Abstract
In long coronavirus disease 2019 (long COVID‐19), involvement of the musculoskeletal system is characterised by the persistence or appearance of symptoms such as fatigue, muscle weakness, myalgia, and decline in physical and functional performance, even at 4 weeks after the onset of acute symptoms of COVID‐19. Muscle injury biomarkers are altered during the acute phase of the disease. The cellular damage and hyperinflammatory state induced by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection may contribute to the persistence of symptoms, hypoxaemia, mitochondrial damage, and dysregulation of the renin‐angiotensin system. In addition, the occurrence of cerebrovascular diseases, involvement of the peripheral nervous system, and harmful effects of hospitalisation, such as the use of drugs, immobility, and weakness acquired in the intensive care unit, all aggravate muscle damage. Here, we review the multifactorial mechanisms of muscle tissue injury, aggravating conditions, and associated sequelae in long COVID‐19.
Keywords: long COVID‐19, muscle, muscle dysfunction, muscle sequelae
Abbreviations
- ACE
angiotensin‐converting enzyme
- Acetyl‐CoA
acetyl coenzyme A
- Ang I
angiotensin I
- Ang II
angiotensin II
- ATP
adenosine triphosphate
- ATR1
angiotensin type 1 receptor
- CFS
chronic fatigue syndrome
- CK
creatine kinase
- CNS
central nervous system
- CO2
carbon dioxide
- COVID‐19
coronavirus disease
- CRP
C‐reactive protein
- DNA
deoxyribonucleic acid
- GBS
Guillain‐Barré syndrome
- GCs
glucocorticoids
- HIF‐1α
hypoxia‐induced factor 1 alpha
- HIF‐2α
hypoxia‐induced factor 2 alpha
- LDH
lactate dehydrogenase
- ME
myalgic encephalomyelitis
- MHC
major histocompatibility complex
- mTORC1
mammalian target of rapamycin complex 1
- NF‐κβ
nuclear factor‐kappa beta
- NK
natural killer cells
- NMBAs
neuromuscular blocking agents
- ICU
intensive care unit
- IFN‐γ
interferon‐gamma
- IGF‐1
type 1 insulin‐like growth factor
- IL
interleukin
- IMV
invasive mechanical ventilation
- PNS
peripheral nervous system
- RAS
renin‐angiotensin system
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- SARS
severe acute respiratory syndrome
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SPPB
short physical performance battery
- TCD4+
helper T cells
- TCD8+
cytotoxic T cells
- TMPRSS2
transmembrane serine protease 2
- TNF‐α
tumour necrosis factor‐alpha
- T2DM
type 2 diabetes mellitus
1. INTRODUCTION
Patients with coronavirus disease 2019 (COVID‐19) may have various signs and symptoms that linger or appear even after 4 weeks of symptom onset, 1 , 2 , 3 including mild and asymptomatic infections. 4 Therefore, this condition has been called long COVID‐19 and classified into two categories: (a) sub‐acute symptomatic or continuous COVID, indicating symptoms lasting between 4 and 12 weeks; and (b) post‐ or post‐chronic COVID syndrome, with the persistence of symptoms beyond 12 weeks. 5 Symptoms are related to complications from various organs and systems, including haematologic (vascular haemostasis and coagulopathy), pulmonary (pulmonary thromboembolism, pneumonia, and pulmonary fibrosis), cardiovascular (atherosclerosis, focal myocardial fibrosis, and acute myocardial infarction), dermatological (psoriasis and lupus), neurological (ischaemic and haemorrhagic stroke), and psychiatric disorders (depression and anxiety). 6 Therefore, symptom permanence is highly heterogeneous, with more than 50 types of sequelae reported in the literature, 7 including dyspnoea and chest pain, 8 , 9 headache, anosmia, amnesia, 10 cardiac arrhythmia, 11 alopecia, 12 insomnia, dementia, 13 and different muscle symptoms.
In long COVID‐19, the involvement of the musculoskeletal system has been evidenced by the persistence of symptoms such as fatigue, 14 muscle weakness, 15 myalgia, 16 , 17 and a decline in physical and functional performance. 18 Muscle cell damage and the hyperinflammatory state induced by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, 19 hypoxaemia, mitochondrial damage, 20 and the dysregulation of the renin‐angiotensin system (RAS) 21 may also contribute to the persistence of symptoms. In addition, the occurrence of cerebrovascular diseases and neuropathies, 22 , 23 negative effects of hospitalisation, such as the use of drugs, immobility during long hospital stays, and weakness acquired in the intensive care unit (ICU), all aggravate muscle sequelae. 19 , 24 , 25 These pathological mechanisms likely establish a persistent muscle dysfunction, initiated in the acute phase of the disease and characterised mainly by the reduction in muscle protein synthesis, resulting in a decrease in muscle mass associated with a state of frailty, leading to loss of autonomy and functionality in activities of daily living in a patient with long COVID‐19. 8 , 26 , 27 , 28
Understanding the pathogenesis of muscle dysfunction in long‐term COVID‐19 can certainly support new studies and muscle management protocols in patients with sequelae associated with COVID‐19. Therefore, in this review, we address the involvement of the skeletal muscles in long COVID‐19, highlighting the mechanisms of tissue damage and associated sequelae.
2. PATHOPHYSIOLOGY OF MUSCLE DAMAGE
SARS‐CoV‐2 infection seems to induce a set of mechanisms that can directly affect the skeletal muscle or worsen muscle injury, establishing effects to muscle tissue characterised as: primary, with possible infection of the muscle cell, leading to cell death and tissue damage; secondary, resulting from damage to other systems, such as the respiratory system (infection of lung cells, causing local inflammation, generating diffuse alveolar damage, hypoxaemia, and consequently, damage to muscle metabolism), neurological system (infection of endothelial cells in the central nervous system [CNS], resulting in hypercoagulation and vasoconstriction, favouring the occurrence of cerebrovascular diseases) and the renin‐angiotensin system (with a decrease in angiotensin‐converting enzyme 2 [ACE2] activity, favouring the expression of inflammatory pathways, muscle atrophy, and fibrosis); tertiary, caused by the cytokine storm (that can induce myopathy and peripheral neuropathy); and quaternary, such as the negative effects of immobility (progressive loss of muscle mass) and hospitalisation (damage of long periods of immobilisation, mechanical ventilation, myopathies and neuropathies resulting from the use of drugs). In addition, these mechanisms lead to muscle dysfunction, characterised by decreased protein synthesis and increased protein degradation, increased oxidative stress, myonuclear apoptosis, and mitochondrial dysfunction (Figure 1).
FIGURE 1.
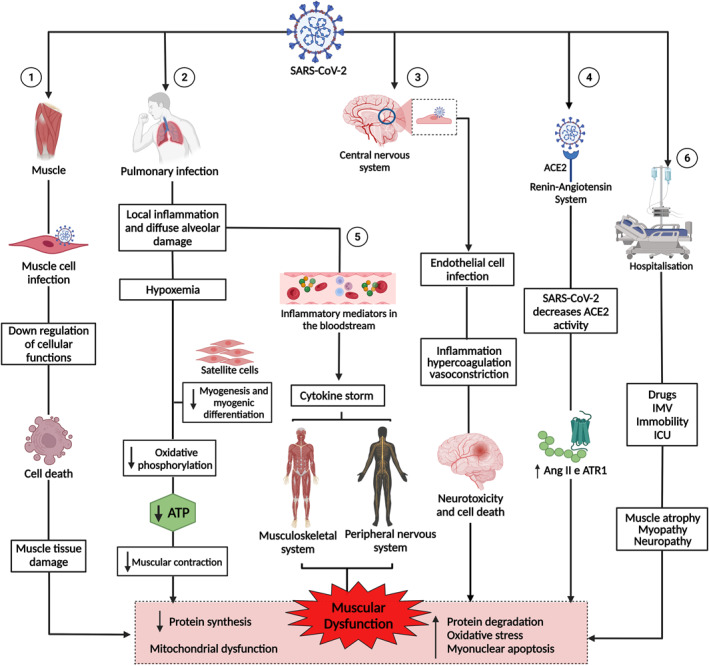
Pathogenesis of muscle dysfunction caused by SARS‐CoV‐2. 1: SARS‐CoV‐2 infects the muscle cell and uses cell machinery for replication, resulting in cell death and tissue damage. 2: SARS‐CoV‐2 infects lung cells, causing a local inflammatory response, diffuse alveolar damage, and hypoxaemia, interfering with myogenesis and myogenic differentiation and muscle metabolism and energy production. 3: SARS‐CoV‐2 infection of endothelial cells results in inflammation, hypercoagulation, and vasoconstriction, leading to neurotoxicity and cell death. 4: The binding of SARS‐CoV‐2 to angiotensin‐converting enzyme 2 (ACE2) negatively regulates the activity of the enzyme, favouring the high expression of angiotensin II (Ang2) and its receptor, angiotensin type 1 receptor (ATR1), leading to muscle atrophy and fibrosis. 5: The exacerbation of inflammation in the lungs increases inflammatory mediators, which are transported by the blood to other organs and systems. In the muscle, inflammatory cytokines increased muscle proteolysis and decreased protein synthesis. In the peripheral nervous system, antibodies attack nerves, causing damage to the axon or myelin. 6: Hospitalisation due to COVID‐19 can cause muscle damage due to the use of drugs and sedatives, as well as mechanical ventilation and immobility. 1, 2, 3, 4, 5, and 6 lead to muscle dysfunction, characterised by decreased synthesis and increased protein degradation, increased oxidative stress, myonuclear apoptosis, and mitochondrial dysfunction. This figure was created with Biorender.com
2.1. Muscle cell damage
It is known that the entry of SARS‐CoV‐2 into the host cell is enabled by angiotensin‐converting enzyme 2 (ACE2) and potentiated by transmembrane serine protease 2 (TMPRSS2). 29 , 30 The literature has well documented that musculoskeletal tissue express ACE2 and TMPRSS2. 26 , 31 , 32 , 33 Therefore, the muscles are susceptible to infection and direct injury by SARS‐CoV‐2. It is likely that once bound to ACE2, the virus enters the muscle cell through endocytosis. 34 , 35 In the endosomal environment, viral proteins are cleaved and activated by TMPRSS2, resulting in the fusion of the viral and cell membranes, causing the release of viral ribonucleic acid (RNA) in the cytoplasm. 36 , 37 Inside the muscle cell, the virus uses the cellular machinery for replication, down‐regulating cellular activities, thus, inducing muscle cell death and injury. 38
2.2. Lung injury aggravating muscle damage
The literature shows that patients with COVID‐19 can develop acute respiratory distress syndrome, characterised by severe hypoxaemia and the need for oxygen therapy and ventilatory support. 39 , 40 This is because the local immune response in the lungs results in diffuse alveolar damage and consequent accumulation of inflammatory exudate, fibrin deposition, hyaline membrane formation, alveolar epithelial desquamation, granulation tissue formation, collagen deposition, and decreased alveolar‐capillary permeability, compromising gas exchange. 41 , 42 , 43 , 44 Hypoxaemia is characterised by a deficit of oxygen in the blood, thereby impairing the oxygen supply to the muscle tissue (hypoxia), compromising biological functions. 45 In vivo studies have demonstrated that hypoxia inhibits muscle protein synthesis and increases degradation, with protein synthesis inhibition being the main mechanism associated with muscle mass reduction. 46 , 47 This is because hypoxia can significantly affect mitochondrial activity, impairing muscle energy generation through oxidative phosphorylation, where oxygen is required as an electron‐terminal receptor, resulting in a decrease in adenosine triphosphate (ATP), which is required for protein synthesis and muscle contraction. 48 , 49 , 50
Hypoxaemia caused by lung injury in COVID‐19 impairs oxidative phosphorylation. Thus, some adaptations occur in tissues to increase oxygen delivery, including induction of erythropoiesis and angiogenesis. These changes are regulated by the hypoxia‐induced factors 1 and 2 alpha (HIF‐1α and HIF‐2α). These transcription factors that coordinate adaptive cellular responses in hypoxic environments then become highly expressed. In addition, HIF‐1α intensifies glycolysis, up‐regulating genes that amplify and transport glucose into cells, thus, increasing energy availability. 51 However, chronic stabilisation of HIF‐1α results in maladaptive and deleterious effects on musculature and increases the expression of profibrotic cytokines. 52 In contrast, hypoxia triggers metabolic reprogramming to regulate cell functions. 49 The energy generation process remains at a smaller scale and is insufficient for functions that require large amounts of ATP, such as protein synthesis and muscle contraction. This process refers to anaerobic glycolysis, where cytosolic pyruvate is converted into lactate by the enzyme lactate dehydrogenase (LDH), favouring the maintenance of the inflammatory process due to excess lactate. 53 Additionally, hypoxia represses myogenesis and myogenic differentiation through mechanisms, such as the activation of the Notch signalling pathway, inhibition of the phosphoinositide 3‐kinase pathway, and degradation of several basic myogenic regulatory factors, such as those of the helix‐loop‐helix family, including Myf5, Myf6, MyoD, and Myog. 54 , 55
The low oxygen supply to skeletal musculature and its consequences on energy generation observed in the acute phase of COVID‐19 seem to extend to the period after infection. In long COVID‐19, the impairment of ATP generation in mitochondria has been evidenced by a marked reduction in peak peripheral oxygen consumption 56 and oxygen extraction by peripheral muscles at rest and during exercise, causing exercise intolerance in patients with the disease. 57
2.3. Cerebrovascular and peripheral nervous system injury
The mechanisms of SARS‐CoV‐2 invasion in the CNS involve the haematogenous route, which involves the binding of the virus to endothelial cells of the blood‐brain barrier, infection of immune cells, olfactory sensory neurons, and the liquor route, where infected lymphocytes attach to endothelial cells present in the cerebrospinal fluid, reaching neurons and glial cells. 41 , 58 , 59 Camargo‐Martínez et al. 22 reported that CNS involvement is the main form of neurological injury during COVID‐19. In addition, patients with more severe conditions had higher levels of D‐dimer, being more predisposed to developing cerebrovascular disease.
Some studies have observed a relationship between COVID‐19 and cerebrovascular complications, with reports of ischaemic and haemorrhagic stroke. 60 , 61 , 62 Ischaemic stroke appears to be the most frequent cerebrovascular complication of COVID‐19. 63 COVID‐19 and its etiological association with a stroke can be explained by the occurrence of coagulopathy due to endothelial inflammation or the displacement of pre‐existing atheromatous plaque. 64 , 65 In this case, the virus binds to ACE2 on endothelial cells, depleting these receptors and increasing angiotensin II (Ang II) levels in the blood. Elevated Ang II levels mainly result in (1) inflammation, leading to clot formation and potential ischaemic stroke and (2) vasoconstriction and fluid retention, with increased blood pressure and potential haemorrhagic stroke. 66
The involvement of the peripheral nervous system (PNS) is characterised by neural damage, neuromuscular junction dysfunction, myopathy, and polyneuropathy. 19 , 35 , 67 The cytokine storm causes microvascular disarray, and metabolic and electrical changes, attracting pathogenic inflammatory cells or mediating neurotoxicity and cell death. 68 Furthermore, it favours hypoxic conditions, which reduce axonal survival factors or increase vascular permeability, causing vasogenic oedema. 19
The appearance of Guillain‐Barré syndrome (GBS) associated with COVID‐19 exemplifies the cytokine storm‐mediated PNS aggression. 69 , 70 Unlike the classic forms associated with other viral infections, characterised by a post‐infectious onset with direct nerve root involvement, GBS associated with SARS‐CoV‐2 infection develops rapidly within a few days of the onset of viral infection. Nerve root damage is not always explicit on magnetic resonance imaging (MRI), indicating that nerve damage may be included in systemic, acute, and severe dysimmune processes associated with the generalised clinical manifestations of COVID‐19. 23 In GBS, the patient has flaccid paralysis, characterised by intense muscle weakness of the lower limbs and loss of deep reflexes and sensory damage. 23 , 71
Cerebrovascular and PNS injuries, which can occur in the acute phase of COVID‐19, can generate musculoskeletal sequelae in most patients, aggravating persistent symptoms observed in long COVID‐19 (Figure 2). For example, stroke survivors can show severe muscle loss (up to 24% in muscle volume 72 ), which is accompanied by significant degradations in fibre type and size distribution and capillary density. However, patients who develop GBS may have severe muscle weakness. Additionally, the literature suggests that recovered patients may remain with latent SARS‐CoV‐2 in the CNS for a long time, which may reactivate and trigger neurological complications observed in long COVID‐19. 22
FIGURE 2.
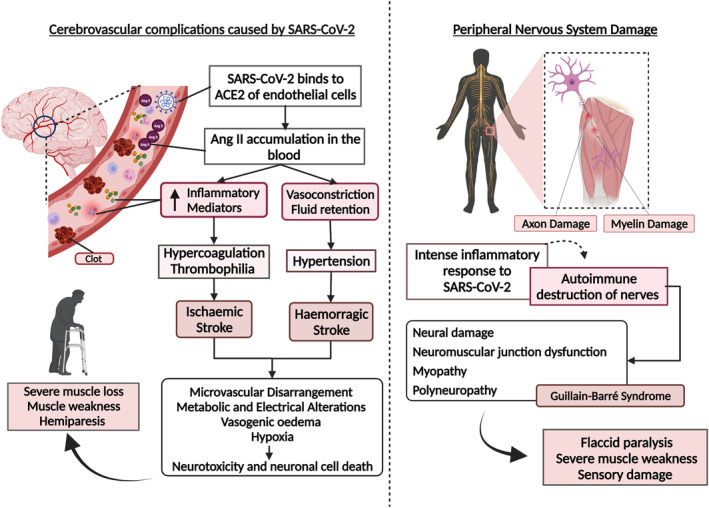
Cerebrovascular complications and neural damage caused by SARS‐CoV‐2 infection and its inflammatory response. Cerebrovascular complications caused by SARS‐CoV‐2: the virus binds to angiotensin‐converting enzyme 2 (ACE2) of endothelial cells, depleting these receptors and increasing the levels of angiotensin II (Ang II) in the blood. Elevated Ang II levels result in inflammation, leading to clot formation and potential ischaemic stroke, vasoconstriction, and fluid retention, with increased blood pressure and potential haemorrhagic stroke. In both events, the consequences include microvascular disarray, hypoxia, metabolic and electrical changes, and vasogenic oedema, leading to neurotoxicity and cell death. The sequelae of cerebrovascular events involve severe muscle wasting, muscle weakness, and hemiparesis. Peripheral nervous system damage: the intense inflammatory response resulting from a viral infection can induce nerve destruction via an autoimmune response, characterised by damage to the axons and myelin sheath. As a result, dysfunction in the neuromuscular junction, myopathies, and polyneuropathies can occur, such as Guillain‐Barré syndrome, generating flaccid paralysis with severe loss of muscle mass and sensory damage. This figure was created with Biorender.com
2.4. Dysregulated renin‐angiotensin system (RAS)
Furthermore, when SARS‐CoV‐2 invades a cell, it downregulates ACE2 expression and induces a soluble form in the serum, decreasing ACE2 activity. These processes can increase the activation of the classical RAS and decrease the expression of the non‐classical one. 21 , 73 RAS is a modulator of muscle mass, and its dysregulation can directly affect skeletal muscle mass and function. 21 Overactivation of the classic RAS pathway has been associated with harmful consequences in the skeletal muscle, such as muscle atrophy and fibrosis, because it can generate a cascade of events in muscle tissue, including increased production of reactive oxygen species (ROS), protein degradation, and decreased protein synthesis. 74 , 75 In contrast, the decrease in non‐classical RAS expression inhibits the protective and maintenance factors of muscle tissue, as its activation is related to the antifibrotic and antiatrophic effects on skeletal muscle. 76
2.5. Hyperinflammatory state in the muscle
In most patients infected with SARS‐CoV‐2, the innate immune response, mediated by the expression of type I interferons that act as signallers of viral infection, recruiting monocytes, macrophages, and dendritic cells, which promote the release of cytokines in response to infection, 77 , 78 and the adaptive response via T and B cells, are sufficient to contain the infection, and the patient recovers. 38 However, there is an atypical and insufficient immune response in some patients, aggravated by the escape of SARS‐CoV‐2 from the immune response, 79 favouring the increase in viral replication and exacerbating inflammation. This results in the production of cytokines and chemokines such as interleukin (IL) 2, IL‐6, IL‐10, interferon‐gamma (IFN‐γ), and tumour necrosis factor‐alpha (TNF‐α), which recruit other immune cells, mainly monocytes and T lymphocytes, to the site of infection. 43 , 80 Activated immune cells express additional cytokines, causing the recruitment of new immune cells, establishing a pro‐inflammatory cycle that results in a cytokine storm, resulting in damage to multiple organs and tissues, including muscle tissue. 81 , 82 Helper T cells (TCD4+) and cytotoxic T cells (TCD8+) are essential for combating SARS‐CoV‐2 and the emergence of autoimmune damage. TCD4+ acts in the production of specific antibodies, cytokines, and interleukins and coordinates the activities of TCD8+. TCD8+ directly eliminates the virus but can be cytotoxic and destroy infected cells. 41 , 78
The pathological increase in IL‐1β, IL‐6, and circulating TNF‐α levels are strongly linked to a loss of muscle mass. 83 IL‐6 participates in pro‐inflammatory processes and regulates immune functions and body metabolism during pathological conditions. 84 Furthermore, it is involved in important autocrine and paracrine signals, controlling myocyte proliferation and differentiation. 85 , 86 The chronicity of high IL‐6 levels is associated with the acceleration of muscle mass loss and damage to metabolic homoeostasis in muscle, in addition to worsening the inflammatory condition, contributing to the severity of comorbidities. 83 , 87 , 88 IL‐1β and TNF‐α are expressed in the muscle and inflammatory cell infiltrates of all inflammatory myopathies. 89
IL‐1β and IL‐6 can induce increased muscle fibroblast activity. 87 IL‐1β and TNF‐α inhibit the differentiation and proliferation of satellite cells, the progenitor cells involved in muscle fibre growth. 17 In vivo studies have demonstrated that the administration of IL‐1β and TNF‐α significantly reduces the synthesis of major muscle proteins. 90 , 91 TNF‐α appears to interfere with skeletal muscle contractility and promote muscle weakness. 92 In vitro studies have reported that TNF‐α inhibits myogenesis and deregulates nuclear factor‐kappa beta (NF‐κβ), a key transcription factor in skeletal muscle atrophy. 93 , 94 Thus, the action of these inflammatory cytokines can decrease muscle mass and, consequently, muscle strength and endurance. 95
In addition to the direct damage that inflammation can cause in skeletal muscle tissue 96 during the acute phase of the disease, it facilitates the appearance of sequelae. Studies 10 , 60 , 97 have shown that SARS‐CoV‐2 can remain detectable in survivors' bodies for up to 4 months after the acute phase, suggesting that the virus may induce a delayed immune response and cause long COVID‐19. 4
Aschman and colleagues 98 evaluated the quadriceps and deltoid tissues from patients who died of severe COVID‐19 and compared them with samples from patients who died of other critical illnesses. They found signs of degenerated muscle fibres more frequently in the COVID‐19 group and lesions indicative of myositis, ranging from mild to severe inflammation. In addition, they observed significant regulation of major histocompatibility complex (MHC) class I and II antigens in myofibres, indicating muscle involvement in the immune response against SARS‐CoV‐2. Natural killer (NK) cells were also identified close to myofibres, suggesting that they participated in the pathogenesis of COVID‐19‐associated myositis. Furthermore, necrotic myofibres were found, but this was not a specific finding in patients with COVID‐19, suggesting that this is a consequence of sepsis or critical illness. Thus, the high expression of cytokines such as IFN‐γ, IL‐1β, IL‐6, IL‐17, and TNF‐α 60 can directly damage the skeletal muscle, inducing fibre proteolysis and decreasing protein synthesis 19 , 22 (Figure 3).
FIGURE 3.
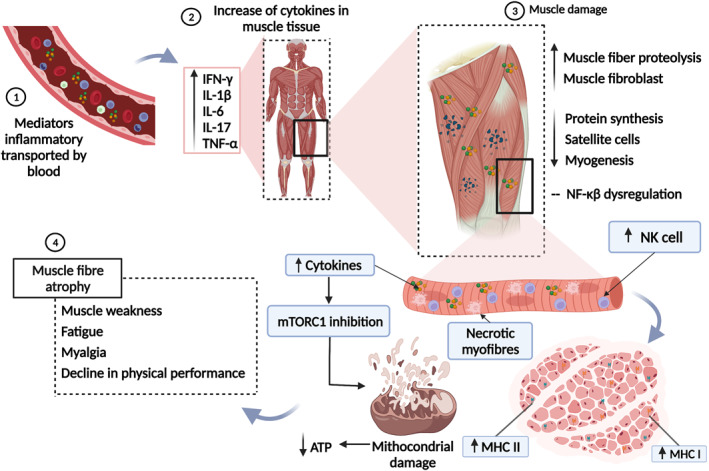
Hyperinflammatory state induces mitochondrial damage and myopathy in long COVID‐19. 1: Inflammatory mediators carried by the bloodstream reach the muscle tissue. 2: Interferon‐gamma (IFN‐γ), interleukin 1 beta (IL‐1β), interleukin 6 (IL‐6), interleukin 17 (IL‐17), and tumour necrosis factor‐alpha (TNF‐α) are at increased levels. 3: Cytokines induce increased proteolysis of muscle fibre and muscle fibroblasts, decreased protein synthesis, differentiation, and proliferation of satellite cells, and decreased myogenesis and dysregulation of nuclear factor‐kappa beta (NF‐Kβ). Muscle injury is characterised by increased infiltrating natural killer (NK) cells, major histocompatibility complex (MHC) class I and II antigens, and necrotic fibres. In addition, the increase in muscle cytokine levels inhibits the activity of the mammalian target of rapamycin complex 1 (mTORC1), resulting in mitochondrial damage, impairing the production of adenosine triphosphate (ATP). 4: The occurrence of symptoms such as muscle weakness, fatigue, myalgia, and the decline in physical performance are consequences of muscle atrophy resulting from tissue damage. This figure was created with Biorender.com
Additionally, the mammalian target of rapamycin complex 1 (mTORC1) is one of the main regulators of muscle protein synthesis. Its inhibition results in the dysregulation of mitochondrial activity, characterised by decreased mitochondrial deoxyribonucleic acid (DNA) production, reduced biogenesis, and increased mitophagy. 20 , 99 Inflammation resulting from SARS‐CoV‐2 infection interacts with the mTORC1 pathway and impairs muscle protein synthesis and mitochondrial activity. 20 Furthermore, mitochondrial damage can facilitate mitochondrial apoptosis, which interferes with the generation of ATP in muscle cells (Figure 3). Thus, mitochondrial function, a regulator of metabolic dysfunction in the skeletal muscle, can be affected by inflammation and has great potential to induce sarcopenia. This is because muscle mitochondria, during inflammation, produce high concentrations of ROS and are prone to autophagy. 99 , 100 , 101
2.6. Hospitalisation and muscle weakness
Many survivors of COVID‐19 have experienced prolonged hospitalisation and immobilisation in the ICU because of severe respiratory impairment that may require oxygen therapy, invasive mechanical ventilation (IMV), or being placed in a prone position. In addition, survivors of critical illnesses have significant skeletal muscle dysfunction, characterised by weakness and atrophy, which result in impaired physical function. 27 , 102 , 103
Mayer et al. 104 demonstrated that prolonged ICU stay is associated with a rapid and significant reduction in the volume of the rectus femoris muscle, at 18.5% on average, until the 7th day of hospitalisation. Furthermore, through ultrasound analysis, they observed a correlation between the size of the rectus femoris and muscle strength and function. The decrease in the density of type II muscle fibres, which are essential in energy generation, seems to be the reason for the changes in muscle strength. Muscle biopsies of patients who require IMV show that type II fibres have a smaller muscle cross‐sectional area and decreased in greater proportion than type I fibres.
Additionally, pathological agents such as viruses can induce the appearance of systemic inflammatory response syndrome, characterised by the activation of cellular and humoural responses. Observations in muscle biopsies include local immune activation constituted by small grouped infiltrates or isolated inflammatory cells, mainly composed of macrophages and TCD4+ that produce changes in the body's microcirculation. 105 Inflammatory mediators such as IL‐1, IL‐2, IL‐6, and TNF‐α have also been observed in the muscle and nervous tissue of patients with ICU‐acquired weakness 106 , 107 , 108 and mediate muscle damage, PNS degeneration/regeneration, and endothelial dysfunction. With the increase in vascular permeability, endoneurial oedema impairs oxygen and energy supply to the neurons, leading to cell death. 106 , 109 This corroborates the electrophysiological findings, which revealed a reduction in the amplitude of muscle action potentials and sensory nerve action potentials, conferring normal or slightly reduced conduction velocity. Furthermore, these findings confirm primary distal axonal degeneration of motor and sensory fibres. 107 , 110 , 111
In addition, pharmacological agents such as glucocorticoids (GCs) have also been used as a therapeutic intervention for COVID‐19 to modulate lung damage resulting from inflammation and prevent the development of respiratory failure. 112 , 113 However, some aspects of this therapy, such as its dosage and duration of use, need further clarification. In general, the prolonged use of GCs causes damage to the skeletal muscles, such as damage to catabolic mechanisms that deregulate proteolytic systems, including ubiquitin‐proteasome, calcium‐dependent cathepsins, and calpains, resulting in the increased proteolysis of myofibrillar proteins, leading to the dissociation between actin and myosin. 24 , 114 , 115 Prolonged GCs use also damages anti‐anabolic mechanisms, inhibits amino acid transport and muscle type 1 insulin‐like growth factor (IGF‐1) production, blocks the transcription factor myogenin, and inhibits protein synthesis and myogenesis. 116 It also induces myocyte apoptosis, reduces serum potassium and phosphate levels, creates a deficit in glycolytic activity, and decreases the expression of calcium ATPase in the sarcoplasmic reticulum, a crucial element in calcium kinetics during muscle relaxation after contraction. 114
Deep sedation allows for the implementation of IMV; however, it results in the impairment of cardiopulmonary independence and a state of absolute immobility, which has negative effects on the skeletal musculature. 117 , 118 Therefore, when sedation is not enough to ensure adequate IMV, neuromuscular blocking agents (NMBAs) are used, favouring patient‐ventilator synchrony. However, NMBAs block the nicotinic acetylcholine receptor in the muscle cell membrane, interrupting the transmission of impulses at the neuromuscular junction. Thus, the persistence of neuromuscular blockade, influenced by the plasma concentration of the agent and its metabolite, leads to prolonged paralysis, myopathy, and generalised muscle weakness. 119 , 120 , 121
Thus, the causes of muscle weakness acquired in the ICU are multifactorial, including pharmacological agents, immobility, and IMV, and associated with prolonged hospitalisation and increased mortality. In addition, affected patients experience limb weakness and atrophy, absence of deep tendon reflexes, sensory loss, changes in muscle contractility, and consequent functional sequelae, such as the loss of muscle strength and endurance, myalgia, fatigue, and reduced aerobic capacity and physical performance. 122 , 123
3. MUSCLE SEQUELAE IN LONG COVID‐19
3.1. Muscle injury biomarkers and symptoms
Patients with muscle damage from COVID‐19 have significantly elevated creatine kinase (CK) levels, regardless of disease severity. In addition, they had higher levels of C‐reactive protein (CRP), lactate dehydrogenase (LDH), cortisol, and ferritin. 60
CK plays an important role in ATP hydrolysis and is normally found in tissues that require high energy levels, such as muscles, and is a marker of muscle damage. 124 , 125 The elevation in serum CK levels seen in patients with COVID‐19 is likely a result of skeletal myopathy. 98 Elevated CRP levels are associated with sarcopenia and an increased risk of muscle weakness. It was observed that CRP levels are significantly higher in patients with sarcopenia and are associated with muscle weakness and reduced physical activity. 126 , 127 , 128 The increased expression of LDH in patients with COVID‐19 signals a state of fatigue, which may indicate increased anaerobic metabolism in the muscle with insufficient production of ATP, as LDH catalyses the interconversion of pyruvate into lactate. 129 The hypercortisolaemia observed in patients with COVID‐19 130 may also indicate muscle damage, as cortisol is a mediator of protein catabolism. During acute illness, its levels increase significantly in the blood, leading to the loss of muscle mass and strength. 131 The high concentrations of ferritin found in patients with COVID‐19 132 may increase the chances of muscle cell damage and death since ferritin is an iron storage protein that can directly interact with energy production in mitochondria, 133 stimulate anaerobic modes of energy production and increase the production of ROS. 27
High levels of CK, CRP, LDH, cortisol, and ferritin reinforce the indication of muscle damage that begins in the acute phase of COVID‐19 and extend into long COVID‐19, as persistent muscle symptoms have been reported. Fatigue is the most recurrent sequela, 4 with a prevalence between 53% and 94.9%, and may be accompanied by myalgia, muscle weakness, and reduced physical performance. 8 , 14 , 15 , 16 , 17 , 18
Muscle dysfunction results from multiple mechanisms and can lead to functional changes, including decreased muscle strength and endurance 27 , 134 ; structural changes, which include a decline in muscle mass, an increase in the percentage of type II fibres, a reduction in the number of capillaries, and changes in bioenergetics, such as mitochondrial dysfunction and the production of oxygen‐free radicals. 99 , 135 Bioenergetic changes affect the quality and quantity of the muscle mass. The loss of muscle mass alters the proper functioning of the body and is associated with other negative consequences, such as loss of strength and physical performance. 20 Thus, the reduction in muscle mass is crucial for a state of frailty, leading to loss of autonomy and functionality in the daily living activities of patients with long COVID‐19. 8 , 36 , 106
Paneroni et al. 134 evaluated the skeletal muscle strength of the quadriceps and biceps femoris of patients who recovered from COVID‐19 at the time of hospital discharge and did not have previous musculoskeletal deficiencies through a test of maximum voluntary contraction and physical performance using the short physical performance battery (SPPB). They found that 86% of patients had muscle weakness in the quadriceps and 73% in the biceps femoris. Regarding the SPPB, 53% of the patients had scores above 10, indicating ‘good’ physical performance. However, 25% had scores below five, indicating low physical autonomy. These findings demonstrate that muscle dysfunction in patients with long COVID‐19 is highly likely.
3.2. Sarcopenia in long COVID‐19
Sarcopenia may be relevant to the functional and physical deterioration in long COVID‐19, induced by multiple factors. For example, the negative effects of immobility are well documented. 28 , 43 Arentson‐Lantz et al. 136 observed that 14 days of rest resulted in a reduction in the cross‐sectional area of muscle fibres, with a preference for type IIa fibres, and a decrease in the number of satellite cells. Kilroe et al. 137 reported that in just 2 days of immobilisation, up to 1.7% of muscle volume can be lost and that this loss can be greater in 7 days, with a 5.5% reduction in muscle volume. In addition, the muscle volume lost during inactivity can lead to a progressive loss of muscle mass and function, as this tissue may not fully recover. 23
Health conditions prior to infection and poor diet are also reported as determinants of sarcopenia. They interact with mitochondrial regulation and dysregulation mechanisms, resulting in mitochondrial dysfunction and autophagy, leading to decreased muscle protein synthesis and increased muscle proteolysis. 23 , 106 For example, type 2 diabetes mellitus (T2DM) and obesity can exacerbate anabolic resistance, which is responsible for muscle atrophy because they increase the levels of inflammation and induce the dysregulation of anabolic hormones. 138
In the acute phase of COVID‐19, anosmia and ageusia are prevalent symptoms; even 1 month after infection, up to 30% and 20% of olfactory and taste dysfunction, respectively, still do not improve. 139 Xerostomia and dysphagia have also been reported with a decrease in masticatory muscle strength. 140 This spectrum of dysfunctions can lead to inadequate nutrition, with reduced intake of important nutrients for increasing and protecting muscle mass, such as essential amino acids, vitamins, macro and microelements, dietary fibre, and hydration. 27 For example, low protein consumption can decrease the hypertrophic response of the muscle. 20
3.3. Chronic fatigue syndrome
In many patients, the persistence of symptoms observed in long COVID‐19 is centred on fatigue, 16 accompanied by cognitive deficits, pain, and dyspnoea, 8 and persist for several months after infection. 15 These symptoms are similar to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a well‐documented post‐viral disease in the literature characterised by severe fatigue after exertion that does not improve with rest, lasting for periods longer than 6 months. It is believed that with the high incidence and prevalence of SARS‐CoV‐2 infections, a significant increase in ME/CFS cases may occur because of the post‐viral fatigue, symptomatically identical to ME/CFS, observed in patients who recover from COVID‐19. 141 In infections by other coronaviruses such as SARS‐CoV, the same reports of a post‐viral syndrome include chronic fatigue, diffuse myalgia, depression, and sleep disturbances, which affect survivors for up to 4 years. 4 , 142 Perrin et al. 143 suggested that patients with long COVID‐19 may have a ‘post‐COVID‐19 syndrome’, similar to SARS.
3.4. Weakness and respiratory conditions
Patients with long COVID‐19 develop intolerance to physical exercise, mainly caused by persistent dyspnoea and fatigue. 20 These symptoms are likely due to the complex interaction between ventilatory limitation, skeletal muscle dysfunction, and cardiac dysfunction.
Pulmonary ventilation is directly affected by the activity of respiratory muscles, especially the diaphragm. 144 Thus, we infer that the muscle injury caused by COVID‐19 is an important factor in exercise intolerance found in long COVID‐19 since it causes a reduction in muscle mass, decreasing the strength and resistance of the respiratory muscles, and impairing the movement of the diaphragm. This reduces vital lung capacity, resulting in difficulty sustaining breathing and causing discomfort.
The assessment of inspiratory and expiratory pressures in patients with SARS sequelae showed values below normal, suggesting respiratory muscle weakness. This finding is considered the main factor for restrictive pulmonary function. 145 In this sense, it is likely that when exposed to daily efforts, patients show an increase in ventilatory demand, which forces them to avoid such activities; thus, they are affected by a chronic sedentary lifestyle, leading to worsening of muscle strength and aerobic capacity, emphasising the cycle dyspnoea‐sedentary lifestyle‐dyspnoea.
3.5. Muscle dysfunction in long‐term COVID‐19 and at‐risk populations
Some populations may have additional risk factors for developing post‐COVID‐19 muscle sequelae. Among these, we highlight patients with obesity, T2DM, and older adults because, prior to infection by SARS‐CoV‐2, they are associated with muscle and functionality impairment from structural and bioenergetic changes in the skeletal muscle.
Patients with obesity may be at greater risk for developing muscle sequelae because they are subjected to a low‐intensity chronic inflammatory state, favoured by the secretion of different inflammatory mediators by adipose tissue, such as TNF‐α, IL‐1, and IL‐6. These inflammatory products are associated with muscle wasting because they disrupt the balance between muscle protein synthesis and breakdown. In addition, these patients are prone to reduced lung ventilation due to limited chest expansion by the accumulation of adipose tissue, which, associated with respiratory muscle weakness, worsens oxygen supply and increases fatigue and muscle weakness. 146
In patients with T2DM, insulin resistance accelerates the loss of muscle mass and strength because it can disrupt the balance between muscle hypertrophy and atrophy, suppressing insulin or insulin‐like growth factor 1 (IGF‐1) signalling, thereby deregulating several processes that favour the increase of protein degradation and decrease of protein synthesis. In addition, T2DM can reduce the activity of the mitochondrial electron transport chain, impairing the bioenergetic efficiency of the muscle. 138 Additionally, patients with diabetes may present metabolic changes that promote immune system dysfunction, favouring the excessive production of pro‐inflammatory cytokines such as TNF‐α and IL‐1β, which can induce muscle dysfunction. 146
Older adults may also be at greater risk of developing post‐COVID‐19 muscle sequelae because ageing induces the decline of several bodily functions, including changes in protein synthesis, impaired neuromuscular function, and hormonal, metabolic, and nutritional changes. These changes favour the loss of muscle mass, characterised by myofibre atrophy (mainly type II) in older adults, increased intramyocellular lipids and collagen, reduced mitochondrial function and biogenesis, altered satellite cell function, and others. 138
4. CLINICAL MANAGEMENT OF MUSCLE SEQUELAE IN LONG COVID‐19
In long COVID‐19, patients can present various symptoms in different organs and systems. Therefore, we emphasise the importance of rehabilitation by an integrated multidisciplinary team involved in the complete recovery of the patient's health and improved quality of life.
When we focus on treating muscle sequelae, we can highlight that loss of muscle mass presents itself as the main outcome of muscle dysfunction induced by SARS‐CoV‐2 infection and is associated with several functional consequences such as muscle weakness, myalgia, and decreased physical and functional performance. In this context, physical training is a key factor in re‐establishing muscle health and includes several strategies that depend on the limitations manifested by the patient and the environment in which he is placed. The procedures generally involve muscle strengthening, mobility exercises, aerobic exercise, and inspiratory muscle training. 147 , 148
Additionally, we highlight the importance of nutritional monitoring, aiming at adequate intake of essential nutrients to increase and protect muscle mass, good functioning of the microbiota, and assistance for the control of comorbidities such as T2DM and obesity, which have a great impact on muscle dysfunction. 20 , 149
Although the need for rehabilitation for patients with COVID‐19 sequelae is clear, the novelty of the viral infection and its constant evolution requires more studies to measure the extent of the functional deficiencies of these patients, evaluate the effects of rehabilitation, and recommend therapeutic approaches. 148
5. CONCLUSIONS
The muscle sequelae observed in long COVID‐19 are due to persistent muscle dysfunction, starting at the acute phase of the disease, mainly characterised by a reduction in muscle protein synthesis due to the hyperinflammatory state, hypoxaemia, muscle cell infection, and dysregulation of RAS. In addition, cerebrovascular diseases resulting from COVID‐19, the involvement of the peripheral nervous system, and harmful effects of hospitalisation aggravate muscle damage. The decrease in the quantity and quality of muscle mass is the main result of muscle dysfunction, causing fatigue and muscle weakness that aggravate respiratory sequelae, resulting in functional limitations, with a consequent reduction in quality of life and the ability to perform daily activities. Understanding the generalised fatigue and muscle weakness observed in patients with long COVID‐19 is essential to guide future research to reduce the current scarcity of studies that assess muscle dysfunction resulting from long COVID‐19, its sequelae, and the impacts on the lives of survivors. Furthermore, studies that recommend evidence‐based rehabilitation related to long‐term COVID‐19 are also needed.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest declared.
AUTHOR CONTRIBUTIONS
Camilla Costa Silva and Luiz Fábio Magno Falcão: conceptualisation, investigation, figures, writing‐original draft and writing – review and editing. Clea Nazaré Carneiro Bichara, Francisca Regina Oliveira Carneiro, Vera Regina da Cunha Menezes Palacios and Juarez Antônio Simões Quaresma: writing‐original draft and writing – review and editing. Ana Virgínia Soares Van den Berg: writing – review and editing. Juarez Antônio Simões Quaresma and Luiz Fábio Magno Falcão: supervision and project administration.
ACKNOWLEDGEMENTS
We would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), Amazon Foundation to Support Studies and Research (FAPESPA) and Secretary of Science, Technology and Higher, Professional and Technological Education of the State of Pará (SECTET‐PA). To the Center for Biological and Health Sciences at the University of the State of Pará and to the Postgraduate Program in Parasitic Biology in the Amazon.
Silva CC, Bichara CNC, Carneiro FRO, et al. Muscle dysfunction in the long coronavirus disease 2019 syndrome: pathogenesis and clinical approach. Rev Med Virol. 2022;e2355. 10.1002/rmv.2355
Juarez Antônio Simões Quaresma and Luiz Fábio Magno Falcão have contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Taribagil P, Creer D, Tahir H. Long COVID syndrome. BMJ Case Rep. 2021;14:e241485. 10.1136/bcr-2020-241485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low‐risk individuals with post‐COVID‐19 syndrome: a prospective, Community‐based study. BMJ Open. 2021;11:e048391. 10.1136/bmjopen-2020-048391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sigfrid L, Drake T, Pauley E. Long Covid in adults discharged from UK hospitals after Covid‐19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yong SJ. Long COVID or post‐COVID‐19 syndrome: putative pathophysiology, risk factors, and treatments. Inf Disp. 2021;53:737‐754. 10.1080/23744235.2021.1924397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade BS, Siqueira S, Soares WRA, et al. Long‐COVID and post‐COVID health complications: an up‐to‐date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. 10.3390/v13040700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2021;11:16144. 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603‐605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weerahandi M, Hochman KA, Simon E, et al. Post‐discharge health status and symptoms in patients with severe COVID‐19. J Gen Intern Med. 2021;36:738‐745. 10.1007/s11606-020-06338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Huang K, Jiang H, et al. Long‐term existence of SARS‐CoV‐2 in COVID‐19 patients: host immunity, viral virulence, and transmissibility. Virol Sin. 2020;35:793‐802. 10.1007/s12250-020-00308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koshi NA, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID‐19. J Cardiovasc Electrophysiol. 2020;31:1003‐1008. 10.1111/jce.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID‐19. Int J Dermatol. 2021;60:122‐124. 10.1111/ijd.15313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatr. 2020;7:611‐627. 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93:1013‐1022. 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 15. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs LG, Paleoudis EG, Lesky‐Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID‐19 infection. PLoS ONE. 2020;15:e0243882. 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goërtz YMJ, Herck MV, Delbressine JM, et al. Persistent symptoms 3 months after a SARS‐CoV‐2 infection: the post‐COVID‐19 syndrome? ERJ Open Res. 2020;6:00542‐02020. 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: a single‐centre longitudinal study. Clin Microbiol Infect. 2021;27:89‐95. 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasan LK, Deadwiler B, Haratian A, Bolia IK, Weber AE, Petrigliano FA. Effects of COVID‐19 on the musculoskeletal system: clinician’s guide. Orthop Res Rev. 2021;13:141‐150. 10.2147/ORR.S321884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirwan R, McCullough D, Butler T, Heredia FP, Davies IG, Stewart C. Sarcopenia during COVID‐19 lockdown restrictions: long‐term health effects of short‐term muscle loss. Geroscience. 2020;42:1547‐1578. 10.1007/s11357-020-00272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez A, Orozco‐Aguilar J, Achiardi O, Simon F, Cabello‐Verrugio C. SARS‐CoV‐2/Renin–Angiotensin system: deciphering the clues for a couple with potentially harmful effects on skeletal muscle. Int J Mol Sci. 2020;21:7904. 10.3390/ijms21217904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camargo‐Martínez W, Lozada‐ Martínez I, Escobar‐Collazos A, et al. Post‐COVID 19 neurological syndrome: implications for sequelae’s treatment. J Clin Neurosci. 2021;88:219‐225. 10.1016/j.jocn.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hussain FS, Eldeeb MA, Blackmore D, Siddiqi ZA. Guillain Barré syndrome and COVID‐19: possible role of the cytokine storm. Autoimmun Rev. 2020;19:102681. 10.1016/j.autrev.2020.102681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarzani R, Spannella F, Giulietti F, Di Pentima C, Giordano P, Giacometti A. Possible harm from glucocorticoid drugs misuse in the early phase of SARS‐CoV‐2 infection: a narrative review of the evidence. Intern Emerg Med. 2021. 10.1007/s11739-021-02860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. English KL, Paddon‐Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;1:34‐39. 10.1097/MCO.0b013e328333aa66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Disser N, De Micheli A, Schonk M, et al. Musculoskeletal consequences of COVID‐19. J Bone Joint Surg Am. 2020;102:197‐1204. 10.2106/JBJS.20.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piotrowicz K, Gąsowski J, Michel J, Veronese N. Post‐COVID‐19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33:2887‐2898. 10.1007/s40520-021-01942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177‐180. [PMC free article] [PubMed] [Google Scholar]
- 29. Basso LGM, Vicente EFEC, Jr , Cilli EM, Costa‐Filho AJ. SARS‐CoV fusion peptides induce membrane surface ordering and curvature. Sci Rep. 2016;6:37131. 10.1038/srep37131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan J, Kok K, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9:221‐236. 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riquelme C, Acuña MJ, Torrejón J, et al. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLOS ONE. 2014;9:e93449. 10.1371/journal.pone.0093449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motta‐Santos D, Dos Santos RAS, Qadri MOF, et al. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res. 2016;39:506‐512. 10.1038/hr.2016.28 [DOI] [PubMed] [Google Scholar]
- 33. Giordani L, He GJ, Negroni E, et al. High‐dimensional single‐cell cartography reveals novel skeletal muscle‐resident cell populations. Mol Cell. 2019;2:609‐621. 10.1016/j.molcel.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 34. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS‐CoV‐2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol. 2020;129:864‐867. 10.1152/japplphysiol.00321.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madia F, Merico B, Primiano G, Cutuli SL, De Pascale G, Servidei S. Acute myopathic quadriplegia in patients with COVID‐19 in the intensive care unit. Neurology. 2020;95:492‐494. 10.1212/WNL.0000000000010280 [DOI] [PubMed] [Google Scholar]
- 36. Hasöksüz M, Kiliç S, Saraç F. Coronaviruses and SARS‐COV‐2. Turk J Med Sci. 2020;50:549‐556. 10.3906/sag-2004-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;16:281‐292. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363‐374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falcão LFM, Pontes LS, Da Silva BGA, et al. The complexity of respiratory disease associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: from immunopathogenesis to respiratory therapy. Rev Med Virol. 2020:e2167. 10.1002/rmv.2167 [DOI] [Google Scholar]
- 42. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi = Chin J Pathol. 2020;49:411‐417. 10.3760/cma.j.cn112151-20200312-00193 [DOI] [PubMed] [Google Scholar]
- 43. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700‐704. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buttignol M, Pires‐Neto RC, Rossi E Silva RC, Albino MB, Dolhnikoff M, Mauad T. Airway and parenchyma immune cells in influenza A(H1N1) pdm09 viral and non‐viral diffuse alveolar damage. Respir Res. 2017;18:147. 10.1186/s12931-017-0630-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cummins EP, Crean D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017;19:210‐221. 10.1016/j.micinf.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 46. Preedy VR, Sugden PH. The effects of fasting or hypoxia on rates of protein synthesis in vivo in subcellular fractions of rat heart and gastrocnemius muscle. Biochem J. 1989;257:519‐527. 10.1042/bj2570519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pettersen EO, Juul NO, Ronning OW. Regulation of protein metabolism of human cells during and after acute hypoxia. Cancer Res 1986;46:4346‐4351. [PubMed] [Google Scholar]
- 48. Beyfuss K, Hood D. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23:100‐117. 10.1080/13510002.2017.1416773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Q, Wang P, Qin Z, et al. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. 2021;38:101815. 10.1016/j.redox.2020.101815C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yun Z, Qun L, Giaccia A. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040‐3055. 10.1128/MCB.25.8.3040-3055.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mason S, Johnson RS. The role of HIF‐1 in hypoxic response in the skeletal muscle. In: Roach RC, Wagner PD, Hackett PH (eds). Hypoxia and the Circulation. Advances in Experimental Medicine and Biology . Springer;618. 10.1007/978-0-387-75434-5_18 [DOI] [PubMed] [Google Scholar]
- 52. Valle‐Tenney R, Rebolledo D, Acuña MJ, et al. HIF‐hypoxia signaling in skeletal muscle physiology and fibrosis. J. Cell Commun. Signal. 2020;14:147‐158. 10.1007/s12079-020-00553-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do. Ann Rheum Dis. 2005;64:971‐980. 10.1136/ard.2004.031641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J Biol Chem. 2004;279:16332‐16338. 10.1074/jbc.M313931200 [DOI] [PubMed] [Google Scholar]
- 55. Wang C, Liu W, Liu Z, Longo C, Liu X, Kuang S. Hypoxia inhibits myogenic differentiation through p53 protein‐dependent induction of Bhlhe40 protein. J Biol Chem. 2015;290:29707‐29716. 10.1074/jbc.M115.688671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID‐19: insights from invasive cardiopulmonary exercise testing. Chest. 2021;161:54‐63. 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baratto C, Caravita S, Faini A, et al. Impact of COVID‐19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. 2021;130:1470‐1478. 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shehata GA, Lord KC, Grudzinski MC, Elsayed M, Abdelnaby R, Elshabrawy HA. Neurological complications of COVID‐19: underlying mechanisms and management. Int J Mol Sci. 2021;22:4081. 10.3390/ijms22084081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Das G, Mukherjee N, Ghosh S. Neurological insights of COVID‐19 pandemic. ACS Chem Neurosci. 2020;11:1206‐1209. 10.1021/acschemneuro.0c00201 [DOI] [PubMed] [Google Scholar]
- 60. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591:639‐644. 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of covid‐19 in the young. N Engl J Med. 2020;382:e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Correia AO, Feitosa PWG, Moreira JLS, Nogueira SAR, Fonseca RB, Nobre MEP. Neurological manifestations of COVID‐19 and other coronaviruses: a systematic review. Neurol Psychiatr Brain Res. 2020;37:27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wijeratne T, Crewther SG, Sales C, Karimi L. COVID‐19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception—a systematic review. Front Neurol. 2021;11:607221. 10.3389/fneur.2020.607221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fraiman P, Junior CG, Moro E, Cavallieri F, Zedde M. COVID‐19 and cerebrovascular diseases: a systematic review and perspectives for stroke management. Front Neurol. 2020;11:574694. 10.3389/fneur.2020.574694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trejo‐Gabriel‐Galán JM. Ictus como complicación y como factor pronóstico de COVID‐19. Neurologia. 2020;35:318‐322. 10.1016/j.nrl.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS‐CoV‐2: evidence and caveats in COVID‐19. Viruses. 2021;13:29. 10.3390/v13010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yachou Y, El Idrissi A, Belapasov V, Benali AS. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS‐CoV‐2: understanding the neurological manifestations in COVID‐19 patients. Neurol Sci. 2020;41:2657‐2669. 10.1007/s10072-020-04575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hosking MP, Lane TE. The role of chemokines during viral infection of the CNS. PLOS Pathog. 2010;6:e1000937. 10.1371/journal.ppat.1000937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang F, Kream RM, Stefano GB. Long‐term respiratory and neurological sequelae of COVID‐19. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e928996. 10.12659/MSM.928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao H, Shen D, Zhou H, Liu J. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19:383‐384. 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID‐19: a systematic review and current update. Acta Neurol Scand. 2020;142:14‐22. 10.1111/ane.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ryan AS, Ivey FM, Prior S, Li G, Hafer‐Macko G. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42:416‐420. 10.1161/STROKEAHA.110.602441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ingraham NE, Barakat AG, Reilkoff R, et al. Understanding the renin–angiotensin–aldosterone–SARS‐CoV axis: a comprehensive review. Eur Respir J. 2020;56:2000912. 10.1183/13993003.00912-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Frantz EDC, Prodel E, Braz ID, et al. Modulation of the renin–angiotensin system in white adipose tissue and skeletal muscle: focus on exercise training. Clin Sci (Lond). 2018;132:1487‐1507. 10.1042/CS20180276 [DOI] [PubMed] [Google Scholar]
- 75. Cabello‐Verrugio C, Cordova G, Salas JD. Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci. 2012;13:560‐569. 10.2174/138920312803582933 [DOI] [PubMed] [Google Scholar]
- 76. Cabello‐Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin‐angiotensin system: an old player with novel functions in skeletal muscle. Med Res Ver. 2015;35:437‐463. 10.1002/med.21343 [DOI] [PubMed] [Google Scholar]
- 77. García LF. Immune response, inflammation, and the clinical spectrum of COVID‐19. Front Immunol. 2020;11:1441. 10.3389/fimmu.2020.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frederiksen LSF, Zhang Y, Foged C, Thakur A. The long road toward COVID‐19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol. 2020;11:1817. 10.3389/fimmu.2020.01817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163‐169. 10.1159/000453066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yan Z, Yang M, Lai C‐L. Long COVID‐19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021;9:966. 10.3390/biomedicines9080966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carson JA, Baltgalvis KA. Interleukin‐6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38:168‐176. 10.1097/JES.0b013e3181f44f11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gao S, Durstine JL, Koh H‐J, Carver WE, Frizzel N, Carson JA. Acute myotube protein synthesis regulation by IL‐6‐related cytokines. Am J Physiol Cell Physiol. 2017;313:C487‐C500. 10.1152/ajpcell.00112.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muñoz‐Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin‐6 myokine signaling in skeletal muscle: a double‐edged sword? FEBS J. 2013;280:4131‐4148. 10.1111/febs.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guerci A, Lahoute C, Hébrad S, et al. Srf‐dependent paracrine signals produced by myofibers control satellite cell‐mediated skeletal muscle hypertrophy. Cell Metab 2012;15:25‐37. 10.1016/j.cmet.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 87. VanderVeen BN, Fix DK, Montalvo RN, Counts BR, Smuder AJ. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated IL‐6. Exp Physiol. 2019;104:385‐397. 10.1113/EP087429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. White JP, Puppa MJ, Sato S, et al. IL‐6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the Apc Min/+ mouse. Skeletl Muscle. 2012;2:14. 10.1186/2044-5040-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Broussard SR, McCusker RH, Novakofski JE, et al. IL‐1β impairs insulin‐like growth factor I‐induced differentiation and downstream activation signals of the insulin‐like growth factor I receptor in myoblasts. J Immunol. 2004;172:7713‐7720. 10.4049/jimmunol.172.12.7713 [DOI] [PubMed] [Google Scholar]
- 90. Cooney RNR, Shumate M. The inhibitory effects of interleukin‐1 on growth hormone action during catabolic illness. Vitam Horm. 2006;74:317‐340. 10.1016/S0083-6729(06)74013-4 [DOI] [PubMed] [Google Scholar]
- 91. Lang CH, Frost RA, Nairn AC, MacLean D, Vary TC. TNF‐α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336‐E347. 10.1152/ajpendo.00366.2001 [DOI] [PubMed] [Google Scholar]
- 92. Hardim BJ, Campbell KS, Smith JD, et al. TNF‐α acts via TNFR1 and muscle‐derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694‐699. 10.1152/japplphysiol.00898.2007 [DOI] [PubMed] [Google Scholar]
- 93. Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A. Tumor necrosis factor‐α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLOS ONE. 2010;5:e13262. 10.1371/journal.pone.0013262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jackman RW, Cornwell EW, Wu C, Kandarian SC. Nuclear factor‐κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98:19‐24. 10.1113/expphysiol.2011.063321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reid MB, Lännergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor‐alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479‐484. 10.1164/rccm.2202005 [DOI] [PubMed] [Google Scholar]
- 96. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269‐270. 10.1038/s41577-020-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Aschman T, Schneider J, Greuel S, et al. Association between SARS‐CoV‐2 infection and immune‐mediated myopathy in patients who have died JAMA Neurol 2021;78:948‐960. 10.1001/jamaneurol.2021.2004 [DOI] [PubMed] [Google Scholar]
- 99. Marzetti E, Calvani R, Cesari M, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288‐2301. 10.1016/j.biocel.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bhatti JS, Bhatti GK, Reddy H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta (BBA)—Mol Basis Dis. 2017;1863:1066‐1077. 10.1016/j.bbadis.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia, Sarcopenia Muscle 2017;8:349‐369. 10.1002/jcsm.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Owen AM, Patel SP, Smith JD, et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. Elife. 2019;3:e49920. 10.7554/eLife.49920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schefold JC, Bierbrauer J, Weber‐Carstens S. Intensive care unit—acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1:147‐157. 10.1007/s13539-010-0010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mayer KP, Bastin MLT, Montgomery‐Yates A, et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24:637. 10.1186/s13054-020-03355-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. De Letter MACJ, Doorn PA van, SavelKoul HFJ, et al. Critical illness polynauropathy and myopathy (CIPNM): evidence for local immune activation by cytokine‐expression in the muscle tissue. J Neuroimmunol. 2000;106:206‐213. 10.1016/s0165-5728(99)00252-0 [DOI] [PubMed] [Google Scholar]
- 106. Witteveen E, Wieske L, Verhamme C, Schultz MJ, Schaik IN, Horn J. Muscle and nerve inflammation in intensive care unit‐acquired weakness: a systematic translational review. J Neurol Sci. 2014;345:15‐25. 10.1016/j.jns.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 107. Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32:140‐163. 10.1002/mus.20304 [DOI] [PubMed] [Google Scholar]
- 108. Cámara‐Lemarroy CR, Guzmán‐de la Garza FJ, Fernández‐Garza NE. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation. 2010;17:314‐324. 10.1159/000292020 [DOI] [PubMed] [Google Scholar]
- 109. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. 10.1186/s13054-015-0993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931‐941. 10.1016/S1474-4422(11)70178-8 [DOI] [PubMed] [Google Scholar]
- 111. Batt J, Dos Santos CC, Cameron JI, Herridge MS. Intensive care unit–acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187:238‐246. 10.1164/rccm.201205-0954SO [DOI] [PubMed] [Google Scholar]
- 112. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid‐19. N Engl J Med. 2021;25:693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307‐1316. 10.1001/jama.2020.1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dirks‐Naylor AJ, Griffiths CL. Glucocorticoid‐induced apoptosis and cellular mechanisms of myopathy. J Steroid Biochem Mol Biol. 2009;117:1‐7. 10.1016/j.jsbmb.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 115. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid‐induced myopathy. J Endocrinol. 2008;197:1‐10. 10.1677/JOE-07-0606 [DOI] [PubMed] [Google Scholar]
- 116. Gupta A, Gupta Y. Glucocorticoid‐induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17:913‐916. 10.4103/2230-8210.117215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tran TT, Beutler SS, Urman RD. Moderate and deep sedation training and pharmacology for nonanesthesiologists. Curr Opin Anaesthesiol. 2019;32:457‐463. 10.1097/aco.0000000000000758 [DOI] [PubMed] [Google Scholar]
- 118. Kress JP. Sedation and mobility. Crit Care Clin. 2013;29:67‐75. 10.1016/j.ccc.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 119. Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107‐1116. 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 120. Segredo V, Caldwell JE, Matthay MA, et al. Persistent paralysis in critically ill patients after long‐term administration of vecuronium. N Engl J Med. 1992;327:524‐528. 10.1056/NEJM199208203270804 [DOI] [PubMed] [Google Scholar]
- 121. Ledowski T, Nißler S, Wenk M, Pogatzki‐Zahn EM, Segelcke D. Efects of muscle relaxants on ischaemia damage in skeletal muscle. Sci Rep. 2018;8:5794. 10.1038/s41598-018-24127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mefford B, Donaldson JC, Bissell BD. To block or not: updates in neuromuscular blockade in acute respiratory distress syndrome. Ann Pharmacother. 2020;54:899‐906. 10.1177/1060028020910132 [DOI] [PubMed] [Google Scholar]
- 123. Barreiro E. Models of disuse muscle atrophy: therapeutic implications in critically ill patients. Ann Transl Med. 2018;6:29. 10.21037/atm.2017.12.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sumien N, Shetty RA, Gonzales EB. Creatine, creatine kinase, and aging. Subcell Biochem. 2018;90:145‐168. 10.1007/978-981-13-2835-0_6 [DOI] [PubMed] [Google Scholar]
- 125. Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Ver. 2008;88:287‐332. 10.1152/physrev.00015.2007 [DOI] [PubMed] [Google Scholar]
- 126. Ferrari R, Caram LM, Faganello MM, Sanchez FF, Tanni SE, Godoy I. Relation between systemic inflammatory markers, peripheral muscle mass, and strength in limb muscles in stable COPD patients. Int J Chronic Obstr Pulm Dis. 2015;10:1553‐1558. 10.2147/COPD.S85954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9‐e17. 10.1016/j.amjmed.2005.10.049 [DOI] [PubMed] [Google Scholar]
- 128. Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455‐461. 10.1093/gerona/gln038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liang X, Liu L, Fu T, et al. Exercise inducible lactate dehydrogenase B regulates mitochondrial function in skeletal muscle. J Biol Chem. 2016;291:25306‐25318. 10.1074/jbc.M116.749424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tan T, Khoo B, Mills EG, et al. Association between high serum total cortisol concentrations and mortality from COVID‐19. Lancet Diabetes Endocrinol. 2020;8:659‐660. 10.1016/S2213-8587(20)30216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Peeters GMEE, Van Schoor NM, Van Rossum EFC, Visser M, Lips P. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol. 2008;69:673‐682. 10.1111/j.1365-2265.2008.03212.x [DOI] [PubMed] [Google Scholar]
- 132. Deng F, Zhang L, Lyu L, et al. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID‐19. Med Clin. 2021;156:324‐331. 10.1016/j.medcli.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Moreira AC, Mesquita G, Gomes MS. Ferritin: an inflammatory player keeping iron at the core of pathogen‐host interactions. Microorganisms. 2020;8:589. 10.3390/microorganisms8040589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Paneroni M, Simonelli C, Saleri M, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID‐19 pneumonia. Am J Phys Med Rehabil. 2021;100(2):105‐109. 10.1097/PHM.0000000000001641 [DOI] [PubMed] [Google Scholar]
- 135. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune‐metabolic viewpoint for age‐related diseases. Nat Rev Endocrinol. 2018;14(10):576‐590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 136. Arentson‐Lantz EJ, English KL, Paddon‐Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol. 2016;120:965‐975. 10.1152/japplphysiol.00799.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kilroe SP, Fulford J, Holwerda AM, et al. Short‐term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab. 2020;318:E117‐E130. 10.1152/ajpendo.00360.2019 [DOI] [PubMed] [Google Scholar]
- 138. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819‐829. 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Marshall M. COVID's toll on smell and taste: what scientists do and don't know. Nature. 2021;589(7842):342‐343. 10.1038/d41586-021-00055-6 [DOI] [PubMed] [Google Scholar]
- 140. Fantozzi PJ, Pampena E, Di Vanna D, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID‐19. Am J Otolaryngol. 2020;41:102721. 10.1016/j.amjoto.2020.102721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dawson C, Capewell R, Ellis S, et al. Dysphagia presentation and management following coronavirus disease 2019: an acute care tertiary centre experience. J Laryngol Otol. 2020;134:981‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post‐SARS syndrome; a case‐controlled study. BMC Neurol. 2011;11:37. 10.1186/1471-2377-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post‐viral syndrome post COVID‐19. Med Hypotheses. 2020;144:110055. 10.1016/j.mehy.2020.110055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ahmed H, Patel K, Greenwood DC, et al. Long‐term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta‐analysis. J Rehabil Med. 2020;52:jrm00063. 10.2340/16501977-2694 [DOI] [PubMed] [Google Scholar]
- 145. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543‐550. 10.1111/j.1440-1843.2010.01720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Michalakis K, Ilias I. SARS‐CoV‐2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metabol Syndr. 2020;14(4):469‐471. 10.1016/j.dsx.2020.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Demeco A, Marotta N, Barletta M, et al. Rehabilitation of patients post‐COVID‐19 infection: a literature review. J Int Med Res;48(8):0300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome‐related coronavirus infection: implications for COVID‐19 rehabilitation. Phys Ther. 2020;100(10):1717‐1729. 10.1093/ptj/pzaa129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high‐risk factors for severe coronavirus disease 2019 (Covid‐19). Diabetes Metab Res Rev. 2021;37:e3377. 10.1002/dmrr.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


