Abstract
Background
This is the study plan of the Karolinska NeuroCOVID study, a study of neurocognitive impairment after severe COVID‐19, relating post‐intensive care unit (ICU) cognitive and neurological deficits to biofluid markers and MRI. The COVID‐19 pandemic has posed enormous health challenges to individuals and health‐care systems worldwide. An emerging feature of severe COVID‐19 is that of temporary and extended neurocognitive impairment, exhibiting a myriad of symptoms and signs. The causes of this symptomatology have not yet been fully elucidated.
Methods
In this study, we aim to investigate patients treated for severe COVID‐19 in the ICU, as to describe and relate serum‐, plasma‐ and cerebrospinal fluid‐borne molecular and cellular biomarkers of immune activity, coagulopathy, cerebral damage, neuronal inflammation, and degeneration, to the temporal development of structural and functional changes within the brain as evident by serial MRI and extensive cognitive assessments at 3–12 months after ICU discharge.
Results
To date, we have performed 51 3‐month follow‐up MRIs in the ICU survivors. Of these, two patients (~4%) have had incidental findings on brain MRI findings requiring activation of the Incidental Findings Management Plan. Furthermore, the neuropsychological and neurological examinations have so far revealed varying and mixed patterns. Several patients expressed cognitive and/or mental concerns and fatigue, complaints closely related to brain fog.
Conclusion
The study goal is to gain a better understanding of the pathological mechanisms and neurological consequences of this new disease, with a special emphasis on neurodegenerative and neuroinflammatory processes, in order to identify targets of intervention and rehabilitation.
Keywords: biomarkers, brain injury, COVID‐19, critical care, magnetic resonance imaging, neurocognitive disorders, patient outcome assessment
1. INTRODUCTION AND AIMS
The COVID‐19 pandemic has posed enormous health challenges to individuals and health‐care systems worldwide. An emerging feature of severe COVID‐19 frequently being reported is that of temporary and extended neurological and cognitive impairment, exhibiting a myriad of symptoms and signs ranging from anosmia to severe encephalopathy and stroke. 1 The cause of this has not yet been fully elucidated, but several mechanisms have been proposed including hyperinflammation and immune responses as well as coagulopathy with endothelial damage leading to micro‐ and macrovascular embolization. 2 There is a growing awareness that cerebral inflammation and glial activation may be a consequence of systemic disease and even surgical trauma. 3 The degree to which COVID‐19 directly affects the brain and cerebral vasculature or triggers secondary effects through systemic inflammation is yet unknown, but the evidence is emerging of high incidences of COVID‐19‐related neurological complications. 4 , 5 Recently, in a case series of COVID‐19 patients with neurological symptoms, there was an unusual pattern of marked CNS inflammation seen in cerebrospinal fluid (CSF). 6 Moreover, MRI of patients surviving COVID‐19 displays a range of pathologies, however here also with possibly distinct pathophysiological profiles related to this new infection. 7 Critical illness neuropathy and myopathy also appear to be significant problems in moderate to severe COVID‐19. 8 There is an urgent need to better understand the mechanisms of injury and pathophysiological processes in COVID‐19 patients and their relations to brain imaging and long‐term cognitive impairment. Identifying blood‐borne biomarkers of neuronal injury, neurodegeneration, and inflammation is expected to be an important part of this process. 1 In this study, we aim to investigate patients treated for severe COVID‐19 in the intensive care unit (ICU) to describe and relate serum‐, plasma‐ and CSF‐borne molecular and cellular biomarkers of inflammation, immune activity and coagulopathy, cerebral damage, neuronal inflammation, and degeneration, to the temporal development of structural and functional changes within the brain as evident by serial MRI and extensive cognitive assessments at 3–12–24 months after ICU discharge. The goal is to gain a better understanding of the pathological mechanisms and neurological consequences of this new disease, with a special emphasis on neurodegenerative and neuroinflammatory processes, as to identify targets of intervention and rehabilitation.
2. MATERIALS AND METHODS
2.1. Study design
This is an observational study of patients treated for severe COVID‐19‐related symptoms to describe and relate serum, plasma‐ and CSF‐borne molecular and cellular biomarkers of inflammation and coagulopathy, cerebral damage, neuronal inflammation and degeneration to MRI findings, and cognition at 3–12 months after ICU discharge.
2.2. Inclusion criteria
All patients ≥18 years of age having required intensive care due to PCR‐positive COVID‐19‐related respiratory distress at the Karolinska University Hospital in Stockholm, Sweden, between March 2020 and June 2021, with biobanked plasma and serum samples during the ICU period and have been able to give study consent after intensive care, are eligible for inclusion.
2.3. Ethics and consent
Initial serum biobanking was performed on patients treated for COVID‐19 in the ICU as per local ethical approval (EPM 2020‐01302). Written informed consent is obtained for study inclusion post‐intensive care to analyze previously biobanked samples and data and perform prospective data collection, MRI, cognitive testing, and follow‐up biosamples at 3–12 months post‐discharge (EPM 2020‐02760, EPM 2020‐03802, EPM 2020‐04282).
2.4. Data collection
Data have been collected structurally during the COVID‐19 pandemic period in a local database at the Karolinska University Hospital from case notes and the local Patient Data Management Systems. This includes baseline characteristics, medication, physiological variables, laboratory samples, and treatments. Additional variables will be collected retrospectively from electronic health records.
2.5. Biosamples and biomarkers
Serial plasma and serum samples have been collected at eight predefined time points during the intensive care and stored locally in the biobank for later analysis. Patients who participate in the prospective follow‐up will provide additional samples at 3‐, 6‐ and 12‐month follow‐up. Whole‐blood, plasma, and serum samples will be serially collected and stored for later molecular, cellular, and genetic analysis. CSF aliquots will be biobanked whenever a lumbar puncture is clinically indicated. Molecular and cellular markers of neuronal injury, neurodegeneration, and neuroinflammation will be analyzed along with markers of systemic inflammation, immune activity, and coagulopathy by comprehensive technical platforms. Biomarkers that will be measured on all plasma samples include neurofilament light and total tau as neuronal injury markers, as well as glial fibrillary acidic protein as an astrocytic activation marker.
2.6. Participant characteristics questions
At the first visit, all participants are asked about the level of education, employment situation, general health, lifestyle, pre‐existing medical conditions, symptoms suggestive of COVID‐19 in the 7 days prior to the baseline visit, physical and cognitive function, and activities of daily living. To gain an understanding of participants' perception of cognitive status, the AD8 is used. 9 AD is a short, practical, and appropriate scale to distinguish between normal cognitive aging, mild cognitive impairment (MCI), or dementia for patients with memory complaints. 10
2.7. Neurological examination
All participants undergo a physical and neurological examination by a board‐certified neurologist with long experience of working with cognitive impairment and dementia disorders. Apart from a general neurological assessment of history and self‐reported symptoms, we have emulated two relevant, validated rating scales that reflect neurological disabilities in functional domains known to be affected in COVID‐19. The Expanded Disability Status Scale (EDSS) 11 is used in Multiple Sclerosis (MS) for grading deficits in vision, brain stem, sensory, motor and bowel/bladder functions, and coordination. We also use Part III, the Motor Examination, of the Unified Parkinson's Disease Rating Scale (UPDRS), which is an adapted version of the Unified Parkinson's Disease Rating Scale for extrapyramidal dysfunction (i.e., involuntary or uncontrollable movements, tremors, and muscle contractions). 12 The UPDRS has a wide utilization and is nearly comprehensive coverage of motor symptoms. 13
Since smell deficits are widely reported in COVID‐19, an in‐house developed smell identification test containing five items (vanilla powder, olive oil, white wine vinegar, ground coffee, and toothpaste) is used to quantify smell functioning in this study, based on recommendations from SmellTracker developed by the Weizmann Institute of Science. 14 In a systematic review and meta‐analysis, the prevalence of self‐reported smell dysfunction in COVID‐19 patients was 41%. 15 Further, the neurological and medical examination follows the “Global COVID‐19: clinical platform: novel coronavirus (COVID‐19): rapid version” Module 4, a minimum data set to examine the medium‐ and long‐term impact of COVID‐19. 16 It is intended to serve as a clinical tool that can be used by member states to document the mid‐ and long‐term sequelae among those affected by COVID‐19 disease. Uniformity in the follow‐up of patients could ensure that long‐term clinical and rehabilitation needs are identified and that patients are directed toward/provided the care they require. Means for gathering standardized information regarding the mid‐ and long‐term sequelae of COVID‐19 are through the WHO Clinical Data Platform. It is relevant to note that the module is designed so that it can be adapted, if needed, and can be applied across different income groups, health systems, and country contexts (e.g., current COVID‐19 status, definitions, and diagnostic criteria).
2.8. Neurocognitive testing and mental status examination
All participants are offered detailed neuropsychological evaluation by a board‐certified neuropsychologist. The testing has been optimized to capture the potential multifaceted cognitive deficits that could present after COVID‐19, based on previous experience of neurodegenerative and neuroinflammatory disorders as well as our current neuroimaging findings and initial reports of cognitive symptoms in the acute phase. 4 The test battery includes:
The Rey Auditory Verbal Learning Test, consisting of 15 unrelated nouns that are read out by the test leader five times. For each round, the patient repeats as many words as they can remember from the list. Approximately 30 min later, a delayed rendering test is administered when the patient repeats the words from memory. Normative data are present for ages 24–81 years. 17
The Rey Complex figure, assessing the visuospatial design and visuospatial memory and distinguishes between different types of disturbances that can affect visuospatial memory. The test consists of a complex figure that the participant should first copy and then draw from memory. According to several studies, both copying and drawing from memory are affected by certain brain injuries, for example, in the executive function of injuries in the frontal lobe and in the overall perception of injuries in the right hemisphere of the brain. Normative values are available for age groups 30–65 and 65–85 years. 18
A phonological Verbal Fluency Test, which measures the flow of words by asking the participant to produce as many words as possible in 1 min beginning with the letters F, A, and S, respectively. 19 Structural and functional imaging studies have shown that both left frontal 20 and temporal 21 lobe regions are involved in verbal fluency performance.
A Category flow test, whereby the participant is asked to produce words belonging to a particular category, such as animals. 22 The fluency tests take little time to complete, are easy to administer, and provide valuable information about cognitive skills and limitations. Previous research has shown that word flow tests have high reliability and are sensitive to cognitive impairments. 23 When analyzing the tests, the number of correct words produced and mental tempo are traditionally measured.
Trail Making Test (TMT) A and B, which give information on visual search, scanning, speed of processing, mental flexibility, and executive functions. In TMT A, the patient needs to draw lines sequentially connecting 25 encircled numbers distributed on a sheet of paper. The requirements for TMT‐B are almost the same, except the patient has to alternate between numbers and letters (e.g., 1, A, 2, B). The amount of time required for each part represents the result. Normative data for ages 18–89 years are available. 24
Coding, a subtest of WAIS‐IV, in which individuals are asked to record associations between different symbols and numbers within time limits. This subtest reflects the psychomotor speed and ability to absorb new material. 25
Digit span, a subtest in WAIS‐IV, which has three parts: Digit Span Forward (the participant tries to repeat digits forward), Digit Span Backward (repeats digits backward), and Digit Span Sequencing (repeats digits in ascending order). This test measures auditory short‐term memory and working memory. 25
Furthermore, the neuropsychological testing is complemented by three self‐reported questionnaires:
The Mental Fatigue Scale (MFS), a validated and sensitive instrument developed to assess mental fatigue after traumatic brain injury, tumors, infections, vascular diseases, and other neurological disorders. Common symptoms include sudden loss of strength during mental activities, impaired attention and attention span, and long recovery that is disproportionate to the level of exercise. 26
The Hospital Anxiety and Depression Scale (HADS), a validated and well‐used self‐assessment form that estimates symptoms of anxiety and depression problems. The scale has a total of 14 questions, seven for anxiety and seven for depression. Overall scores are given for anxiety and depression scores separately. 27
RAND‐36, which comprises 36 items that assess eight health concepts: physical functioning, role limitations caused by physical health problems, role limitations caused by emotional problems, social functioning, emotional well‐being, energy/fatigue, pain, and general health perceptions. Physical and mental health summary scores are also derived from the eight RAND‐36 scales. 28
2.9. Imaging
All participants are offered advanced MRI assessment of the brain and lungs at the 3‐ and 12‐month follow‐ups, which complements routine clinical chest X‐ray and computed tomography. All MRIs are performed on a Siemens Skyra 3 Tesla MRI scanner with a wide bore (70 cm), and participants will be able to listen to music and watch relaxing videos in order to improve patient comfort and reduce motion artifacts. This setup has been very much appreciated by the participants and is now synergistically also being implemented more broadly at our department clinically.
The brain imaging protocol includes:
High‐spatial resolution 3D anatomical T1‐weighted and T2‐weighted FLAIR imaging.
Dedicated coronal STIR imaging for the olfactory bulbs and optic nerves.
High‐angular resolution diffusion imaging for tractography.
Quantitative MRI (simultaneous PD, T1, and T2 mapping). 29
Myelin quantification (Rapid Estimation of Myelin for Diagnostic Imaging, REMyDI). 30
3D Arterial Spin Labeling cerebral blood flow measurements.
When possible (in relation to renal function):
An ultra‐high‐temporal resolution (670 ms) multi‐band accelerated T2*‐based dynamic susceptibility contrast‐enhanced perfusion scan developed in‐house.
Post‐contrast 3D high‐spatial resolution anatomical imaging with a special focus on leptomeningeal inflammation.
And most importantly, based on our published data on COVID‐19 31 and preliminary results from the 3‐month follow‐up, a novel sequence providing microscopic scale resolution for microvascular pathology:
Ultra‐high‐resolution (650 μm isotropic) 3D echo‐planar susceptibility‐weighted imaging.
All imaging are reported in a structured manner by board‐certified radiologists and neuroradiologists, including assessments of atrophy, 32 white matter changes, 33 and SWI abnormalities. 34
A novel thoracic protocol MRI protocol is also applied, including short breath‐hold sequences:
Anatomical 2D T2‐weighted imaging.
Ultra‐short echo time 3D imaging.
Look‐locker T1‐mapping (pre‐ and post‐contrast, when possible).
An ultra‐high‐temporal resolution (430 ms) accelerated T1‐based contrast‐enhanced perfusion scan.
2.10. Cohort description and sample size
Up to 100, and with a minimum goal of 40, patients are to be included and are expected to consist of a male and female mix related to the clinical cohort where approximately 75% are males. Mirroring the clinical cohort, the mean age is expected to be close to 60 years of age. The study is exploratory and hypothesis generating a formal power analysis is not readily applicable. However, as the analysis plan includes dimensionality reduction with methods such as PCA analysis and clustering methods, the number of composite variables related to outcome measures will be reduced to statistically tractable numbers.
2.11. Statistical analysis
This study is an observational study with an in‐depth biochemical, neuropsychological analysis and radiological follow‐up. Thus, given the limited number of patients, this study is hypothesis generating. Methods of data reduction and composite pattern recognition will need to be employed to limit the degrees of freedom and risk of type I error. This will include PCA and cluster analysis. Composite patterns or clusters of biomarkers will be related to MR findings and outcome assessments using regression techniques.
2.12. Time plan
Q1 2020–Q2 2021: Patients treated for COVID‐19 in the ICU.
Q2 2020: Ethical approval obtained. Biofluid samples from the acute phase biobanked.
Q3 2020: First 3‐month follow‐ups (neuropsychological assessment, neurological examination, neuroimaging, and blood sampling).
Q4 2020: First 6‐month follow‐up (blood sampling).
Q2 2021–Q2 2022: 12‐month follow‐ups (neuropsychological assessment, neurological examination, neuroimaging, and blood and CSF sampling).
Q3 2022: Batch analysis of biobanked plasma, serum, and CSF.
Q4 2022: Final analysis phase and publication.
3. PRELIMINARY RESULTS
As of December 31, 2021, 56 patients are included in the study and at different stages of follow‐up. The demographics of this preliminary cohort are shown in Table 1. Despite that this study, by design, includes only survivors of ICU and no mortality has yet been encountered, it can be seen that the cohort has had severe disease, with nearly 38% of patients requiring mechanical ventilation. The mean age was 59% and 72% were male. Fifty‐two percent had hypertension and 36% had diabetes mellitus. Age, sex, and comorbidity profiles thus appear similar to other COVID‐19 ICU cohorts 35 which will support the generalizability of results.
TABLE 1.
Demographics of the 56 participants included in the study population
| Variable (N = 56) | % | Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. |
|---|---|---|---|---|---|---|---|
| Age | 23 | 50 | 59 | 57.6 | 66.2 | 79 | |
| Sex, Female | 28% | ||||||
| BMI | 20.3 | 27.4 | 29.9 | 31.1 | 34.9 | 42.3 | |
| Smoker/previous smoker | 39% | ||||||
| Charlson Index (non‐Age) | 0 | 0 | 1 | N/A | 2 | 8 | |
| Hypertension | 52% | ||||||
| Ischemic Heart Disease | 12% | ||||||
| COPD | 1.8% | ||||||
| Diabetes Mellitus | 36% | ||||||
| Obesity | 46% | ||||||
| Heart Failure | 5.4% | ||||||
| Atrial Fibrillation | 8.9% | ||||||
| SAPS III score | 37 | 45 | 50 | 50 | 54 | 70 | |
| PFI on ICU arrival | 6.2 | 9.2 | 10.6 | 11.5 | 12.9 | 29 | |
| Mechanical Ventilation | 38% | ||||||
| NIV prior ICU | 5.4% | ||||||
| HFOC prior ICU | 34% | ||||||
| Tracheostomy | 7.1% | ||||||
| ICU days | 1 | 4.1 | 6.4 | 8.3 | 9.9 | 51 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HFOC, high‐flow oxygen cannula; NIV, non‐invasive ventilation; PFI, partial pressure of oxygen/fraction inspired oxygen index; SAPS, simplified acute physiology score.
To date, we have performed 51 3‐month follow‐up MRIs in the ICU survivors. Of these, two patients (~4%) have had incidental findings on brain MRI findings requiring activation of the Incidental Findings Management Plan and re‐admittance to the hospital for treatment and additional clinical workup. One of these patients had a subacute hemorrhage in the right thalamus (i.e. a bleeding occurring post‐discharge and about 2–3 weeks prior to the imaging) and one patient had several subacute cortical microinfarcts (exemplified in Figure 1) related to a subtotal occlusion of an internal carotid artery.
FIGURE 1.
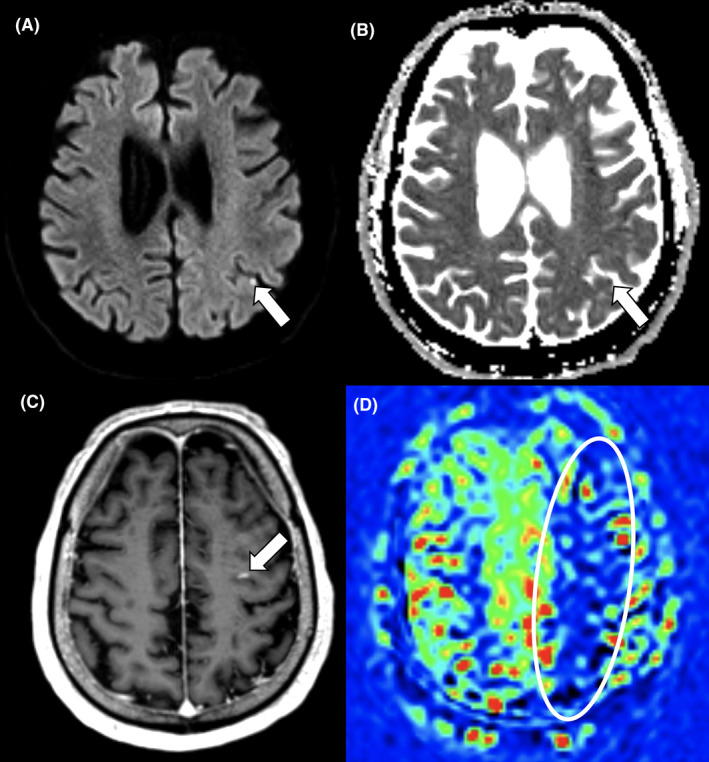
Cortical microinfarcts as an incidental imaging finding. Brain MRI at 3‐month follow‐up in a 68‐year‐old male COVID‐19 critical care survivor revealing four asymptomatic cortical microinfarcts of varying age and a hypoperfusion syndrome in the watershed areas of the left middle cerebral artery territory. Diffusion‐weighted b1000 image (A) and Apparent Diffusion Coefficient map (B) revealing an acute cortical microinfarct in the left parietal lobe. Subacute cortical microinfarct with blood–brain barrier disruption revealed on a contrast‐enhanced 3D T1‐weighted image (C). Reduced relative cerebral blood flow in the watershed areas of the left middle cerebral artery territory detected by Arterial Spin Labeling (D)
The neuropsychological and neurological examinations have so far revealed varying and mixed patterns. Several patients expressed cognitive and/or mental concerns and fatigue, complaints closely related to brain fog. Further examinations may reveal relevant remediation measures.
4. DISCUSSION AND CONCLUSION
Here, we present the study plan of the ongoing Karolinska NeuroCOVID study. Understanding the mechanisms of neurocognitive manifestations in COVID‐19 is needed to identify potential targets of intervention. This, in turn, could lead to better long‐term treatments and rehabilitation, thus possibly contributing to reducing the long‐term personnel and socioeconomic burden of this pandemic. This granular study is anticipated to help gain specific knowledge on the pathophysiological mechanisms, and radiological and long‐term cognitive manifestations, of severe COVID 19 disease. These initial reports from MRI imaging also stress the need for having management plans for incidental findings in these kinds of studies and highlight the clinical value of close monitoring of the often vulnerable COVID‐19 critical care survivors.
CONFLICTS OF INTEREST
TG is a recipient of the Grant for Multiple Sclerosis Innovation Award from Merck; not related. HZ has served at scientific advisory boards for Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, NervGen, AZTherapies, and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program (outside submitted work). FP has received research grants from Sanofi‐Genzyme, Merck KGaA and UCB, and fees for serving as Chair of DMC in clinical trials with Parexel Lundbeck and Roche.
AUTHOR CONTRIBUTIONS
All authors have been involved in both study planning, data collection, and manuscript writing.
STUDY REGISTRATION
ClinicalTrials.gov Identifier: NCT04578197.
Nelson DW, Granberg T, Andersen P, et al. The Karolinska NeuroCOVID study protocol: Neurocognitive impairment, biomarkers and advanced imaging in critical care survivors. Acta Anaesthesiol Scand. 2022;66:759–766. doi: 10.1111/aas.14062
Funding information
This study was made possible thanks to a generous donation from Cecilia and Björn Rosengren, Stockholm, Sweden. It is further supported by Alzheimersfonden. DWN is supported by Region Stockholm ALF Medicine grants. TG is supported by Alzheimersfonden, Karolinska Institutet (StratNeuro StartUp grant, CIMED junior grant), Region Stockholm (ALF Medicine grants, ALF clinical postdoc grant), and the Swedish Society for Medical Research (Big grant). JK is supported by ALF clinical researcher grant and The Swedish Medical Association, MK is supported by Stiftelsen Stockholms Sjukhem, Wallenberg Clinical grant, and VR Theme Aging platform grant. PA, MA, GH, and HW by Region Stockholm ALF medicine grants. CT and NL by CIMED and FORTE.
REFERENCES
- 1. DeKosky ST, Kochanek PM, Valadka AB, et al. Blood Biomarkers for detection of brain injury in COVID‐19 patients. J Neurotrauma. 2021;38(1):1‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mondello C, Roccuzzo S, Malfa O, et al. Pathological findings in COVID‐19 as a tool to define SARS‐CoV‐2 pathogenesis. A systematic review. Front Pharmacol. 2021;12:614586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danielson M, Wiklund A, Granath F, et al. Association between cerebrospinal fluid biomarkers of neuronal injury or amyloidosis and cognitive decline after major surgery. Br J Anaesth. 2021;126(2):467‐476. [DOI] [PubMed] [Google Scholar]
- 4. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shahjouei S, Tsivgoulis G, Farahmand G, et al. SARS‐CoV‐2 and stroke characteristics: a report from the multinational COVID‐19 Stroke Study Group. Stroke. 2021;STROKEAHA120032927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edén A, Kanberg N, Gostner J, et al. CSF biomarkers in patients with COVID‐19 and neurological symptoms: a case series. Neurology. 2020. [DOI] [PubMed] [Google Scholar]
- 7. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cabañes‐Martínez L, Villadóniga M, González‐Rodríguez L, et al. Neuromuscular involvement in COVID‐19 critically ill patients. Clin Neurophysiol. 2020;131(12):2809‐2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galvin JE, Roe CM, Coats MA, Morris JC. Patient's rating of cognitive ability: using the AD8, a brief informant interview, as a self‐rating tool to detect dementia. Arch Neurol. 2007;64(5):725‐730. [DOI] [PubMed] [Google Scholar]
- 10. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559‐564. [DOI] [PubMed] [Google Scholar]
- 11. Meyer‐Moock S, Feng Y‐S, Maeurer M, Dippel F‐W, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129‐2170. [DOI] [PubMed] [Google Scholar]
- 13. Movement disorder society task force on rating scales for Parkinson's disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738‐750. [DOI] [PubMed] [Google Scholar]
- 14. Snitz K, Perl O, Honigstein D, et al. SmellSpace: an odor‐based social network as a platform for collecting olfactory perceptual data. Chem Senses. 2019;44(4):267‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori‐Asenso R. Smell and taste dysfunction in patients with COVID‐19: a systematic review and meta‐analysis. Mayo Clin Proc. 2020;95(8):1621‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO . Global COVID‐19 Clinical Data Platform for clinical characterization and management of patients with suspected or confirmed COVID‐19 [Internet]. 2021. Available from: https://www.WHO.int/teams/health‐care‐readiness‐clinical‐unit/covid‐19/data‐platformWHO
- 17. Van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J. Rey's verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290‐302. [DOI] [PubMed] [Google Scholar]
- 18. Fastenau PS, Denburg NL, Hufford BJ. Adult norms for the Rey‐Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. Clin Neuropsychol. 1999;13(1):30‐47. [DOI] [PubMed] [Google Scholar]
- 19. Barry D, Bates ME, Labouvie E. FAS and CFL forms of verbal fluency differ in difficulty: a meta‐analytic study. Appl Neuropsychol. 2008;15(2):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Birn RM, Kenworthy L, Case L, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self‐paced overt response fMRI study of verbal fluency. NeuroImage. 2010;49(1):1099‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Libon DJ, Xie SX, Wang X, et al. Neuropsychological decline in frontotemporal lobar degeneration: a longitudinal analysis. Neuropsychology. 2009;23(3):337‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test‐retest reliability. Br J Clin Psychol. 2000;39(2):181‐191. [DOI] [PubMed] [Google Scholar]
- 24. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203‐214. [DOI] [PubMed] [Google Scholar]
- 25. Wechsler D. Wechsler adult intelligence scale‐Fourth Edition [Internet]. American Psychological Association. 2012. Available from 10.1037/t15169-000 [DOI] [Google Scholar]
- 26. Johansson B, Starmark A, Berglund P, Rödholm M, Rönnbäck L. A self‐assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2010;24(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 27. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 28. Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473‐483. [PubMed] [Google Scholar]
- 29. Granberg T, Uppman M, Hashim F, et al. Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. AJNR: Am J Neuroradiol. 2016;37(6):1023‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouellette R, Mangeat G, Polyak I, et al. Validation of rapid magnetic resonance myelin imaging in multiple sclerosis. Ann Neurol. 2020;87(5):710‐724. [DOI] [PubMed] [Google Scholar]
- 31. Klironomos S, Tzortzakakis A, Kits A, et al. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297(3):E324‐E334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasquier F, Leys D, Weerts JG, Mounier‐Vehier F, Barkhof F, Scheltens P. Inter‐ and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36(5):268‐272. [DOI] [PubMed] [Google Scholar]
- 33. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR: Am J Roentgenol. 1987;149(2):351‐356. [DOI] [PubMed] [Google Scholar]
- 34. Gregoire SM, Chaudhary UJ, Brown MM, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73(21):1759‐1766. [DOI] [PubMed] [Google Scholar]
- 35. COVID‐ICU Group on behalf of the REVA Network and the COVID‐ICU Investigators. Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47(1):60‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]


