Abstract
COVID‐19 is a world disaster. In response to COVID‐19 quarantine, stress, anxiety, and depression may easily develop which negatively affect immunity and decrease the patient's response against the COVID‐19 virus. This study investigated the effect of an integrated intervention combining cognitive‐behavioural stress management (CBSM) and progressive muscle relaxation (PMRs) on immune biomarkers and disease severity and progression in patients with COVID‐19 and the period to which these changes last. Thirty patients with mild or moderate COVID‐19 were randomly distributed into intervention and control groups. The intervention group performed an integrated intervention combining CBSM and PMRs. There were three outcome measures including blood immune markers, salivary immunoglobulin A, and Wisconsin scale (WIS). Two‐week post‐intervention, there were significant differences between groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A. While there were non‐significant differences between both groups in Interleukin‐10 and TNF‐α. The significant differences between groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A significantly continued 1 week as a follow‐up. This study concluded that performing an integrated intervention combining CBSM and PMRs for 2 weeks significantly increases immune biomarkers mainly Leucocytes, Lymphocytes, Interleukin‐10, and Interleukin‐6 along with S‐IgA. Also, this protocol significantly decreases disease severity and associated stress, anxiety, and depression; and enhances the quality of life in patients with COVID‐19. The study was retrospectively registered with NCT04998708.
Keywords: COVID‐19, COVID‐19 progression, COVID‐19 severity, immune biomarkers, relaxation protocol
1. INTRODUCTION
COVID‐19 is a world disaster. According to the World Health Organization, COVID‐19 has infected more than 200,840,180 cases, including 4,265,903 deaths on 5 August 2021 (World Health Organization. Corona‐ Virus Disease [COVID‐19] Outbreak, 2020). COVID‐19 is known as an enclosed RNA beta‐coronavirus‐type (Gorbalenya et al., 2020). The most followed protocol in the management of COVID‐19 is a quarantine for 14 days. In response to this quarantine, stress, anxiety, and depression may easily develop in patients with COVID‐19 (Rajkumar, 2020). Symptoms of anxiety and depression commonly develop as a response to COVID‐19 quarantine (Rajkumar, 2020). A recent meta‐analysis demonstrated that stress, anxiety, and depression transiently develop as a response to COVID‐19 quarantine. The prevalence of transit stress, anxiety, and depression are 29.6%, 31.9%, and 33.7% respectively (Salari et al., 2020).
Stress suppresses the activity of the hypothalamic‐pituitary‐adrenal (HPA) axis identified as the chief stress regulator in humans. Consequently, a decrease in the normal control of the neuro‐endocrine system occurs. The hypothalamus releases corticotropin‐releasing factors (CRFs; Mohamed & Alawna, 2020b; Zamani‐Alavijeh et al., 2018). These CRFs bind to specific receptors on the anterior pituitary gland to trigger the release of adrenocorticotropic hormone (ACTH). Adrenocorticotropic hormone releases cortisol hormone via binding to specific receptors on the adrenal cortex. In stressful conditions, serum cortisol increases leading to suppression of immune function along with the release of key body inflammation substances (prostaglandins and leukotrienes). This reduces the activity and function of immune cells (eosinophil, basophil, macrophages, neutrophil, mast cells T‐lymphocytes, and B‐lymphocytes; Geraghty & Kaufer, 2015; Goppelt‐Struebe et al., 1989; Mohamed & Alawna, 2020b). Thus, a decrease in the body's defense to COVID‐19 infections and an increase in COVID‐19 progression and severity occur (Alawna & Mohamed, 2020; Mohamed & Alawna, 2020b).
The body acts to resolve stress, anxiety, and depression by promoting complex and interconnected cellular, neuroendocrine, and molecular infrastructures. This occurs via several adaptations in both peripheral and central nervous systems (Mohamed & Alawna, 2020b; Tsigos et al., 2000). Thus, immune strength is crucial in the management of COVID‐19 infections. Consequently, therapies that improve immune function are important to be performed during the quarantine period (Mohamed & Alawna, 2021b; Mohamed & Alawna, 2020a; Mohamed et al., 2020). Previously, we investigated the effect of aerobic exercise as a therapy to improve immune function (Mohamed & Alawna, 2021a). We found that aerobic exercise significantly increased immune function. In addition, it significantly decreased COVID‐19 disease progression and severity.
Different studies were conducted earlier to demonstrate the important role of relaxation techniques for patients with COVID‐19 (Ozamiz‐Etxebarria et al., 2020; Özlü et al., 2021; Xiao et al., 2020). They mainly investigate the effect of relaxation techniques on anxiety, sleep quality, and negative emotions. All these studies did not investigate the effect of these relaxation techniques on neither immune function nor COVID‐19 progression and severity which are crucial and specified to COVID‐19. Progressive muscle relaxation (PMRs) techniques were commonly used in COVID‐19 related studies. These techniques have a high controversy on their effect on immune function. Ikemata and Momose investigated the effects of PMRs on activities of daily living, dementia symptoms, and immune function in group home residents with dementia in Japan (Ikemata & Momose, 2017). They found that PMRs enhance behavioural and psychological symptoms of dementia and activities of daily living in group home residents with dementia but does not affect their immune function. In contrast, Pawlow and Jones investigated the effects of brief PMRs on salivary cortisol and salivary immunoglobulin A (Pawlow & Jones, 2005). They found that brief PMRs significantly reduce salivary cortisol and increase salivary immunoglobulin A concentration and secretion rate. A recent systematic and meta‐analysis review supported the effectiveness of stress‐reducing interventions in enhancing immunity in studies that tested immune function by methods of incorporating an in vitro, in vivo, or psychophysiological challenge (Schakel et al., 2019).
Cognitive‐behavioural stress management (CBSM) is a programme that combines stress management training with relaxation training. The effect of CBSM has been demonstrated in several studies unrelated to COVID‐19. McGregor et al. investigated the effect of CBSM on immune function among women with early‐stage breast cancer (McGregor et al., 2004). They found that CBSM improved emotional responses to their breast cancer experience in parallel with later improvement in cellular immune function. Antoni et al. investigated the effects of CBSM on distress responses and immunologic changes Following Notification of HIV‐1 Seropositivity (Antoni et al., 1991). They found that CBSM significantly increased helper‐inducer (CD4) and natural killer (CD56) cell counts in addition to a slight increase in proliferative responses to phytohaemagglutinin.
Improving immune function is a key in the management of COVID‐19. Interventions that improve the psychological status of patients may be helpful in counter fight COVID‐19. Thus, we conducted this study to investigate the effect of an integrated intervention combining CBSM and PMRs on immune biomarkers and disease severity and progression in patients with COVID‐19 and the period to which these changes last.
2. MATERIALS AND METHODS
2.1. Study procedures
A randomized controlled design was used. The study was double blinded in which both patients and assessors were blinded to study procedures. Each patient was asked to sign an informed consent before participation in this study. This study was approved by the ethics committee of the School of Health Sciences, Istanbul Gelisim University, Turkey. Patients were recruited from December 2020 to April 2021. The patients were collected by reviewing the hospital's data in Istanbul, Turkey. Then, we called patients with COVID‐19 to ask them about their welling to participate in this study. Patients who accepted to participate were interviewed through video calls using Zoom App to discuss study procedures with them. Patients who accepted to engage in this study were visited by a researcher at their home while they are in quarantine. Through the initial visit, the consent form was signed, and blood samples were taken. Any patient must have permission to perform our intervention from the physician who supervises him/her. The clinical protocol of this study was retrospectively registered at the https://www.clinicaltrials.govplatform with a registration number of NCT04998708. The study was registered retrospectively for several reasons including the vulnerability of the whole research team to have COVID‐19, participants being hospitalized, lab closure, city lockdown…. etc. which significantly can affect the dates and data of the trial. There were no deviations from the registered trial protocol.
The suitable sample size for this study was determined using a priori power test. The G*POWER programme (ver. 3.1.9.2, Heinrich‐Heine‐University) was used. The criteria entered into the programme included a multivariate analysis of repeated measurements (MANOVA) test using 2 groups, 2 measurements, a power level of 80%, a significance level of 0.05, and a medium effect size (dz = 0.25; Faul et al., 2007). Based on the previous assumptions, the total sample size needed for this study was 30 patients. A minimum power of 80% or more is acceptable in most studies (Kadam & Bhalerao, 2010). The flow of patients throughout the study is shown in Figure 1.
FIGURE 1.
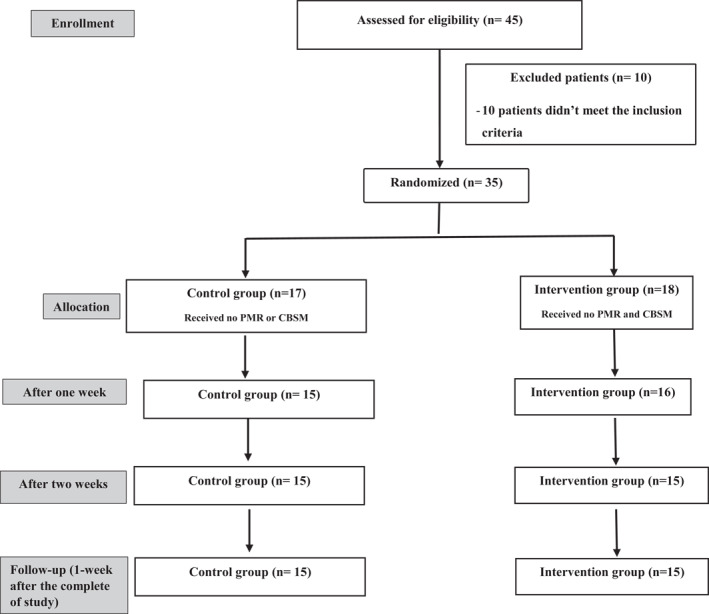
Flow chart for the patients through the study
Initially, 45 patients accepted the participation in this study. Ten patients did not meet the study inclusion criteria and additional five patients did not complete the whole study because they did not like doing exercise. Finally, 30 patients with COVID‐19 completed the whole study (2 weeks intervention and 3 weeks post‐intervention ‘follow‐up’). The study ended after the completion of the intervention.
The patients' age ranged from 25 to 45 years old. The inclusion criterion included that the patient had a recent mild or moderate COVID‐19 with no or low‐grade fever 99.5–100.94°F (37.5–38.3°C; Affronti et al., 2010; Zhuang et al., 2020). Mild grade COVID‐19 was determined by having symptoms of acute upper respiratory tract infection (fever, cough, myalgia, runny nose, fatigue, sore throat, and sneezing) or gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhoea). Moderate grade COVID 19 was determined by having mild symptoms plus pneumonia (cough, frequent fever) with no obvious hypoxaemia, the presence of lesions on chest CT (Yuki et al., 2020). The exclusion criteria included that the patient was indicated for hospitalization, the patient has high‐grade fever >100.94°F (>38.3°C), or the patient had other chronic diseases such as heart problems, hypertension, or diabetes. Women on contraceptives were excluded because contraceptives decrease immune function increase the risk of various autoimmune disorders (Williams, 2017).
2.2. Evaluative procedures
There were three main dependent variables including blood immune markers, salivary immunoglobulin A, and Wisconsin Upper Respiratory Symptom Survey. These measurements were collected at the baseline, 2 weeks post‐intervention, and 1 week as a follow‐up.
A lab technician and one researcher were asked to visit the patient at home during the quarantine. The technician collected blood and saliva samples and the researcher conducted the Wisconsin Upper Respiratory Symptom Survey. They wore special protective equipment recommended by WHO (WHO, 2020b; World Health Organization (WHO), 2020b). Three visits were performed including the beginning of the research procedures, 24‐h post‐intervention (2 weeks), and 1 week as a follow‐up.
2.2.1. Blood sample collection
Blood samples were taken in the morning (8:30–9:30) by collecting 10 ml of venous blood. Patients were asked to stop any exercise for at least 24 h before blood sampling. Also, patients were asked to stop eating or drinking 8 h before collecting samples. Samples were collected in vacutainer tubes with sodium ethylenediami‐netetraacetic acid (EDTA) for plasma separation. The blood was centrifuged at 3000 rpm for 15 min at 4°C. The total lymphocytes, leucocytes, and monocytes were measured utilizing a multichannel haemocyte analysis system (SE‐9000; Sysmex Corp.; Lira et al., 2017; Shimizu et al., 2011). The concentrations of IL‐6, IL‐10, and TNF‐α were analyzed by using ELISA commercial kits assay (R&D Systems). The manufacturer's instructions for analysis of IL‐6, IL‐10, and TNF‐α on an EZ‐Reader microplate reader at 450 nm were followed (de Souza et al., 2018; Lira et al., 2017). The samples were stored at −20°C for further analysis.
2.2.2. Saliva sample collection
The saliva sample was taken without any saliva stimulation methods. The patient was asked to rinse their mouths with distilled water and evacuate their mouth just before the collection. We used the passive drainage method for this collection. The patient slightly flexed their head forward to allow the saliva to move into a sterilized and pre‐weighed Falcon tube for 5 min. The weight of tubes was measured again following the collection to estimate the volume and the saliva flow rate. The tubes were weighed with 0.1 mg accuracy with the proposed saliva density proposed at 1.0 g ml−1. The samples were stored at −80°C for further analysis. The S‐IgA concentration was analyzed utilizing commercial ELISA kits (IgA Salivary, DRG). The IgA‐S secretion rate (ng/min) was measured by multiplying the whole concentration of IgA‐S present in the mucosal surface per unit of time by the saliva flow rate (ml/min; de Souza et al., 2018).
2.2.3. Wisconsin Upper Respiratory Symptom Survey
The Wisconsin Upper Respiratory Symptom Survey is an empirically derived patient‐oriented illness‐specific quality‐of‐life evaluative outcomes instrument (Barrett & Barrington, 2005). The development process of this survey was described in detail by Barrett et al. (2018). The Wisconsin Upper Respiratory Symptom Survey is designed to evaluate the negative effect of acute upper respiratory infection on the quality of life. It is a validated and reliable measurement for evaluating the change in the quality of life over time including influenza‐like illness symptoms of headache, body aches, and fever (B. Barrett et al., 2018). The patients were asked to fill the survey at the baseline, 2 weeks post‐intervention, and 1 week as a follow‐up.
2.3. Treatment procedures
Patients were assigned randomly into intervention, and control groups. Patients were randomly allocated to four permuted blocks using computer software to have two equal sample sizes. The randomization was performed by a college staff member who was not involved in this study. All patients in the groups followed the WHO procedures of quarantine (WHO, 2020a) and took standardized medications administered by the Turkish Ministry of Health (Hydroxychloroquine Sulphate 200 Mg).
The intervention group performed an integrated intervention combining CBSM and PMRs. We used these two interventions because it has been shown their positive effects on immune function. The two relaxation techniques included PMR training (Xiao et al., 2020) and CBSM (McGregor et al., 2004). We used two types of relaxation techniques to produce maximum effects of relaxation techniques within the limited period of COVID‐19 quarantine (2 weeks). The control groups did not receive any relaxation techniques during the study.
2.4. Progressive muscle relaxation training
Progressive muscle relaxation training has been reported to decrease anxiety, negative emotions, and enhance sleep quality in patients with COVID‐19 (Özlü et al., 2021; Xiao et al., 2020). The patients performed PMR in their bed for 30 min in the morning after waking up and at night before sleeping. We recorded a detailed video for PMR to be watched by all patients before participating in this study. The PMR order was initiated by doing isometric contractions of muscles with a concentration on the sensation of tension for 3–5 s. Then, they take a rest for 10–15 s. Next, the patients were instructed to perform the PMR in this sequence foot, leg, hip and waist, chest, arm, shoulder, and face. The specific instructions for each exercise were demonstrated in the study of Özlem et al. (2021). The patient should at least complete 12 days of PMRs to be included in the statistical analysis.
2.5. Cognitive‐behavioural stress management (CBSM)
Cognitive‐behavioural stress management has been reported to significantly decrease depression, anxiety, and stress in patients with COVID‐19 (Li et al., 2020). All included patients were taken a 2‐h structured group session to illustrate the CBSM in detail before starting the study. All sessions were conducted by a hired psychologist who was blinded to study procedures. The CBSM consisted of a 2‐h structured group session performed two times/week. Each session focussed on various CBSM techniques (e.g., coping skills training, cognitive restructuring, assertion training, and stress management). The patients also were asked to do homework assignments (e.g., relaxation monitoring cards and a cognitive restructuring worksheet) given each week. The patient should attend all 2‐h structured group sessions and do all assignments to be included in the statistical analysis.
2.6. Data analysis
Patients' files were encoded by a university administrator who did not join this study (Taylor & Murphy, 2010). Intention‐to‐treat and general linear models of analysis were followed. A MANOVA test was used to measure within each group interactions, while an independent MANOVA test was used to measure between‐groups comparisons. The outcome measures were measured at the baseline, 2 weeks post‐intervention, and 1 week as a follow‐up. All results were compared to the baseline results. The baseline characteristics of patients in both groups were compared using Pearson chi‐squared tests for categorical variables (gender, previous lung infection history) and t‐test for continuous variables (age and body mass index). The significance level was set at p < 0.05. The SPSS (ver. 25, IBM Inc.) was used for statistical analysis.
3. RESULTS
At the baseline, there were non‐significant differences between groups in age, and body mass index (p > 0.05). All measurements were normally distributed in both groups (Shapiro‐Wilk test, p > 0.05; Rochon et al., 2012). The demographic and physical features of all patients at the baseline are demonstrated in Table 1. All included patients in the intervention group performed PMRs for at least 12 days. Also, all included patients attended all 2‐h structured group sessions and do all assignments.
TABLE 1.
Physical characteristics of patients in both groups
| Items | Control group | Intervention group | p |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (yrs.) | 33.45 ± 3.96 | 37.56 ± 3.25 | >0.05 |
| BMI | 24.85 ± 1.21 | 26.65 ± 1.31 | >0.05 |
| Male | 8 | 7 | >0.05 |
| Female | 7 | 8 | >0.05 |
| Smoking | 6 | 7 | >0.05 |
| Non‐smoking | 8 | 8 | >0.05 |
Abbreviations: cm, centimetre; p, probability; SD, standard deviation; yrs, years; *, significant.
3.1. Between‐groups comparisons
At baseline measurements, there were non‐significant differences between both groups in the Wisconsin scale (WIS) total score, Lymphocytes, Leucocytes, Interleukin‐6, Interleukin‐10, TNF‐α, and Immunoglobulin‐A (p > 0.05). Two weeks post‐intervention, there were significant differences between groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A (p < 0.05). While there were non‐significant differences between both groups in Interleukin‐10 and TNF‐α. The significant differences between groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A continued 1 week as a follow‐up (p > 0.05). Between‐groups comparisons are shown in Table 2 and Figure 2.
TABLE 2.
Independent measure multivariate analysis of repeated measurements (MANOVA) between groups of immune markers and Wisconsin scale (WIS) between the baseline, and 2 weeks after the intervention, and 1‐week follow‐up
| Baseline | After 2 Weeks | Follow‐up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD±SE | 95%CI | p | F | Po | MD±SE | 95%CI | p | F | Po | MD ± S E | 95%CI | p | F | Po | |
| Wisconsin S. | 3.53 ± 2.02 | −0.60, 7.67 | 0.091 | 3.06 | 0.39 | 13.00 ± 1.94 | 9.02, 16.98 | >0.01* | 44.82 | 1.00 | 13.87 ± 2.59 | 8.57, 19.16 | >0.01* | 28.77 | 0.99 |
| Leucocytes | 0.20 ± 0.487 | −0.80, 1.20 | 0.68 | 0.17 | 0.68 | 1.55 ± 0.52 | 0.48, 2.62 | >0.01* | 8.77 | 0.81 | 1.55 ± 0.55 | 0.42, 2.68 | >0.01* | 7.95 | 0.78 |
| Lymphocytes | 0.01 ± 0.11 | −0.21, 0.24 | 0.92 | 0.01 | 0.05 | 0.80 ± 0.08 | 0.64:.96 | >0.01* | 100.19 | 1.00 | 0.65 ± 0.10 | 0.45, 0.85 | >0.01* | 43.60 | 1.00 |
| Inter‐6 | 0.32 ± 4.2 | −8.33, 8.97 | 0.94 | 0.01 | 0.05 | 3.89 ± 4.25 | −4.82, 12.60 | 0.03* | 0.84 | 0.14 | 3.73 ± 4.26 | −4.99, 12.44 | 0.04* | 0.77 | 0.14 |
| Inter‐10 | 0.01 ± 0.41 | −0.84 0.85 | 0.98 | 0.00 | 0.05 | 0.74 ± 0.41 | −0.09, 1.58 | 0.07 | 3.35 | 0.42 | 0.69 ± 0.40 | −0.14, 1.51 | 0.10 | 2.93 | 0.38 |
| IgA | 0.11 ± 0.14 | −0.17, 0.40 | 0.42 | 0.66 | 0.12 | 1.04 ± 0.16 | 0.72, 1.36 | >0.01* | 43.88 | 1.00 | 0.84 ± 0.16 | 0.52, 1.16 | >0.01* | 28.35 | 0.99 |
| TNF‐α | 0.34 ± 0.25 | −0.17, 0.85 | 0.19 | 1.85 | 0.26 | 0.34 ± 0.25 | −0.17, 0.85 | 0.18 | 1.86 | 0.26 | 0.34 ± 0.25 | −0.17, 0.86 | 0.18 | 1.86 | 0.26 |
Abbreviations: p, significance level; Po, power; *, significant.
FIGURE 2.
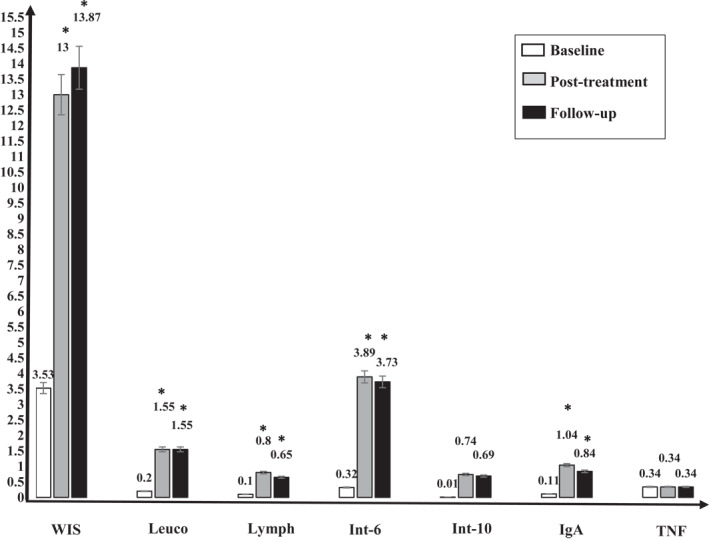
Independent measure multivariate analysis of repeated measurements (MANOVA) between groups at the baseline after 2 weeks of the intervention, and 1 week after the study (follow‐up). At baseline measurements, there were non‐significant differences between both groups in the Wisconsin scale (WIS) total score, Lymphocytes, Leucocytes, Interleukin‐6, Interleukin‐10, TNF‐α, and Immunoglobulin‐A (p > 0.05). After 2 weeks of intervention, there were significant differences between both groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A (p < 0.05). While there were non‐significant differences between both groups in Interleukin‐10 and TNF‐α. One‐week post‐intervention of the study, the significant differences between both groups in the WIS total score, Leucocytes, Lymphocytes, Interleukin‐6, and Immunoglobulin‐A which occurred after the study continued significant (p > 0.05). Abbreviations: IgA, Immunoglobulin‐A; Int‐10, Interleukin‐10; Int‐6, Interleukin‐6; Leuco, Leucocytes; Lymph, Lymphocytes; TNF, TNF‐α.; WIS, Wisconsin scale; *, significance
3.2. Within‐groups comparisons
The WIS total score significantly reduced in control and intervention groups 2 weeks post‐intervention (p < 0.05). This significant decrease continued for 1 week as a follow‐up. Leucocytes, Lymphocytes, and Interleukin‐10 significantly increased in control and intervention groups 2 weeks post‐intervention (p < 0.05). These significant increases continued for 1 week as a follow‐up. Immunoglobulin‐A and Interleukin‐6 significantly increased in the intervention group (p < 0.05) only, while they non‐significantly increased in the control group 2 weeks post‐intervention (p > 0.05). These results continued the same 1 week as a follow‐up. TNF‐α non‐significantly increased in both groups 2 weeks post‐intervention (p < 0.05). This non‐significant increase continued for 1 week as a follow‐up. Within‐group comparisons are demonstrated in Table 3 and Figures 3 and 4.
TABLE 3.
Repeated measure multivariate analysis of repeated measurements (MANOVA) of immune biomarkers and Wisconsin scale (WIS) between the baseline, and 2 weeks after the intervention, and 1 week as a follow‐up
| Group | Baseline | After 2 Weeks | Follow‐up | |||||
|---|---|---|---|---|---|---|---|---|
| M±SD | MD±SD | 95%CI | p | MD±SD | 95%CI | p | ||
| Wisconsin scale | Control | 110.3 ± 5.64 | 104.27 ± 5.85 | 4.61, 7.39 | >0.01* | 105.67 ± 5.51 | 3.48, 5.72 | >0.01* |
| Intervention | 106.73 ± 5.42 | 91.27 ± 4.73 | 13.45, 17.48 | >0.01* | 91.80 ± 8.36 | 10.65, 19.22 | >0.01* | |
| Leucocytes, ×109/L | Control | 5.267 ± 1.37 | 5.84 ± 1.43 | 0.46, 0.68 | >0.01* | 5.66 ± 1.45 | 0.26, 0.52 | >0.01* |
| Intervention | 5.4667 ± 1.30 | 7.3847 ± 1.43 | 1.69, 2.15 | >0.01* | 7.21 ± 1.54 | 1.45, 2.03 | >0.01* | |
| Lymphocytes, ×109/L | Control | 0.93 ± 0.31 | 1.29 ± 0.23 | 0.20, 0.54 | >0.01* | 1.23 ± 0.23 | 0.15, 0.45 | 0.01* |
| Intervention | 0.9367 ± 0.30 | 2.0933 ± 0.21 | 0.99, 1.32 | >0.01* | 1.88 ± 0.30 | 0.75, 1.14 | >0.01* | |
| Interleukin‐6, pg/mL | Control | 22.73 ± 11.53 | 23.01 ± 11.45 | −0.07, 0.61 | 0.11 | 22.95 ± 11.40 | −0.10, 0.52 | 0.16 |
| Intervention | 23.01 ± 11.60 | 26.90 ± 11.83 | 3.20, 4.50 | >0.01* | 26.67 ± 11.90 | 3.01, 4.24 | >0.01* | |
| Interleukin‐10, pg/mL | Control | 6.16 ± 1.15 | 6.31 ± 1.169 | 0.026, 0.27 | 0.02* | 6.27 ± 1.170 | 0.01, 0.21 | 0.03* |
| Intervention | 6.17 ± 1.11 | 7.05 ± 1.055 | 0.72, 1.05 | >0.01* | 6.96 ± 1.03 | 0.61, 0.97 | >0.01* | |
| Immunoglobulin A, g/L | Control | 2.20 ± 0.37 | 2.39 ± 0.51 | 0.06, 0.31 | 0.07 | 2.38 ± 0.50 | 0.053, 0.30 | 0.08 |
| Intervention | 2.32 ± 0.39 | 3.42 ± 0.34 | 0.89, 1.33 | >0.01* | 3.22 ± 0.35 | 0.72, 1.09 | >0.01* | |
| TNF‐α | Control | 10.23 ± 0.79 | 10.23 ± 0.79 | 0.00, 0.01 | 0.13 | 10.23 ± 0.79 | 0.00, 0.01 | 0.22 |
| Intervention | 10.57 ± 0.56 | 10.57 ± 0.56 | 0.00, 0.01 | 0.07 | 10.57 ± 0.56 | 0.00, 0.01 | 0.13 | |
Abbreviations: CI, confidence interval; M, mean; p, probability; SD, standard deviation; *, significant.
FIGURE 3.
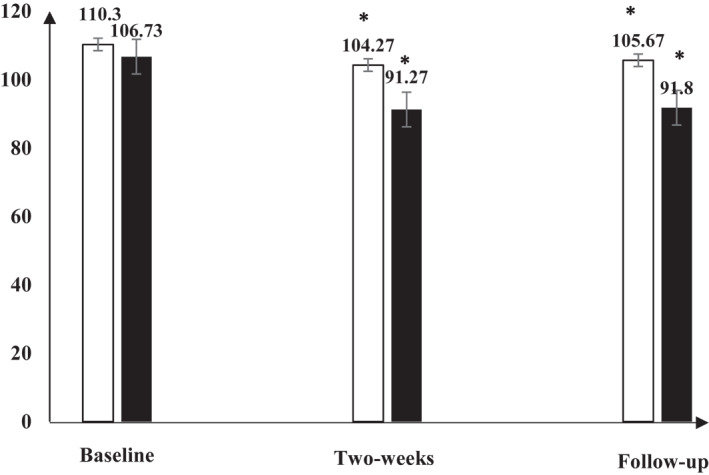
Repeated measure multivariate analysis of repeated measurements (MANOVA) for the Wisconsin scale (WIS) in both groups at the baseline, after 2 weeks of the intervention, and 1 week after the study (Follow‐up). There was significant difference in the WIS in both groups and this significant difference continued till 1‐week follow‐up. Abbreviation: *, significance
FIGURE 4.
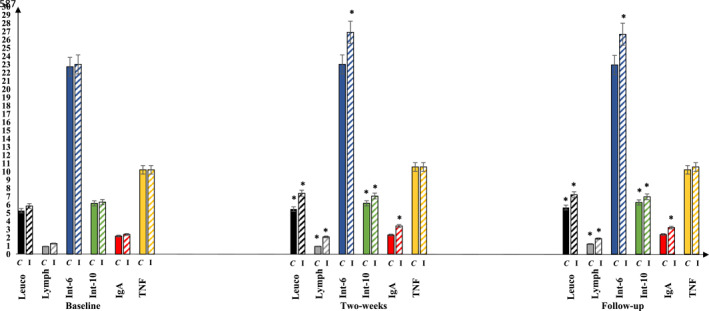
Repeated measure multivariate analysis of repeated measurements (MANOVA) in both groups for the leucocytes, Lymphocytes, Interleukin‐6, Interleukin‐10, Immunoglobulin‐A, and TNF at the baseline, after 2 weeks of the intervention, and 1 week after the study (Follow‐up). Leucocytes, Lymphocytes, and Interleukin‐10 significantly increased in control and intervention groups after the intervention (p < 0.05), and they continued significantly increased for 1‐week follow‐up. After 2 weeks of the intervention, Immunoglobulin‐A and Interleukin‐6 significantly increased in the intervention group (p < 0.05) only, while they non‐significantly increased in the control group (p > 0.05); these results continued the same for 1‐week follow‐up. TNF‐α non‐significantly increased in both groups (p < 0.05), and it continued non‐significantly increased for 1‐week follow‐up. Abbreviations: C, control group; I, intervention group; IgA, Immunoglobulin‐A; Int‐10, Interleukin‐10; Int‐6, Interleukin‐6; Leuco, Leucocytes; Lymph, Lymphocytes; TNF, TNF‐α; *, significance
4. DISCUSSION
This study investigated the effect of following an integrated intervention combining CBSM and PMRs on immune biomarkers and COVID‐19's progression and symptoms severity. This study is unique as it is the first clinical trial that demonstrated that relaxation techniques should be followed during COVID‐19 quarantine and could be a successful adjunct intervention for patients with COVID‐19 to enhance their immune response against COVID‐19 infection.
In this study, Leucocytes, Lymphocytes, and Interleukin‐10 significantly increased in intervention and control groups. In addition, Interleukin‐6 and IgA significantly increased only in the intervention group. IgA is a key immune cell against COVID‐19 because IgA is the primary antibody in saliva secretions that wash mucosal surfaces of the respiratory system. Saliva secretions offer a defensive role against respiratory tract infections (Cunningham‐Rundles, 2009; Hines et al., 1996; Rodríguez et al., 2005). Interestingly, the human body exerts much energy in the secretion of IgA. The daily secretion of IgA is more than the secretion of all the other antibody cells together. Thus, therapies that significantly increase the amount of IgA predominantly in saliva should be recommended.
Additionally, IgA is a vital serum immunoglobulin that mediates different protective roles via interacting with specified receptors and immune mediators. IgA has a crucial role in fighting respiratory infections due to its unique existence in respiratory saliva (the location of COVID‐19 virus invasion). IgA deficiency is one of the common immunodeficiencies with up to 1 in 400 subjects influenced in people with no clear symptoms of susceptibility to viral infection (Hammarström et al., 2000).
The increase in IgA response due to relaxation techniques that occurred in this study came in accordance with the results of other studies. Green et al. investigated the effect of a 20‐min relaxation session performed for 3 weeks on salivary immunoglobulins (S‐IgA; Green et al., 1988). They found that relaxation techniques significantly increased S‐IgA. Taniguchi et al. studied the effect of one session of relaxation techniques on S‐IgA among Japanese women medical co‐workers (Taniguchi et al., 2007). They found that only 10 min of relaxation training significantly increased S‐IgA levels after 10 min of relaxation training. Pereira et al. examined the effect of relaxation techniques on S‐IgA among undergraduate university students (Perera et al., 1998). They found that 30 min of watching relaxation video, shown on two separate days, significantly increased S‐IgA.
The increase in Leucocytes, Lymphocytes, Interleukin‐6, and Interleukin‐10 that occurred after relaxation techniques in this study came in accordance with the results of other studies. Infante et al. investigated the effect of relaxation techniques on immune biomarkers in transcendental meditation doctors (Infante et al., 2014). They found that regular relaxation techniques significantly increased lymphocytes, B‐lymphocytes, CD3+CD4−CD8+, and natural killer cells. Kang et al. examined the effect of 8 weeks of relaxation techniques (CBSM) on immune biomarkers (Kang et al., 2011). They found CBSM significantly increased lymphocyte proliferation, natural killer cell activity, Interleukin‐4, and Interleukin −10 responses with no significant effect on IFN‐g, Interleukin −2, and Interleukin −6. Jang et al. investigated the effects of relaxation techniques on cytokines levels in mind‐body trainers (Jang et al., 2017). They found that regular mind‐body training significantly increased Interleukin‐10 and Interleukin‐10 +IFN‐gamma levels and non‐significantly increased TNF‐alpha and Interleukin‐6. Mind‐body therapies are health and fitness interventions that are supposed to work on a physical and mental level such as controlled breathing and/or focussed meditation/attention interventions whereby participants must actively move their joints and muscles (Gendron et al., 2018). This came in accordance with the non‐significant increase in TNF‐alpha that occurred in this study. The increase in Interleukin‐6, contrary to other studies, might be attributed to the use of two types of relaxation techniques performed daily for 2 weeks.
The severity and progression of upper respiratory tract infection symptoms along with the quality of life measured by the WIS total score significantly decreased more in the intervention group than the control group. This difference occurred due to several mechanisms. Relaxation techniques have been reported to significantly normalize HPA, CRF, ACTH, and cortisol function. These results come in accordance with the results of several studies. Utrecht et al. examined the influence of relaxation techniques on endocrine, anxiety, and cardiovascular performance in pregnant women (Urech et al., 2010). They found that ACTH and cortisol levels significantly declined after just 1 session of relaxation techniques (guided imagery and PMR). Vandana et al. examined the influence of relaxation techniques on adrenaline and cortisol levels in healthy individuals (Vandana et al., 2011). They found that both cortisol and adrenaline levels significantly declined after just 1 session of PMR and this significant decline was sustained up to 8 months with continual performing of this exercise. Jones et al. studied the influence of relaxation techniques on serum cortisol in HIV‐ seropositive females (Jones et al., 2014). They found that 30 min of relaxation techniques (autogenic training, guided imagery, and PMR) performed for 10 weeks significantly declined serum cortisol. Ma et al. studied the influence of diaphragmatic breathing relaxation on serum cortisol levels in normal grownups (Ma et al., 2017). They found that 10 weeks of relaxation techniques significantly declined serum cortisol.
Previous studies found that both PMR and CBSM are effective in decreasing COVID‐19 associated stress, anxiety, and depression. Özlü et al. investigated the effect of PMR on anxiety and sleep quality in patients with COVID‐19 (Özlü et al., 2021). They found that PMR significantly decreased anxiety and enhanced sleep quality of patients with COVID‐19. Also, Xiao et al. investigated the effect of PMR on negative emotions and sleep quality in patients with COVID‐19 (Xiao et al., 2020). They found that PMR significantly decreased anxiety and depression and enhanced sleep quality in patients with COVID‐19 patients during quarantine. Li et al. investigate the effect of CBSM on anxiety, depression, and stress in patients with COVID‐19 (Li et al., 2020). They found that CBSM significantly decreased anxiety, depression, and stress in patients with COVID‐19. No participant reported harms during the study or during the follow up period.
There were several limitations in this study. First, the inability to delineate whether CBSM or PMRs is responsible for the effects because this study is preliminary, and it intended to maximally improve immune function and quality of life throughout the quarantine. Second, the investigators were unable to supervise the participants directly through the study due to the high risk of viral infection that can occur with any direct contact with the participants. Third, relaxation protocol was performed for 2 weeks since this is the mentioned quarantine time by the WHO for any patient with COVID‐19; thus, this study was designed to study the short‐term effect of the integrated protocol on patients with COVID‐19. Fourth, the study did not include severe COVID‐19 cases because patients with severe COVID‐19 usually go to hospitals for oxygen therapy and close monitoring. Fifth, this study did not examine the variability between men and women to relaxation exercise because there are no significant gender differences present among patients with COVID‐19 (Griffith, 2020). Future research should investigate the long‐term effect of relaxation protocol on COVID‐19 associated disorders and death rates.
5. CONCLUSION
This study concluded that performing an integrated intervention combining CBSM and PMRs for 2 weeks significantly increases immune biomarkers mainly Leucocytes, Lymphocytes, Interleukin‐10, and Interleukin‐6 along with S‐IgA. Also, this intervention significantly decreases disease severity and associated stress, anxiety, and depression; and enhances the quality of life in patients with COVID‐19.
CONFLICT OF INTEREST
No conflict of interest was declared for this study.
Supporting information
Supplementary Material S1
Supplementary Material S2
ACKNOWLEDGEMENT
No funding was declared for this study.
Alawna, M. , & Mohamed, A. A. (2022). An integrated intervention combining cognitive‐behavioural stress management and progressive muscle relaxation improves immune biomarkers and reduces COVID‐19 severity and progression in patients with COVID‐19: A randomized control trial. Stress and Health, 1–11. 10.1002/smi.3151
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, (Ayman Mohamed). The data are not publicly available due to containing information that could compromise the privacy of research participants. The data could be obtained by emailing the corresponding author on dr_ayman_pt@hotmail.com. The data will be available after 6 months of the publication till 3 years.
REFERENCES
- Affronti, M. , Mansueto, P. , Soresi, M. , Abbene, A. M. , Affronti, A. , Valenti, M. , Giannitrapani, L. , & Montalto, G. (2010). Low‐grade fever: How to distinguish organic from non‐organic forms. International Journal of Clinical Practice, 64(3), 316–321. 10.1111/j.1742-1241.2009.02256.x [DOI] [PubMed] [Google Scholar]
- Alawna, M. , & Mohamed, A. A. (2020). Effects of increasing aerobic capacity on improving psychological problems seen in patients with COVID‐19: A review. Diabetes & Metabolic Syndrome: Clinical Research Reviews, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni, M. H. , Baggett, L. , Ironson, G. , LaPerriere, A. , August, S. , Klimas, N. , Schneiderman, N. , & Fletcher, M. A. (1991). Cognitive‐behavioral stress management intervention buffers distress responses and immunologic changes following notification of HIV‐1 seropositivity. Journal of Consulting and Clinical Psychology, 59(6), 906–915. 10.1037//0022-006x.59.6.906 [DOI] [PubMed] [Google Scholar]
- Barrett, A. W. , & Barrington, L. W. (2005). Bias in newspaper photograph selection. Political Research Quarterly, 58(4), 609–618. 10.1016/j.jclinepi.2004.11.019 [DOI] [Google Scholar]
- Barrett, B. , Hayney, M. S. , Muller, D. , Rakel, D. , Brown, R. , Zgierska, A. E. , Barlow, S. , Hayer, S. , Barnet, J. H. , Torres, E. R. , & Coe, C. L. (2018). Meditation or exercise for preventing acute respiratory infection (MEPARI‐2): A randomized controlled trial. PLoS One, 13(6), e0197778. 10.1371/journal.pone.0197778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham‐Rundles, C. (2009). Lung disease, antibodies and other unresolved issues in immune globulin therapy for antibody deficiency. Clinical and Experimental Immunology, 157(SUPPL. 1), 12–16. 10.1111/j.1365-2249.2009.03952.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, D. C. , Matos, V. A. F. , dos Santos, V. O. A. , Medeiros, I. F. , Marinho, C. S. R. , Nascimento, P. R. P. , Dorneles, G. P. , Peres, A. , Müller, C. H. , Krause, M. , Costa, E. C. , & Fayh, A. P. T. (2018). Effects of high‐intensity interval and moderate‐intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Frontiers in Physiology, 9(MAY), 1–9. 10.3389/fphys.2018.00567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A.‐G. , & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Gendron, L. M. C. , Nyberg, A. , Saey, D. , Maltais, F. , & Lacasse, Y. (2018). Active mind‐body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews, 2018(10), CD012290. 10.1002/14651858.CD012290.PUB2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty, A. C. , & Kaufer, D. (2015). Effects of glucocorticoids in the immune system. Advances in Experimental Medicine & Biology, 872, 217–233. 10.1007/978-1-4939-2895-8 [DOI] [PubMed] [Google Scholar]
- Goppelt‐Struebe, M. , Wolter, D. , & Resch, K. (1989). Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo‐oxygenase/PGE isomerase. British Journal of Pharmacology, 98(4), 1287–1295. 10.1111/j.1476-5381.1989.tb12676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya, A. E. , Baker, S. C. , Baric, R. S. , de Groot, R. J. , Drosten, C. , Gulyaeva, A. A. , Haagmans, B. L. , Lauber, C. , Leontovich, A. M. , Neuman, B. W. , Penzar, D. , Perlman, S. , Poon, L. L. M. , Samborskiy, D. V. , Sidorov, I. A. , Sola, I. , & Ziebuhr, J. (2020). The species severe acute respiratory syndrome‐related coronavirus: Classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5(4), 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. L. , Green, R. G. , & Santoro, W. (1988). Daily relaxation modifies serum and salivary immunoglobulins and psychophysiologic symptom severity. Biofeedback and Self‐Regulation, 13(3), 187–199. 10.1007/BF00999169 [DOI] [PubMed] [Google Scholar]
- Griffith, D. M. , Sharma, G. , Holliday, C. S. , Enyia, O. K. , Valliere, M. , Semlow, A. R. , Stewart, E. C. , & Blumenthal, R. S. (2020). Men and COVID‐19: A biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Preventing Chronic Disease, 17, E63. 10.5888/PCD17.200247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström, L. , Vorechovsky, I. , & Webster, D. (2000). Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clinical and Experimental Immunology, 120(2), 225–231. 10.1046/J.1365-2249.2000.01131.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, M. T. , Schott, H. C. , Bayly, W. M. , & Leroux, A. J. (1996). Exercise and immunity: A review with emphasis on the horse. Journal of Veterinary Internal Medicine, 10(10), 280–289. 10.1111/j.1939-1676.1996.tb02063.x [DOI] [PubMed] [Google Scholar]
- Ikemata, S. , & Momose, Y. (2017). Effects of a progressive muscle relaxation intervention on dementia symptoms, activities of daily living, and immune function in group home residents with dementia in Japan. Japan Journal of Nursing Science: JJNS, 14(2), 135–145. 10.1111/JJNS.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante, J. , Peran, F. , Rayo, J. , Serrano, J. , Garcia, L. , Roldan, A. , Dominguez, M. , & Duran, C. (2014). Levels of immune cells in transcendental meditation practitioners. International Journal of Yoga, 7(2), 147–151. 10.4103/0973-6131.133899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J. H. , Park, H. Y. , Lee, U. S. , Lee, K. J. , & Kang, D. H. (2017). Effects of mind‐body training on cytokines and their interactions with catecholamines. Psychiatry Investigation, 14(4), 483–490. 10.4306/pi.2017.14.4.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. , Owens, M. , Kumar, M. , Cook, R. , & Weiss, S. M. (2014). The effect of relaxation interventions on cortisol levels in HIV‐ sero‐positive women. Journal of the International Association of Physicians in AIDS Care, 13(4), 318–323. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam, P. , & Bhalerao, S. (2010). Sample size calculation. International Journal of Ayurveda Research, 1(1), 55–57. 10.4103/0974-7788.59946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, D.‐H. , Mcardle, T. , Park, N.‐J. , Weaver, M. T. , Smith, B. , & Carpenter, J. (2011). Dose effects of relaxation practice on immune responses in women newly diagnosed with breast cancer. An Exploratory Study, 38(3), 240. [DOI] [PubMed] [Google Scholar]
- Li, J. , Li, X. , Jiang, J. , Xu, X. , Wu, J. , Xu, Y. , Lin, X. , Hall, J. , Xu, H. , Xu, J. , & Xu, X. (2020). The effect of cognitive behavioral therapy on depression, anxiety, and stress in patients with COVID‐19: A randomized controlled trial. Frontiers in Psychiatry, 11(October), 1–12. 10.3389/fpsyt.2020.580827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira, F. S. , dos Santos, T. , Caldeira, R. S. , Inoue, D. S. , Panissa, V. L. G. , Cabral‐Santos, C. , Campos, E. Z. , Rodrigues, B. , & Monteiro, P. A. (2017). Short‐term high‐ and moderate‐intensity training modifies inflammatory and metabolic factors in response to acute exercise. Frontiers in Physiology, 8(OCT), 1–8. 10.3389/fphys.2017.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Yue, Z. Q. , Gong, Z. Q. , Zhang, H. , Duan, N. Y. , Shi, Y. T. , Wei, G. X. , & Li, Y. F. (2017). The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Frontiers in Psychology, 8(JUN), 1–12. 10.3389/fpsyg.2017.00874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor, B. A. , Antoni, M. H. , Boyers, A. , Alferi, S. M. , Blomberg, B. B. , & Carver, C. S. (2004). Cognitive‐behavioral stress management increases benefit finding and immune function among women with early‐stage breast cancer. Journal of Psychosomatic Research, 56(1), 1–8. 10.1016/S0022-3999(03)00036-9 [DOI] [PubMed] [Google Scholar]
- Mohamed, A. , & Alawna, M. (2021a). The effect of aerobic exercise on immune biomarkers and symptoms severity and progression in patients with COVID‐19: A randomized control trial. Journal of Bodywork and Movement Therapies, 28, 425–432. 10.1016/J.JBMT.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A. , & Alawna, M. (2021b). Enhancing oxygenation of patients with coronavirus disease 2019: Effects on immunity and other health‐related conditions. World Journal of Clinical Cases, 9(19), 4939–4958. 10.12998/WJCC.V9.I19.4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A. A. , & Alawna, M. (2020a). Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID‐19): A review. Diabetes & Metabolic Syndrome: Clinical Research Reviews, 14(4), 489–496. 10.1016/j.dsx.2020.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A. A. , Alawna, M. , Amro, M. , & Mohame, A. A. (2020). Aerobic exercises recommendations and specifications for patients with COVID‐19: A systematic review. Physical Therapy, 24(24), 13049–13055. [DOI] [PubMed] [Google Scholar]
- Mohamed, A. A. M. , & Alawna, M. (2020b). Important role of relaxation techniques on immune functions, glycemic control, and stress in diabetic patients with COVID‐19: A review. Current Diabetes Reviews. 10.2174/1573399816999201012200109 [DOI] [PubMed] [Google Scholar]
- Ozamiz‐Etxebarria, N. , Santamaría, M. D. , Munitis, A. E. , & Gorrotxategi, M. P. (2020). Reduction of COVID‐19 anxiety levels through relaxation techniques: A study carried out in Northern Spain on a sample of young university students. Frontiers in Psychology, 11. 10.3389/FPSYG.2020.02038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özlü, İ. , Öztürk, Z. , Özlü, Z. K. , Tekin, E. , & Gür, A. (2021). The effects of progressive muscle relaxation exercises on the anxiety and sleep quality of patients with COVID‐19: A randomized controlled study. Perspectives in Psychiatric Care, 57, 1791–1797. 10.1111/PPC.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlow, L. A. , & Jones, G. E. (2005). The impact of abbreviated progressive muscle relaxation on salivary cortisol and salivary Immunoglobulin A (sIgA). Applied Psychophysiology and Biofeedback, 30(4), 375–387. 10.1007/s10484-005-8423-2 [DOI] [PubMed] [Google Scholar]
- Perera, S. , Sabin, E. , Nelson, P. , & Lowe, D. (1998). Increases in salivary lysozyme and IgA concentrations and secretory rates independent of salivary flow rates following viewing of a humorous videotape. International Journal of Behavioral Medicine, 5(2), 118–128. 10.1207/s15327558ijbm0502_3 [DOI] [PubMed] [Google Scholar]
- Rajkumar, R. P. (2020). COVID‐19 and mental health: A review of the existing literature. Asian Journal of Psychiatry, 52, 102066. 10.1016/j.ajp.2020.102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon, J. , Gondan, M. , & Kieser, M. (2012). To test or not to test: Preliminary assessment of normality when comparing two independent samples. BMC Medical Research Methodology, 12, 81. 10.1186/1471-2288-12-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Tjärnlund, A. , Ivanji, J. , Singh, M. , García, I. , Williams, A. , Marsh, P. D. , Troye‐Blomberg, M. , & Fernández, C. (2005). Role of IgA in the defense against respiratory infections: IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine, 23(20), 2565–2572. 10.1016/j.vaccine.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Salari, N. , Hosseinian‐Far, A. , Jalali, R. , Vaisi‐Raygani, A. , Rasoulpoor, S. , Mohammadi, M. , Rasoulpoor, S. , & Khaledi‐Paveh, B. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: A systematic review and meta‐analysis. Globalization and Health, 16(1), 1–11. 10.1186/S12992-020-00589-W/TABLES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakel, L. , Veldhuijzen, D. S. , Crompvoets, P. I. , Bosch, J. A. , Cohen, S. , Van Middendorp, H. , Joosten, S. A. , Ottenhoff, T. H. M. , Visser, L. G. , & Evers, A. W. M. (2019). Effectiveness of stress‐reducing interventions on the response to challenges to the immune system: A meta‐analytic review. Psychotherapy and Psychosomatics, 88, 274–286. 10.1159/000501645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, K. , Suzuki, N. , Imai, T. , Aizawa, K. A. , Nanba, H. , Hanaoka, Y. , Kuno, S. , Mesaki, N. , Kono, I. , & Akamal, T. (2011). Monocyte and T‐CELL responses to exercise training in elderly subjects. The Journal of Strength & Conditioning Research, 25(9), 2565–2572. 10.1519/JSC.0b013e3181fc5e67 [DOI] [PubMed] [Google Scholar]
- Taniguchi, T. , Hirokawa, K. , Tsuchiya, M. , & Kawakami, N. (2007). The immediate effects of 10‐minute relaxation training on salivary immunoglobulin A (s‐IgA) and mood state for Japanese female medical co‐workers. Acta Medica Okayama, 61(3), 139–145. 10.18926/AMO/32902 [DOI] [PubMed] [Google Scholar]
- Taylor, H. H. , & Murphy, B. (2010). Altered central integration of dual somatosensory input after cervical spine manipulation. Journal of Manipulative and Physiological Therapeutics, 33(3), 178–188. 10.1016/j.jmpt.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Tsigos, C. , Kyrou, I. , Kassi, E. , & Chrousos, G. P. (2000). Stress, endocrine physiology and pathophysiology. In Endotext. [Google Scholar]
- Urech, C. , Fink, N. S. , Hoesli, I. , Wilhelm, F. H. , Bitzer, J. , & Alder, J. (2010). Effects of relaxation on psychobiological wellbeing during pregnancy: A randomized controlled trial. Psychoneuroendocrinology, 35(9), 1348–1355. 10.1016/j.psyneuen.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Vandana, B. , Vaidyanathan, K. , Saraswathy, L. A. , Sundaram, K. R. , & Kumar, H. (2011). Impact of integrated amrita meditation technique on adrenaline and cortisol levels in healthy volunteers. Evidence‐based Complementary and Alternative Medicine, 2011, 1–6. 10.1155/2011/379645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2020a). Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID‐19). WHO. [Google Scholar]
- WHO . (2020b). Rational use of personal protective equipment for coronavirus disease 2019 (COVID‐19) and considerations during severe shortages. WHO. [Google Scholar]
- Williams, W. V. (2017). Hormonal contraception and the development of autoimmunity: A review of the literature. The Linacre Quarterly, 84(3), 275–295. 10.1080/00243639.2017.1360065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2020a). Corona‐ virus disease (COVID‐19) outbreak. [Google Scholar]
- World Health Organization (WHO) . (2020b). Rational use of personal protective equipment for coronavirus disease 2019 (COVID‐19). WHO. [Google Scholar]
- Xiao, C. X. , Lin, Y. J. , Lin, R. Q. , Liu, A. N. , Zhong, G. Q. , & Lan, C. F. (2020). Effects of progressive muscle relaxation training on negative emotions and sleep quality in COVID‐19 patients: A clinical observational study. Medicine, 99(47), e23185. 10.1097/MD.0000000000023185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki, K. , Fujiogi, M. , & Koutsogiannaki, S. (2020). COVID‐19 pathophysiology: A review. Clinical Immunology, 215, 108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani‐Alavijeh, F. , Araban, M. , Koohestani, H. R. , & Karimy, M. (2018). The effectiveness of stress management training on blood glucose control in patients with type 2 diabetes. Diabetology & Metabolic Syndrome, 10(1), 1–9. 10.1186/s13098-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, S.‐F. , Hu, J. , Qiao, N. , Lan, Z.‐H. , Lai, J.‐Y. , Wu, J.‐G. , & Wu, X.‐Y. (2020). Low‐grade fever during COVID‐19 convalescence: A report of 3 cases. World Journal of Clinical Cases, 8(12), 2655–2661. 10.12998/wjcc.v8.i12.2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, (Ayman Mohamed). The data are not publicly available due to containing information that could compromise the privacy of research participants. The data could be obtained by emailing the corresponding author on dr_ayman_pt@hotmail.com. The data will be available after 6 months of the publication till 3 years.


