ABSTRACT
Objective
There is little evidence related to the effects of the Omicron severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variant on pregnancy outcomes, particularly in unvaccinated women. This study aimed to compare pregnancy outcomes of unvaccinated women infected with SARS‐CoV‐2 during the pre‐Delta, Delta and Omicron waves.
Methods
This was a retrospective cohort study conducted at two tertiary care facilities: Sancaktepe Training and Research Hospital, Istanbul, Turkey, and St George's University Hospitals NHS Foundation Trust, London, UK. Included were women who tested positive for SARS‐CoV‐2 by real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) during pregnancy, between 1 April 2020 and 14 February 2022. The cohort was divided into three periods according to the date of their positive RT‐PCR test: (i) pre‐Delta (1 April 2020 to 8 June 2021 in Turkey, and 1 April 2020 to 31 July 2021 in the UK), (ii) Delta (9 June 2021 to 27 December 2021 in Turkey, and 1 August 2021 to 27 December 2021 in the UK) and (iii) Omicron (after 27 December 2021 in both Turkey and the UK). Baseline data collected included maternal age, parity, body mass index, gestational age at diagnosis and comorbidities. The primary outcome was the need for oxygen supplementation, classified as oxygen support via nasal cannula or breather mask, non‐invasive mechanical ventilation with continuous positive airway pressure (CPAP) or high‐flow oxygen, mechanical ventilation with intubation, or extracorporeal membrane oxygenation (ECMO). Inferences were made after balancing of confounders, using an evolutionary search algorithm. Selected confounders were maternal age, body mass index and gestational age at diagnosis of infection.
Results
During the study period, 1286 unvaccinated pregnant women with RT‐PCR‐proven SARS‐CoV‐2 infection were identified, comprising 870 cases during the pre‐Delta period, 339 during the Delta wave and 77 during the Omicron wave. In the confounder‐balanced cohort, infection during the Delta wave vs during the pre‐Delta period was associated with increased need for nasal oxygen support (risk ratio (RR), 2.53 (95% CI, 1.75–3.65); P < 0.001), CPAP or high‐flow oxygen (RR, 2.50 (95% CI, 1.37–4.56); P = 0.002), mechanical ventilation (RR, 4.20 (95% CI, 1.60–11.0); P = 0.003) and ECMO (RR, 11.0 (95% CI, 1.43–84.7); P = 0.021). The maternal mortality rate was 3.6‐fold higher during the Delta wave compared to the pre‐Delta period (5.3% vs 1.5%, P = 0.010). Infection during the Omicron wave was associated with a similar need for nasal oxygen support (RR, 0.62 (95% CI, 0.25–1.55); P = 0.251), CPAP or high‐flow oxygen (RR, 1.07 (95% CI, 0.36–3.12); P = 0.906) and mechanical ventilation (RR, 0.44 (95% CI, 0.06–3.45); P = 0.438) with that in the pre‐Delta period. The maternal mortality rate was similar during the Omicron wave and the pre‐Delta period (1.3% vs 1.3%, P = 0.999). The need for nasal oxygen support during the Omicron wave was significantly lower compared to the Delta wave (RR, 0.26 (95% CI, 0.11–0.64); P = 0.003). Perinatal outcomes were available for a subset of the confounder‐balanced cohort. Preterm birth before 34 weeks' gestation was significantly increased during the Delta wave compared with the pre‐Delta period (15.4% vs 4.9%, P < 0.001).
Conclusions
Among unvaccinated pregnant women, SARS‐CoV‐2 infection during the Delta wave, in comparison to the pre‐Delta period, was associated with increased requirement for oxygen support (including ECMO) and higher maternal mortality. Disease severity and pregnancy complications were similar between the Omicron wave and pre‐Delta period. SARS‐CoV‐2 infection of unvaccinated pregnant women carries considerable risks of morbidity and mortality regardless of variant, and vaccination remains key. Miscommunication of the risks of Omicron infection may impact adversely vaccination uptake among pregnant women, who are at increased risk of complications related to SARS‐CoV‐2. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: COVID‐19, effectiveness, maternal, variant of concern, wild‐type
CONTRIBUTION —
What are the novel findings of this work?
Infection of unvaccinated pregnant women with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) during the Delta wave, compared with the pre‐Delta period (wild‐type strain and Alpha variant of SARS‐CoV‐2), was associated with higher rate of maternal death and greater need for oxygen supplementation, mechanical ventilation and extracorporeal membrane oxygenation. However, disease severity and pregnancy complications during the Omicron wave were similar to those observed during the pre‐Delta period. This finding was limited by the small sample size.
What are the clinical implications of this work?
Among unvaccinated women, infection during the Delta wave was associated with worse maternal outcomes and preterm birth before 34 weeks' gestation compared with the pre‐Delta period. The severity of infection and pregnancy outcomes during the Omicron wave were similar to those in the pre‐Delta period, with no evidence of milder disease associated with the Omicron variant. To minimize the risks of SARS‐CoV‐2 infection during pregnancy, SARS‐CoV‐2 vaccination remains critically important.
INTRODUCTION
During the coronavirus disease 2019 (COVID‐19) pandemic, there have been several peaks of infection, caused mostly by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants of concern, labelled alphabetically in chronological order. The first few pandemic peaks were caused by the wild‐type strain and the Alpha variant (B.1.1.7), against which available vaccines render strong immunity. The Delta variant (B.1.617) was more virulent than the Alpha variant, and demonstrated some immune escape from vaccines, making them somewhat less effective 1 . The more recent Omicron variant (B.1.1.529) has shown even greater immune escape from vaccines, leading to record case numbers (but not increased mortality) in every country in which it has been detected 2 , 3 . This has been widely interpreted to mean that the Omicron variant is causing less severe disease; however, health authorities have advised caution, as data on Omicron infection have been derived largely from countries with high vaccination rates 4 .
SARS‐CoV‐2 infection is associated with excess maternal and perinatal mortality and morbidity 5 . Some evidence suggests that the Delta variant (compared with the wild‐type strain and the Alpha variant) causes more severe disease in pregnancy and a heightened risk of stillbirth 6 . However, there is little evidence related to the effect of the Omicron variant on pregnancy outcomes, particularly in unvaccinated women. This study aimed to compare pregnancy outcomes of SARS‐CoV‐2‐infected, unvaccinated women during the pre‐Delta, Delta and Omicron periods.
METHODS
This was a retrospective cohort study conducted at two tertiary care facilities: Sancaktepe Training and Research Hospital, Istanbul, Turkey, and St George's University Hospitals NHS Foundation Trust, London, UK. Included were women who were unvaccinated against COVID‐19 and who tested positive for SARS‐CoV‐2 on real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) during pregnancy, between 1 April 2020 and 14 February 2022. The study cohort was divided into three periods according to the date of their positive RT‐PCR test and official resources delineating the periods of dominance of the Delta and Omicron variants of concern in each country 7 : (i) pre‐Delta, from 1 April 2020 to 8 June 2021 in Turkey, and 1 April 2020 to 31 July 2021 in the UK, (ii) Delta, from 9 June 2021 to 27 December 2021 in Turkey, and 1 August 2021 to 27 December 2021 in the UK, and (iii) Omicron, after 27 December 2021 in both Turkey and the UK.
Testing indications for SARS‐CoV‐2 included admission for obstetric indication (e.g. admission for delivery), SARS‐CoV‐2 symptomatology, and exposure to someone with confirmed SARS‐CoV‐2 infection. The management of infected women was similar in both centers. Oxygen supplementation with nasal cannula was started for women with oxygen saturation below 95%, severe shortness of breath or tachypnea. When target oxygen saturation was not maintained on standard flow, the level of oxygen support was escalated to breather mask with reservoir and high‐flow oxygen. Women who required high‐flow oxygen support to maintain a target oxygen saturation were started on steroid treatment. Non‐invasive mechanical ventilation with continuous positive airway pressure (CPAP), mechanical ventilation with intubation, and extracorporeal membrane oxygenation (ECMO) were utilized in sequential manner in women who failed to maintain target oxygen saturation levels. Processes of care that were not included as outcomes were admission to a maternal intensive care unit and therapies such as convalescent plasma, remdesivir and interleukin inhibitors. Indications for their use varied between the study centers and during the various stages of the pandemic.
Study data were obtained from electronic records, and included baseline characteristics such as maternal age, parity, body mass index (BMI), gestational age at diagnosis, comorbidities and maternal complications. Perinatal outcomes were available in a subset of participants. The main outcome measure was the need for respiratory support, classified as oxygen support via nasal cannula or breather mask, CPAP or high‐flow oxygen, or mechanical ventilation with intubation or ECMO. Other outcomes assessed included rates of pre‐eclampsia, maternal death and perinatal outcomes.
Statistical analysis
Baseline characteristics and outcomes were compared using the chi‐square test, Fisher's exact test, Student's t‐test or the Mann–Whitney U‐test, as appropriate. Exposure groups (pre‐Delta vs Delta and pre‐Delta vs Omicron) were matched for confounders using propensity score matching. Selected confounders were maternal age, BMI and gestational age at diagnosis which were chosen from a set of candidate variables (maternal age, BMI, parity, gestational age at diagnosis, comorbidities) described in a previous study based on a similar cohort 8 . Balance was optimized for the confounders, and the balance checks were performed using propensity score histograms and randomization checks for Mahalanobis distance. Standardized mean differences in baseline characteristics between the groups were calculated with the aim of reducing differences below 10%. The matching ratio was selected with the aim of minimizing discarded subjects (i.e. unmatched individuals) and reaching optimal confounder balance.
Sensitivity analyses were performed using smaller and larger matching ratios to check the robustness of estimates. After matching, the effects were estimated using generalized estimating equations, thereby avoiding potential bias from exclusion of cases for which confounder‐matching could not be achieved; match identifiers were treated as random effects, while variant wave and other predictor variables were treated as fixed effects. The stability of estimates was tested using generalized linear mixed‐effects models using both match identifiers and treatment centers as random effects, but these results were not reported if both analyses had similar results.
Effect estimates were reported as risk ratios and 95% CI. All analyses were performed using R for Statistical Computing Software (Vienna, Austria), and P‐values < 0.05 were considered statistically significant.
RESULTS
During the study period, a total of 1286 unvaccinated pregnant women with RT‐PCR‐proven SARS‐CoV‐2 infection were identified, comprising 870 women infected during the pre‐Delta period, 339 women infected during the Delta wave and 77 women infected during the Omicron wave. The majority of the cases were from Turkey (n = 1160) and the remainder were from the UK (n = 126).
In our study population, the rates of mechanical ventilation (invasive or non‐invasive) increased during the Delta wave and decreased again during the Omicron wave. The bimonthly average proportion of positive cases requiring mechanical ventilation reached a peak towards the second half of the Delta wave (22.1%), followed by a decrease during the Omicron wave (to 4.7%) (Figure 1).
Figure 1.
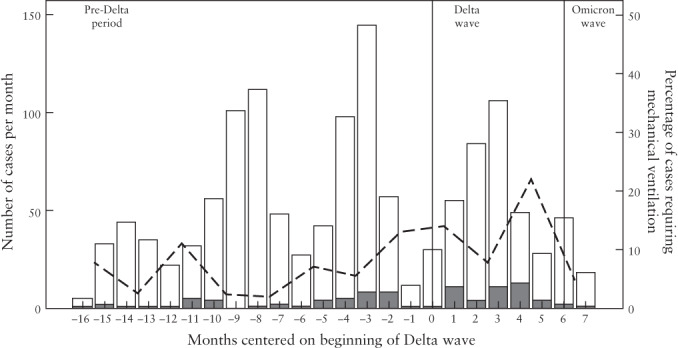
Total monthly number of women infected by SARS‐CoV‐2 and proportion requiring mechanical ventilation during the pre‐Delta period, Delta wave and Omicron wave. Dashed line shows bimonthly average rate of cases requiring non‐invasive or invasive mechanical ventilation.  , with mechanical ventilation;
, with mechanical ventilation;  , without mechanical ventilation.
, without mechanical ventilation.
Delta wave vs pre‐Delta period
Pregnant women infected with SARS‐CoV‐2 during the Delta wave vs the pre‐Delta period were slightly older and had higher BMI and later gestational age at diagnosis (including diagnosis in the third trimester) (Table 1), all of which are factors that are known to increase the risk of severe COVID‐19 8 . After 1:1 matching for baseline characteristics, the confounder‐balanced cohort showed standardized mean differences in baseline factors that were small and within 10% (Table 1, Figure S1a).
Table 1.
Comparison of baseline characteristics between SARS‐CoV‐2‐infected pregnant women during Delta wave vs pre‐Delta period, in whole cohort and in matched cohorts
| Variable | Delta wave (n = 339) | Pre‐Delta (n = 870) | SMD* | P * | Pre‐Delta matched cohort (n = 339) | SMD† | P † |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | 30.3 ± 5.45 | 29.1 ± 5.62 | 0.218 | < 0.001 | 30.2 ± 5.35 | 0.017 | 0.820 |
| Maternal age | 0.143 | 0.004 | 0.038 | 0.823 | |||
| < 25 years | 54 (15.9) | 212 (24.4) | 55 (16.2) | ||||
| ≥ 25 to < 38 years | 251 (74.0) | 587 (67.5) | 255 (75.2) | ||||
| ≥ 38 years | 34 (10.0) | 71 (8.2) | 29 (8.6) | ||||
| Maternal BMI (kg/m2) | 26.7 (24.1–29.0) | 25.3 (23.8–27.9) | 0.120 | < 0.001 | 26.0 (24.0–28.3) | −0.006 | 0.586 |
| Maternal BMI | 0.186 | < 0.001 | 0.000 | 0.999 | |||
| < 25 kg/m2 | 117 (34.5) | 416 (47.8) | 117 (34.5) | ||||
| ≥25 to < 30 kg/m2 | 169 (49.9) | 316 (36.3) | 169 (49.9) | ||||
| ≥ 30 kg/m2 | 53 (15.6) | 138 (15.9) | 53 (15.6) | ||||
| Gestational age at diagnosis (weeks) | 29.6 ± 8.54 | 27.3 ± 10.1 | 0.248 | < 0.0001 | 29.3 ± 8.7 | 0.038 | 0.612 |
| Parous | 232 (68.4) | 584 (67.1) | 0.028 | 0.662 | 243 (71.7) | −0.070 | 0.356 |
| Trimester at diagnosis | 0.251 | 0.0005 | 0.061 | 0.619 | |||
| First | 24 (7.1) | 115 (13.2) | 25 (7.4) | ||||
| Second | 98 (28.9) | 291 (33.4) | 109 (32.1) | ||||
| Third | 217 (64.0) | 464 (53.3) | 205 (60.5) | ||||
| Comorbidity | |||||||
| Pregestational diabetes | 4 (1.2) | 10 (1.1) | 0.003 | 0.964 | 6 (1.8) | 0.062 | 0.524 |
| Pulmonary disease | 6 (1.8) | 11 (1.3) | 0.041 | 0.502 | 2 (0.6) | 0.109 | 0.154 |
| Chronic hypertension | 5 (1.5) | 10 (1.1) | 0.028 | 0.646 | 6 (1.8) | −0.023 | 0.761 |
Data are presented as mean ± SD, n (%) or median (interquartile range).
Delta vs pre‐Delta whole cohort.
Delta vs pre‐Delta matched cohort.
BMI, body mass index; SMD, standardized mean difference.
In the confounder‐balanced cohort, SARS‐CoV‐2 infection during the Delta wave vs the pre‐Delta period was associated with an increased need for oxygen supplementation, defined by increased need for nasal oxygen support, CPAP or high‐flow oxygen, mechanical ventilation and ECMO (Table 2). In addition, during the Delta wave, compared with the pre‐Delta period, maternal death was 3.6‐fold higher (5.3% vs 1.5%, P = 0.010), and very preterm birth (prior to 34 + 0 weeks' gestation) was significantly increased (15.4% vs 4.9%, P < 0.001). The rates of preterm birth before 37 + 0 weeks' gestation and stillbirth did not differ significantly between the Delta and pre‐Delta periods, although absolute rates were higher in the Delta‐dominant period.
Table 2.
Maternal and perinatal adverse outcomes in pregnant women infected during SARS‐CoV‐2 Delta wave compared with confounder‐matched cohort infected during pre‐Delta period
| Adverse outcome | Pre‐Delta matched cohort | Delta wave | RR (95% CI)† | P |
|---|---|---|---|---|
| Maternal | ||||
| n | 339 | 339 | ||
| Nasal O2 support | 34 (10.0) | 86 (25.4) | 2.53 (1.75–3.65) | < 0.001 |
| CPAP or high‐flow O2 | 14 (4.1) | 35 (10.3) | 2.50 (1.37–4.56) | 0.002 |
| Mechanical ventilation | 5 (1.5) | 21 (6.2) | 4.2 (1.60–11.0) | 0.003 |
| ECMO | 1 (0.3) | 11 (3.2) | 11.0 (1.43–84.7) | 0.021 |
| Pre‐eclampsia | 12 (3.5) | 8 (2.4) | 0.66 (0.27–1.61) | 0.367 |
| Maternal death | 5 (1.5) | 18 (5.3) | 3.60 (1.35–9.58) | 0.010 |
| Perinatal* | ||||
| n | 308 | 123 | ||
| Preterm birth < 37 weeks | 55 (17.9) | 31 (25.2) | 1.41 (0.96–2.08) | 0.081 |
| Preterm birth < 34 weeks | 15 (4.9) | 19 (15.4) | 3.17 (1.67–6.04) | < 0.001 |
| Stillbirth | 3 (1.0) | 4 (3.3) | 2.10 (0.51–8.74) | 0.304 |
Data are presented as n (%), unless indicated otherwise.
Perinatal data not available in all cases.
Risk ratio (RR) calculated using generalized estimating equations with log‐binomial link function using matching ID as clusters.
CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; O2, oxygen.
Omicron wave vs pre‐Delta period
Pregnant women infected during the Omicron wave vs the pre‐Delta period had higher BMI and were infected at a later gestational age (Table 3). As such, women infected during the Delta wave, compared to those infected during the pre‐Delta period, had a much higher baseline risk of severe COVID‐19. A 4:1 matching of cases infected in the pre‐Delta period and Omicron wave was required for adequate balancing in the confounder‐balanced cohort, achieving standardized mean differences in baseline characteristics between the groups within 10% (Table 3, Figure S1b).
Table 3.
Comparison of baseline characteristics between SARS‐CoV‐2‐infected pregnant women during Omicron wave vs pre‐Delta period, in whole cohort and in matched cohorts
| Variable | Omicron wave (n = 77) | Pre‐Delta (n = 870) | SMD* | P * | Pre‐Delta matched cohort (n = 308) | SMD† | P † |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | 29.8 ± 6.04 | 29.1 ± 5.62 | 0.132 | 0.284 | 29.4 ± 5.31 | 0.067 | 0.608 |
| Maternal age | 0.114 | 0.532 | 0.057 | 0.373 | |||
| < 25 years | 18 (23.4) | 212 (24.4) | 68 (22.1) | ||||
| ≥ 25 to < 38 years | 50 (64.9) | 587 (67.5) | 218 (70.8) | ||||
| ≥ 38 years | 9 (11.7) | 71 (8.2) | 22 (7.1) | ||||
| Maternal BMI (kg/m2) | 27.4 (25.0–29.4) | 25.3 (23.8–27.9) | 0.315 | < 0.001 | 26.7 (25.0–29.4) | 0.022 | 0.869 |
| Maternal BMI | 0.379 | 0.002 | −0.035 | 0.999 | |||
| < 25 kg/m2 | 19 (24.7) | 416 (47.8) | 76 (24.7) | ||||
| ≥ 25 to < 30 kg/m2 | 40 (51.9) | 316 (36.3) | 160 (51.9) | ||||
| ≥ 30 kg/m2 | 18 (23.4) | 138 (15.9) | 72 (23.4) | ||||
| Gestational age at diagnosis (weeks) | 36.0 (28.0–38.6) | 29.4 (20.1–36.1) | 0.452 | < 0.001 | 35.2 (28.2–38.3) | 0.023 | 0.856 |
| Parous | 58 (75.3) | 584 (67.1) | 0.181 | 0.140 | 213 (69.2) | 0.137 | 0.288 |
| Trimester at diagnosis | 0.412 | 0.008 | 0.000 | 0.999 | |||
| First | 6 (7.8) | 115 (13.2) | 24 (7.8) | ||||
| Second | 13 (16.9) | 291 (33.4) | 52 (16.9) | ||||
| Third | 58 (75.3) | 464 (53.3) | 232 (75.3) | ||||
| Comorbidity | |||||||
| Pregestational diabetes | 0 (0.0) | 10 (1.1) | −0.152 | 0.715 | 1 (0.3) | −0.080 | 0.999 |
| Pulmonary disease | 0 (0.0) | 11 (1.3) | −0.159 | 0.661 | 2 (0.6) | −0.114 | 0.999 |
| Chronic hypertension | 0 (0.0) | 10 (1.1) | −0.152 | 0.715 | 4 (1.3) | −0.161 | 0.706 |
Data are presented as mean ± SD, n (%) or median (interquartile range).
Omicron vs pre‐Delta whole cohort.
Omicron vs pre‐Delta matched (4:1) cohort.
BMI, body mass index; SMD, standardized mean difference.
In the confounder‐balanced cohort, infection during the Omicron wave vs the pre‐Delta period was associated with a similar need for oxygen supplementation (however defined), and there were no differences in the rates of maternal death or pre‐eclampsia (Table 4). For the subset of the cohort for whom delivery outcomes were available, there were no differences in the rate of preterm birth or stillbirth during the Omicron wave vs during the pre‐Delta period. However, the 95% CIs were wide, and we cannot rule out Omicron being milder compared to pre‐Delta variants.
Table 4.
Maternal and perinatal adverse outcomes in pregnant women infected during SARS‐CoV‐2 Omicron wave compared with confounder‐matched cohort infected during pre‐Delta period
| Adverse outcome | Pre‐Delta matched cohort | Omicron wave | RR (95% CI)† | P |
|---|---|---|---|---|
| Maternal | ||||
| n | 308 | 77 | ||
| Nasal O2 support | 32 (10.4) | 5 (6.5) | 0.62 (0.25–1.55) | 0.251 |
| CPAP or high‐flow O2 | 15 (4.9) | 4 (5.2) | 1.07 (0.36–3.12) | 0.906 |
| Mechanical ventilation | 9 (2.9) | 1 (1.3) | 0.44 (0.06–3.45) | 0.438 |
| ECMO | 2 (0.6) | 0 (0.0) | NE | — |
| Pre‐eclampsia | 11 (3.6) | 5 (6.5) | 1.81 (0.65–5.08) | 0.253 |
| Maternal death | 4 (1.3) | 1 (1.3) | 1.00 (0.11–8.82) | 0.999 |
| Perinatal* | ||||
| n | 287 | 36 | ||
| Preterm birth < 37 weeks | 46 (16.0) | 3 (8.3) | 0.52 (0.17–1.59) | 0.250 |
| Preterm birth < 34 weeks | 14 (4.9) | 1 (2.8) | 0.57 (0.08–4.20) | 0.580 |
| Stillbirth | 2 (0.7) | 0 (0.0) | 1.99 (0.18–21.7) | 0.571 |
Data are presented as n (%), unless indicated otherwise.
Perinatal data not available in all cases.
Risk ratio (RR) calculated using generalized estimating equations with log‐binomial link function using matching ID as clusters.
CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; NE, not estimable; O2, oxygen.
Omicron wave vs Delta wave
Table S1 shows that, in the confounder‐balanced cohort, infection during the Omicron wave vs during the Delta wave was associated with a 74% reduced risk of nasal oxygen support (6.5% vs 24.7%). Although, in the Omicron wave vs in the Delta wave, the rate of invasive respiratory support (1.3% vs 4.5%) and maternal death (1.3% vs 3.2%) were lower, differences did not reach statistical significance.
Sensitivity analyses
Without excluding any data, and adjusting for any effects of confounders via multiple log‐binomial regression, our results are in agreement with the findings of the propensity score matching analysis that infection during the Delta wave was associated with a significantly greater need for advanced respiratory support (invasive or non‐invasive mechanical ventilation) compared with infection during the pre‐Delta period (adjusted RR, 2.19 (95% CI, 1.44–3.35); P < 0.001), while no significant difference was observed during the Omicron wave vs during the pre‐Delta period (adjusted RR, 0.78 (95% CI, 0.23–1.95); P = 0.644) (Table 5). The factors associated with requirement for advanced respiratory support (non‐invasive or invasive mechanical ventilation) using generalized estimating equations with a log‐binomial link function using treatment centers as clusters are shown in Table 5.
Table 5.
Factors associated with requirement for advanced respiratory support (non‐invasive or invasive mechanical ventilation)
| Variable | RR (95% CI)* | P * | aRR (95% CI)† | P † |
|---|---|---|---|---|
| Maternal age (years) | 1.32 (1.02–1.63) | 0.006 | 1.21 (0.99–1.50) | 0.060 |
| Maternal BMI (kg/m2) | 1.25 (1.03–1.51) | 0.016 | 1.18 (0.97–1.42) | 0.078 |
| Gestational age at diagnosis (weeks) | 1.44 (1.14–1.85) | 0.002 | 1.36 (1.08–1.75) | 0.012 |
| Comorbidity, any | NE | — | NE | — |
| Infection during pre‐Delta period | Reference | — | Reference | — |
| Infection during Delta wave | 2.45 (1.61–3.74) | < 0.001 | 2.19 (1.44–3.35) | < 0.001 |
| Infection during Omicron wave | 1.04 (0.30–2.47) | 0.993 | 0.78 (0.23–1.95) | 0.644 |
Risk ratio (RR) calculated using generalized estimating equations with log‐binomial link function using treatment centers as clusters.
Adjusted RR (aRR) calculated by adjusting for effects of confounders. BMI, body mass index; NE, not estimable.
DISCUSSION
Summary of key study findings
Among unvaccinated pregnant women, SARS‐CoV‐2 infection during the Delta wave, vs during the pre‐Delta period, was associated with increased requirement for oxygen support (including ECMO) and higher maternal mortality. Conversely, infection severity and complications during the Omicron wave were similar, and not significantly milder, compared with the pre‐Delta period. Our findings were consistent among confounder‐matched cohort estimates and multiple regression‐adjusted estimates. While we cannot rule out smaller effects resulting from a milder disease course during the Omicron wave, SARS‐CoV‐2 infection in unvaccinated pregnant women still carried considerable risks of both morbidity and mortality.
Strengths and limitations
The main strengths of this study are that it provides data related to maternal and perinatal outcomes associated with SARS‐CoV‐2 infection during the Delta and Omicron waves and compares these outcomes between three time periods covering the Omicron, Delta and pre‐Delta variants. The study centers in the two countries were similar in terms of size, delivery capacity and stringency of government restrictions during the course of the pandemic. However, variant sequencing data were unavailable, and a proxy (i.e. time of infection) was used to indicate the predominant variant. Data related to previous SARS‐CoV‐2 infection were not obtained, given that the majority of cases could have been asymptomatic. Collider bias is a common issue in the COVID‐19 literature and testing indications may have influenced our findings. Inclusion of asymptomatic individuals who were tested based on the admission protocol may have affected the estimates. However, the true asymptomatic rate is likely to be underestimated in the sample, and thus we may have overestimated the severity of Omicron infection in our cohort. The Omicron‐wave cohort was small, and estimates smaller than an approximately 3‐fold change would not be statistically significant, hence we cannot rule out the possibility of a milder disease course with Omicron. Finally, the severity of the disease may be confounded by the availability of antiviral treatment during the period when Omicron was the dominant variant, resulting in milder COVID‐19.
Interpretation of study findings and comparison with literature
In our study, we observed a more severe clinical course in pregnant women infected during the Delta wave and maternal mortality was increased in comparison with the pre‐Delta period. These findings are consistent with the limited literature on SARS‐CoV‐2 variants of concern and greater severity of maternal disease during the Delta wave 6 , 9 . All related studies followed our approach of using the date of SARS‐CoV‐2 infection as a surrogate for the viral variant, as genotyping each infection is not commonly available. While the difference in the absolute rate of stillbirth between the Delta wave and pre‐Delta period did not reach statistical significance in our study, the report from the US Centers for Disease Control and Prevention found that infections during the Delta wave were associated with an increased incidence of stillbirth compared with previous waves (i.e. the wild‐type strain and Alpha variant) 6 ; the occurrence of more severe infections during the Delta wave raises the possibility that disease severity is a mediator of stillbirth. Our findings are not consistent with widely reported observations that outside pregnancy the Omicron variant is associated with less severe clinical infection.
Clinical and research implications
As yet, there are no data on the severity of infection by the Omicron variant in unvaccinated pregnant women compared with previous variants. A recent report from our group showed that vaccinated women infected during the Omicron wave had neither moderate nor severe disease, while unvaccinated pregnant women were more likely to need oxygen support 10 . This emphasizes the importance of vaccination of pregnant women, even during the Omicron wave, during which the efficacy of vaccination against SARS‐CoV‐2 infection is diminished. Vaccination with an mRNA vaccine is safe and effective, but is still underutilized in pregnant women 11 , 12 , 13 . Pregnant women already have high levels of vaccine hesitancy, and miscommunication of the protective effects of the vaccine or risk associated with SARS‐CoV‐2 infection may have dire consequences 12 . While there has been widespread media coverage regarding the reduced virulence of the Omicron SARS‐CoV‐2 variant, our data suggest that this is not the case for unvaccinated pregnant women. This should be emphasized in public messaging. Moreover, further research is required to assess the effectiveness of available vaccines (e.g. mRNA, viral vector) for preventing SARS‐CoV‐2 infection and related morbidities in pregnant women.
Conclusions
In unvaccinated pregnant women, SARS‐CoV‐2 infection during the Delta wave was associated with increased need for oxygen support (including ECMO) and higher maternal mortality compared with infection during the pre‐Delta wave; however, there was no clear evidence of reduced disease severity during the Omicron wave (vs the pre‐Delta period). Miscommunication of the risks of the Omicron variant may influence adversely the vaccination rate among pregnant women, who are at increased risk of adverse maternal and perinatal events related to COVID‐19.
Supporting information
Figure S1 Propensity score histograms and Mahalanobis distance density plot checking for complete randomization. Groups were balanced regarding propensity scores for Delta (a) and Omicron (b) waves, and randomization checks using Mahalanobis distance for Delta (c) and Omicron (d) waves showed successful matching (P = 0.908 and 0.965 for Delta and Omicron waves, respectively).
Table S1 Maternal and perinatal adverse outcomes in pregnant women infected during SARS‐CoV‐2 Omicron wave compared with confounder‐matched cohort infected during Delta wave
Contributor Information
E. Kalafat, Email: mail@erkankalafat.com.
A. Khalil, asmakhalil79@googlemail.com, Email: akhalil@sgul.ac.uk.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, Datir R, Collier DA, Albecka A, Singh S, Pandey R, Brown J, Zhou J, Goonawardane N, Mishra S, et al. Whittaker C, Mellan T, Marwal R, Datta M, Sengupta S, Ponnusamy K, Radhakrishnan VS, Abdullahi A, Charles O, Chattopadhyay P, Devi P, Caputo D, Peacock T, Wattal C, Goel N, Satwik A, Vaishya R, Agarwal M; Indian SARS‐CoV‐2 Genomics Consortium (INSACOG); Genotype to Phenotype Japan (G2P‐Japan) Consortium; CITIID‐NIHR BioResource COVID‐19 Collaboration , Mavousian A, Lee JH, Bassi J, Silacci‐Fegni C, Saliba C, Pinto D, Irie T, Yoshida I, Hamilton WL, Sato K, Bhatt S, Flaxman S, James LC, Corti D, Piccoli L, Barclay WS, Rakshit P, Agrawal A, Gupta RK. SARS‐CoV‐2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021; 599: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui Z, Liu P, Wang N, Wang L, Fan K, Zhu Q, Wang K, Chen R, Feng R, Jia Z, Yang M, Xu G, Zhu B, Fu W, Chu T, Feng L, Wang Y, Pei X, Yang P, Xie XS, Cao L, Cao Y, Wang X. Structural and functional characterizations of infectivity and immune evasion of SARS‐CoV‐2 Omicron. Cell 2022; 185: 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madhi SA, Kwatra G, Myers JE, Jassat W, Dhar N, Mukendi CK, Nana AJ, Blumberg L, Welch R, Ngorima‐Mabhena N, Mutevedzi PC. Population immunity and Covid‐19 severity with Omicron variant in South Africa. N Engl J Med 2022; 386: 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Update on Omicron. https://www.who.int/news/item/28‐11‐2021‐update‐on‐omicron [Accessed 26 February 2022].
- 5. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol‐Urganci I, O'Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P, Magee L, Khalil A. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Global Health 2021; 9: e759–772. Erratum in: Lancet Global Health 2021; 9: e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney‐Delman D, Ellington SR. Risk for stillbirth among women with and without COVID‐19 at delivery hospitalization – United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UK Government . Variants: distribution of case data, 23 December 2021. https://www.gov.uk/government/publications/covid‐19‐variants‐genomically‐confirmed‐case‐numbers/variants‐distribution‐of‐case‐data‐23‐december‐2021 [Accessed 21 February 2022].
- 8. Kalafat E, Prasad S, Birol P, Tekin AB, Kunt A, Di Fabrizio C, Alatas C, Celik E, Bagci H, Binder J, Le Doare K, Magee LA, Mutlu MA, Yassa M, Tug N, Sahin O, Krokos P, O'brien P, von Dadelszen P, Palmrich P, Papaioannou G, Ayaz R, Ladhani SN, Kalantaridou S, Mihmanli V, Khalil A. An internally validated prediction model for critical COVID‐19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol 2022; 226:403.e1–403.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vousden N, Ramakrishnan R, Bunch K, Morris E, Simpson NAB, Gale C, O'Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ, Knight M. Severity of maternal infection and perinatal outcomes during periods of SARS‐CoV‐2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Med 2022; 1(1): e000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birol Ilter P, Prasad S, Berkkan M, Mutlu MA, Tekin AB, Celik E, Ata B, Turgal M, Yildiz S, Turkgeldi E, O'Brien P, von Dadelszen P, Magee LA, Kalafat E, Tug N, Khalil A. Clinical severity of SARS‐CoV‐2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet Gynecol 2022; 59: 560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalafat E, O'Brien P, Heath PT, Le Doare K, von Dadelszen P, Magee L, Ladhani S, Khalil A. Benefits and potential harms of COVID‐19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol 2021; 57: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, Magee LA, O'Brien P, Rezvani A, von Dadelszen P, Khalil A. COVID‐19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol 2022; 226: 236.e1–236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalafat E, Magee LA, von Dadelszen P, O'Brien P, Khalil A. SARS‐CoV‐2 vaccination in pregnancy: a unique opportunity for equity. Lancet 2021; 398: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Propensity score histograms and Mahalanobis distance density plot checking for complete randomization. Groups were balanced regarding propensity scores for Delta (a) and Omicron (b) waves, and randomization checks using Mahalanobis distance for Delta (c) and Omicron (d) waves showed successful matching (P = 0.908 and 0.965 for Delta and Omicron waves, respectively).
Table S1 Maternal and perinatal adverse outcomes in pregnant women infected during SARS‐CoV‐2 Omicron wave compared with confounder‐matched cohort infected during Delta wave
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


