Abstract
In dengue‐endemic regions, the co‐infection with SARS‐CoV‐2 and dengue is a significant health concern. Therefore, we performed a literature search for relevant papers in seven databases on 26 Spetember 2021. Out of 24 articles, the mortality rate and intensive care unit (ICU) admission were 19.1% and 7.8%, respectively. The mean hospital stay was 11.4 days. In addition, we identified two pregnancies with dengue and COVID‐19 co‐infection; one ended with premature rupture of membrane and intrauterine growth restriction fetus, while the other one ended with maternal mortality and intrauterine fetal death. COVID‐19 and dengue co‐infection had worse outcomes regarding mortality rates, ICU admission, and prolonged hospital stay. Thus, wise‐decision management approaches should be adequately offered to these patients to enhance their outcomes. Establishing an early diagnosis might be the answer to reducing the estimated significant burden of these conditions.
Keywords: co‐infection, COVID‐19, dengue, SARS‐CoV‐2, systematic review
Abbreviations
- COVID‐19
coronavirus disease
- ICU
intensive care unit
- ISI
Web of Science
- IUFD
intrauterine foetal death
- IUGR
intrauterine growth restriction
- NYAM
The New York Academy of Medicine
- PROM
premature rupture of membrane
- SIGLE
System for information on Grey Literature in Europe
- VHL
Virtual Health Library
1. INTRODUCTION
In late 2019, the world faced one of the most dangerous epidemics since the outbreak of the Spanish flu. 1 This epidemic was caused by coronavirus disease (COVID‐19) that infected more than 300 million individuals, and more than 5 million patients died due to the infection itself or complications. 2 In addition, approximately more than 400 million cases are infected with dengue fever each year, transmitted by the Aedes aegypti mosquito. 3 The high burden of dengue and COVID‐19 infections increases the possibility of co‐infection, which is a significant health concern due to the overlapped symptomatology and similar laboratory findings of the two conditions in dengue‐endemic regions. 4 , 5 This overlap would make reaching the correct diagnosis and, subsequently, the proper management challenging for both diseases. 5 Furthermore, there are previous studies about the co‐infection of dengue and COVID‐19 during the current pandemic, with reported worse clinical manifestations and higher complications. 6 , 7 Nevertheless, there is scarce evidence on the outcomes and associated prognosis, which is essential to implement convenient public health policies. Therefore, we conducted this systematic review to give a summary of the available literature regarding the outcomes of dengue and COVID‐19 co‐infection.
2. METHOD
2.1. Search strategy and study selection
We aimed to identify relevant original papers reporting dengue patients suffering Covid‐19 infection. On 26 September 2021, we conducted a systematic search following the recommendations of PRISMA's checklist to detect our included studies. We used the search term ‘(Dengue Fever OR Dengue) AND (COVID‐19 OR COVID 19 OR novel coronavirus OR SARS CoV 2)’ through seven databases including PubMed, Google Scholar, Scopus, Web of Science (ISI), Virtual Health Library, The New York Academy of Medicine, and System for information on Grey Literature in Europe. Our search results were collected using EndNote software, where duplications were removed. Subsequently, we moved the results to Microsoft Excel sheets, and two phases of title and abstract screening followed by full‐text screening were done. Two authors screened the results independently, and we included the study upon their agreement. A senior reviewer was added to solve any discrepancy in case of disagreement. We included all articles that reported dengue and COVID‐19 co‐infection and excluded reviews, conference papers, and no available full texts. In addition, a manual search was performed through references of included articles and relevant articles in Google Scholar and PubMed (Figure 1).
FIGURE 1.
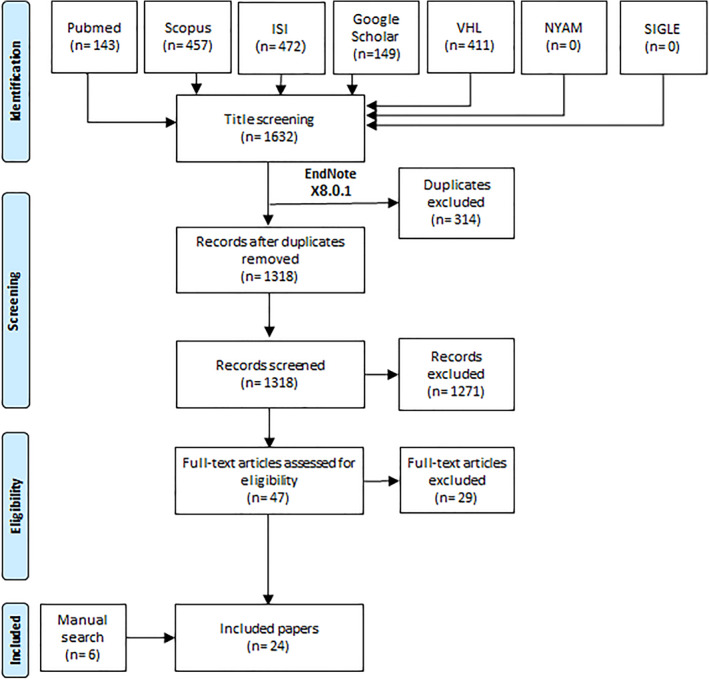
Flow diagram or the study process
2.2. Data extraction and quality assessment
We made an excel sheet containing our outcomes of interest. The characteristics of each study included (study design, sample size, country, and sex) while the patients' outcomes were (mortality, intensive care unit (ICU) admission, hospital stay length, obstetric and foetal outcomes). We calculated the prevalence of mortality and ICU admission by dividing the number of deaths and ICU admissions, respectively, by the total number of cases. The extraction was done by three reviewers separately, and the final results were considered upon consensus. A senior author was added for the discussion if needed. Moreover, we did a quality assessment for our included papers using the Joanna Briggs Institute Critical Appraisal tool for case report papers and The National Institute of Health tool for cohort studies (Table S1 and S2).
3. RESULTS
After screening 1318 papers, 47 papers were eligible for another phase of full text screening. Out of these, we included 18 papers together with additional 6 papers from manual search trials. Finally, we had 24 articles with a sample size of 89 patients with dengue and COVID‐19 co‐infection with a male prevalence of 63% (Table 1, Figure 1). Regarding study design, there were 22 case reports and two retrospective cohort studies. In terms of countries of patients, Peru, followed by Argentina, Saudi Arabia, and India, had the highest numbers of dengue‐COVID‐19 co‐infected patients (Figure 2).
TABLE 1.
Characteristics of the included studies
| Study ID | Country | Sample size | Age | Gender | Diagnosis of dengue | Diagnosis of COVID‐19 | Outcome | ICU admission | Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|
| Irwinda‐2021 | Indonesia | 1 | 23 | F | NS1 antigen or IgM DENV | PCR | Died | Yes | 5 |
| Alam‐2021 | Indonesia | 1 | 10 months | F | NS1 antigen | PCR | Survived | No | 16 |
| Hariadi‐2021 | Indonesia | 1 | 68 | F | DENV IgM and IgG | PCR | Survived | No | 11 |
| Verduyn‐2020 | France | 1 | 18 | M | NS1 antigen | PCR | Survived | No | 7 |
| Khalil‐2020 | Saudi Arabia | 1 | 63 | M | NS1 antigen and IgG DENV | PCR | Survived | No | 6 |
| 1 | 53 | F | DENV IgM, IgG and PRC | PCR | Survived | No | 5 | ||
| 1 | 48 | F | NS1 antigen, IgM and IgG DENV | PCR | Survived | No | 4 | ||
| 1 | 46 | M | NS1 antigen and PCR | PCR | Survived | No | 0 | ||
| Malibari‐2020 | Saudi Arabia | 1 | 58 | M | NS1 antigen, IgM and IgG DENV | PCR | Survived | No | 7 |
| Reyes‐Ruiz‐2021 | Mexico | 1 | 42 | F | PCR | PCR | Survived | No | 18 |
| Wahiduzzaman‐2021 | Bangladesh | 1 | 34 | M | NS1 antigen | PCR | Survived | No | ‐ |
| Gupta‐2021 | India | 1 | 65 | M | DENV IgM | PCR | Survived | No | 17 |
| Kasi‐2020 | India | 1 | 9 months | F | NS1 antigen, IgM DENV | PCR | Survived | No | 14 |
| Kariyappa‐2021 | India | 1 | 5 months | F | DENV IgM and ELISA | PCR | Survived | No | 21 |
| Mahajan‐2020 | India | 1 | 22 | F | NS1 antigen | PCR | Survived | No | 9 |
| Krishna‐2021 | India | 1 | 28 | F | NS1 antigen, IgM DENV | PCR | Survived | No | 7 |
| Estofolete‐2020 | Brazil | 1 | 60 | F | NS1 antigen, IgM and IgG DENV | PCR | Died | Yes | 5 |
| Bicudo‐2020 | Brazil | 1 | 56 | F | NS1 antigen, IgM and IgG DENV | PCR | Survived | No | 6 |
| Pontes‐2020 | Brazil | 1 | 39 | M | PCR | PCR | Survived | No | ‐ |
| Rojas‐2021 | Colombia | 1 | 24 | F | NS1 antigen and PCR | PCR | Survived | Yes | 6 |
| 1 | 59 | M | DENV IgM and IgG | IgG SARS‐CoV‐2 | Died | Yes | 63 | ||
| Villamil‐Gómez‐2021 | Colombia | 1 | 52 | M | DENV IgM and IgG | PCR | Survived | No | 7 |
| Nasomsong‐2021 | Thailand | 1 | 50 | F | PCR | PCR | Survived | No | 11 |
| Roso‐2021 | Argentina | 1 | 57 | F | PCR | PCR | Survived | No | 5 |
| Carosella‐2021 | Argentina | 13 | 37 (29–50) a | 6 F, 7 M | NS1 antigen, PCR or serologic conversion | PCR | All survived | 0 patients | 12 (10–14) a |
| Radisic‐2020 | Argentina | 1 | 25 | M | NS1 antigen, IgM DENV | PCR | Survived | No | 8 |
| Saipen‐2021 | Philippines | 1 | 62 | F | NS1 antigen, IgG DENV | PCR | Survived | No | 9 |
| Mejía‐Parra‐2021 | Peru | 50 | 55.5 (40.5–65) a | 11 F, 39 M | PCR, NS1 antigen, IgM and IgG DENV | PCR, IgM and IgG SARS‐CoV‐2 | 14 died | 3 patients | ‐ |
Abbreviations: M, male; F, female.
Median (IQR).
FIGURE 2.
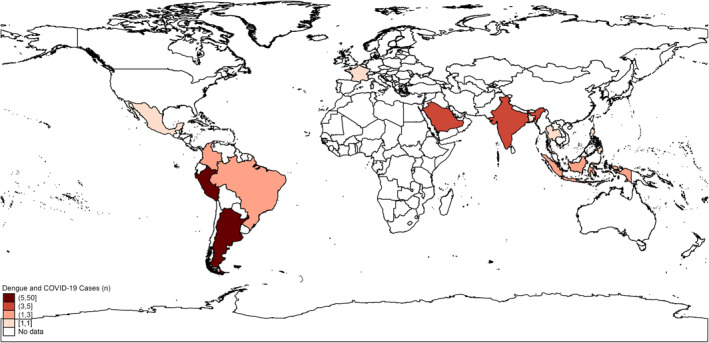
The worldwide distribution of the reported cases of dengue and COVID‐19 co‐infection
3.1. Mortality
Out of 89 cases identified with dengue and COVID‐19 co‐infection, 17 patients passed away, with a prevalence of 19.1%. In addition, the mortality rates were higher among males than females, with a prevalence of 10.1% and 9%, respectively.
3.2. Intensive care unit admission
Seven cases needed ICU admission, assuming a prevalence of 7.8%.
3.3. Hospital stay
Twenty‐one papers reported the hospital stay duration. The mean hospital stay for the included 37 patients was 11.4 days.
3.4. Obstetric and foetal outcomes
We identified two pregnancies with dengue and COVID‐19 co‐infection. One ended with premature rupture of membrane (PROM) and intrauterine growth restriction (IUGR) foetus, while the other one ended with maternal mortality and intrauterine foetal death (IUFD).
4. DISCUSSION
In the present systematic review, we mainly aimed to determine the clinical outcomes of patients suffering from dengue and COVID‐19 co‐infection. Our results indicated the high mortality rate among these patients (19.1%), based on cumulative evidence from relevant studies. It should be noted that this rate is remarkably higher than the estimated global rates for dengue and COVID‐19 patients (1.3% and 2.04%, respectively). 8 , 9 Moreover, we noted that the prevalence of co‐infection and mortality was higher among males. This is consistent with previous dengue investigations, which indicated that the prevalence of dengue infection is higher among males. 10 , 11 , 12 , 13 , 14 Many reasons have been proposed for these differences, including using fully covered dresses by females, potential differences in healthcare services, and prioritising provisions of male individuals in societies where dengue is endemic. 15 , 16
To the best of our knowledge, advanced age is considered one of the major risk factors in patients with dengue fever or COVID‐19 infections. 17 , 18 In our study, two patients from two case reports who experienced death event had an age of 59 and 60 years. 19 , 20 Moreover, in the case series of Parra and colleagues, mortality rate was 28% in patients with dengue and COVID‐19 co‐infection whose median age was 55.5 years. 21 Due to the limited sample size in our study, we can not confirm the role of age in predicting mortality from dengue and COVID‐19 co‐infection. Therefore, more studies with bigger sample size and controlling of other confouders are needed for studying this association.
Our results also show a high rate of ICU admissions secondary to COVID‐19 and dengue infection, being 7.8%. However, it should be noted that this rate is lower than the estimated one for COVID‐19 patients, being 9.8%. 22 This might be attributed to the different population characteristics and quality of care offered to both populations. The rate of patients requiring ICU admission secondary to severe dengue is also high. Previous dengue‐related studies indicated that the mortality rate among patients admitted to the ICU secondary to severe dengue manifestations might be up to 23.1%. 23 , 24 , 25 , 26 Such differences are usually attributed to the method of defining severe disease in these patients and the degree of severity of included participants, as reported among these studies. We furtherly found that our population had a mean hospital stay of 11.4 days. In the literature, the estimated median hospital stay for individual COVID‐19 and dengue populations are 5 and 6 days, respectively. 27 , 28 Previous dengue studies reported that hospital stay was remarkably longer among infants than children and adults. This has been attributed to the frequency of complications, which might affect this age group at a potentially higher rate secondary to the infection. 15 , 29 , 30
Many factors can contribute to severe COVID‐19, including co‐infection. 31 , 32 , 33 , 34 , 35 , 36 The current literature reveals a solid association between COVID‐19 and co‐infection with different viruses and bacteria. As a result, these patients usually have severe outcomes due to remarkable deterioration in their health status. 31 A previous meta‐analysis reported that the prevalence of bacterial co‐infection with COVID‐19 was 5.1%, while secondary infection accounted for 13.1%. Moreover, it has been shown that the rate of ICU admissions was significantly associated with bacterial infections among COVID‐19 patients. 37 In addition, it is widely known that COVID‐19 is associated with a high rate of complications that are usually life‐threatening. 38 , 39 , 40 Many previous studies also showed that among patients with severe dengue infection, up to 55.9% suffered from a concurrent bacterial infection. 23 , 26 Dengue infection might also lead to serious morbidities such as dengue shock syndrome and dengue haemorrhagic fever, 41 , 42 , 43 , 44 , 45 , 46 contributing to the estimated high rates of mortality and ICU admissions in the current study.
Regarding obstetric and foetal outcomes, we only found two pregnancies with COVID‐19 and dengue co‐infection occurred, both of which were complicated; one with PROM and IUGR and the other with maternal mortality and IUFD. Evidence from various studies in the literature indicates that neonatal dengue infection is attributed to vertical transmission of the virus from an affected mother. Based on these investigations, the diagnosis of dengue in these neonates was related to maternal dengue infection (occurring amid delivery by 10 days). 47 , 48 , 49 Many relevant studies also demonstrated that dengue infection in this population is usually associated with premature birth, stillbirth, or low birth weight. 48 , 50 , 51 , 52 , 53 On the other hand, some studies suggested that vertical transmission of protective antibodies from infected mothers to their infants during placental transfer might reduce the severity of the disease and enhance the outcomes. 28 However, it has been demonstrated that these antibodies are age‐dependent and usually decrease with advancing age. 54 Accordingly, the current evidence needs further elaboration by more relevant studies. Moreover, only minimal cases with maternal co‐infection are reported. Therefore, further studies with larger sample sizes are needed to provide more solid evidence.
Establishing an early diagnosis of dengue infection in these patients is critical in the management process. In this context, some studies indicate that conducting the NS1 rapid test might provide an early diagnosis of dengue infection in the newborns of mothers that acquired the infection within the perinatal period. Besides, detecting IgM Dengue Antibody (MAC‐ELISA) is very useful in these cases and might be a good alternative to the NS1 rapid test. However, it should be noted that the sensitivity of these tests is not consistent among these studies and depends on the course and duration of illness. 28 , 55 It should also be noted that once the diagnosis of dengue has been established, closely monitoring (for severe haemorrhagic events and warning signs) of patients is favourable to enhance the prognosis. However, such an approach is difficult to conduct because of COVID‐19‐related strict isolation measures, as these patients usually have limited visits to reduce the rates of transmitting the rapidly spreading SARS‐CoV‐2 viral infection. 56 , 57 Furthermore, it has been previously demonstrated that the rate of misdiagnosis of neonatal dengue is considerable. 58 , 59 Therefore, some authors suggested that a differential diagnosis in critical settings should be considered with sepsis because of the similar clinical manifestations.
Although the current systematic review provides cumulative evidence regarding COVID‐19 and dengue co‐infection outcomes, the reported results might have limitations. The main limitation is the limited studies in this context and the minimal sample size in the included studies. This has been a limitation to conducting a proper analysis to identify the potential predictors of this co‐infection and potentially enhance the interventional and management approaches.
5. CONCLUSION
COVID‐19 and dengue co‐infection had worse outcomes regarding mortality rates, ICU admission, and prolonged hospital stay. Thus, wise‐decision management approaches should be adequately offered to these patients to enhance their outcomes. Establishing an early diagnosis might be the answer to reducing the estimated significant burden of these conditions. Shedding more light on the management and prevention of COVID‐19 in areas where other diseases are endemic is also encouraged, owing to the remarkable burden over these populations. Finally, further studies are needed due to the limitations of the currently available data.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Amr Ehab El‐Qushayri was responsible for the idea and the study design. All authors shared in screening, extraction, and writing of the full text. Sherief Ghozy supervised all steps, and all authors approved the final version before submission.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
We would like to thank Abdelaziz Abdelaal (Faculty of Medicine, Tanta University, Tanta, Egypt) for his efforts and help in the current work. The authors received no funding for this study.
El‐Qushayri AE, Kamel AMA, Reda A, Ghozy S. Does dengue and COVID‐19 co‐infection have worse outcomes? A systematic review of current evidence. Rev Med Virol. 2022;32(5):e2339. 10.1002/rmv.2339
Contributor Information
Amr Ehab El‐Qushayri, Email: amrehab11111@gmail.com.
Sherief Ghozy, Email: Ghozy.Sherief@mayo.edu.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Franchini AF, Auxilia F, Galimberti PM, Piga MA, Castaldi S, Porro A. COVID 19 and Spanish flu pandemics: all it changes, nothing changes. Acta Bio Medica Atenei Parm. 2020;91(2):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.worldometers.info/coronavirus/
- 3. Tian Y‐S, Zhou Y, Takagi T, Kameoka M, Kawashita N. Dengue virus and its inhibitors: a brief review. Chem Pharm Bull. 2018;66(3):191‐206. [DOI] [PubMed] [Google Scholar]
- 4. Plasencia‐Dueñas R, Failoc‐Rojas VE, Rodriguez‐Morales AJ. Impact of the COVID‐19 pandemic on the incidence of dengue fever in Peru. J Med Virology. 2021;94(1):393‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Wangdi K. Clinical features and outcomes of COVID‐19 and dengue co‐infection: a systematic review. BMC Infect Dis. 2021;21(1):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kembuan GJ. Dengue serology in Indonesian COVID‐19 patients: coinfection or serological overlap? IDCases. 2020;22:e00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo C, Zhou Z, Wen Z, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta‐analysis. Frontiers in cellular and infection microbiology. 2017;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Global Worldometer . Prevalence of Coronavirus Cases. 2021. https://www.worldometers.info/coronavirus/. Last updated on October 9, 2021. [Google Scholar]
- 10. Afroze S, Shakur S, Wahab A, Shakur S. Clinical profile of dengue and predictors of its severity among children. Am J Pediatr. 2019;5:219‐223. [Google Scholar]
- 11. Shultana K, Rahman A, Al Baki A, et al. Dengue infection in children: clinical profile and outcome in Dhaka City. Am J Pediatr. 2019;5(3):111. [Google Scholar]
- 12. Ramabhatta S, Palaniappan S, Hanumantharayappa N, Begum SV. The clinical and serological profile of pediatric dengue. Indian J Pediatr. 2017;84(12):897‐901. [DOI] [PubMed] [Google Scholar]
- 13. Gulati S, Maheshwari A. Atypical manifestations of dengue. Trop Med Int health TM IH. 2007;12(9):1087‐1095. [DOI] [PubMed] [Google Scholar]
- 14. Anker M, Arima Y. Male‐female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pacific surveillance and response. J WPSAR. 2011;2(2):17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mishra S, Ramanathan R, Agarwalla SK. Clinical profile of dengue fever in children: a study from Southern Odisha, India. Scientifica. 2016;2016:6391594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed SM, Adams AM, Chowdhury M, Bhuiya A. Gender, socioeconomic development and health‐seeking behaviour in Bangladesh. Soc Sci Med. 2000;51(3):361‐371. (1982). [DOI] [PubMed] [Google Scholar]
- 17. Karunakaran A, Ilyas WM, Sheen SF, Jose NK, Nujum ZT. Risk factors of mortality among dengue patients admitted to a tertiary care setting in Kerala, India. J Infect & Publ Health. 2014;7(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 18. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med. 2020;288(4):469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Estofolete CF, Machado LF, Zini N, et al. Presentation of fatal stroke due to SARS‐CoV‐2 and dengue virus coinfection. J Med Virology. 2021;93(3):1770‐1775. [DOI] [PubMed] [Google Scholar]
- 20. Agudelo Rojas OL, Tello‐Cajiao ME, Rosso F. Challenges of dengue and coronavirus disease 2019 coinfection: two case reports. J Med case Rep. 2021;15(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mejía‐Parra JL, Aguilar‐Martinez S, Fernández‐Mogollón JL, et al. Characteristics of patients coinfected with severe acute respiratory syndrome coronavirus 2 and dengue virus, lambayeque, Peru, May–August 2020. A Retrospective Analysis. Travel Medicine and Infectious Disease. 2021;43:102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID‐19: a systematic review and meta‐analysis. Epidemiol Infect. 2020:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen CM, Chan KS, Yu WL, et al. The outcomes of patients with severe dengue admitted to intensive care units. Med Baltim. 2016;95(31):e4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juneja D, Nasa P, Singh O, Javeri Y, Uniyal B, Dang R. Clinical profile, intensive care unit course, and outcome of patients admitted in intensive care unit with dengue. J Crit care. 2011;26(5):449‐452. [DOI] [PubMed] [Google Scholar]
- 25. Chandralekha, Gupta P , Trikha A, Trikha A. The north Indian dengue outbreak 2006: a retrospective analysis of intensive care unit admissions in a tertiary care hospital. Trans R Soc Trop Med Hyg. 2008;102(2):143‐147. [DOI] [PubMed] [Google Scholar]
- 26. Amâncio FF, Heringer TP, de Oliveira Cda C, et al. Clinical profiles and factors associated with death in adults with dengue admitted to intensive care units, Minas Gerais, Brazil. PloS one. 2015;10(6):e0129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rees EM, Nightingale ES, Jafari Y, et al. COVID‐19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen TM, Huan VT, Reda A, et al. Clinical features and outcomes of neonatal dengue at the children's hospital 1, Ho Chi Minh, Vietnam. J Clin Virology: The Official Publication of the Pan American Society for Clinical Virology. 2021;138:104758. [DOI] [PubMed] [Google Scholar]
- 29. Hause AM, Perez‐Padilla J, Horiuchi K, et al. Epidemiology of dengue among children aged < 18 Months‐Puerto Rico, 1999‐2011. Am J Trop Med Hyg. 2016;94(2):404‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammond SN, Balmaseda A, Pérez L, et al. Differences in dengue severity in infants, children, and adults in a 3‐year hospital‐based study in Nicaragua. Am J Trop Med Hyg. 2005;73(6):1063‐1070. [PubMed] [Google Scholar]
- 31. Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID‐19. J Med virology. 2021;93(9):5310‐5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malekifar P, Pakzad R, Shahbahrami R, et al. Viral coinfection among COVID‐19 patient groups: an update systematic review and meta‐analysis. BioMed Res Int. 2021;2021:5313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh V, Upadhyay P, Reddy J, Granger J. SARS‐CoV‐2 respiratory co‐infections: incidence of viral and bacterial co‐pathogens. Int J Infect Dis: IJID: Official Publication of the International Society for Infectious Diseases. 2021;105:617‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lansbury L, Lim B, Baskaran V, Lim WS. Co‐infections in people with COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):266‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramadan HK, Mahmoud MA, Aburahma MZ, et al. Predictors of severity and Co‐infection resistance profile in COVID‐19 patients: first report from upper Egypt. Infect Drug Resist. 2020;13:3409‐3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Aidaoui K, Haoudar A, Khalis M, et al. Predictors of severity in covid‐19 patients in Casablanca, Morocco. Cureus. 2020;12(9):e10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langford BJ, So M, Leung V, et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID‐19: living rapid review update and meta‐regression. Clin Microbiol & Infect: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badraoui R, Alrashedi MM, El‐May MV, Bardakci F. Acute respiratory distress syndrome: a life threatening associated complication of SARS‐CoV‐2 infection inducing COVID‐19. J Biomol Struct Dynam. 2021;39(17):6842‐6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19 associated mucormycosis: a life‐threatening complication in patients admitted with severe to critical COVID‐19 from Pakistan. Clin Microbiol & Infect: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021;27(11):1704‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guven BB, Erturk T, Kompe Ö, Ersoy A. Serious complications in COVID‐19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous emphysema and haemothorax. Epidemiol Infect. 2021;149:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya‐Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta‐analysis. Infect Dis Poverty. 2021;10(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carod‐Artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Neurology. 2013;12(9):906‐919. [DOI] [PubMed] [Google Scholar]
- 43. Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mok Y, Quah J, Siau C. A rare but potentially lethal complication of dengue. Asian Pacific J Trop Med. 2013;6(6):500‐501. [DOI] [PubMed] [Google Scholar]
- 45. Laul A, Laul P, Merugumala V, Pathak R, Miglani U, Saxena P. Clinical profiles of dengue infection during an outbreak in Northern India. J Trop Med. 2016;2016:5917934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khan MAS, Al Mosabbir A, Raheem E, et al. Clinical spectrum and predictors of severity of dengue among children in 2019 outbreak: a multicenter hospital‐based study in Bangladesh. BMC Pediatr. 2021;21(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiwanitkit V. Unusual mode of transmission of dengue. J Infect Dev Countries. 2009;4(1):51‐54. [DOI] [PubMed] [Google Scholar]
- 48. Sirinavin S, Nuntnarumit P, Supapannachart S, Boonkasidecha S, Techasaensiri C, Yoksarn S. Vertical dengue infection: case reports and review. Pediatr Infect Dis J. 2004;23(11):1042‐1047. [DOI] [PubMed] [Google Scholar]
- 49. Basurko C, Matheus S, Hildéral H, et al. Estimating the risk of vertical transmission of dengue: a prospective study. Am J Trop Med Hyg. 2018;98(6):1826‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Friedman EE, Dallah F, Harville EW, et al. Symptomatic Dengue infection during pregnancy and infant outcomes: a retrospective cohort study. Plos Neglected Trop Dis. 2014;8(10):e3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paixão ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: the causes and threats of new and re‐emerging arboviral diseases. BMJ Glob health. 2018;3(Suppl 1):e000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ismail NA, Kampan N, Mahdy ZA, Jamil MA, Razi ZR. Dengue in pregnancy. Southeast Asian J Trop Med public health. 2006;37(4):681‐683. [PubMed] [Google Scholar]
- 53. Tan PC, Soe MZ, Si Lay K, Wang SM, Sekaran SD, Omar SZ. Dengue infection and miscarriage: a prospective case control study. PLoS neglected Trop Dis. 2012;6(5):e1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jain A, Chaturvedi UC. Dengue in infants: an overview. FEMS Immunol Med Microbiol. 2010;59(2):119‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hunsperger EA, Muñoz‐Jordán J, Beltran M, et al. Performance of dengue diagnostic tests in a single‐specimen diagnostic algorithm. J Infect Dis. 2016;214(6):836‐844. [DOI] [PubMed] [Google Scholar]
- 56. Organization WH, UNICEF . Handbook for Clinical Management of Dengue; 2012. [Google Scholar]
- 57. Organization WH, SPf Research , Diseases TiT , Diseases WHODoCoNT , Epidemic WHO , Alert P. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization; 2009. [PubMed] [Google Scholar]
- 58. Nigam A, Varun N, Saxena P. Dengue in pregnancy. Indian J Med Specialities. 2016;7(4):145‐148. [Google Scholar]
- 59. Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


