < 0.001], mTICI [adjusted OR, 0.80(95% CI, 0.67-0.95), P<0.001], and PC-CS score [adjusted OR, 0.77(95% CI, 0.66-0.88), P<0.001]. Similarly, our results detected that the neutrophil count [adjusted OR, 0.83(95% CI, 0.76-0.91), P<0.001], NIHSS [adjusted OR, 0.83(95% CI, 0.76-0.91), P<0.001], and mTICI [adjusted OR, 1.74(95% CI, 1.31-2.37), P<0.001] were still associated with a favorable long-term outcome (90-day mRS ≤ 3).
Table 3.
Predictors for MCE and favorable outcome (mRS ≤ 3) in enrolled ABAO patients with cerebellar infarctions treated by EVT.
| MCE | Favorable outcome (mRS ≤ 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| OR (95%CI) | p-value | Adjusted OR (95%CI) | p-value | OR (95%CI) | p-value | Adjusted OR (95%CI) | p-value | ||
| Age | 0.99 (0.97-1.01) | 0.435 | 0.99 (0.97-1.02) | 0.515 | |||||
| Sex | 1.63 (0.93-2.94) | 0.095 | 0.62 (0.36-1.10) | 0.099 | |||||
| Dyslipidemia | 1.10 (0.68-1.76) | 0.701 | 1.27 (0.76-2.11) | 0.355 | |||||
| Diabetes mellitus | 0.85 (0.49-1.45) | 0.561 | 0.61 (0.32-1.11) | 0.120 | |||||
| Hypertension | 1.10 (0.67-1.81) | 0.717 | 1.21 (0.71-2.10) | 0.496 | |||||
| Atrial fibrillation | 0.89 (0.48-1.59) | 0.689 | 1.23 (0.65-2.55) | 0.510 | |||||
| Lymphocyte | 0.90 (0.64-1.23) | 0.510 | 1.29 (0.92-1.80) | 0.130 | |||||
| Neutrophil count | 1.18 (1.11-1.27) | <0.001 | 1.17 (1.09-1.25) | <0.001 | 0.80 (0.73-0.86) | <0.001 | 0.83 (0.76-0.91) | <0.001 | |
| NLR | 1.01 (1.00-1.04) | 0.10 | 0.94 (0.89-0.97) | 0.003 | |||||
| SBP | 1.01 (0.99-1.01) | 0.187 | 1.00 (0.99-1.01) | 0.467 | |||||
| DBP | 1.00 (1.00-1.04) | 0.637 | 0.99 (0.98-1.01) | 0.298 | |||||
| TOAST | |||||||||
| LAA | Reference | NA | Reference | NA | |||||
| CE | 0.63 (0.35,1.10) | 0.109 | 1.59 (0.89,2.80) | 0.111 | |||||
| SOE | 0.62 (0.09,2.95) | 0.573 | 0.51 (0.03,3.07) | 0.536 | |||||
| SUE | 0.72 (0.27,1.79) | 0.498 | 1.43 (0.52,3.56) | 0.463 | |||||
| Occlusion Sites | |||||||||
| BA distal | Reference | NA | Reference | NA | Reference | NA | |||
| BA middle | 1.32 (0.73,2.39) | 0.360 | 0.44 (0.24,0.80) | 0.008 | 0.28 (0.12, 0.63) | 0.003 | |||
| BA proximal | 1.35 (0.66,2.72) | 0.403 | 0.44 (0.20,0.91) | 0.031 | 0.48 (0.17,1.25) | 0.140 | |||
| V4 | 2.23 (1.17,4.28) | 0.015 | 0.31 (0.14,0.63) | 0.002 | 0.29 (0.11,0.74) | 0.011 | |||
| Thrombolysis treatment | 0.98 (0.70-1.34) | 0.883 | 1.05 (0.74-1.48) | 0.762 | |||||
| Preonset mRS | 0.82 (0.49-1.32) | 0.428 | 0.69 (0.36-1.18) | 0.201 | |||||
| Onset to recanalization time | 1.00 (1.00-1.01) | 0.003 | 1.00 (1.00-1.00) | 0.005 | 1.00 (1.00-1.00) | 0.184 | |||
| NIHSS baseline | 1.06 (1.03-1.09) | <0.001 | 1.04 (1.01-1.07) | 0.013 | 0.91 (0.88,0.93) | <0.001 | 0.92 (0.89-0.95) | <0.001 | |
| PC-ASPECTS | 0.83 (0.72-0.96) | 0.011 | 1.70 (1.42,2.07) | <0.001 | 1.62 (1.29-2.06) | <0.001 | |||
| mTICI | 0.76 (0.65-0.88) | <0.001 | 0.80 (0.67-0.95) | 0.011 | 1.83 (1.46,2.35) | <0.001 | 1.74 (1.31-2.37) | <0.001 | |
| PC-CS score | 0.74 (0.65-0.84) | <0.001 | 0.77 (0.66-0.88) | <0.001 | 1.32 (1.16,1.5) | <0.001 | 1.22 (1.01,1.47) | 0.050 | |
PC-CS, posterior circulation collateral system score; BA, basilar artery; mTICI, modified thrombolysis in cerebral infarction; PCA, posterior cerebral artery; V4, V4 segment of vertebral artery; CE, cardioembolism; NIHSS, National Institutes of Health Stroke Scale; PC-ASPECTS, posterior circulation Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; DBP, diastolic blood pressure; SOE, stroke of other determined cause; SUE, stroke of undetermined cause; TIA, transient ischemic attack; TOAST, Trial of ORG 10172 in Acute Stroke Treatment, NA, Not available.
Association of Neutrophil Count With Malignant Cerebellar Edema and Long-Term Outcome
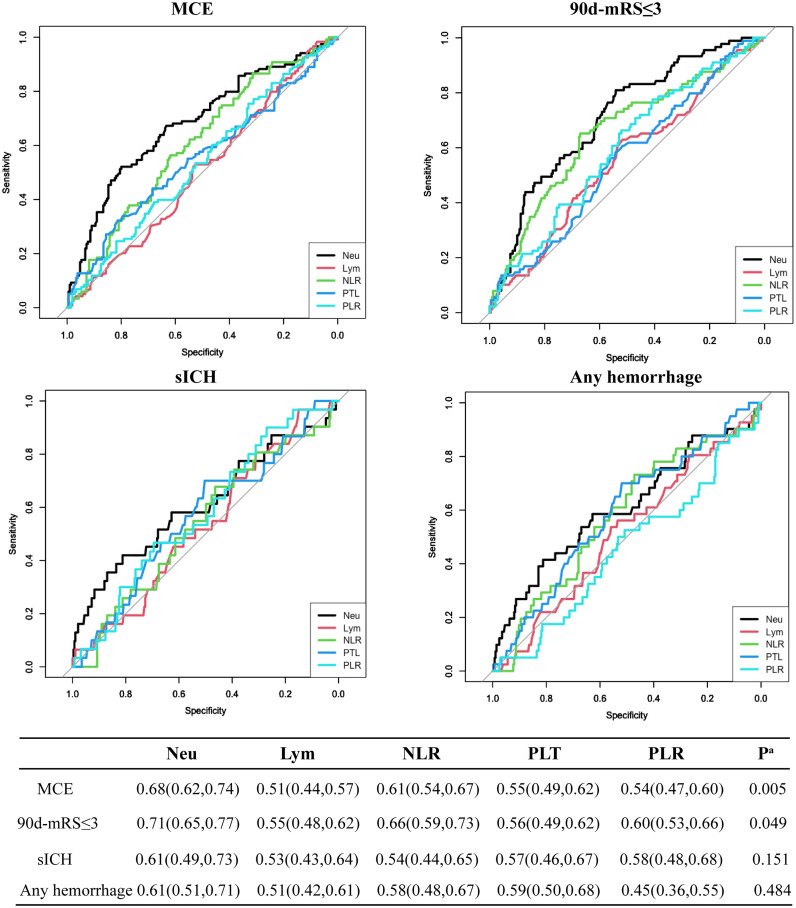
With the development of immunotherapy, inflammation cells might act as novel potential therapeutic targets for stroke at admission, compared to other unchangeable predictors for MCE identified above, such as NIHSS, the circulation level, and mTICI status (7, 8, 15). Thus, we compared the predictive values of inflammation factors for 4 endpoints and found that the neutrophil count achieved the highest AUC value when predicting MCE and 90-day mRS ≤3 [MCE: neutrophils vs. NLR, 0.68(0.62, 0.74) vs. 0.61(0.54,0.67), P=0.005 by using DeLong’s test; favorable outcome: neutrophils vs. NLR, 0.71(0.65, 0.77) vs. 0.66(0.59, 0.73), P=0.049 by using the DeLong’s test; Figure 1 ].
Figure 1.
Predictive values of neutrophil count (Neu), leukocyte count (Lym), platelet count (PTL), PLR, and NLR for 4 endpoints including MCE, long-term favorable outcome, sICH, and any hemorrhage.
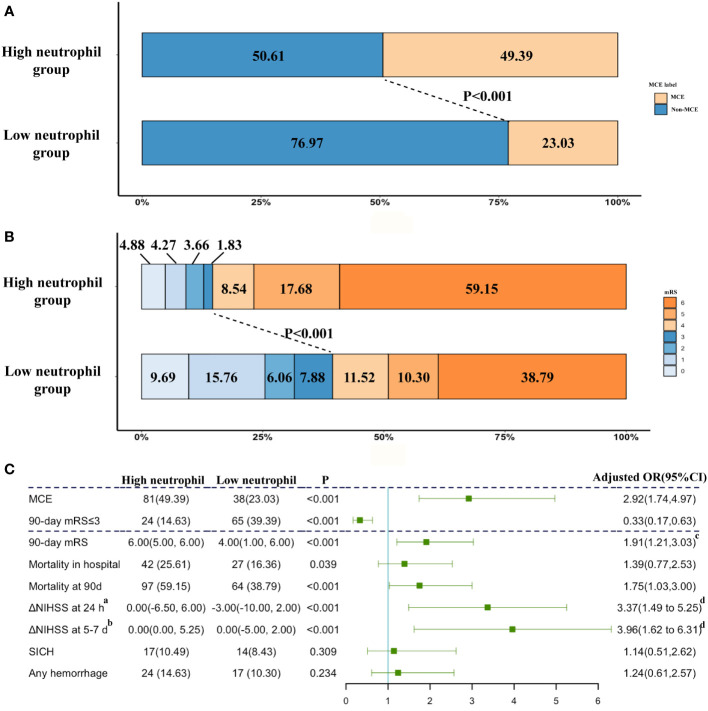
These ABAO patients with cerebellar infarctions after EVT were further divided into the high neutrophil group and low neutrophil group by the median value of neutrophils (9.87×109/L) in enrolled patients ( Supplemental Table 2 ). The ratio of MCE significantly increased, while the incidence of favorable outcome obviously reduced in patients with a high neutrophil status (high neutrophil group vs. low neutrophil group: MCE: 49.39% vs. 23.03%, Figure 2A ; favorable outcome: 14.63% vs. 39.39%, Figure 2B ). As shown in Figure 2C , based on multivariable analysis, the high neutrophil level was remarkably related with 90-day mRS ≤ 3 [adjusted OR, 0.33(95% CI, 0.17-0.63)] and MCE [adjusted OR, 2.92(95% CI, 1.74-4.97)].
Figure 2.
The impacts of neutrophil count on clinical outcome. (A) Distribution of the MCE across patients with different neutrophil count status; (B) Distribution of the mRS scores at 90 days according to the trichotomized neutrophil count status. (C) Adjusted logistic regression models evaluated the associations between neutrophil level with outcomes. a Change from NIHSS at admission from NIHSS at 24 h after EVT. b Change from NIHSS at admission from NIHSS at 5-7 days after EVT. cAdjusted common OR; adjusted estimates of outcome were calculated using multiple regression, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, neutrophil count, mTICI, PC-CS score, occlusion sites, and onset to recanalization time. dß-values were estimated from a multivariable linear regression model; adjusted estimates of outcome were calculated using multiple regression, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, neutrophil count, mTICI, PC-CS score, occlusion sites, and onset to recanalization time. NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale score at 90 days.
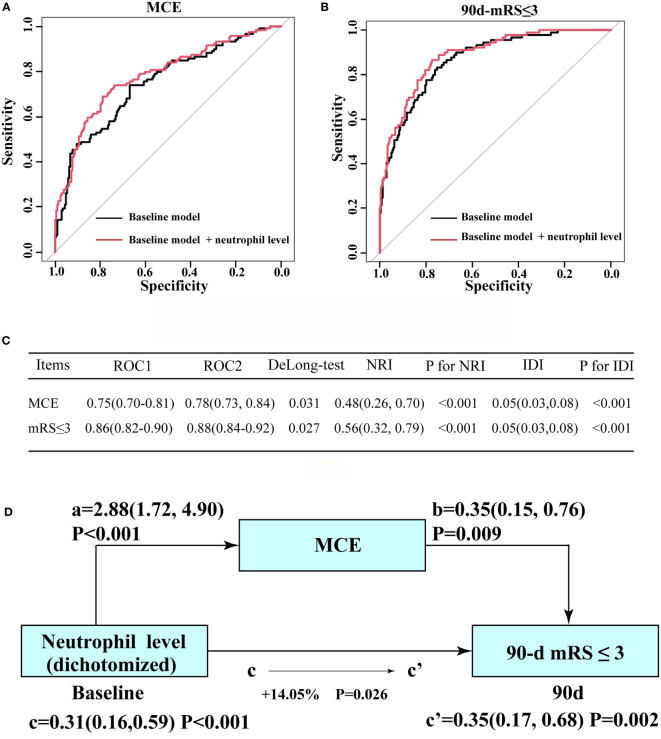
The addition of the neutrophil status significantly increased the prediction ability of the baseline model for MCE in enrolled individuals and yielded a statistically elevated AUC value [baseline model vs. baseline model + high neutrophil status (dichotomized): 0.75(95% CI: 0.70–0.81) vs. 0.78(95% CI: 0.73–0.84), P=0.031 by using DeLong’s test; Figure 3A ]. Significant improvements in risk reclassification and discrimination were also detected after adding the neutrophil status (dichotomized) into the baseline model, with an NRI of 0.48[95% CI: 0.26–0.70] and an IDI of 0.05 [95% CI: 0.03–0.08] ( Figure 3C ). The improved roles of neutrophils for the baseline model in predicting a long-term outcome was also observed [baseline model vs. baseline model + high neutrophil status (dichotomized): 0.86(95% CI: 0.82–0.90) vs. 0.88(95% CI: 0.84–0.92), P=0.027 by using the DeLong’s test; Figure 3B ]. After including MCE as a mediator, we observed a significant partial mediation effect for MCE on higher neutrophil count (dichotomized)-related effects on the 90-day functional outcome in enrolled patients (estimated proportion mediated by MCE: 14.05%, P=0.026, Figure 3D ).
Figure 3.
Incremental effect of the neutrophil count level on the predictive value of the baseline model for favorable outcome and MCE. (A) ROC curves for baseline model and novel model count level when predicting long-term outcome. (B) ROC curves for baseline model (ROC1) and novel model (ROC2: adding neutrophil count level into the baseline model) when predicting MCE. (C) Results from DeLong’s test, the NRI, and IDI by comparing ROC curves in (A, B), separately. (D) Mediation analysis by MCE of the associations between neutrophil count level and clinical outcome. a, regression coefficient of the association between neutrophil count and MCE; b, regression coefficient of the association between MCE and clinical outcome; c, regression coefficient of the association between neutrophil count level and clinical outcome; c’, regression coefficient of the association between neutrophil count level and clinical outcome, using neutrophil count level and MCE as additional independent variables. The percentage difference of the coefficients (1-c/c’) is shown. a, b, c, and c’ were evaluated by adjusted OR, taking the following variables into account: baseline NIHSS score, baseline PC-ASPECTS, mTICI, PC-CS score, occlusion sites, and onset to recanalization time.
Subgroup and Sensitive Analysis
None of the interactions by variables including age, sex, NIHSS, mTICI, PC-ASPECTS, and PC-CS score between the neutrophil count level and MCE and the long-term outcome were statistically significant ( Supplemental Figures 2A, B ). Sensitivity analysis showed no modifications of the effects of neutrophils on outcomes after the exclusion of symptomatic hemorrhagic, transformation, and unsuccessful revascularization. The details of the analysis are shown in Supplemental Table 3 . Given that the proportion of men was significantly higher than that of women, we further explored the prognostic value of neutrophils in different genders in Supplemental Figure 3 . The high neutrophil count was positively related with MCE and poor outcome in both female and male patients. Neutrophil counts were not significantly altered between female MCE patients and male MCE patients and between female poor outcome patients and male poor outcome patients.
Among all ABAO patients after EVT in the BASILAR registry, the impacts of the neutrophil status on outcomes were also evaluated. As shown in Supplemental Table 4 , the frequency of a favorable outcome was obviously decreased in the high neutrophil group [low neutrophil group vs. high neutrophil group: 133(40.06) vs. 56(22.05), P <0.001]. Based on multivariate analysis, the high neutrophil group was still an independent predictor for a 90-day favorable outcome [adjusted OR with 95% CI: 0.49 (0.30-0.78), P=0.002] in all ABAO patients. To further investigate the clinical value of findings in the present study, we classified enrolled patients into the normal range group (2-8 × 10(9)/L), elevated group (8-9.87 × 10(9)/L), and extremely high group (≥9.87 × 10(9)/L) in Supplemental Tables 5, 6 . No patients enrolled in the present research had a neutrophil count below 2 × 10(9)/L. In Supplemental Table 5 , we compared the baseline characteristics among the three groups. As shown in Supplemental Table 6 , we found that the elevated neutrophil count group had similar effects on clinical outcomes compared with the normal range group (all P>0.05), while the extremely high neutrophil counts significantly reduced the proportion of favorable prognosis [aOR 0.37, 95%CI (0.18, 0.75), P=0.006] and increased the ratio of MCE [aOR 2.36, 95%CI (1.29, 4.40), P=0.006].
Discussion
Based on a multi-centered cohort derived from the BASILAR registry, this is, to our knowledge, the first study to explore the associations between MCE and clinical outcomes, and investigate predictors for MCE in ABAO patients with cerebellar infarctions treated by EVT. Our results revealed that 1) the presence of MCE was associated with 0.35-folds for favorable outcome and 3.24-folds for mortality among these patients; 2) the neutrophil count was a more reliable predictor of MCE compared with other inflammatory markers; 3) the prognostic roles of a high neutrophil status for a poor long-term outcome could be partially explained by its roles in promoting MCE; 4) adding a neutrophil level to a conventional model obviously improved its prediction ability for MCE and outcome in enrolled ABAO patients.
The incidence of MCE was estimated to range from 4% to 39% among all cases of pure cerebellar infarctions, while the MCE in ABAO with cerebellar infarctions were less intensively explored, who were usually excluded in previous literatures (5, 6, 16). In our study, the rate of MCE in ABAO with cerebellar infarctions was 36.17%, which was comparable to the previously reported incidence of MCE in pure cerebellar infarctions (5, 16). However, the rates for poor outcome and mortality in ABAO individuals with MCE were 89.92% and 74.79%, respectively, which were much higher than ABAO without MCE and pure cerebellar infarctions with MCE (16). Aside from the concomitant brain stem injury after occluding basilar artery, the relative low incidence of decompression craniectomy (N=11) might also be the important reason for the worse prognosis of ABAO with MCE in present research (4, 5). In addition, compared with previous literatures focusing on brain stem infarcts and illustrating that brainstem damage degree determined the outcomes for these populations, our results provided a new evidence of involving the cerebellum as a reliable prognosticator of poor outcome due to the posterior fossa mass effects of MCE (17, 18). Patients with cerebellar infarcts may require prompt detection during pretreatment imaging, closely monitoring for the early detection of MCE, and even considerations for pre-emptive neurosurgery (6).
As a marker of systemic inflammation, the neutrophil count is easily obtained from routine laboratory data without an additional technique or cost. In line with findings in previous researches on the anterior circulation stroke, our research found that an elevated neutrophil count was a strong prognostic factor for worse clinical outcomes in ABAO patients after EVT (19, 20). Physically, the neutrophils in infarcted regions would obviously block the microvascular network, reduce the brain blood flow of microvessels, and aggravate tissue damage (21). Both in vitro and in vivo experiments also demonstrated that the myeloperoxidase and neutrophil extracellular traps from neutrophils could directly increase neuronal death and lead to augmented final infarction volumes (22). The elaborated functions of neutrophils in ischemic lesions have led neutrophils to be promising therapeutic targets to reduce damage after stroke (23).
Mediation analysis in our study suggested that MCE might be an important mechanism linking peripheral inflammation to a poor outcome for ABAO patients after EVT. The blood–brain barrier (BBB) was disrupted early after stroke, which would facilitate the infiltration of peripheral immune cells into the infarct regions to further potentiate damages for constituents in BBB. Among these immune cells, the neutrophil count in infarction tissues displayed an exponential increase within a few hours after the symptom onset and remained elevated for 1 week, while lymphocytes extravasated into these regions in a smaller count and reached maximum accumulation several days later (24). Thus, due to the fact that brain tissue edema most likely occurred within 3 days after EVT according to previous reports (5, 16), the fast accumulation of neutrophils might play critical roles in promoting injury. In contrast with findings from the Daniela group, which demonstrated that neutrophils and the neutrophil-to-lymphocyte ratio (NLR) showed identical predictive performances in forecasting cerebral edema, our results detected much better prognostic abilities of neutrophils than NLR for MCE (7). Compared with cerebrum, the differential response manners of cerebellar BBB to inflammatory stimulation may be an important reason for this phenomenon (7). An in vitro experiment demonstrated the lower expression of major tight junction components of occludin, claudin-1 and claudin-3 in cerebellar endothelial cells compared with the cerebrum (25). A stronger elevation of VCAM-1 and ICAM-1 expression in cerebellar endothelial cells suggested a higher interaction between inflammatory cells and vessels, which might lead the cerebellum to be more sensitive to neutrophils, the fastest-accumulated cells in infarcted regions after stroke (26). The products of neutrophils including reactive oxide species, proteases (matrix proteinase 3, elastase), lipocalin-2, and neutrophil extracellular traps would directly degrade junction proteins among endothelial cells in BBB (27). The cytokines such as interleukin-1β, interleukin-6, and interleukin-8 from neutrophils also played detrimental roles in BBB integrity and amplified the inflammatory response (28). Taken together, our findings revealed that the neutrophil count was an early independent predictor for the malignant edema of cerebellum and functional outcome in ABAO patients treated by EVT.
This study has several limitations. First, the ABAO patients included in current research were from the national registry of China, and thus, the associations between inflammatory composites and outcomes need to be tested in non-Chinese populations. Second, the acute stress, chemoradiotherapy and the applications of medication, such as corticosteroids and antibiotics, might lead to the modulations of count and functions of neutrophils. We did not assess their impacts on neutrophils but attempted to minimize their effects by only using the baseline neutrophil count at admission and excluding patients with prior medication use and relevant disease history. Third, though initial infarction volumes were reported to be related with brain edema, we did not assess it due to the fact that we included patients through preoperative or postoperative imaging examinations, and there were no preoperative CTP or MRI imaging data for all patients. In addition, the technical differences between CTP and MRI might also introduce a technique bias when assessing infarct volumes (6). To lower the relevant impacts, NIHSS, PC-ASPECTS, collateral circulation status, and onset to treatment time, which were important influential factors for infarct volumes, were included in the present analysis (29, 30). Fourth, recent studies have illustrated the gender differences on the inflammation response. Although our results indicated that there was no significant difference in the prognostic role of neutrophils in ABAO patients, further analysis was limited due to the relatively small number of female patients (31). Due to the real-world design of the present study, we cannot specifically control for gender ratio when enrolling patients. Based on your valuable suggestion, we will strictly control the gender proportion in the national RCT BASILAR2 (Clinical Trial Registry identifier: ChiCTR2000040902), which is being carried out by our group, and try to explore the characteristics of inflammatory response pattern in female ABAO patients in the BASILAR2 trail. Fifth, compared with measuring inflammatory cells once, the dynamic alterations of these factors might provide additional prognostic information. We will try to monitor the longitudinal alterations of inflammatory factors and explore their roles in determining MCE and outcomes in the multi-center random clinical trial of BASILAR2.
Conclusions
MCE acted essential roles in worsening the prognosis for ABAO after EVT, and thus, additional treatments before, during, and after EVT, such as reducing MCE, were needed to improve clinical outcome for ABAO patients. These 5 factors including the onset to recanalization time, baseline NIHSS score, collateral circulation, neutrophil count at admission, and recanalization status were critical contributors to MCE. Compared with these unchangeable factors including stroke severity and the collateral circulation status, the admission neutrophil count could not only act as an easily accessible biomarker from a blood routine examination to be incorporated into the clinical decision-making system but also be a target to guide immunomodulation therapy for ABAO patients.
Code Availability
The analyzing codes of this study are available from the corresponding author upon reasonable request.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Xinqiao Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CL: Drafting/revision of the manuscript for content, including medical writing for content, major role in the acquisition of data, analysis or interpretation of data. FL: Study concept or design, analysis, or interpretation of data. SL: Study concept or design. QC: Major role in the acquisition of data. HS: Study concept or design, analysis, or interpretation of data. QY: Drafting/revision of the manuscript for content, including medical writing for content, study concept or design. KZ: Drafting/revision of the manuscript for content, including medical writing for content, Study concept or design. WZ: Drafting/revision of the manuscript for content, including medical writing for content, major role in the acquisition of data, study concept or design. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 82001264, 82071323, 82001265, 81901236), Chongqing Natural Science Foundation (No. cstc2020jcyj-msxmX0926), Clinical Medical Research Talent Training Program of Army Medical University (No. 2019XLC2008 and 2019XLC3016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.835915/full#supplementary-material
References
- 1. Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar Artery Occlusion. Lancet Neurol (2011) 10(11):1002–14. doi: 10.1016/S1474-4422(11)70229-0 [DOI] [PubMed] [Google Scholar]
- 2. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular Treatment Versus Standard Medical Treatment for Vertebrobasilar Artery Occlusion (BEST): An Open-Label, Randomised Controlled Trial. Lancet Neurol (2020) 19(2):115–22. doi: 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 3. Langezaal LCM, van der Hoeven E, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular Therapy for Stroke Due to Basilar-Artery Occlusion. N Engl J Med (2021) 384(20):1910–20. doi: 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 4. Yoon W, Baek BH, Lee YY, Kim SK, Kim JT, Park MS. Association of Pretreatment Pontine Infarction With Extremely Poor Outcome After Endovascular Thrombectomy in Acute Basilar Artery Occlusion. J Neurointerv Surg (2021) 13(2):136–40. doi: 10.1136/neurintsurg-2020-015930 [DOI] [PubMed] [Google Scholar]
- 5. Fabritius MP, Thierfelder KM, Meinel FG, Othman AE, Dorn F, Sabel BO, et al. Early Imaging Prediction of Malignant Cerebellar Edema Development in Acute Ischemic Stroke. Stroke (2017) 48(9):2597–600. doi: 10.1161/STROKEAHA.117.018237 [DOI] [PubMed] [Google Scholar]
- 6. Mourand I, Mahmoudi M, Dargazanli C, Pavillard F, Arquizan C, Labreuche J, et al. DWI Cerebellar Infarct Volume as Predictor of Outcomes After Endovascular Treatment of Acute Basilar Artery Occlusion. J Neurointerv Surg (2020) 13(11):995–1001. doi: 10.1136/neurintsurg-2020-016804 [DOI] [PubMed] [Google Scholar]
- 7. Ferro D, Matias M, Neto J, Dias R, Moreira G, Petersen N, et al. Neutrophil-to-Lymphocyte Ratio Predicts Cerebral Edema and Clinical Worsening Early After Reperfusion Therapy in Stroke. Stroke (2021) 52(3):859–67. doi: 10.1161/STROKEAHA.120.032130 [DOI] [PubMed] [Google Scholar]
- 8. Enzmann G, Kargaran S, Engelhardt B. Ischemia-Reperfusion Injury in Stroke: Impact of the Brain Barriers and Brain Immune Privilege on Neutrophil Function. Ther Adv Neurol Disord (2018) 11:1756286418794184. doi: 10.1177/1756286418794184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strbian D, Sairanen T, Silvennoinen H, Salonen O, Kaste M, Lindsberg PJ. Thrombolysis of Basilar Artery Occlusion: Impact of Baseline Ischemia and Time. Ann Neurol (2013) 73(6):688–94. doi: 10.1002/ana.23904 [DOI] [PubMed] [Google Scholar]
- 10. Reinhard M, Schork J, Allignol A, Weiller C, Kaube H. Cerebellar and Cerebral Autoregulation in Migraine. Stroke (2012) 43(4):987–93. doi: 10.1161/STROKEAHA.111.644674 [DOI] [PubMed] [Google Scholar]
- 11. Writing Group for the BG. Zi W, Qiu Z, Wu D, Li F, Liu H, et al. Assessment of Endovascular Treatment for Acute Basilar Artery Occlusion Via a Nationwide Prospective Registry. JAMA Neurol (2020) 77(5):561–73. doi: 10.1001/jamaneurol.2020.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Hoeven EJ, McVerry F, Vos JA, Algra A, Puetz V, Kappelle LJ, et al. Collateral Flow Predicts Outcome After Basilar Artery Occlusion: The Posterior Circulation Collateral Score. Int J Stroke (2016) 11(7):768–75. doi: 10.1177/1747493016641951 [DOI] [PubMed] [Google Scholar]
- 13. Jauss M, Muffelmann B, Krieger D, Zeumer H, Busse O. A Computed Tomography Score for Assessment of Mass Effect in Space-Occupying Cerebellar Infarction. J Neuroimaging (2001) 11(3):268–71. doi: 10.1111/j.1552-6569.2001.tb00045.x [DOI] [PubMed] [Google Scholar]
- 14. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke (2015) 46(10):2981–6. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 15. Zhang SR, Phan TG, Sobey CG. Targeting the Immune System for Ischemic Stroke. Trends Pharmacol Sci (2021) 42(2):96–105. doi: 10.1016/j.tips.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 16. Broocks G, Elsayed S, Kniep H, Kemmling A, Flottmann F, Bechstein M, et al. Early Prediction of Malignant Cerebellar Edema in Posterior Circulation Stroke Using Quantitative Lesion Water Uptake. Neurosurgery (2021) 88(3):531–7. doi: 10.1093/neuros/nyaa438 [DOI] [PubMed] [Google Scholar]
- 17. Mourand I, Machi P, Nogue E, Arquizan C, Costalat V, Picot MC, et al. Diffusion-Weighted Imaging Score of the Brain Stem: A Predictor of Outcome in Acute Basilar Artery Occlusion Treated With the Solitaire FR Device. AJNR Am J Neuroradiol (2014) 35(6):1117–23. doi: 10.3174/ajnr.A3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang P, Jia X, Zhang M, Cao Y, Zhao Z, Shan Y, et al. Correlation of Longitudinal Gray Matter Volume Changes and Motor Recovery in Patients After Pontine Infarction. Front Neurol (2018) 9:312. doi: 10.3389/fneur.2018.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher Neutrophil Counts Before Thrombolysis for Cerebral Ischemia Predict Worse Outcomes. Neurology (2015) 85(16):1408–16. doi: 10.1212/WNL.0000000000002029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boisseau W, Desilles JP, Fahed R, Kyheng M, Zuber K, Sabben C, et al. Neutrophil Count Predicts Poor Outcome Despite Recanalization After Endovascular Therapy. Neurology (2019) 93(5):e467–e75. doi: 10.1212/WNL.0000000000007859 [DOI] [PubMed] [Google Scholar]
- 21. Chen C, Huang T, Zhai X, Ma Y, Xie L, Lu B, et al. Targeting Neutrophils as a Novel Therapeutic Strategy After Stroke. J Cereb Blood Flow Metab (2021) 41(9):2150–61. doi: 10.1177/0271678X211000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, et al. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl Stroke Res (2020) 11(1):108–21. doi: 10.1007/s12975-019-00694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wanrooy BJ, Wen SW, Wong CH. Dynamic Roles of Neutrophils in Post-Stroke Neuroinflammation. Immunol Cell Biol (2021) 99(9):924–35. doi: 10.1111/imcb.12463 [DOI] [PubMed] [Google Scholar]
- 24. Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, et al. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front Immunol (2021) 12:678744. doi: 10.3389/fimmu.2021.678744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silwedel C, Forster C. Differential Susceptibility of Cerebral and Cerebellar Murine Brain Microvascular Endothelial Cells to Loss of Barrier Properties in Response to Inflammatory Stimuli. J Neuroimmunol (2006) 179(1-2):37–45. doi: 10.1016/j.jneuroim.2006.06.019 [DOI] [PubMed] [Google Scholar]
- 26. Engelhardt B, Ransohoff RM. The Ins and Outs of T-lymphocyte Trafficking to the CNS: Anatomical Sites and Molecular Mechanisms. Trends Immunol (2005) 26(9):485–95. doi: 10.1016/j.it.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 27. Ludewig P, Sedlacik J, Gelderblom M, Bernreuther C, Korkusuz Y, Wagener C, et al. Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Inhibits MMP-9-mediated Blood-Brain-Barrier Breakdown in a Mouse Model for Ischemic Stroke. Circ Res (2013) 113(8):1013–22. doi: 10.1161/CIRCRESAHA.113.301207 [DOI] [PubMed] [Google Scholar]
- 28. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as Protagonists and Targets in Chronic Inflammation. Nat Rev Immunol (2017) 17(4):248–61. doi: 10.1038/nri.2017.10 [DOI] [PubMed] [Google Scholar]
- 29. Al-Dasuqi K, Payabvash S, Torres-Flores GA, Strander SM, Nguyen CK, Peshwe KU, et al. Effects of Collateral Status on Infarct Distribution Following Endovascular Therapy in Large Vessel Occlusion Stroke. Stroke (2020) 51(9):e193–202. doi: 10.1161/STROKEAHA.120.029892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng B, Forkert ND, Zavaglia M, Hilgetag CC, Golsari A, Siemonsen S, et al. Influence of Stroke Infarct Location on Functional Outcome Measured by the Modified Rankin Scale. Stroke (2014) 45(6):1695–702. doi: 10.1161/STROKEAHA.114.005152 [DOI] [PubMed] [Google Scholar]
- 31. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The Impact of Sex and Gender on Stroke. Circ Res (2022) 130(4):512–28. doi: 10.1161/CIRCRESAHA.121.319915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.