Abstract
Six hundred microorganisms were isolated from sugar beets collected from different parts of Finland to study their slime production. A total of 170 of them produced exopolysaccharides, of which 35% were heteropolysaccharides. The yield of heteropolysaccharides from sucrose was lower than that of dextrans. Five isolates, which were chosen for closer study, were identified as Leuconostoc mesenteroides (two species), Rahnella aquatilis (two species), and Enterobacter amnigenus.
During sugar beet storage and processing, sucrose losses due to microbial activity occur. One of the reasons is the formation of slimy microbial polysaccharides, which cause severe processing and quality problems (1–3, 5, 15). The first report on slime production in sugar-containing liquid caused by small cocci dates back to 1861 (8). Leuconostoc sp. was suggested to be the reason for slime production in sugar factories in 1878 (16). Several studies deal with this dextran (glucose polymer) producer (2, 3, 14). Two major levan-forming (fructose polymer) bacteria, Pseudomonas fluorescens and Corynebacterium beticola, have been isolated from both intact and deteriorated sugar beets (11–13). The analysis of polysaccharide problems in sugar factories has concentrated mainly on dextran (1–3, 15), and some work on levan has also been done (9, 10, 14). Reports of other harmful polysaccharides are rare.
Several suggestions for solving the problems caused by dextran have been made (14, 16, 17), including enzymatic hydrolysis with dextranase (2, 3). Results obtained with commercial dextranases in both cane and beet factories have been promising. An endo-levanase from a Bacillus sp. has been reported recently (7), but suitable levanases are not yet commercially available.
In this paper, we report the results of the screening of soil microbes associated with sugar beet spoilage and slime production in the most northern sugar beet fields in the world. Microorganisms were isolated from both intact and deteriorated sugar beets harvested from different parts of Finland (Salo, Janakkala, Mikkeli, and Orimattila). Samples from either the surface or inside of the beet were taken and were streaked onto agar plates having the following composition (in grams per liter): soft brown sugar, 40; MgSO4 · 7H2O, 0.2; K2HPO4, 9; KH2PO2, 3; yeast extract, 2; agar, 15. The samples were then incubated at 28°C. This medium was used in all experiments, omitting agar when appropriate.
In the first isolation round, 170 of 600 microbes were detected as exopolysaccharide (EPS) producers based on slimy colony morphology. These isolates were chosen for closer study. The slime was peeled from 9-cm-diameter agar plates and weighed. The polymer yields per plate were typically 3 to 4 g (wet weight), with moisture contents of 86 to 94%.
The monosaccharide composition of the polysaccharides of the slime-producing microorganisms was first determined from 17 randomly chosen isolates. These isolates were cultured in 20 ml of the growth medium on a rotary shaker at 160 rpm and 28°C for 2 to 3 days, and the cells were harvested by centrifugation (8,000 × g for 20 min at 5°C) and heated at 105°C overnight for dry weight measurement. The polysaccharide material from the culture medium was precipitated with 2 volumes of isopropanol and 2 ml of 2.5 M sodium acetate buffer, pH 6.0. The precipitate was collected by centrifugation (31,186 × g for 30 min at 5°C), washed with 70% (vol/vol) ethanol, and freeze-dried. A total of 30 mg of the freeze-dried polysaccharide was hydrolyzed with 1 N H2SO4 at 120°C in an autoclave for 1 h, neutralized with 5 M NaOH, and filtered (0.2 μm pore size) for monosaccharide analysis.
Sucrose concentration was determined from filtered (0.45 μm pore size) culture. Sucrose and the monosaccharide composition of the isolated EPS after acid hydrolysis were analyzed with Dionex high-pressure liquid chromatography equipment (4500I) with a pulsed electrochemical detector (6). A CarboPac PA1TM anion-exchange column (25 cm by 4.6 mm; Dionex) was used. Water and 0.2 M NaOH were used as eluents with a slightly modified gradient. Sucrose, l-arabinose, d-fructose, l-fucose, d-galactose, d-glucose, d-mannose, d-xylose, and l-rhamnose purchased from Sigma Chemical Co., St. Louis, Mo., were used as standards. Six isolates (35.3%) of the 17 microorganisms produced heteropolymers, indicating a higher than expected frequency of occurrence (14).
A further analysis of 95 randomly chosen strains gave similar results, with 34% being heteropolymer producers. Glucose was the dominant sugar in the polysaccharides, and fructose was the first or second most dominant sugar. Galactose, mannose, and small amounts of rhamnose, fucose, and arabinose were present in most polymers. No xylose was detected. Of all the measured monosaccharides, 71% was glucose and 18% was fructose. This indicates that dextrans were present in large amounts, although many heteropolysaccharide formers were present as shown by the 5% mannose and 5% galactose of total sugars and their presence in most of the tested samples. One percent of the total sugars was fucose.
Five isolates (no. 1 through 5) were selected for closer growth study on the basis of either their high viscosity or monosaccharide composition. They were identified by the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). Isolate no. 1 was identified as Enterobacter amnigenus (heteropolysaccharide) having 98% similarity to Enterobacter partial 16S rDNA genes and a fatty acid profile typical of E. amnigenus. Physiological tests ruled out the genus Klebsiella. Determination of partial sequences of isolates 2 and 3 revealed 100% similarity to the most variable region of 16S rDNA of Leuconostoc mesenteroides (dextran). Partial rDNA sequencing of amplified PCR products from isolates 4 (heteropolysaccharide) and 5 (levan) showed 96.6% similarity to Rahnella aquatilis and 99.6% similarity to each other. The fatty acid profiles were those of Rahnella, Serratia, and Pantoea, and physiological tests pointed to R. aquatilis.
The growth and EPS formation by these microorganisms were studied at five different temperatures (Table 1). All the tested isolates grew at 5°C, although slowly. E. amnigenus clearly produced less EPS than the other strains (data not shown). All strains except E. amnigenus produced about the same amount of EPS at temperatures between 5 and 30°C, although a tendency for a higher EPS yield below 13°C could be seen. This is important since the beets in Finland are stored in conditions where the temperature is often close to 5°C. At these low temperatures, the microbial growth is slow, but a large amount of the available sucrose is transformed into various polymeric sugars. The amount of EPS produced was almost 10 times higher than the obtained cell mass, except for the strain E. amnigenus, which showed the best EPS production at 30°C.
TABLE 1.
Yield of EPS and cell mass obtained from four selected isolates at five different temperatures
| Temp (°C) | Yield (g/liter) from isolate no.:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |||||
| EPS | Cell mass | EPS | Cell mass | EPS | Cell mass | EPS | Cell mass | |
| 5 | 26.3 | 2.4 | 28.3 | 4.2 | 18.9 | 1.9 | 26.4 | 3.1 |
| 13 | 27.8 | 2.5 | 24.6 | 2.5 | 20.1 | 3.2 | 23.8 | 2.9 |
| 23 | 20.8 | 1.2 | 20.2 | 1.5 | 17.7 | 3.0 | 19.3 | 3.5 |
| 30 | 22.5 | 2.2 | 25.3 | 2.6 | 20.0 | 3.3 | 21.3 | 3.0 |
| 37 | 8.7 | 0.6 | 7.4 | 0.7 | 7.1 | 1.0 | 8.7 | 1.2 |
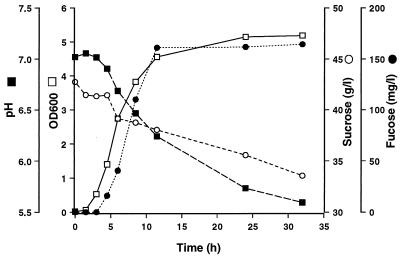
E. amnigenus, which produced a heteropolymer with glucose, galactose, fucose, and mannose as structural monosaccharides, was grown at 30°C to determine the relationship between substrate consumption, cell growth, and polymer formation (Fig. 1). The EPS was precipitated from culture filtrate, freeze-dried, and dissolved in 1 N H2SO4. After acid hydrolysis at 100°C for 3 h, the samples were neutralized with 5 N NaOH. The amount of EPS was calculated from the fucose concentration (32% of total monosaccharides, as previously determined) after acid hydrolysis. The fucose yield was 160 mg/liter, which equals 0.5 g of polymer/liter. The heteropolysaccharide formation by this organism was related to cell growth resulting in pH drop. Only 21% of the sugar was consumed, and about 6% of it was converted to polysaccharide material.
FIG. 1.
Time course of fucose-containing EPS production by isolate 1 (E. amnigenus) at 30°C during 270-ml shake flask cultivation. Amount of fucose, consumption of sucrose, pH, and cell mass at an optical density of 600 nm (OD600) are shown.
E. amnigenus or Rahnella sp., belonging to the family Enterobacteriaceae, have not previously been detected in deteriorated sugar beets. Previous reports have concentrated on either dextran or levan producers (4, 10–13). The isolation methods in these studies were similar to ours, but the numbers of isolates were much smaller than in our study (34 isolates were used in the study reported in reference 4 and 73 isolates were used in the studies reported in references 10–13). Only one study (10) investigated the monosaccharide composition of the formed polymers.
The results obtained in this study show that highly viscous polysaccharides produced by sugar beet spoilage due to microorganisms consist of many different types of sugars. Of the 600 microbes isolated from sugar beet surfaces and interiors, 170 produced extracellular slimes, of which about 35% were heteropolymer producers. This result differs from data published by other authors which suggest that 95% of polysaccharides produced by sugar beet spoilage organisms are dextran or levan (14).
The very different climatic and soil conditions in northern Europe might explain the differences between our results and previous results obtained in different latitudes (2, 3, 9, 10, 14). Beets in Finland are stored in the field in small clamps before processing. Freezing and thawing often occur, leading to beet deterioration more often than in warmer climatic conditions and in larger clamps. One must, however, remember that results obtained in liquid cultures do not necessarily give a true picture of the situation on the surface or interior of the sugar beet.
Sugar factories try to avoid the problems caused by microbial polymers by discarding spoiled beet loads. In addition, commercially available dextranase has been used to aid in filtration problems. Levan may cause problems similar to those caused by dextran. However, according to the results obtained in this study, sugar beet spoilage microbes can form many different types of heteropolysaccharides, which explains why the processing problems are not systematically avoided by the use of available degrading enzymes.
Further work to characterize some of the isolated polysaccharide-forming organisms, their growth conditions, enzymes involved in polymer formation, and the structure of the formed polymers is being carried out in our laboratory.
Acknowledgments
We thank Asta Tervila-Wilo and Ossi Pastinen for high-pressure liquid chromatography analysis.
REFERENCES
- 1.Atkins P J, McCowage R J. Proceedings of the 1984 Sugar Processing Research Conference. New Orleans, La: Sugar Processing Research Institute; 1984. Dextran—an overview of the Australian experience; pp. 108–140. [Google Scholar]
- 2.Barfoed S, Mollgaard A. Dextranase löst Dextranprobleme in DDS-Zuckerfabriken. Zuckerindustrie. 1987;112:391–395. [Google Scholar]
- 3.Clarke M A, Edye L A, Cole F, Kitchar J L. Sugarcane factory trials with dextranase enzyme (from Chaetomium gracile) Sugar J. 1997;59:20–22. [Google Scholar]
- 4.De Lucca A J, Kitchen R A, Clarke M A, Goynes W R. Mesophilic and thermophilic bacteria in a cane sugar refinery. Zuckerindustrie. 1992;117:237–240. [Google Scholar]
- 5.Greenfield P F, Geronimos G L. Effect of dextrans on the viscosity of sugar solutions and molasses. Int Sugar J. 1982;80:67–72. [Google Scholar]
- 6.Kerherve P, Charriere B, Gadel F. Determination of marine monosaccharides by high-pH anion-exchange chromatography with pulsed amperometric detection. J Chromatogr A. 1995;718:283–289. [Google Scholar]
- 7.Miasnikov A N. Characterization of a novel endo-levanase and its gene from Bacillus sp. L7. FEMS Microbiol Lett. 1997;154:23–28. doi: 10.1111/j.1574-6968.1997.tb12619.x. [DOI] [PubMed] [Google Scholar]
- 8.Pasteur L. Sur la fermentation visquese et la fermentation butyrique. Bull Soc Chim Paris. 1861;11:30–31. [Google Scholar]
- 9.Reinefield E, Biesener K-M, Reinefeld A, Rexilius L. Proceedings of the 15th General Assembly of CITS. Vienna, Austria: CITS; 1975. Gaschromatographische Untersuchungen zum Verhalten von Nichtzuckerstoffen beim Technischen Prozess der Zuckergewinnung; pp. 124–145. [Google Scholar]
- 10.Schneider F. Eigenschaften und Verhalten frostgeschädigter Rüben bei der Zuckerfabrikation. Zucker. 1957;10:375–383. [Google Scholar]
- 11.Schneider F, Hoffmann-Wahlbeck H P, Abdou M A-F. Über Polysaccharidbildner in der Zuckerfabrikation, I. Mitteilung Zucker. 1969;23:652–657. [Google Scholar]
- 12.Schneider F, Hoffmann-Wahlbeck H P, Abdou M A-F. Über Polysaccharidbildner in der Zuckerfabrikation, II. Mitteilung Zucker. 1968;17:465–473. [Google Scholar]
- 13.Schneider F, Hoffmann-Wahlbeck H P, Abdou M A-F. Über Polysaccharidbildner in der Zuckerfabrikation, III. Mitteilung Zucker. 1969;20:561–566. [Google Scholar]
- 14.Shore M, Dutton J V, Houghton B J. 26th Technical Conference of British Sugar 1982. Eastbourne, United Kingdom: British Sugar; 1982. Evaluation of deteriorated beet; pp. F1–F34. [Google Scholar]
- 15.Sidebotham R L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- 16.Van Tieghem P. Sur la gomme de sucrerie. Ann Sci Natur (Bot) 1878;6:180–203. [Google Scholar]
- 17.Vukov K, Falvay A, editors. Physics and chemistry of sugar-beet in sugar manufacture. 1977. p. 386. , 425, 463. Elsevier Scientific Publishers, Amsterdam, The Netherlands. [Google Scholar]