Abstract
Objective
To correlate clinical outcomes to pathology in SARS‐CoV‐2 infected placentas in stillborn and live‐born infants presenting with fetal distress.
Design
Retrospective, observational.
Setting
Nationwide.
Population
Five stillborn and nine live‐born infants from 13 pregnant women infected with SARS‐CoV‐2 seeking care at seven different maternity units in Sweden.
Methods
Clinical outcomes and placental pathology were studied in 14 cases (one twin pregnancy) of maternal SARS‐CoV‐2 infection with impaired fetal outcome. Outcomes were correlated to placental pathology in order to investigate the impact of virus‐related pathology on the villous capillary endothelium, trophoblast and other cells.
Main outcome measures
Maternal and fetal clinical outcomes and placental pathology in stillborn and live‐born infants.
Results
Reduced fetal movements were reported (77%) and time from onset of maternal COVID‐19 symptoms to signs of fetal distress among live‐born infants was 6 (3–12) days and to diagnosis of stillbirth 11 (2–25) days. Two of the live‐born infants died during the postnatal period. Signs of fetal distress led to emergency caesarean section in all live‐born infants with umbilical cord blood gases and low Apgar scores confirming intrauterine hypoxia. Five stillborn and one live‐born neonate had confirmed congenital transmission. Massive perivillous fibrinoid deposition, intervillositis and trophoblast necrosis were associated with SARS‐CoV‐2 placental infection and congenital transmission.
Conclusions
SARS‐CoV‐2 can cause rapid placental dysfunction with subsequent acute fetal hypoxia leading to intrauterine fetal compromise. Associated placental pathology included massive perivillous fibrinoid deposition, intervillositis and trophoblast degeneration.
Keywords: chronic histiocytic intervillositis, coronavirus, COVID‐19, COVID‐19 maternal‐fetal transmission, fetal distress, maternal floor infarction, placental endothelial cells, placental pathology, SARS‐CoV‐2, SARS‐CoV‐2 placental infection, vertical SARS‐CoV‐2 transmission, villous macrophages
Tweetable abstract
SARS‐CoV‐2 can cause rapid placental dysfunction and intrauterine fetal compromise.
Linked article: This article is commented on by Yves Ville, pp. 1375 in this issue. To view this minicommentary visit https://doi.org/10.1111/1471-0528.17162.
 This article includes Author Insights, a video abstract available at: https://vimeo.com/bjogabstracts/authorinsights17132
This article includes Author Insights, a video abstract available at: https://vimeo.com/bjogabstracts/authorinsights17132
1. INTRODUCTION
Following the emergence of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic, there was limited scientific knowledge on the impact of COVID‐19 in pregnant women. 1 , 2 , 3 Recent studies have suggested that SARS‐CoV‐2 infection can lead to an increased risk of maternal death 4 , 5 and/or severe maternal morbidity. 2 , 3 , 4 , 5 , 6 , 7 , 8 Pregnant women with SARS‐CoV‐2 infection have been found to have higher rates of preterm delivery and caesarean section (CS) compared with non‐infected pregnant women. 2 , 3 , 4 , 5 , 8 , 9 , 10 Evidence suggests that rates of stillbirth may have changed substantially during the pandemic, 11 , 12 with Khalil et al. 13 demonstrating a four‐fold increase in the stillbirth rate in a cohort of mothers in Britain. These findings were reiterated in a multinational meta‐analysis by Chmielewska et al., 11 which even emphasised that some outcomes, such as stillbirth, had shown considerable disparity between high‐resource and low‐resource settings. In Sweden, recent studies have showed an increased risk for preterm delivery but not for stillbirth. 14 , 15
Congenital transmission of SARS‐CoV‐2 has been convincingly reported in several case reports and series, 16 , 17 , 18 and thus placental pathology is of key interest. 19 , 20 , 21 SARS‐CoV‐2 antigens and nucleic acid have been identified most frequently in the placental villous syncytiotrophoblast, 22 , 23 but have also been identified to a lesser extent in the villous cytotrophoblast and in villous stromal macrophages (Hofbauer cells) and capillary endothelial cells. 24 , 25 , 26 , 27 For SARS‐CoV‐2, angiotensin‐converting enzyme 2 (ACE2) is the undisputed receptor for cellular entry, and studies have shown strong and diffuse ACE2 staining in the cytotrophoblast and syncytiotrophoblast cells of placental villi, as well as in extravillous trophoblasts. 28 , 29 A handful of studies have reported that SARS‐CoV‐2 infection can lead to rapid placental dysfunction with fetal distress and imminent intrauterine demise of the fetus. 16 , 17 , 26 However, as congenital transmission is a rare event and not all infected pregnant women have poor obstetrical and neonatal outcomes, the possibility of fetal compromise due to COVID‐19 in the mother is still debated.
Using a national Swedish case series of 13 women presenting with signs of fetal distress and COVID‐19, we studied the clinical outcomes and placental pathology of 14 placentas (one twin pregnancy) infected by SARS‐CoV‐2 with and without congenital transmission. Our aim was to correlate clinical outcomes to placental pathology in stillborn and live‐born infants presenting with fetal distress. To the best of our knowledge, this study represents the largest case series of placentas infected with SARS‐CoV‐2 among patients presenting with intrauterine fetal death after 22 weeks of gestation or having acute fetal distress.
2. METHODS
The inclusion criteria for enrolment into this retrospective case series included women having pregnancies complicated by positive maternal testing for SARS‐CoV‐2; having an infant with an impaired perinatal outcome including stillbirth after 22 weeks of gestation or signs of severe fetal compromise leading to immediate delivery; and having the placenta testing positive for SARS‐CoV‐2. Cases were collected throughout Sweden from 1 August 2020 to 16 December 2021. All the women had a positive test result for SARS‐CoV‐2 using reverse transcriptase quantitative polymerase chain reaction (RT‐qPCR) prior to delivery (with the exception of Case 5, see details in Results). The cases were enrolled from seven different Maternity and Delivery Units at tertiary or university hospitals in Sweden (Malmö, Lund, Helsingborg, Varberg, Stockholm, Uppsala and Linköping). Clinicians and pathologists involved were personally contacted by one of the authors (M.Z.) requesting confirmation of the clinical, laboratory and pathology findings. Informed written consent was obtained from all women involved in the study. Case numbers 1, 3, 4, 6, 8, 9, 12, 13 and 14 were included through the COVID‐19 in Pregnancy and Early Childhood (COPE) study 30 using individual patient consent forms. Case numbers 2, 5, 7, 10 and 11 were included separately and individual consent forms were obtained for these patients as well. Additional consent was obtained from the women’s partner where appropriate (in live‐born cases). The study was performed in agreement with principles of the Declaration of Helsinki for Human Research. Patients were not involved in the development of the research reported in the study.
2.1. Sampling
Blood samples, nasopharynx (NPH) and throat swabs from mothers, stillborn infants and neonates were collected according to clinical praxis. Neonatal nasopharyngeal swabs were taken within 24 hours of birth. If positive, testing was continued every 24 hours until the neonate was negative, whereafter the neonate was taken out of isolation. The incision into the placental disc parenchyma to obtain a placental tissue sample for RT‐qPCR test in Cases 1–10 and 14 was performed after disinfection of the fetal and maternal surfaces as described by Baud et al. 31 The placental tissue was obtained by a triangulate section reaching the core of the placental disc and the remaining placenta was kept in formalin. For Cases 11–13, the standard practice of the pathology laboratory analysing the samples did not include disinfecting placental surfaces. A new blade, however, was used and no reports of any problem with contamination have been reported. Sample collection, processing and laboratory testing followed guidance from national guidelines and recommendations from the Swedish Public Health Authority. 32
2.2. Histopathology and immunohistochemistry
Placentas were subjected to gross pathological evaluation and any abnormalities were noted. Trimmed placental weights were recorded and compared with normal values adjusted for gestational age. 34 Following careful dissection of the placentas, representative tissues for microscopic examination were selected and submitted for routine histological processing. Haematoxylin and eosin staining was performed according to routine procedures. Immunohistochemistry was performed using standard methods with a polyclonal antibody to the SARS‐CoV‐2 nucleocapsid protein (40143‐T62; Sino Biological) at a 1:2000 dilution. Appropriate positive and negative immunohistochemistry controls were performed. In Sweden, ACE2 detection and in situ RNA detection was only available in a few research environments. The available methods were therefore RT‐qPCR and immunohistochemistry for SARS‐CoV‐2 nucleoprotein in Cases 1–10 and 14. For Cases 11–13, an RNA scope was available at that particular laboratory. Following staining, microscopic examination and diagnosis of the umbilical cord, extraplacental membranes and placental discs was conducted. Results of placental findings were reviewed by three specialists in placental pathology (D.A.S., D.G., N.P.).
2.3. Clinical correlation
In each case, maternal and fetal/infant clinical data were obtained from hospital records. Body mass index (BMI) at the first antenatal care visit was recorded and the presence of significant placental pathology was assessed. Results from SARS‐CoV‐2 testing, including possible maternal‐fetal transmission as defined by Shah et al., 35 was also noted. Congenital infection with intrauterine fetal death/stillbirth or in live‐born neonates was categorised into ‘confirmed’ and ‘probable’ cases. 35 Case 9 16 has been published previously, otherwise none of the other cases have been presented in the literature prior to submission of this paper.
3. RESULTS
Five women (5/13, 38%) gave birth to stillborn infants (mean gestational age 33+0, range 24+4 to 38+2 weeks). Eight mothers (8/13, 62%) gave birth to nine live‐born infants (including one set of twins) (mean gestational age 32+3, range 24+1 to 36+0 weeks). Two of the nine live‐born infants died later during the postnatal period, a neonatal mortality rate of 22%. There were no full‐term (37–42 weeks of gestation) infants delivered to women in this cohort. The maternal and fetal characteristics including placental pathology from the pregnant women presenting with stillbirth are reported in Table 1 and for live‐born infants in Table 2. The villous syncytiotrophoblast from all placentas stained positively for SARS‐CoV‐2 nucleocapsid protein using immunohistochemistry, confirming that the placentas from all cases were infected with SARS‐CoV‐2 based on published immunohistochemical and molecular pathology criteria for determining placental infection. 36 , 37
TABLE 1.
Maternal, fetal and infant characteristics from five pregnant women with SARS‐CoV‐2 infection and stillbirth in which the placenta was confirmed to be infected with the coronavirus
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Maternal and fetal characteristics | |||||
| Maternal age (years) | 31 | 26 | 25 | 25 | 37 |
| Gestational age (weeks) at | |||||
| Onset of maternal symptoms | 34+4 | 24+2 | 32+5 | 34+5 | 31+0 |
| Positive maternal RT‐qPCR | 34+5 | 23+3 | 33+1 | 34+6 | (Please see Results) |
| IUFD | 35+1 | 24+4 | 34+1 | 38+2 | 33+0 |
| Parity | 1 | 1 | 0 | 0 | 1 |
| Body mass index (BMI) kg/m2 at antenatal care visit | 21 | 20 | 31 | 27 | 24 |
| Maternal comorbidities | None | None | None | Hypothyroidism, Idiopathic thrombocytopenia purpura | None |
| Mother born in Sweden | Yes | Yes | No | No | No |
| Presenting symptoms at time of seeking healthcare services |
Reduced fetal movements for 1 day Painful uterine contractions for 1 day |
Fever 40 °C for 1 day Uterine contractions for 1 day |
Reduced fetal movements for 1 day Uterine contractions, back pain, small bleeding for 1 day Difficulties in breathing, cough, fever on and off, nausea for 10 days |
Reduced fetal movements for 1 day |
Reduced fetal movements for 2 days Fever for 14 days Headache for 14 days Nausea/reduced apetite for 14 days |
| Fetal SARS‐CoV‐2 status using RT‐qPCR a | Positive | Negative | Positive | Negative | Negative (Cycle Threshold just above threshold for positive test) |
| Birthweight (g) | 2200 | 686 | 2190 | 2665 | 1920 |
| Small‐for‐gestational age (SGA) 37 | No | No | No | No | No |
| Placental features and autopsy findings | |||||
| Vertical transmission (congenital infection with intrauterine fetal death/stillbirth) 34 | Confirmed | Confirmed | Confirmed | Confirmed | Confirmed (Note: fetal SARS‐CoV‐2 cycle threshold = 36.5, just above threshold for a positive test) |
| Placental weight (g) | N/A | 236 | 376 | 401 | 305 |
| Placental weight according to gestational week 33 | N/A | Normal | Normal | Underweight | Underweight |
| Trophoblast staining for SARS‐CoV‐2 | ++ | ++ | ++ | ++ | ++ |
| Fibrinoid deposits—type and percentage coverage of placental parenchyma | Massive perivillous fibrinoid deposition, 90% | Borderline perivillous fibrinoid deposition, 25% | Massive perivillous fibrinoid deposition, 80% | Massive perivillous fibrinoid deposition, 80% | Massive perivillous fibrinoid deposition, 85% |
| Intervillositis |
|
Acute intervillositis | Acute intervillositis | Chronic intervillositis | Chronic intervillositis |
| Trophoblast necrosis | ++ | ++ | ++ | − | − |
| Maternal vascular malperfusion | + | + | − | − | + |
| Karyorrhexis phenomena | + | + | − | − | − |
| Choriangiosis | + | − | − | − | − |
|
Placental tissue SARS‐CoV‐2 RT‐qPCR |
+ | + | + | + | + |
| Other important pathological features | Calcification | Umbilical cord: hypospiralised | Nucleated erythrocytes | Late development of chorionic villi, nucleated erythrocytes, sub‐chorionitis | Necrosis of decidua basalis and capsularis |
| Fetus gender | Boy | Girl | Girl | Girl | Girl |
| Autopsy findings | Mild maceration. SARS‐CoV‐2 positive lung tissue. No congenital anomalies seen. Brain and liver size larger than reference values for gestational week 35. Blood trombosis in right atrium and umbilical cord vein. Petechial bleeding in epicardium | Appropriate for gestational age fetus with no visible congenital anomalies. Fetal lung tissue showing grooves of epithelial cell indicating suspected aspiration. No bacterial or virus infection could be shown. Normal genetic screening | Parents declined consent. No gross anomalies seen. Heart puncture RT‐qPCR positive for SARS‐CoV‐2. | Parents declined consent. No gross anomalies seen. | Left hand was malformed with a shortening of two fingers and suspected absence of two metacarpal bones |
Reverse transcription quantitative polymerase chain reaction (RT‐qPCR) using nasopharyngeal or heart puncture swab from fetus.
TABLE 2.
Characteristics of pregnant women with SARS‐CoV‐2 infection presenting with signs of fetal distress and characteristics of their live‐born infants including placental features
| Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12,13 Diamniotic dichorionic twins | Case 14 | ||
|---|---|---|---|---|---|---|---|---|---|
| Maternal characteristics | |||||||||
| Maternal age (years) | 28 | 38 | 30 | 27 | 31 | 30 | 31 | 33 | |
| Gestational age (in weeks) at | |||||||||
| Onset of maternal symptoms | 34+4 | 21+3 | 34+2 | 34+1 | 32+4 | 23+0 | 33+0 | 28+4 | |
| Positive maternal RT‐qPCR | 34+2 (contact tracing) | 21+6 | 34+5 | 34+4 | 32+5 | 23+4 | 33+4 | 28+4 | |
| Time of delivery | 35+1 | 31+6 | 36+0 | 34+4 | 33+5 | 24+1 | 34+0 | 29+3 | |
| Parity | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | |
| BMI (kg/m2) | 31 | 23 | 27 | 27 | 23 | 35 | 40 | 22 | |
| Maternal comorbidities | Depression, Gastric by‐pass, gestational diabetes | None | None | None | None | Polycystic ovaries, Pregnant after ovulation stimulation | Depression, Asthma | Migraine | |
| Mother born in Sweden | Yes | Yes | Yes | No | Yes | Yes | Yes | No | |
| Presenting symptoms | Reduced fetal movement for 1 day | Planned ultrasound scan due to bad obstetric history (Previous pregnancy: oligo‐hydramnios, chorio‐amnionitis with premature delivery) | Reduced fetal movements for 1 day |
Reduced fetal movement for 1 day Dry cough‐1 day Fever for 3 days Abdominal pain for 3 days |
Reduced fetal movement for 3 days Maternal fever, 2–4 days ago |
Reduced fetal movement for 2 days Fever for 8 days Blocked nose for 8 days |
Reduced fetal movements‐1 day |
Cough for 6 days Sore throat for 6 days Uterine contractions for 1 day |
|
| Cardiotocograph(CTG) classification 38 | Pathological
|
Normal | Pathological
|
Pathological
|
Pathological
|
Pathological
|
Normal 12 hours prior to delivery | Normal 12 hoursprior to delivery | Pathological
|
| Mode of delivery | Emergency caesarean section | Semi‐acute caesarean section | Emergency caesarean section | Emergency caesarean section | Emergency caesarean section | Emergency caesarean section | Emergency caesarean section | Emergency caesarean section | |
| Indication for delivery | Pathological CTG pattern | Fetal blood flow velocimetry deterioration | Pathological CTG pattern | Pathological CTG pattern | Pathological CTG pattern | Pathological CTG pattern and BFC 2 | Preeclampsia (high blood pressure and thrombocytopenia) | Pathological CTG pattern | |
| Infant characteristics | |||||||||
| Apgar Score (1, 5, 10 minutes) | 2, 4, 7 | 9, 10, 10 | 4, 8, 9 | 1, 4, 8 | 3, 5, 8 | 2, 5, 7 | 0, 0, 1 | 4, 7, 8 | 1, 5, 8 |
|
Umbilical cord blood gases (Units: lactate in mmol/litre, base excess [BE] in mEq/litre) |
Arterial pH: 7.15 Arterial lactate: 11.5 |
NA | NA |
Arterial pH: 7.20 Arterial lactate: 11.0 Vein pH: 7.22 Vein lactate: 10.1 |
Arterial pH: 7.21 Arterial BE: −7.6 Arterial lactate: 10 Vein pH: 7.26 |
Vein pH: 7.07 Vein BE: −15.5 |
Vein pH: 6.69 Vein BE: −22 |
Vein pH: 7.29 Vein BE: – 11 |
NA |
| Neonatal SARS‐CoV‐2 status (RT‐qPCR using naso‐pharyngeal swab within 24 hours of birth) | Negative | Negative | NA | Positive (Infant had no contact with parents prior to SARS‐CoV‐2 testing) | NA | Negative | Negative | Negative | Positive (Infant had no contact with parents prior to SARS‐CoV‐2 testing) |
| Birthweight (g) | 2064 | 1200 | 2708 | 2310 | 1675 | 570 | 2182 | 1846 | 1370 |
| Small‐for‐gestational age (SGA) 37 | Yes | Yes | No | No | Yes | Yes | No | Yes | No |
| Infant gender | Girl | Boy | Girl | Boy | Girl | Girl | Girl | Boy | Boy |
| Infant health at discharge | Thrombocytopenia before delivery, normalised 4 days postpartum | Healthy | Healthy | No spontaneous breathing directly after birth. Continuous positive airway pressure (CPAP) for 24 mins. No extra support was needed thereafter. Neonate discharged 14 days after birth. | Manual ventilation for 5 minutes directly after birth. There‐after CPAP for 9 hrs. Due to prematurity + SGA, neonate was kept at the Neonatal ward for 8 days. Dis‐charged after neo‐natal homecare for another 6 weeks. | Moved to the neonatal intensive care unit (NICU) due to a massive intra‐cerebral bleed. Life support turned off day 2. Post‐mortem showed intra‐ventricular haem‐orrhage (IVH) (grade 4) and fresh bleeding in the adrenal glands. | The neonate suffered from hypoxic ischaemic encephalopathy (HIE) grade III and intensive care was discontinued 6 days after birth. The neonate died 8 days after delivery. | Healthy | No spontaneous breathing after birth. Intubated with respirator day 1. Day 2: CPAP and developed IVH (grade 1). Recovering at the neonatal ward with high flow nasal cannula (HFNC) Day 18. |
| Placental features | |||||||||
| Vertical transmission (Congenital infection) 34 | Probable | Probable | Probable | Confirmed | Probable | Probable | Probable | Probable | Probable |
| Placental weight (grams) | 325 | 256 | 594 | 342 | 294 | 156 | 400 | 283 | 300 |
| Placental weight according to gestational week 33 | Underweight | Underweight | Overweight | Normal weight | Underweight | Underweight | Normal weight | Underweight | Normal weight |
| Trophoblast staining for SARS‐CoV‐2 | + | + | + | + | + | + | + | + | + |
| Fibrinoid deposits, percentage distribution within placental parenchyma | Borderline massive perivillous fibrinoid deposition, 40% | − | Borderline massive perivillous fibrinoid deposition, 40% | Massive intervillous fibrinoid deposition, 75% | Massive intervillous fibrinoid deposition, 75% | Massive perivillous fibrinoid deposition, 90% | Massive perivillous fibrinoid deposition and infarcts, >90% | Borderline massive perivillous fibrinoid deposition with infarcts, 40% | Perivillous fibrinoid deposition with infarcts, 10% |
| Intervillositis | Acute intervillositis | − | Focal acute intervillositis | Acute intervillositis | Widespread focal acute‐chronic inter‐villousitis with ‘Nuclear dust’ | Widespread chronic histiocyte intervillositis | Widespread chronic histiocytic intervillositis | Widespread chronic histiocytic intervillositis | Chronic histiocytic intervillositis |
| Trophoblast necrosis | + | − | + | + | + | + | + | + | + |
| Maternal vascular malperfusion | + | − | + | − | − | − | + | − | − |
| Choriangiosis | − | − | − | + | + | − | + | + | − |
| Other placental findings | Agglutination of villi with inflammatory cells, increased villous maturation but no signs of maternal vasculopathy. Umbilical cord was hyperspiralised |
Intraparenchymal infarctions covering 10% of the placenta tissue, plasma cell inflammation of the decidua capsularis and basalis. Immuno‐chemistry for SARS‐CoV‐2 was positive focally |
Acute villitis, maternal vasculopathy of hypertrophy type. The umbilical cord was hyper‐spiralised with a marginal insertion. | Multiple regions of dense intervillous infiltrates of neutrophilic granulocytes and macrophages. SARS‐CoV‐2 nucleoprotein was strongly positive in the cytoplasm + nucleus of villous cytotropho‐blasts and syncytio‐trophoblasts in areas with intervillositis and fibrinoid depositions | Fibrinoid deposition focally transmural with accentuation on the maternal side of the placenta | Immunochemistry for SARS‐CoV‐2 was weakly positive in trophoblast cells but molecular analysis of (mRNA COVN and COVS) strongly positive in trophoblast cells and in placental parenchyma | Acute aterosis and thrombosis, villous necrosis and agglutination of villi with inflammatory cells. The umbilical cord was hyper‐spiralised. Calcified areas were found within the parenchyma | Few maternal vessels with necrosis of blood vessel walls, agglutination of villi with inflammatory cells and calcified areas with the parenchyma | Strong, diffuse positivity for SARS‐CoV‐2 nucleoprotein in villous trophoblasts. Areas with intervillositis are dominated with MPO+ granulocytes, CD68+ macrophages/histiocytes and to a lesser extent CD3+ T‐lymphocytes and minimally CD20+ B‐cells |
The majority of the women presented in their third trimester (11 of 13 women, 85%). Three of the five women (60%) presenting with intrauterine fetal demise (IUFD) were not born in Sweden, whereas six of the eight women (75%) with live‐born infants were Swedish‐born. Experiencing reduced fetal movements was the major reason for seeking healthcare in ten of the 13 cases (77%). High fever (>39°C) was reported in four of 13 cases (31%). All women had tested positive for SARS‐CoV‐2 using RT‐qPCR, except for Case 5, where the mother had a negative nasopharyngeal swab. However, as her partner was COVID‐19‐positive, serum antibodies for SARS‐CoV‐2 confirmed COVID‐19 in the mother.
Histopathological examination of the placentas revealed a spectrum of pathologically increased fibrinoid deposition that varied in severity up to massive perivillous fibrinoid deposition (MPFD) in 13 of the 14 placentas (93%), which varied in estimated mean percentage covering the placental parenchyma in stillborn cases (72%, range: 25%–90%) and live‐born cases (58%, range: 10%–90%). The only case without fibrinoid deposition (Case 7) was a mother who had recovered from COVID‐19 infection by the time of delivery, 10 weeks after confirmed infection. Another finding was the presence of intervillositis with acute inflammatory cells mixed with chronic inflammatory cells dominated by histiocyte‐like cells (CD68+), which was present in almost all placentas (13 of 14 placentas, 93%), Case 7 again being the exception (described in detail below). The degree of intervillous inflammation, which was of mixed inflammatory cell type (11 of 14 cases, 79%), varied between the different cases and also geographically within the parenchyma of each placental disc.
3.1. Stillborn infants
Four of the five women (80%) presented in the third trimester and had experienced reduced fetal movements, and three of these women (60%) had even experienced painful uterine contractions (Table 1). The majority of the mothers had no significant comorbidities and only one had an abnormal BMI (Case 3: 31 kg/m2). All five women had been concomitantly diagnosed with COVID‐19 using an RT‐qPCR except for Case 5, who had positive IgG antibodies for SARS‐CoV‐2. Two of the stillborn infants tested positive for SARS‐CoV‐2 (Case 1: positive lung tissue at autopsy; Case 3: positive swab obtained from heart puncture), while the RT‐qPCR from the infant in Case 5 showed a cycle threshold value of 36.5, just above the threshold for a positive test. Of note, none of the stillborn infants was small‐for‐gestational age (SGA). 38 The onset of maternal signs and symptoms preceded IUFD diagnosis from 2 to 25 days, with a mean of 11 days, prior to diagnosis of fetal demise.
RT‐qPCR for SARS‐CoV‐2 in placental tissue was positive in all cases (100%). Gross examination of the placentas from three of the five stillbirths (60%) showed that they were underweight in relation to the gestational week of the pregnancy (placental weight <10th percentile for gestational age). 34 The syncytiotrophoblast of all five cases (100%) stained positive for SARS‐CoV‐2 nucleocapsid protein. Furthermore, all cases showed extensive fibrinoid deposition in the intervillous spaces (100%), from borderline to massive parenchyma involvement (Table 1; Figure 1). All five placentas demonstrated intervillositis (also termed chronic histiocytic intervillositis) composed of a mixture of acute and chronic inflammation, with some placentas showing a predominance of histiocytes (Cases 1, 4 and 5) and the others having predominantly neutrophils in the intervillous inflammatory infiltrate (Cases 2 and 3; Figure 1). Trophoblast necrosis was present in all five placentas (100%). Of note, cases varied with regard to the degree of intervillous inflammation. This was illustrated in the placentas from Cases 1, 4 and 5 where there was MPFD and the extent of chronic histiocytic intervillositis appeared to be more severe compared with Cases 6 and 8 (live‐born infants, see below) (Figure 1), where borderline fibrinoid deposition was associated with less severe intervillositis.
FIGURE 1.
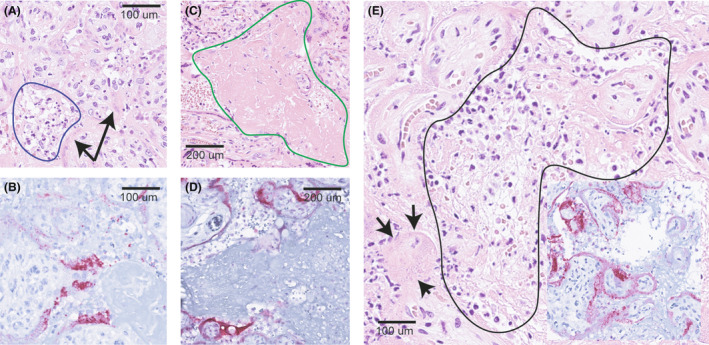
Histopathology of SARS‐CoV‐2 positive placentas. (A) Placental parenchyma stained with haematoxylin–eosin (HE) from Case 8 (live‐born) showing acute intervillositis manifested by a mixed inflammatory infiltrated dominated by polymorphonuclear granulocytes in the intervillous space (blue demarcation) along with degeneration of villous trophoblasts (arrows). (B) Positive stain for SARS‐CoV‐2 nucleocapsid protein in villous trophoblasts in the same region as in (A) from Case 8. (C) HE‐stain from Case 6 (live‐born) demonstrating massive fibrinoid deposition in the intervillous space (green demarcation). (D) Positive stain for SARS‐CoV‐2 nucleocapsid protein in villous trophoblasts from the same region as in (C) in Case 6. (E) HE‐stain of Case 3 (stillborn) showing acute intervillositis among reticulate deposition of fibrinoid (black demarcation) surrounded by degenerating villous trophoblasts (arrows). Inset shows positivity for SARS‐CoV‐2 nucleocapsid protein in the same region
Maternal vascular malperfusion including abnormalities such as placental hypoplasia, 34 placental infarction and retroplacental haemorrhage were found in Cases 1, 2 and 5 (60%). Villous stromal‐vascular karyorrhexis, represented by erythrocyte extravasation and fragmentation, was noted in two cases (Cases 1 and 2). Similarly, nucleated fetal erythrocytes signalling fetal hypoxia were observed in the fetal chorionic vasculature of two cases (Cases 3 and 4).
3.2. Live‐born infants
Six of the nine (67%) women with live‐born infants presented with reduced fetal movements as their major reason for seeking healthcare. Cardiotocography (CTG) tracings from Cases 6, 9, 11 and 14 (Figure S1) showed decreased variability in the fetal heart rate tracing. Late, prolonged decelerations portraying a pathological pattern 39 were seen in six of the nine live‐born infants (67%) leading to emergency CS in which intrauterine hypoxia was confirmed by umbilical cord blood sampling (Table 2). Of note, all pregnancies were terminated before term with CS (100%). Low Apgar scores were also noted in eight of the nine live‐born cases (89%). Routine SARS‐CoV‐2 testing in infants using RT‐qPCR within 24 hours of birth showed a positive result in Cases 9 and 14. Intrauterine (congenital) transmission was confirmed in Case 9 using viral genomic testing of the mother and neonate. 16
At birth, five of the live‐born infants (56%) were SGA. The timing from onset of maternal COVID‐19 symptoms to emergency CS due to signs of fetal distress varied from 3 to 12 days (mean: 6 days), with the exception of Case 7 (73 days, details below).
Similar to stillborn infants, five of the nine placentas were underweight (56%) and all showed borderline to massive fibrinoid deposition (mean 51%). Varying grades of intervillositis were seen. Focal intervillositis in which the inflammatory infiltrate was predominantly neutrophilic was seen in Cases 8 and 10, both female infants born by emergency CS in the third trimester of pregnancy due to pathological CTG changes. Intervillositis with a predominance of histiocytes was present in Cases 11, 12, 13 and 14 (Figure S2). Trophoblast necrosis was present in eight of nine placentas (89%).
Case 7 had both a unique clinical history as well as a distinct placental pathology. Ultrasonography evaluation at 24+6 weeks gestation showed severe oligohydramnios and intrauterine growth restriction (IUGR) with an estimated fetal weight of 496 g (−37%), pathological umbilical artery blood flow class (BFC) 3A 40 and signs of fetal brain sparing. 41 Maternal uterine artery blood flow was, however, unaffected. Three weeks previously, the mother had tested positive for SARS‐CoV‐2 with minor symptoms (fever). She was admitted to the prenatal unit for further testing and fetal observation. There were no CTG changes of concern, but two doses of 12 mg betamethasone were administered with a 24‐hour interval to enhance fetal lung maturity. Serology for TORCH agents including cytomegalovirus, toxoplasmosis, rubella, herpes simplex and parvovirus were negative. Repeated blood flow velocimetry 2 days later showed slight improvement, with BFC 2, which was expected secondary to betamethasone administration, returning to BFC 3A after another 3 days. Chorionic villus sampling was performed as part of clinical routine to investigate possible genetic/chromosomal abnormalities as a cause of the very low estimated fetal weight and a normal Array‐CGH (Comparative Genomic Hybridisation) was confirmed. The patient was discharged at 27+2 weeks with planned biweekly check‐ups. Fetal growth had improved slightly at 28+6 weeks with an estimated fetal weight of 945 g (−33%). Umbilical artery blood flow had also improved to BFC 1 at 29+2 and remained stable until 31+2, when it deteriorated to BFC 2. The patient was readmitted and received a 12‐mg rescue dose of betamethasone at 31+2 weeks, and 6 g magnesium sulphate for fetal neuroprotection, whereafter an uncomplicated CS was performed. The placenta was small for gestational week 31 but lacked fibrinoid depositions. Instead, old infarcts were observed with plasma cell infiltration in both the decidua capsularis and basalis. Additionally, immunohistochemistry for SARS‐CoV‐2 was focally positive in the placenta.
Cases 12 and 13 relate to a woman spontaneously pregnant with diamniotic dichorionic twins. With a prior history of pre‐eclampsia, she contacted the local maternity centre complaining of reduced fetal movements and cold‐like symptoms, and a positive SARS‐CoV‐2 test. After an assessment of the mother's well‐being and a normal CTG of the fetuses, she was sent home with a prescription for low molecular weight heparin (7500 IE per day) for 2 weeks as per the recommendation of the Swedish Society of Obstetrics and Gynaecology. 42 Four days after her positive SARS‐CoV‐2 test, the patient’s blood pressure had risen to 145/100 and laboratory tests revealed that her platelet count had fallen from 46 × 109/litre to 26 × 109/litre, 7 hours later. It was decided to perform a CS with general anaesthesia following administration of a platelet concentrate. However, during the surgery, the patient experienced an acute fall in her oxygen saturation to 80% (suspected bronchospasm secondary to asthma and COVID‐19 infection). After the delivery of the infants, the woman was given betamethasone to induce bronchorelaxation. Twin 1 was a female born without signs of spontaneous life and having Apgar scores of 0, 0 and 1 at 1, 5 and 10 minutes, respectively. Cord blood gases, presumably from the umbilical vein, revealed a pH of 6.69 and base excess −22. Twin 2, a boy, had Apgar scores of 4, 7 and 8 at 1, 5 and 10 minutes, respectively. Twin 1 suffered from hypoxic ischaemic encephalopathy grade III and intensive care was discontinued 6 days after birth. Placental pathology from Twin 1 demonstrated MPFD with necrosis of chorionic villi that constituted more than 90% of the placental volume. Widespread chronic histiocytic intervillositis and signs of vascular malperfusion with acute atherosis and thrombosis in maternal decidual vessels was also observed. The placenta of Twin 2 was less affected, showing an estimated 40% of the placental volume covered by perivillous fibrinoid deposits (Table 2).
3.3. Congenital transmission
Categories of SARS‐CoV‐2 transmission were defined according to Shah et al.,35 with congenital infection with intrauterine fetal death ‘confirmed’ in Cases 1–5 where detection of the virus by RT‐qPCR from placental tissue was seen in all cases and from fetal tissue seen in Cases 1 and 3 (Table 1). Similarly, congenital infection in live‐born neonates was ‘confirmed’ in Cases 9 (viral genomic testing) and ‘probable’ infection in Cases 6–8 and 10–14 (Table 2). Associating the placental findings from the six confirmed cases of congenital transmission (Case 1–5 and 9) included a triad of MPFD, intervillositis and trophoblast necrosis. The intensity of fibrinoid deposition in the placental parenchyma varied from 25 to 90%. Maternal vascular malperfusion and karyorrhexis was seen in Cases 1, 2 and 5.
4. DISCUSSION
4.1. Main findings
This nationwide Swedish case series demonstrated the clinical outcomes and placental pathology in a unique series of women with SARS‐CoV‐2 infection in pregnancy presenting with fetal compromise. The majority of the women presented with a cardinal clinical symptom, namely, reduced fetal movements in the third trimester. In the case of a living fetus at the time of presentation, signs of fetal distress included pathological CTG patterns or umbilical blood flow changes that led to immediate termination of the pregnancies by CS. SARS‐CoV‐2 infection was the causative agent associated with fetal compromise in all cases leading to rapid placental dysfunction and intrauterine demise in five of the 14 cases reported, with two neonatal deaths. A triad of placental features were associated with the infection, namely, trophoblast degeneration, intervillositis and massive perivillous fibrinoid deposits.
4.2. Strengths and limitations
To the best of our knowledge this is one of the largest case series on placental dysfunction in SARS‐CoV‐2 mothers with fetal compromise and congenital transmission. Another strength was that cases were collected from maternity centres throughout Sweden. This was of considerable importance, as the study was reporting on rare events such as congenital transmission. The findings from six confirmed cases of SARS‐CoV‐2 congenital transmission were reported, giving us the unique opportunity to stratify clinical outcomes and placental features in this special group of patients. Similarly, COVID‐19 infection was confirmed in all cases using RT‐qPCR, leaving little doubt about the cause of placental dysfunction.
Limitations of this study include its retrospective nature and the lack of a control group of mothers that had presented with COVID‐19 without fetal impairment. Lack of biological samples including cord blood and amniotic fluid could also have aided in the classification of the ‘probable’ cases of congenital transmission. The authors also acknowledge that the vast majority of women presenting with SARS‐CoV‐2 infection during pregnancy have a normal perinatal outcome. 8 , 11 However, the aim of this study was to focus on the small subset of women who do suffer adverse perinatal outcomes and to highlight that SARS‐CoV‐2 can lead to or aggravate concurrent placental dysfunction with intrauterine hypoxia and stillbirth in rare cases.
4.3. Interpretation
Reduced fetal movements have been associated with a higher rate placental lesions and adverse pregnancy outcome as compared with controls. 43 Additionally, accelerating fetal growth in the third trimester puts higher demands on optimal placental function. The fetus may therefore be more vulnerable to acute placental dysfunction in these weeks. 44 The pathological CTG changes seen included decreased variability, repeated episodes of late decelerations or bradycardia and the absence of accelerations, all of which have been associated with impaired uteroplacental function and intrauterine fetal hypoxia. 45 Similarly, low Apgar scores, abnormal umbilical cord arterial and venous pH and lactate confirmed hypoxia prior to birth. 46 , 47 SARS‐CoV‐2 infection was therefore the causative agent associated with fetal compromise in all cases, including the twin pregnancy (Cases 12 and 13) where the death of the Twin 1 may be associated to massive fibrin deposits extending over 90% of the placental parenchyma. Pregnant women with COVID‐19 have been reported to develop a pre‐eclampsia‐like syndrome, which may account for the high blood pressure and falling thrombocyte count noted in the mother with the twin pregnancy. 48
The cases included in the study help elucidate the timing of fetal compromise after the first onset of maternal signs and symptoms of COVID‐19 infection. We noted that, on average, IUFD was diagnosed 11 days and signs of fetal distress were identified 6 days after the onset of maternal COVID‐19 symptoms. This would indicate that placental dysfunction can be triggered in a matter of a few days after COVID‐19 infection, a recently reported finding. 49 Similarly, the placentas of both stillborn and live‐born infants exhibited extensive fibrinoid deposits accompanied by villous necrosis characteristic of MPFD and intervillositis. Indeed, MPFD lesions have been strongly associated with intrauterine growth restriction (IUGR) and stillbirth, 50 , 51 as fibrin deposits obstruct the intervillous spaces so that maternal blood can no longer perfuse the chorionic villi, resulting in villous ischaemia and necrosis. Thus, the pathological features seen in placentas well explain the clinical outcomes of the stillborn and live‐born infants included in the current study. Furthermore, these findings are indicative of a rapid, progressive and widespread destruction of functional placental tissue leading to acute fetal distress and the risk of IUFD within days of COVID‐19 infection in the mother.
A wide spectrum of other pathological features was also seen that included intervillositis in 13 of 14 placentas, as well as findings resulting from maternal vascular malperfusion and trophoblast necrosis. Areas of more severe fibrinoid deposition were associated with a more severe inflammatory response with a mixed population of inflammatory cells being seen in the intervillous spaces. This feature was dissimilar to previous data where placentas with massive fibrinoid deposition appeared to have a less severe onslaught of intervillositis than in cases where the fibrinoid deposition was not as prominent. 23 In light of the spectrum of microscopic abnormalities present in SARS‐CoV‐2 infected placentas, it may be proposed that the changes illustrated may represent a form of villous repair in response to viral involvement of the trophoblast, which was present in all cases in this study.
When comparing placental features, there was no clear evidence that either Hofbauer cells or any other cell type was associated with a greater probability of maternal‐fetal transmission. Similar to Schwartz et al., 27 it seems probable that transplacental infection of the fetus with SARS‐CoV‐2 can occur in the absence of Hofbauer cell involvement. However, all cases having congenital transmission exhibited MPFD that was associated with intervillositis.
Some studies have indicated an increase in the stillbirth rate during the pandemic, 11 , 12 , 13 and newer virus strains have been associated with a significant increase of severe COVID‐19 illness in mothers. 52 This study adds to growing data showing evidence of fetal compromise secondary to SARS‐CoV‐2 related placental dysfunction. 16 , 17 , 18 , 49 In addition to direct virus‐related injury, indirect effects such as limitations in access to antenatal care during the pandemic or reluctance to go to the hospital when needed, for fear of contracting infection, might have caused the increase in stillbirths. Studies on the quality of maternal healthcare services during the pandemic, confirmed disparities in access to antenatal services across Europe 53 and the pandemic hanging as a ‘shadow’ over pregnant women 54 . However, population‐based studies have only confirmed an increase in the number of medically indicated preterm births and shown no increase in spontaneous preterm births or stillbirths in Scandinavia. 14 , 15
Congenital transmission is thankfully a rare event and the placental syncytiotrophoblast layer may therefore act as an effective barrier preventing the cascade of inflammatory events seen with congenital transmission of SARS‐CoV‐2.
5. CONCLUSION
This study showed that SARS‐CoV‐2 infection in pregnant women can lead to placental dysfunction with consequent intrauterine hypoxia, fetal distress and, in some cases, stillbirth. The major symptom for seeking healthcare for women included into this study was reduced fetal movements in the third trimester. In women presenting with living fetuses, monitoring with CTG or umbilical cord blood flow showed abnormal findings indicating intrauterine hypoxia.
Of the placental features, trophoblast degeneration, intervillositis and massive perivillous fibrinoid deposits appear to be associated with placental SARS‐CoV‐2 infection, especially in cases with congenital transmission. Further studies are warranted to explore the mechanisms of virion transfer into and through the placenta and to understand why placental dysfunction occurs only in a small subset of pregnancies.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interest forms are available to view online as supporting information.
AUTHOR CONTRIBUTIONS
MZ and VS conceived the project, MZ performed the literature search, prepared the tables, figures, merged and interpreted all the data and wrote the manuscript draft. MZ, DG, AS, AKW, EW, LI, MN, MB, NP, SH and ÅS contributed with recruitment and description of cases from their departments. DAS contributed to the pathology discussion. DG and NP performed the pathological examination and prepared the figures. All authors critically reviewed the manuscript for important intellectual content and approved it in its final version.
ETHICS APPROVAL
Women provided written informed consent to publication, available upon request. Additional consent was obtained from the women’s partner were appropriate (in live‐born cases). Cases 1, 3, 4, 6, 8, 9, 12, 13 and 14 are part of the COPE study and have given additional specific consent for publication of this case series. The COPE study has been granted national ethical approval by the Swedish Ethical Review Authority (dnr 2020‐02189 and amendments 2020‐02848, 2020‐05016, 2020‐06696, and 2021‐00870). The case study was performed in agreement with principles of the Declaration of Helsinki.
Supporting information
Figure S1
Figure S2
Legends
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ACKNOWLEDGEMENTS
The authors sincerely thank the participating women and partners for giving consent and allowing for the publication of details regarding the cases included in the current study. The authors would like to thank the COVID‐19 in Pregnancy and Early Childhood (COPE) study for helping to recruit the cases presented in this study. The authors also gratefully acknowledge Dr Rodrigo Mitev Munoz, Professor Laszlo Szekely and other researchers from the originating pathology laboratories responsible for analysing the placenta specimens and sharing pictures and analysis data.
Zaigham M, Gisselsson D, Sand A, Wikström A‐K, von Wowern E, Schwartz DA, et al. Clinical‐pathological features in placentas of pregnancies with SARS‐CoV‐2 infection and adverse outcome: case series with and without congenital transmission. BJOG: Int J Obstet Gy. 2022;129:1361–1374. 10.1111/1471-0528.17132
Linked article: This article is commented on by Yves Ville, pp. 1375 in this issue. To view this minicommentary visit https://doi.org/10.1111/1471-0528.17162.
 This article includes Author Insights, a video abstract available at: https://vimeo.com/bjogabstracts/authorinsights17132
This article includes Author Insights, a video abstract available at: https://vimeo.com/bjogabstracts/authorinsights17132
Funding informationMZ was financed by grants from the Swedish state under an agreement between the Swedish government and the county councils, the ALF‐agreement YF0054; and the South Hospital Region Project Grant (2021‐2020‐0689). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker KF, O’Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 2020;127(11):1324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chinn J, Sedighim S, Kirby KA, Hohmann S, Hameed AB, Jolley J, et al. Characteristics and outcomes of women with COVID‐19 giving birth at US academic centers during the COVID‐19 pandemic. JAMA Netw Open. 2021;4(8):e2120456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ortiz‐Prado E, Simbana‐Rivera K, Gomez‐Barreno L, Rubio‐Neira M, Gauman L, Kyriakidis N, et al. Clinical, molecular, and epidemiological characterization of the SARS‐CoV‐2 virus and the coronavirus disease 2019 (COVID‐19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98(1):115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019‐nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karimi L, Vahedian‐Azimi A, Makvandi S, Sahebkar A. A systematic review of 571 pregnancies affected by COVID‐19. Adv Exp Med Biol. 2021;1321:287–98. [DOI] [PubMed] [Google Scholar]
- 10. Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, et al. Pregnancy and neonatal outcomes of COVID‐19: coreporting of common outcomes from PAN‐COVID and AAP‐SONPM registries. Ultrasound Obstet Gynecol. 2021;57(4):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol‐Urganci I, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9(6):e759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalil A, von Dadelszen P, Kalafat E, Sebghati M, Ladhani S, Ugwumadu A, et al. Change in obstetric attendance and activities during the COVID‐19 pandemic. Lancet Infect Dis. 2021;21(5):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stephansson O, Pasternak B, Ahlberg M, Hervius Askling H, Aronsson B, Appelqvist E, et al. SARS‐CoV‐2 and pregnancy outcomes under universal and non‐universal testing in Sweden: register‐based nationwide cohort study. BJOG. 2022;129:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magnus MC, Oakley L, Gjessing HK, Stephansson O, Engjom HM, Macsali F, et al. Pregnancy and risk of COVID‐19: a Norwegian registry‐linkage study. BJOG. 2022;129(1):101–9. 10.1111/1471-0528.16969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaigham M, Holmberg A, Karlberg ML, Lindsjö OK, Jokubkiene L, Sandblom J, et al. Intrauterine vertical SARS‐CoV‐2 infection: a case confirming transplacental transmission followed by divergence of the viral genome. BJOG. 2022;128(8):1388–94. 10.1111/1471-0528.16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun. 2020;11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Facchetti F, Bugatti M, Drera E, Tripodo C, Sartori E, Cancila V, et al. SARS‐CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59:102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoenmakers S, Snijder P, Verdijk RM, Kuiken T, Kamphuis SSM, Koopman LP, et al. Severe acute respiratory syndrome coronavirus 2 Placental infection and inflammation leading to fetal distress and neonatal multi‐organ failure in an asymptomatic woman. J Pediatric Infect Dis Soc. 2021;10(5):556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts DJ, Edlow AG, Romero RJ, Coyne CB, Ting DT, Hornick JL, et al. A standardized definition of placental infection by SARS‐CoV‐2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS‐CoV‐2 Placental Infection Workshop. Am J Obstet Gynecol 2021;225(6)593.e1–593.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naidu SAG, Clemens RA, Pressman P, Zaigham M, Davies KJA, Naidu AS. COVID‐19 during pregnancy and postpartum. J Diet Suppl. 2020;8:1–37. [DOI] [PubMed] [Google Scholar]
- 22. Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz DA, Levitan D. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infecting pregnant women and the fetus, intrauterine transmission, and Placental pathology during the coronavirus disease 2019 (COVID‐19) pandemic: It’s complicated. Arch Pathol Lab Med. 2021;145(8):925–8. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz DA, Baldewijns M, Benachi A, Bugatti M, Collins RRJ, De Luca D, et al. Chronic Histiocytic Intervillositis with Trophoblast necrosis is a risk factor associated with Placental infection from coronavirus disease 2019 (COVID‐19) and intrauterine maternal‐fetal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission in live‐born and stillborn infants. Arch Pathol Lab Med. 2021;145(5):517–28. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz DA, Morotti D. Placental pathology of COVID‐19 with and without fetal and neonatal infection: Trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS‐CoV‐2. Viruses. 2020;12(11):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poisson TM, Pierone G Jr. Placental pathology and fetal demise at 35 weeks of gestation in a woman with SARS‐CoV‐2 infection: a case report. Case Rep Womens Health. 2021;30:e00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz DA, Baldewijns M, Benachi A, Bugatti M, Bulfamante G, Cheng K, et al. Hofbauer cells and coronavirus disease 2019 (COVID‐19) in pregnancy: molecular pathology analysis of villous macrophages, endothelial cells, and placental findings from 22 placentas infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) with and without fetal transmission. Arch Pathol Lab Med. 2021;45(11):1328–40. [Google Scholar]
- 28. Beesley MA, Davidson JR, Panariello F, Shibuya S, Scaglioni D, Jones BC, et al. COVID‐19 and vertical transmission: assessing the expression of ACE2/TMPRSS2 in the human fetus and placenta to assess the risk of SARS‐CoV‐2 infection. BJOG. 2022;129(2):256–66. 10.1111/1471-0528.16974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gengler C, Dubruc E, Favre G, Greub G, de Leval L, Baud D. SARS‐CoV‐2 ACE‐receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27(3):489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlsson Y, Bergman L, Zaigham M, Linden K, Andersson O, Veje M, et al. COVID‐19 in pregnancy and early childhood (COPE): study protocol for a prospective, multicentre biobank, survey and database cohort study. BMJ Open. 2021;11(9):e049376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. JAMA. 2020;323(21):2198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Swedish Public Health Authority . Recommendations for laboratory handling of patient samples with COVID‐19. Updated 2020‐09‐22. Available at: https://www.folkhalsomyndigheten.se/smittskydd‐beredskap/utbrott/aktuella‐utbrott/covid‐19/information‐till‐varden/rekommendationer‐for‐laboratoriehantering‐av‐patientprover‐med‐avseende‐pa‐covid‐19/
- 33. Kraus F, Redline R, Gersell D, Nelson M, Dicke J. Placental Pathology. American Registry of Pathology (2005).
- 34. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz DA, Morotti D, Beigi B, Moshfegh F, Zafaranloo N, Patanè L. Confirming vertical fetal infection with coronavirus disease 2019: neonatal and pathology criteria for early onset and Transplacental transmission of severe acute respiratory syndrome coronavirus 2 from infected pregnant mothers. Arch Pathol Lab Med. 2020;144(12):1451–6. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz DA, Thomas KM. Characterizing COVID‐19 maternal‐fetal transmission and placental infection using comprehensive molecular pathology. EBioMedicine. 2020;60:102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–8. [DOI] [PubMed] [Google Scholar]
- 38. SFOG . CTG kort slutversion Sweden: SFOG; 2009. Available from: https://www.sfog.se/media/17094/ctg_kort__slutversion.pdf. Accessed September 21, 2021.
- 39. Laurin J, Lingman G, Marsál K, Persson PH. Fetal blood flow in pregnancies complicated by intrauterine growth retardation. Obstet Gynecol. 1987;69(6):895–902. [PubMed] [Google Scholar]
- 40. Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol. 2016;594(5):1215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swedish Society for Obstetrics and Gynecology (SFOG) . Recommendations for care of pregnant women with confirmed or suspected COVID‐19 (Article in Swedish). Available from: https://www.sfog.se/media/337503/sfog‐raad‐avseende‐gravida‐under‐raadande‐covid‐19‐pandemi‐210604.pdf. Accessed September 21, 2021
- 42. Levy M, Kovo M, Izaik Y, Luwisch Cohen I, Schreiber L, Ganer Herman H, et al. Reduced fetal movements at term in singleton low risk pregnancies‐is there an association with placental histopathological findings? Acta Obstet Gynecol Scand. 2020;99(7):884–90. [DOI] [PubMed] [Google Scholar]
- 43. Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chandraharan E, Arulkumaran S. Prevention of birth asphyxia: responding appropriately to cardiotocograph (CTG) traces. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):609–24. [DOI] [PubMed] [Google Scholar]
- 45. Zaigham M, Källén K, Olofsson P. Gestational age‐related reference values for Apgar score and umbilical cord arterial and venous pH in preterm and term newborns. Acta Obstet Gynecol Scand. 2019;98(12):1618–23. [DOI] [PubMed] [Google Scholar]
- 46. Wiberg N, Källén K, Herbst A, Olofsson P. Relation between umbilical cord blood pH, base deficit, lactate, 5‐minute Apgar score and development of hypoxic ischemic encephalopathy. Acta Obstet Gynecol Scand. 2010;89(10):1263–9. [DOI] [PubMed] [Google Scholar]
- 47. Mendoza M, Garcia‐Ruiz I, Maiz N, Rodo C, Garcia‐Manau P, Serrano B, et al. Pre‐eclampsia‐like syndrome induced by severe COVID‐19: a prospective observational study. BJOG. 2020;127(11):1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bouachba A, Allias F, Nadaud B, Massardier J, Mekki Y, Bouscambert Duchamp M, et al. Placental lesions and SARS‐Cov‐2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katzman PJ, Genest DR. Maternal floor infarction and massive Perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev. 2002;5:159–64. [DOI] [PubMed] [Google Scholar]
- 50. Andres RL et al. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163:935–8. [DOI] [PubMed] [Google Scholar]
- 51. Donati S, Corsi E, Maraschini A, Salvatore MA, ItOSS‐COVID‐19 Working Group . SARS‐CoV‐2 infection among hospitalised pregnant women and impact of different viral strains on COVID‐19 severity in Italy: a national prospective population‐based cohort study. BJOG. 2022;129(2):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lazzerini M, Benedetta C, Ilaria M, Zalka D, Maryse A, Ingvild HN, et al. Quality of facility‐based maternal and newborn care around the time of childbirth during the COVID‐19 pandemic: online survey investigating maternal perspectives in 12 countries of the WHO European Region. Lancet Regional Health Europe. 2022;13:100268. 10.1016/j.lanepe.2021.100268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zaigham M, Linden K, Sengpiel V, Mariani I, Valente EP, Covi B, Lazzerini M, Elden H. IMAgiNE EURO Study Group . Large gaps in the quality of healthcare experienced by Swedish mothers during the COVID‐19 pandemic: A cross‐sectional study based on WHO standards. Women Birth 2022: Jan 24:S1871‐5192(22)00010‐5. 10.1016/j.wombi.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Linden K, Domgren N, Zaigham M, Sengpiel V, Andersson ME, Wessberg A. Being in the shadow of the unknown ‐ Swedish women’s lived experiences of pregnancy during the COVID‐19 pandemic, a phenomenological study. Women Birth 2021:S1871‐5192(21)00159–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Legends
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


