Abstract
Among coronavirus disease 2019 (COVID‐19) patients, physically active individuals may be at lower risk of fatal outcomes. However, to date, no meta‐analysis has been carried out to investigate the relationship between physical activity (PA) and fatal outcomes in patients with COVID‐19. Therefore, this meta‐analysis aims to explore the hospitalisation, intensive care unit (ICU) admissions, and mortality rates of COVID‐19 patients with a history of PA participation before the onset of the pandemic, and to evaluate the reliability of the evidence. A systematic search of MEDLINE/PubMed, Cumulative Index to Nursing and Allied Health Literature, Scopus, and medRxiv was conducted for articles published up to January 2022. A random‐effects meta‐analysis was performed to compare disease severity and mortality rates of COVID‐19 patients in physically active and inactive cases. Twelve studies involving 1,256,609 patients (991,268 physically active and 265,341 inactive cases) with COVID‐19, were included in the pooled analysis. The overall meta‐analysis compared with inactive controls showed significant associations between PA with reduction in COVID‐19 hospitalisation (risk ratio (RR) = 0.58, 95% confidence intervals (CI) 0.46–0.73, P = 0.001), ICU admissions (RR = 0.65, 95% CI 0.52–0.81, P = 0.001) and mortality (RR = 0.47, 95% CI 0.38–0.59, P = 0.001). The protective effect of PA on COVID‐19 hospitalisation and mortality could be attributable to the types of exercise such as resistance exercise (RR = 0.27, 95% CI 0.15–0.49, P = 0.001) and endurance exercise (RR = 0.41, 95% CI 0.23–0.74, P = 0.003), respectively. Physical activity is associated with decreased hospitalisation, ICU admissions, and mortality rates of patients with COVID‐19. Moreover, COVID‐19 patients with a history of resistance and endurance exercises experience a lower rate of hospitalisation and mortality, respectively. Further studies are warranted to determine the biological mechanisms underlying these findings.
Keywords: COVID‐19, exercise, meta‐analysis, physical activity
Abbreviations
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- ICU
Intensive care unit
- MET
Metabolic Equivalent of Task
- PA
Physical activity
- PRISMA
Preferred Reporting Items for Systematic Review and Meta‐Analyses
- RR
risk ratio
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The rapid spread of coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus two (SARS‐CoV‐2) has led to 282 million confirmed cases and 5.4 million deaths. 1 A high rate of transmission of SARS‐CoV‐2 and mortality owing to COVID‐19 is mainly due to emerging new variants, which make efforts less effective in the fight against the virus. 2 However, a variety of public health interventions such as government policy, mask‐wearing, and vaccination have been implemented worldwide to mitigate and control the spread of the outbreak of COVID‐19 disease. 3 Restrictive measures to prevent the spread have resulted in difficulties for the population to maintain healthy lifestyles such as the engagement in recommended levels of physical activity (PA). 4 Meyer et al. 5 reported 30% reduction in PA during COVID‐19 quarantine independent of sex and age. Specific home‐based PA recommendations have been recently published in an attempt to take advantage of both quarantine and staying physically active. 6 , 7 Adherence to government PA guidelines during the COVID‐19 pandemic has been strongly recommended. Studies have shown that potential outcomes from leading an unhealthy lifestyle, such as hypertension, diabetes, obesity, and cardiovascular disease (CVD) increase the risk of SARS‐CoV‐2 infection as well as the severity and mortality rate. 8 Importantly, obesity and hypertension were the most prevalent disorders reported in hospitalised and deceased patients due to COVID‐19. 9 , 10 , 11
Several studies have shown that a baseline sedentary lifestyle increases the mortality of hospitalised patients with COVID‐19. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Moreover, engaging in healthy lifestyle behaviours may protect against the most severe consequences of COVID‐19 disease including systemic inflammation, and reduced quality of life. 12 , 20 , 21 Importantly, an unhealthy lifestyle has been considered a risk factor for COVID‐19 hospital admission. 15 Different mechanisms may explain the protective effect of PA on COVID‐19 outcomes and disease severity. 14 Regular PA improves immune function, and regularly active individuals have a lower incidence, intensity of symptoms, and mortality from COVID‐19 and other various viral infections. 22 , 23 , 24 , 25 Moreover, regular PA reduces the risk of systemic inflammation, which is considered the primary contributor to lung damage in COVID‐19 patients. 26 Additionally, it has a protective impact on COVID‐19 risk factors such as obesity and hypertension. 9 , 14 Furthermore, we previously reported that a sedentary lifestyles increase the risk of COVID‐19 severity and mortality. 16 Further, high hospitalisation rates have been reported in patients with less cardiorespiratory fitness. 27
Given this mortality risk in physically inactive COVID‐19 patients, this meta‐analysis aims to explore the hospitalisation, intensive care unit (ICU) admissions, and mortality rates of COVID‐19 patients with a history of PA participation before the onset of the pandemic.
2. METHODS
The present study was carried out in accordance with methodological guidelines from the Cochrane Handbook for Systematic Reviews. 28 The present study's findings were reported in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analyses statement (Supplementary Material S1). 29
2.1. Search strategy
Relevant studies were systematically searched in electronic databases including MEDLINE/PubMed, Cumulative Index to Nursing and Allied Health Literature, Scopus, and medRxiv by two researchers (MA and FM) up to January 2022. The search strategy was as follows: (“severe acute respiratory syndrome coronavirus 2” or “novel coronavirus” or “COVID‐19” or “2019‐nCoV” or “SARS‐CoV‐2”) and (“survival” or “fatal outcome” or “mortality” or “death” or “hospitalisation” or “intensive care”) and (“physical activity,” or “exercise training,” or “physical training,” or “exercise activity”; Supplementary Material S2). Furthermore, we searched all reference lists of included studies for any other eligible articles. Language restriction was not considered.
2.2. Eligibility criteria
The Eligibility criteria followed the PICOs question. 30 In prospective and cross‐sectional studies, we included studies that examine the relationship between PA and COVID‐19 clinical outcomes and have reported at least one of the following outcomes: COVID‐19 related mortality, hospitalisation, and ICU admission. Furthermore, editorials, letters, commentaries, and abstracts with insufficient data were excluded from the present meta‐analysis.
2.3. Data extraction
First, titles and abstracts of all retrieved articles were screened by two investigators (M.A., F.M.) for relevance. Second, the relevant full‐text articles were reviewed for inclusion and the following data were extracted from eligible studies, where available: study design, country, PA documentation, age and gender, relative outcomes, and comorbidity factors. In all stages, discrepancies were resolved through discussion before conducting meta‐analysis.
2.4. Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of studies. The NOS for cohort studies includes 3 domains (quality of selection, comparability, quality of outcome, and adequacy of follow‐up), with a maximum score of nine points. 31 Studies with NOS scores of 0–3, 4–6, and 7–9 were considered low, moderate, and high quality, respectively. 32
2.5. Subgroup analysis
We also performed a subgroup analysis to determine the effect of PA levels on our study outcomes based on Metabolic Equivalent of Task (MET) minutes per week. Low and moderate‐vigorous PA levels were classed as achieving less than or equal to 500 and higher than 500 MET‐min per week, respectively. 13 , 16 , 33 Additionally, we performed another subgroup analysis to determine the effect of PA induced‐adaptation on our study outcomes based on types of exercise related to endurance exercise, resistance exercise, and combined training adaptations.
2.6. Statistical analyses
All meta‐analyses were conducted using Review manager (Version 5.4, The Nordic Cochrane Centre, Copenhagen, The Cochrane Collaboration, 2014). Dichotomous outcomes were pooled and expressed as risk ratios (RRs) with 95% confidence intervals (CI). 34 The pooled analysis results were classified based on study types into two categories, prospective cohorts and cross‐sectional and the pooled RRs were estimated using the random‐effect model. Heterogeneity was calculated using Cochran's Q statistics and I2. I2 from zero to 24%, 25%–49%, 50%–74% and 75%–100% were interpreted as low, moderate, substantial and considerable heterogeneity. 34 Funnel plots with Egger weighted regression test were used for assessing publication bias using STATA version 16. Finally, the overall pooled prevalence of the respective outcomes was re‐estimated by the one study removed methods to perform sensitivity analysis.
3. RESULTS
3.1. Study identification and characteristics
A total of 1956 potentially relevant articles were identified in our literature search. Four hundred and 60 studies remained after removing duplicates. After screening titles and abstracts, 1397 research articles were excluded. Of 33 obtained research articles, another 21 articles were excluded (no sufficient data (n = 8); editorial or news (n = 2) and reviews (n = 11); Supplementary Table S2). 35 Finally, 12 articles met the eligibility criteria and were included in the meta‐analysis (Figure 1). The characteristics of the included studies are listed in Table 1. Twelve studies involving 1,256,609 cases (991,268 physically active cases and 265,341 physically inactive cases) were included in the meta‐analysis. Publication ranged from 2020 to 2021 and the majority of these were from European countries, Iran, China, US, Korea, and Brazil; Characteristics of comorbidity for different groups among the included studies were reported in four studies 16 , 17 , 18 , 19 and are listed in Table 2. All included studies were of high quality with NOS scores equal to or greater than 7 (Table 3). The designs of the included studies were as follows: cohort (n = 6) and cross‐sectional (n = 6) and we performed a subgroup analysis based on different study types.
FIGURE 1.

PRISMA flow diagram of study selection
TABLE 1.
General characteristics of included studies
| Study | Design | Country | Physical activity documentation | Age (year) | Gender | COVID‐19 diagnosis | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Group (n) | Hospitalisation, n (%) | ICU admissions, n (%) | Mortality, n (%) | |||||||
| Ahmadi et al. 2021 12 | Community‐based cohort | UK | International physical activity questionnaire | 56.5 ± 8.1 | F = 255,838 | RT‐PCR | Inactive (92,221) | NR | NR | 112 (12%) |
| M = 212,731 | Insufficient (140,609) | 115 (0.08%) | ||||||||
| Sufficient (232,603) | 160 (0.06%) | |||||||||
| Cho et al. 2021 13 | Nationwide case‐control | Korea | Self‐reported questionnaire | 50.7 ± 14.3 | F = 3832 | RT‐PCR | Physically inactive (1313) | NR | NR | 31 (33.7%) |
| M = 2456 | Light (1752) | 27 (29.3%) | ||||||||
| Moderate (861) | 4 (4.3%) | |||||||||
| Vigorous (2362) | 13 (14.1%) | |||||||||
| Moderate to vigorous (3223) | 17 (18.5%) | |||||||||
| de Souza et al. 2021 36 | Cross‐sectional | Brazil | International physical activity questionnaire | 18–80 | F = 658 | RT‐PCR | None (485) | 36 (13.8%) | NR | NR |
| M = 371 | 1 times/week (192) | 19 (9.9%) | ||||||||
| ≥2 times/week (261) | 36 (7.4%) | |||||||||
| Ekblom‐Bak et al. 2021 14 | Case‐control | Sweden | Self‐reported questionnaire | 49.9 ± 10.7 | F = 254 | RT‐PCR | Never/irregular (293) | 181 (36%) | 67 (43%) | 45 (36%) |
| M = 603 | 1–2 times/week (254) | 157 (32%) | 49 (31%) | 48 (38%) | ||||||
| ≥3 times/week (232) | 159 (32%) | 41 (26%) | 32 (26%) | |||||||
| Halabchi et al. 2021 8 | Cross‐sectional | Iran | Electronic health record | 492.3 ± 11.9 | F = 2629 | RT‐PCR | Inactive (4445) | 820 (18.4) | 58 (1.3) | 79 (1.8) |
| M = 2065 | Active (249) | 28 (11.2) | 2 (0.8) | 0 (0) | ||||||
| Hamer et al. 2020 15 | Community‐based cohort | UK | International physical activity questionnaire | 57.1 ± 9.0 | F = 173,038 | RT‐PCR | None (68,913) | 186 (27%) | NR | NR |
| M = 214,071 | Insufficient (108,707) | 192 (17%) | ||||||||
| Sufficient (209,489) | 382 (18%) | |||||||||
| Hamrouni et al. 2021 38 | Prospective cohort | UK | International physical activity questionnaire | 37–73 | F = 135,884 | RT‐PCR | Low (47,827) | NR | NR | 109 (27%) |
| M = 123,603 | Moderate (105,564) | 150 (38%) | ||||||||
| High (106,006) | 138 (34%) | |||||||||
| Lee et al. 2021 16 | Nationwide cohort | Korea | Personal medical interview | 20–60 | F = 37,272 | RT‐PCR | Insufficient training (41,293) | NR | 273 (21.1) | 32 (2.5) |
| M = 39,123 | Resistance training (18,994) | 25 (16.7) | 0 (0.0) | |||||||
| Endurance training (5036) | 109/561 (19.4) | 11 (2.0) | ||||||||
| Combined training (11,072) | 39/291 (13.4) | 2 (0.7) | ||||||||
| Maltagliati et al. 2021 37 | Cross‐sectional | 27 European countries | Self‐reported questionnaire | 69.3 ± 8.5 | F = 1763 | RT‐PCR | Hardly ever or never (1167) | 36 (54%) | NR | NR |
| M = 1376 | 1 times/week (541) | 10 (15%) | ||||||||
| >1 times/week (1161) | 15 (23%) | |||||||||
| 1–3 times/month (270) | 5 (/%) | |||||||||
| Salgado‐Aranda et al. 2021 17 | Retrospective cohort | Spain | Rapid physical activity questionnaire | 54.3 ± 10.7 | F = 236 | RT‐PCR | Inactive (297) | NR | 26 (8.8%) | 41 (13.8%) |
| M = 284 | Active (223) | 14 (6.3%) | 4 (1.8%) | |||||||
| Sallis et al. 2021 18 | Retrospective observational cohort | US | Electronic health record | 47.5 ± 16.97 | F = 29 992 | RT‐PCR | Consistently inactive (6984) | 732 (10.5%) | 195 (2.8%) | 170 (2.4%) |
| M = 18 447 | Some activity (38 338) | 3405 (8.9%) | 972 (2.5%) | 590 (1.5%) | ||||||
| Consistently meeting PA guidelines (3118) | 99 (3.2%) | 32 (1%) | 11 (0.4%) | |||||||
| Yuan et al. 2021 19 | Cross‐sectional | China | Personal medical interview | 61.8±13.6 | F = 80; M = 84 | RT‐PCR | Inactive (103) | NR | 26 (25.2) | 6 (5.8) |
| Active (61) | 3 (4.9) | 0 (0.0) | ||||||||
Abbreviations: NR, not reported; RT‐PCR, reverse transcription polymerase chain reaction.
TABLE 2.
Characteristics of comorbidity for different groups among the included studies
| Study | Comorbidity factor | ||||||
|---|---|---|---|---|---|---|---|
| Group (n) | BMI, mean (SD) | Diabetes, n (%) | CVD, n (%) | Hypertension, n (%) | COPD, n (%) | Smoker, n (%) | |
| Lee et al. 2021 16 | Insufficient training (41,293) | 23.8 (3.9) | 3738 (9.1) | 1372 (3.3) | 8245 (20.0) | NR | 7130 (17.3) |
| Strength training (18,994) | 23.7 (3.3) | 355 (7.1) | 151 (3.0) | 832 (16.5) | 934 (18.6) | ||
| Aerobic training (5036) | 24.1 (3.8) | 1745 (9.2) | 601 (3.2) | 3866 (20.4) | 3382 (17.8) | ||
| Combined training (11,072) | 24.1 (3.5) | 680 (6.1) | 233 (2.1) | 1585 (14.3) | 2230 (20.1) | ||
| Salgado‐Aranda et al. 2021 17 | Inactive (297) | NR | 44 (14.8) | 10 (3.4) | 107 (36) | 20 (6.7) | 20 (6.7) |
| Active (223) | 25 (11.2) | 6 (2.7) | 55 (24.7) | 5 (2.2) | 8 (3.6) | ||
| Sallis et al. 2021 18 | Consistently inactive (6984) | 32.2 (7.39) | 2665 (14.9) | 689 (16.5) | 1682 (15.6) | 788 (14.5) | 1558 (15.5) |
| Some activity (38,338) | 31.3 (7.06) | 15,133 (81.1) | 3410 (81.6) | 8827 (81.7) | 4449 (81.7) | 8008 (79.6) | |
| Consistently meeting PA guidelines (3118) | 28.2 (5.45) | 851 (3.4) | 82 (2) | 297 (2.7) | 210 (3.9) | 492 (4.9) | |
| Yuan et al. 2021 19 | Inactive (103) | NR | 19 (18.4) | 14 (13.6) | 37 (35.9) | 10 (9.7) | 9 (8.7) |
| Active (61) | 12 (19.7) | 4 (6.6) | 15 (24.6) | 2 (3.3) | 8 (13.1) | ||
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; NR, not reported.
TABLE 3.
Summary of the Newcastle‐Ottawa scale for bias assessment of included studies
| Cohort study | Selection (4) | Comparability (2) | Outcome (3) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Representativeness of exposed cohort | Selection of non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of study | Study control for age and sex | Additional factors; controlled for ≥ 2 variables including comorbidities | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow up of cohorts | 9 |
| Ahmadi et al. 2021 12 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Hamer et al. 2020 15 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Hamrouni et al. 2021 38 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Lee et al. 2021 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Salgado‐Aranda et al. 2021 17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sallis et al. 2021 18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Cross‐sectional study | Selection (5) | Comparability (2) | Outcome (3) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Author | Representativeness of the sample | Sample size | Non‐respondents | Ascertainment of exposure | The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled. | Assessment of the outcome | Statistical test | 10 |
| de Souza et al. 2021 36 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| Maltagliati et al. 2021 37 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Yuan et al. 2021 19 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 8 |
| Cho et al. 2021 13 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Ekblom‐Bak et al. 2021 14 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| Halabchi et al. 2021 8 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
3.2. Physical activity and the risk of coronavirus disease 2019 hospitalisation
Six studies involving 441,651 cases (360,605 physically active cases and 81,046 control cases) reported COVID‐19 hospitalisation. 8 , 14 , 15 , 18 , 36 , 37 Overall, PA was significantly associated with a reduction in COVID‐19 hospitalisation compared with control (RR = 0.58, 95% CI 0.46–0.73, P = 0.00001). Significant heterogeneity was observed among the included studies (I 2 = 92%, P = 0.00001; Figure 2a). According to the study types, the pooled main effect of PA on COVID‐19 hospitalisation in cohort and cross‐sectional studies were RR, 0.58 (95% CI: 0.38, 0.89; P = 0.01) and RR, 0.57 (95% CI: 0.43, 0.77; P = 0.0003), respectively. Subgroup analysis of PA‐induced adaptation according to the type of exercise showed that endurance exercise positively affected COVID‐19 hospitalisation, but it did not reach a statistically significant difference (RR = 0.90, 95% CI 0.35–2.34, P = 0.83). Resistance exercise was significantly associated with reduction in COVID‐19 hospitalisation (RR = 0.27, 95% CI 0.15–0.49, P = 0.0001; Figure 2b).
FIGURE 2.
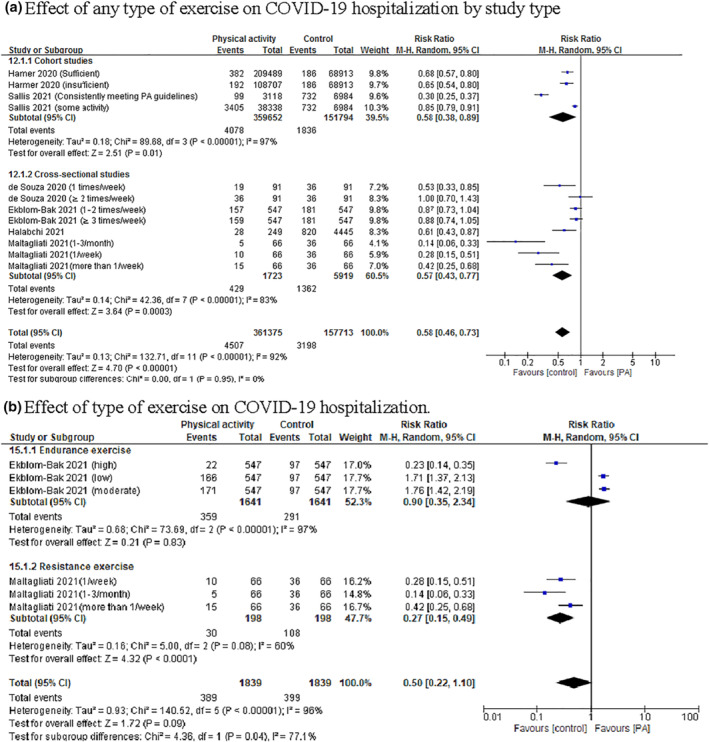
Forest plot of the relationship between physical activity (PA) and the risk of coronavirus disease 2019 (COVID‐19) hospitalisation based on different (a) study type and (b) PA‐induced adaptations
3.3. Physical activity and risk of coronavirus disease 2019 intensive care unit admissions
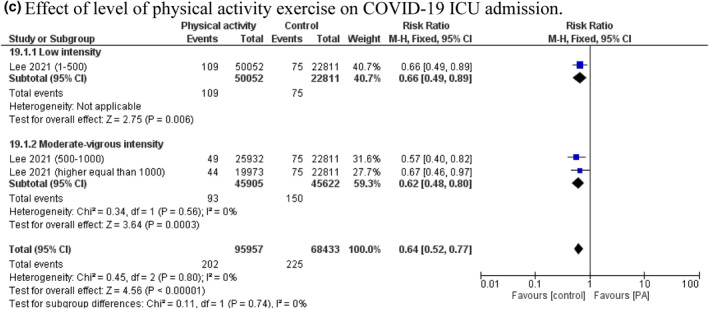
Six studies involving 130,774 cases (77,435 physically active cases and 53,339 control cases) were included. 8 , 14 , 16 , 17 , 18 , 19 The random‐effect model showed that PA was associated with reduction in COVID‐19 ICU admissions compared with control (RR = 0.65, 95% CI 0.52–0.81, P = 0.0001). The value of I 2 = 73% indicated that significant heterogeneity exists in the included studies (P = 0.0001; Figure 3a). The pooled main effects were comparable for the different study designs: RR = 0.67, 95% CI: 0.51, 0.89; P = 0.005 (cohort studies) and RR = 0.60, 95% CI: 0.43, 0.86; P = 0.004 (cross‐sectional studies). Subgroup analyses that stratified studies based on different PA‐induced adaptation showed that the positive effects of endurance and resistance exercises on COVID‐19 ICU admissions did not reach a statistically significant difference (RR = 0.78, 95% CI 0.45–1.35, P = 0.38 and RR = 0.75, 95% CI 0.50–1.13, P = 0.17; respectively). Whereas aerobic plus muscle strength training was significantly associated with a reduction in COVID‐19 ICU admissions (RR = 0.53, 95% CI 0.38–0.74, P = 0.0002; Figure 3b). Subgroup analyses that stratified studies based on different PA levels, showed no difference between low and moderate‐vigorous levels on the decreased risk of COVID‐19 ICU admissions (RR = 0.66, 95% CI 0.49–0.89, P = 0.006 and RR = 0.62, 95% CI 0.48–0.80, P = 0.0003, respectively). Although, stratifying studies based on different PA levels decreased heterogeneity to I 2 = 0% (P = 0.80, Figure 3c).
FIGURE 3.
Forest plot of the relationship between physical activity (PA) and the risk of coronavirus disease 2019 (COVID‐19) intensive care unit (ICU) admissions based on (a) study type, (b) PA‐induced adaptations and (c) PA levels

3.4. Physical activity and risk of coronavirus disease 2019 mortality
In total, nine studies involving 867,978 cases (670,357 physically active cases and 197,621 control cases) were included within this meta‐analysis. 8 , 12 , 13 , 14 , 16 , 17 , 18 , 19 , 38 There was a statistically significant association between PA with reduction in COVID‐19 mortality compared with control (RR = 0.47, 95% CI 0.38–0.59, P = 0.00001). The heterogeneity between studies was high, I 2 = 78% (P = 0.00001; Figure 4a). The RRs observed in the cohort and cross‐sectional studies were 0.50 (95% CI: 0.39, 0.64, P = 0.00001), and 0.41 (95% CI: 0.23, 0.72, P = 0.002), respectively (Figure 4b). Subgroup analyses of PA‐induced adaptation demonstrated a positive association between endurance exercise with reduction in COVID‐19 mortality (RR = 0.41, 95% CI 0.23–0.74, P = 0.003). In addition, resistance exercise did not have a significant effect on reducing COVID‐19 mortality (RR = 0.13, 95% CI 0.01–2.06, P = 0.15). The positive effect of combined training in reducing COVID‐19 mortality, did not reach a statistically significant level (RR = 0.23, 95% CI 0.06–0.97, P = 0.05), (Figure 4c). Subgroup analyses that stratified studies based on different PA levels in cohort and cross‐sectional studies showed no difference between low and moderate‐vigorous levels on the risk of COVID‐19 mortality (in cohort studies: RR = 0.67, 95% CI 0.54–0.84, P = 0.0004 and RR = 0.56, 95% CI 0.49–0.64, P = 0.00001, respectively; in cross‐sectional studies: RR = 0.42, 95% CI 0.24–0.75, P = 0.003 and RR = 0.34, 95% CI 0.21–0.54, P = 0.00001, respectively). By stratifying studies based on different PA levels, heterogeneity decreased to I 2 = 0% in both cohort (P = 0.43) and cross‐sectional studies (P = 0.95, Figure 4d).
FIGURE 4.
Forest plot of the relationship between physical activity (PA) and the risk of coronavirus disease 2019 (COVID‐19) mortality based on (a) study type, (b) PA‐induced adaptations and PA levels in cohort (c) and (d) cross‐sectional studies


3.5. Sensitivity analysis and publication bias
In sensitivity analyses, the overall pooled estimates of the respective outcomes obtained in each analysis closely resembled the preliminary associations. Further, funnel plots were checked for the included studies, which suggested no noticeable bias in the present meta‐analysis (Figure 5). Additionally, Begg's correlation rank and Egger's regression did not show significant publication bias (Table 4).
FIGURE 5.

Funnel plots for publication bias on fatal outcomes of COVID‐19
TABLE 4.
Results of the subgroup analysis based on fatal outcomes of COVID‐19.
| Risk factors | Effect measures | Number of study | Effect size (95% CI) | Heterogeneity | Begg’s test P‐value | Egger’s test P‐value | |
|---|---|---|---|---|---|---|---|
| I2 | P‐value | ||||||
| Hospitalization rate | RR | 6 | 0.58 (0.46‐0.73) | 92% | 0.00001 | 1.98 | 0.657 |
| Hospitalization rate based on Type of exercise | RR | 2 | 0.50 (0.22‐1.10) | 96% | 0.00001 | 1.93 | 0.102 |
| ICU admissions rate | RR | 6 | 0.65 (0.52‐0.81) | 73% | 0.0001 | 1.92 | 0.534 |
| ICU admissions rate based on PA levels | RR | 1 | 0.64 (0.52‐0.77) | 0% | 0.80 | 1.74 | 0.217 |
| ICU admissions rate based on Type of exercise | RR | 4 | 0.74 (0.50‐1.09) | 85% | 0.00001 | 1.70 | 0.86 |
| Mortality rate | RR | 9 | 0.47 (0.38‐0.59) | 78% | 0.00001 | 1.85 | 0.141 |
| Mortality rate based on type of exercise | RR | 3 | 0.38 (0.22‐0.67) | 83% | 0.00001 | 1.78 | 0.819 |
| Mortality rate based on PA levels in cohort studies | RR | 4 | 0.59 (0.53‐0.66) | 0% | 0.43 | 1.77 | 0.309 |
| Mortality rate based on PA levels in cross‐sectional studies | RR | 1 | 0.37 (0.25‐0.52) | 0% | 0.95 | 1.26 | 0.367 |
4. DISCUSSION
In this study, we performed pooled analyses to estimate the hospitalisation, ICU admissions, and mortality rates of COVID‐19 patients based on prior PA engagement. This study is the first meta‐analysis to comprehensively compare disease severity in COVID‐19 patients according to previous PA levels. The present meta‐analysis indicates that PA decreases the risk of hospitalisation, ICU admissions, and mortality rates of patients with COVID‐19. Moreover, patients with low PA intensity had comparable outcomes with those who had moderate to vigorous activities, suggesting any amount of PA may be beneficial. Furthermore, subgroup analysis showed that the protective effect of PA on COVID‐19 hospitalisation and mortality is strongest for resistance exercise and endurance exercise, respectively.
Previous studies have demonstrated that PA reduces the incidence of non‐communicable and chronic diseases and the mortality in infectious diseases. 39 , 40 The beneficial effects of regular PA on the immune system have been considered one of the main underlying mechanisms in reducing severe outcomes in both chronic and infectious diseases and their subsequent hospitalisation. 41 , 42 , 43 Additionally, regular PA has been shown to boost innate immune system responses, including the production of macrophages, natural killer cells, and neutrophils. 25 , 44 More importantly, there is an improvement in acquired immune system function including T cells and antibody responses following regular PA. 45 , 46 In addition to the direct effects of PA on the immune system, the metabolic regulation as a result of participating in regular PA can also improve the innate immune system's response to pathogens. 47 Taken together, these mechanisms partly explain the relationship between PA and COVID‐19 severe outcomes in the present meta‐analysis.
In addition to the beneficial effects on the immune system, PA also brings cardiorespiratory and musculoskeletal adaptations. 48 According to the present results, increased muscle strength was associated with a reduced risk of COVID‐19 hospitalisation. Considering the effects of age on increasing hospitalisation 49 and the observed anti‐sarcopenia effects of PA, 50 participating in regular PA can promote muscle strength while maintaining muscle mass, which effectively prevents the occurrence of severe cases of disease. 50 , 51 Interaction between exercised skeletal muscle and the immune system may be owing to the production of anti‐inflammatory cytokines such as IL‐6. 52 Moreover, in some progressive diseases such as some types of cancer, the maintenance of muscle mass has been associated with more effective immune responses to fight against the severe outcomes of the disease. 53 , 54 Taken together, the present findings and discussed mechanisms indicate that improved muscle strength may be protective from hospitalisation in COVID‐19 disease. However, more studies are needed to investigate this issue.
In the present meta‐analysis, PA was associated with reducing the risk of ICU admission and mortality in COVID‐19 patients. Moreover, the risk of mortality was associated with a lower baseline physical fitness. It has been suggested that preexisting health conditions are a major cause of mortality in COVID‐19. 55 Christensen et al. (2021) have also suggested that although cardiorespiratory fitness may not predict COVID‐19 infection, it was a predictor of disease progression and mortality. 56 The current study results also support the relationship between the rate of mortality and aerobic fitness. Also, based on the present meta‐analysis results, combined exercises may reduce ICU admission rate, which may effectively reduce mortality risk. It seems that cardiorespiratory and muscular adaptations following regular combined exercise training can effectively prevent severe cases and mortality from COVID‐19 disease.
Based on the present meta‐analysis results, there is no significant difference between low and high levels of PA in ICU admission and mortality rates in COVID‐19 patients. Although some studies suggested a link between higher levels of PA and a reduction in COVID‐19 mortality, according to the European CVD Prevention Guidelines, 500‐100 MET per week is enough to reduce the risk of cardiovascular diseases. 57 Moreover, according to the J‐shaped theory of the immune system, long‐term high‐intensity exercise training can also effectively suppress immune system responses and develop upper respiratory infections. 58 Taken together, even moderate to low levels of PA can reduce the risk of severe COVID‐19 and mortality. Although, more studies in this field can be helpful.
An important issue raised just after the outbreak of COVID‐19 is the decline in PA levels. A population‐based study has shown that PA decreased by up to about 27.3% just 30 days after the onset of the COVID‐19 pandemic. 59 The potential risks of decreased PA in communities and new variants of the virus (e.g. delta and omicron) requires attention, as the present meta‐analysis results indicate that PA is associated with the risk of COVID‐19 severe outcomes. General recommendations should continue to seek to improve the level of PA to counteract with possible new strains.
Findings from the present meta‐analysis must be interpreted in light of its limitations. First, because most of the studies included in our analysis did not report comorbidities associated with severe COVID‐19 outcomes, the association of PA with adverse COVID‐19 outcomes may be more exaggerated than indicated by the estimates. More prospective and well‐organised studies are warranted to determine the leading cause of hospitalisation and mortality in COVID‐19 patients and evaluate the impact of different aetiologies and clinical factors on prognosis. Second, most of the included studies used the International PA Questionnaire to measure PA behaviour and have not provided enough information about the types of PA and the possibility to reduce COVID‐19 outcomes. Third, overall pooled analyses indicated a relationship between PA and COVID‐19 severe outcomes. However, our results did not reach statistically significant levels in some analyses likely owing to the paucity of included studies in relation to PA type and COVID‐19 severe outcomes. Therefore, further studies should consider evaluating the impact of specific types of PA on COVID‐19 outcomes. Finally, definitions used for the intensity of PA varied between studies and should be consistent in future studies.
5. CONCLUSION
In this meta‐analysis, we showed that PA decreases the hospitalization, ICU admission, and mortality rates of COVID‐19 patients. Additionally, COVID‐19 patients with a history of resistance and endurance exercises experience a lower rate of hospitalization and mortality, respectively. The findings of this meta‐analysis suggest that public health authorities should continue to encourage people to participate in recommended levels of PA during the COVID‐19 pandemic while following public health safety guidelines.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Masoud Rahmati and Jae Il Shin developed the idea and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Masoud Rahmati and Fatemeh Malakoutinia ran the search strategy; Masoud Rahmati, Fatemeh Malakoutinia, Mahdieh Molanouri Shamsi and Kayvan Khoramipour selected articles and extracted data; Masoud Rahmati evaluated the quality of the literature. Masoud Rahmati, Mahdieh Molanouri Shamsi and Kayvan Khoramipour wrote the manuscript, and Wongi Woo, Seoyeon Park, Dong K Yon, Seung Won Lee, Jae Il Shin and Lee Smith edited it. All listed authors reviewed and approved the final manuscript.
Supporting information
Supporting Information S1
Supporting Information S2
Table S1
ACKNOWLEDGEMENTS
This research was supported by the Lorestan University, Khorramabad, Iran.
Rahmati M, Shamsi MM, Khoramipour K, et al. Baseline physical activity is associated with reduced mortality and disease outcomes in COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2022;32(5):e2349. 10.1002/rmv.2349
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as supplementary information. The data are available by accessing the published studies listed in Table 1.
REFERENCES
- 1. Roser M, Ritchie H, Ortiz‐Ospina E, Hasell J. Coronavirus disease (COVID‐19)–Statistics and research. Our World in data. 2020;4. [Google Scholar]
- 2. Karim SSA, Karim QA. Omicron SARS‐CoV‐2 variant: a new chapter in the COVID‐19 pandemic. Lancet. 2021;398(10317):2126‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parodi SM, Liu VX. From containment to mitigation of COVID‐19 in the US. JAMA. 2020;323(15):1441‐1442. [DOI] [PubMed] [Google Scholar]
- 4. Khoramipour K, Basereh A, Hekmatikar AA, Castell L, Ruhee RT, Suzuki K. Physical activity and nutrition guidelines to help with the fight against COVID‐19. J Sports Sci. 2021;39(1):101‐107. [DOI] [PubMed] [Google Scholar]
- 5. Meyer J, McDowell C, Lansing J, et al. Changes in physical activity and sedentary behavior in response to COVID‐19 and their associations with mental health in 3052 US adults. Int J Environ Res public health. 2020;17(18):6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medicine ACoS. Staying Active during the Coronavirus Pandemic. American College of Sports Medicine; 2020. [Google Scholar]
- 7. Organization WH. Stay Physically Active during Self‐Quarantine. World Health Organization; 2020. [Google Scholar]
- 8. Halabchi F, Mazaheri R, Sabeti K, et al. Regular sports participation as a potential predictor of better clinical outcome in adult patients with COVID‐19: a Large Cross‐sectional Study. J Phys Activity Health. 2020;18(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 9. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID‐19: a systematic review and meta‐analysis. Aging (Albany NY). 2020;12(13):12493‐12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao M, Piernas C, Astbury NM, et al. Associations between body‐mass index and COVID‐19 severity in 6· 9 million people in England: a prospective, community‐based, cohort study. Lancet Diabetes & Endocrinol. 2021;9(6):350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamer M, Gale CR, Kivimäki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID‐19: a community‐based cohort study of adults in the United Kingdom. Proc Natl Acad Sci. 2020;117(35):21011‐21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmadi MN, Huang B‐H, Inan‐Eroglu E, Hamer M, Stamatakis E. Lifestyle risk factors and infectious disease mortality, including COVID‐19, among middle aged and older adults: evidence from a community‐based cohort study in the United Kingdom. Brain, Behavior, and Immunity. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho D‐H, Lee SJ, Jae SY, et al. Physical activity and the risk of COVID‐19 infection and mortality: a nationwide population‐based case‐control study. J Clin Med. 2021;10(7):1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ekblom‐Bak E, Väisänen D, Ekblom B, et al. Cardiorespiratory fitness and lifestyle on severe COVID‐19 risk in 279,455 adults: a case control study. Int J Behav Nutr Phys Activity. 2021;18(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS‐CoV‐2 infection, severe COVID‐19 illness and COVID‐19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021. [DOI] [PubMed] [Google Scholar]
- 17. Salgado‐Aranda R, Pérez‐Castellano N, Núñez‐Gil I, et al. Influence of baseline physical activity as a modifying factor on COVID‐19 mortality: a single‐center, retrospective study. Infect Dis Ther. 2021;10(2):801‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID‐19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021. [DOI] [PubMed] [Google Scholar]
- 19. Yuan Q, Huang H‐y, Chen X‐l, et al. Does pre‐existent physical inactivity have a role in the severity of COVID‐19? Ther Adv Respir Dis. 2021;15:17534666211025221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nobari H, Fashi M, Eskandari A, Pérez‐Gómez J, Suzuki K. Potential improvement in rehabilitation quality of 2019 novel coronavirus by isometric training system; Is there “muscle‐lung cross‐talk”. Int J Environ Res Public Health. 2021;18(12):6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nobari H, Fashi M, Eskandari A, Villafaina S, Murillo‐Garcia Á, Pérez‐Gómez J. Effect of COVID‐19 on health‐related quality of life in adolescents and children: a systematic review. Int J Environ Res Public Health. 2021;18(9):4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burtscher J, Millet GP, Burtscher M. Low cardiorespiratory and mitochondrial fitness as risk factors in viral infections: implications for COVID‐19. Br J Sports Med. 2021;55:413‐415. [DOI] [PubMed] [Google Scholar]
- 23. da Silveira MP, da Silva Fagundes KK, Bizuti MR, Starck É, Rossi RC, de Resende E Silva DT. Physical exercise as a tool to help the immune system against COVID‐19: an integrative review of the current literature. Clin Exp Med. 2021;21(1):15‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nieman DC. Does Exercise Alter Immune Function and Respiratory Infections? President's Council on Physical Fitness and Sports Research Digest; 2001. [Google Scholar]
- 25. Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young DR, Coleman KJ, Ngor E, Reynolds K, Sidell M, Sallis RE. Associations between Physical Activity and Cardiometabolic Risk Factors Assessed in a Southern California Health Care System, 2010–2012; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brawner CA, Ehrman JK, Bole S, et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Paper presented at: Mayo Clin Proc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 30. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc JMLA. 2018;106(4):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 32. Dessie ZG, Zewotir T. Mortality‐related risk factors of COVID‐19: a systematic review and meta‐analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Committee IR. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)‐short and Long Forms; 2005. https://ugc.futurelearn.com/uploads/files/bc/c5/bcc53b14‐ec1e‐4d90‐88e3‐1568682f32ae/IPAQ_PDF.pdfs [Google Scholar]
- 34. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101‐129. [Google Scholar]
- 35. Wu Z‐h, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID‐19: a meta‐analysis. Acta Diabetol. 2021;58(2):139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Souza FR, Motta‐Santos D, dos Santos Soares D, et al. Association of physical activity levels and the prevalence of COVID‐19‐associated hospitalization. J Sci Med Sport. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maltagliati S, Sieber S, Sarrazin P, et al. Muscle strength explains the protective effect of physical activity against COVID‐19 hospitalization among adults aged 50 years and older. J Sports Sci. 2021:1‐8. [DOI] [PubMed] [Google Scholar]
- 38. Hamrouni M, Roberts MJ, Thackray A, Stensel DJ, Bishop N. Associations of obesity, physical activity level, inflammation and cardiometabolic health with COVID‐19 mortality: a prospective analysis of the UK Biobank cohort. BMJ Open. 2021;11(11):e055003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee I‐M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. lancet. 2012;380(9838):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nieman DC, Henson DA, Austin MD, Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45(12):987‐992. [DOI] [PubMed] [Google Scholar]
- 41. Luben R, Hayat S, Wareham N, Pharoah P, Khaw K‐T. Usual physical activity and subsequent hospital usage over 20 years in a general population: the EPIC‐Norfolk cohort. BMC Geriatr. 2020;20(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryrsø CK, Faurholt‐Jepsen D, Ritz C, et al. The impact of physical training on length of hospital stay and physical function in patients hospitalized with community‐acquired pneumonia: protocol for a randomized controlled trial. Trials. 2021;22(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shamsi MM, Hassan ZM, Gharakhanlou R. Exercise‐induced chaperokine activity of hsp70: possible role in chronic diseases. Chaperokine Activity of Heat Shock Proteins. 2019:193‐209. [Google Scholar]
- 44. Jee Y‐S. Physical exercise for strengthening innate immunity during COVID‐19 pandemic: 4th series of scientific evidence. J Exerc Rehabil. 2020;16(5):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ledo A, Schub D, Ziller C, et al. Elite athletes on regular training show more pronounced induction of vaccine‐specific T‐cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav Immun. 2020;83:135‐145. [DOI] [PubMed] [Google Scholar]
- 46. Turner JE, Brum PC. Does regular exercise counter T cell immunosenescence reducing the risk of developing cancer and promoting successful treatment of malignancies? Oxidative medicine and cellular longevity . 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015:21. [PubMed] [Google Scholar]
- 48. Rivera‐Brown AM, Frontera WR. Principles of exercise physiology: responses to acute exercise and long‐term adaptations to training. Pm&r. 2012;4(11):797‐804. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta‐analysis. BMC Geriatr. 2018;18(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta‐analysis. Clin Interventions aging. 2017;12:835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID‐19)–associated hospitalization: COVID‐19–associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72(11):e695‐e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rogeri PS, Gasparini SO, Martins GL, et al. Crosstalk between skeletal muscle and immune system: which roles do IL‐6 and glutamine play? Front Physiology. 2020;11:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shamsi MM, Chekachak S, Soudi S, et al. Effects of exercise training and supplementation with selenium nanoparticle on T‐helper 1 and 2 and cytokine levels in tumor tissue of mice bearing the 4 T1 mammary carcinoma. Nutrition. 2019;57:141‐147. [DOI] [PubMed] [Google Scholar]
- 54. Shamsi MM, Chekachak S, Soudi S, et al. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL‐15 and IL‐10/TNF‐α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine. 2017;90:100‐108. [DOI] [PubMed] [Google Scholar]
- 55. Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID‐19. Sci Rep. 2021;11(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christensen RA, Arneja J, Cyr StK, Sturrock SL, Brooks JD. The association of estimated cardiorespiratory fitness with COVID‐19 incidence and mortality: a cohort study. Plos one. 2021;16(5):e0250508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Kardiologia Pol Pol Heart J. 2016;74(9):821‐936. [DOI] [PubMed] [Google Scholar]
- 58. Chamorro‐Viña C, Fernandez‐del‐Valle M, Tacón AM. Excessive exercise and immunity: the J‐shaped curve The Active Female. 2014:357‐372. [Google Scholar]
- 59. Tison GH, Avram R, Kuhar P, et al. Worldwide effect of COVID‐19 on physical activity: a descriptive study. Ann Intern Med. 2020;173(9):767‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Table S1
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. The data are available by accessing the published studies listed in Table 1.


