Abstract
Background
Survival among critically ill COVID‐19 patients varies between countries and time periods. Mortality rates up to 60% have been reported in intensive care units (ICUs). Standard‐of‐care has evolved throughout the pandemic. The purpose of the study was to explore management and mortality of COVID‐19 ICU‐patients during the first pandemic wave and assess their post‐ICU health status.
Methods
We conducted an exploratory observational ambidirectional population‐based study of ICU‐patients with COVID‐19 in a Swedish county during 1 March‐30 June 2020. Primary outcome was 60‐day mortality with secondary outcomes including treatments, complications, self‐reported general health and dyspnoea post‐discharge. Patients were consecutively divided into equal tertiles with cut‐offs on April 4 and April 20, 2020, to analyse time trends.
Results
One hundred patients, median age was 63 years, were included, and 60‐day mortality rate was 22%. Ninety‐one percent had moderate/severe ARDS and 88% required mechanical ventilation. In the first tertile of patients 60‐day mortality was 33%, declining to 15% and 18% in the following two. This reduction paralleled increased use of thromboprophylaxis, less steep rise of treated ICU‐patients per day and expanded ICU resources. Four months post‐discharge, 63% of survivors reported self‐assessed decline in general health retrospectively compared to prior COVID‐19.
Conclusions
In this cohort, the initial 60‐day mortality quickly declined, despite continuous admittance of critically ill patients. This was parallel to adaptation to increased workload and more intense thromboembolic prophylaxis. A majority of survivors reported declined general health four months after discharge. Further studies on long‐term health status of ICU‐survivors are indicated.
Editorial Comment.
In a retrospective study of 100 consecutive Covid‐19 ICU patients from one Swedish county, the overall 90‐day mortality declined over time, but reduced self‐reported quality of life was reported by most patients 4 months after discharge.
1. BACKGROUND
The novel coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has posed a challenge to health care systems across the world. The first case of COVID‐19 in the County of Östergötland, one of 21 Swedish healthcare regions, was confirmed on March 9th, 2020. At that stage, no evidence‐based treatment for COVID‐19 existed, but early reports of increased incidence of venous thromboembolism in patients with COVID‐19 urged an implementation of expanded thromboprophylaxis. 1 One of the fatal complications of COVID‐19 is ARDS, 2 which can impair general health and breathing up to 5 years post‐discharge. 3 Several studies on cohorts of critically ill patients with COVID‐19 have been published, reporting ICU mortality rates between 30%–60%. 4 , 5 , 6 Research on changes in mortality rate over time, management, complications and post‐discharge health among COVID‐19 ICU‐patients is limited. The purpose of this study was therefore to explore the possible temporal changes in clinical characteristics, management, post‐ICU health status and mortality in a Swedish population of ICU‐treated COVID‐19 patients during the first wave of the pandemic.
2. METHODS
2.1. Ethical approval
The study was approved by the Ethical Review Board in Sweden (2020–03888, 2020–02080 and 2020–03029, 2020–04443). In accordance with the ethics approval, and because the 4‐month telephone follow‐up was part of a clinical follow‐up, a written informed consent was not required.
2.2. Study setting
The study was conducted within the county of Östergötland, Sweden. The county has a population of approximately 450,000 inhabitants and is served by three hospitals; a tertiary care university hospital with 400 beds and four ICUs, and two general hospitals, one with 241 beds and an ICU and the other with 76 beds. A total of 30 ICU beds were supported at the beginning of the pandemic. All COVID‐19 patients admitted to an ICU within the county during the study period were included, and all hospitalized patients with symptoms of COVID‐19 during the study period were tested for SARS‐CoV‐2 infection.
2.3. Data collection
We conducted an exploratory observational ambidirectional population‐based study of patients with COVID‐19 admitted to ICUs in a Swedish county during 1 March‐30 June 2020. Clinical data were obtained retrospectively from hospital medical records and additional data were retrieved from the Swedish Intensive Care Registry. The primary outcome was 60‐day mortality, with secondary outcomes including baseline characteristics, complications, treatments, and self‐rated health. Clinical data, interventions, complications and 30‐, 60‐ and 90‐day mortality after ICU admission were registered as well as follow‐up data for survivors at median four months post‐discharge from hospital (telephone interview). To further analyse temporal changes, the study cohort was split into three tertiles with 33, 34 and 33 consecutively admitted patients to ICU, respectively. The time period for each tertile began on March 16, April 4, and April 20, 2020. No analysis of missing data was performed.
2.4. Definitions
Infection with SARS‐CoV‐2 was confirmed by a positive Real Time Polymerase Chain Reaction (RT‐PCR) assay of nasopharyngeal swabs or a typical clinical picture together with a positive serological test detecting SARS‐CoV‐2 antibodies. Sixty‐day mortality was defined as all‐cause mortality within 60 days after admission to ICU. Expected 30‐day mortality rate (EMR) was calculated using the Simplified Acute Physiology Score 3 (SAPS 3) score adapted to the Swedish setting. 7 , 8 , 9
The definition used for ARDS was the Berlin definition. 10 The Kidney Disease Improving Global Outcomes (KDIGO) definition of acute kidney injury (AKI) was used. 11 Septic shock was defined by the sepsis‐3 criteria. 12 Bleeding events was defined in accordance with International Society on Thrombosis and Haemostasis (ISTH). 13 The definition of ventilator‐associated pneumonia (VAP) followed the current definitions issued by the Swedish Intensive Care Registry. 14 Iatrogenic immunosuppression was defined as treatment with chemotherapy or immunosuppressants upon admission.
Low‐dose Low Molecular Weight Heparin (LMWH) was defined as 4500 IU tinzaparin or 75 IU/kg once daily. High‐dose LMWH was defined as 4500 IU or 75 IU/kg twice daily. Full dose LMWH was defined as a total dose of 175 IU/kg daily. Corticosteroid treatment was given in some instances on indications such as septic shock or ARDS. The subgroup that did receive at least an equivalent anti‐inflammatory dosage of corticosteroids to that of dexamethasone 6 mg once daily, was defined as ‘high‐dose corticosteroid’. At the telephone follow‐up, dyspnoea was recorded according to the modified Medical Research Council (mMRC) dyspnoea scale. 15 , 16 They were also asked to rate their general health status at the time on a five‐point Likert scale from very good to very bad, similar to the general question regarding overall health in the WHO health survey, 17 and to estimate their general health on the same scale prior to COVID‐19. The questionnaire was part of a clinical follow‐up, with a primary focus to identify long term rehabilitation needs, with specific focus on cognition, physical functioning and activity level, described further in a publication by Divanoglou et al. 18 For the purposes of this study questions regarding self‐rated general health and dyspnoea are included for analysis.
2.5. Statistical analysis
Continuous data are presented as median and interquartile ranges (IQR) unless indicated. IBM SPSS Statistics for Windows 25.0 was used for analysis (IBM Corp, Armonk, NY, USA). Mann–Whitney U‐test and Kruskal‐Wallis test were used for continuous variables and the χ2 test or Fisher's exact test for categorical variables. A p‐value of ≤ .05 was considered statistically significant.
3. RESULTS
3.1. Study population
Out of 113 patients with COVID‐19 admitted to ICU during the study period, 10 (8.8%) patients were excluded as COVID‐19 was not the reason for admission and another 3 (2.7%) patients as they were transferred to another county (Figure 1). Among the 100 patients included in the analyses, 98 (98%) were confirmed SARS‐CoV‐2 PCR positive and 2% were included based on a typical clinical picture in combination with a positive serological test. The median age was 63 years and 75 (75%) were male (Table 1). The most frequent comorbidities were hypertension (53%), diabetes mellitus (29%), and ischemic heart disease (18%). On admission to ICU, median SOFA‐score was 4. Most of the patients had moderate or severe ARDS (91%) and 88% required mechanical ventilation. Limitation of the level of care, that is, withholding ventilator support, dialysis or cardiopulmonary resuscitation was decided for 18 patients during their time in the ICU.
FIGURE 1.
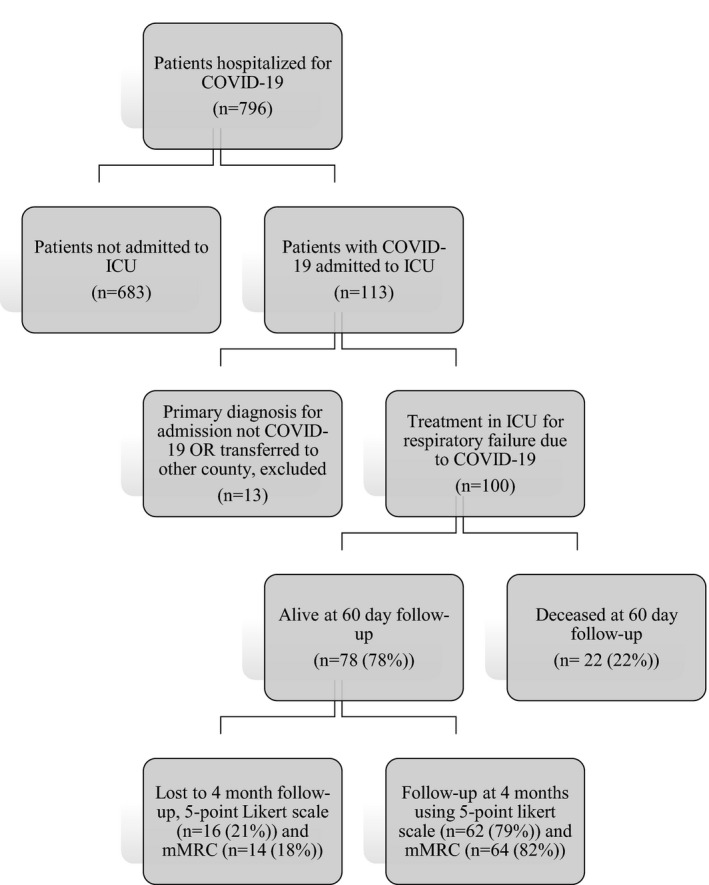
Study flowchart of inclusion and final outcome (60‐day mortality): Patients hospitalised for COVID‐19 and admitted to ICU in Region Östergötland 1st of March to 30th of June 2020
TABLE 1.
Baseline patient characteristics, total and stratified by survivors and non‐survivors (60 days after ICU admission)
| Characteristics | N | All (n = 100) | Survivors (n = 78) | Deceased (n = 22) |
|---|---|---|---|---|
| Female, n (%) | 100 | 25 (25%) | 18 (23%) | 7 (31%) |
| Age years, median (IQR) | 100 | 63 (56–70) | 61 (54–68) | 70 (62–75) |
| Distribution | 100 | |||
| 18‐49 year | 14 (14%) | 12 (15%) | 2 (9%) | |
| 50‐64 year | 40 (40%) | 35 (45%) | 5 (23%) | |
| >64 year | 46 (46%) | 31 (40%) | 15 (68%) | |
| Active smoker | 91 | 5 (5%) | 2 (3%), n = 71 | 3 (15%), n = 20 |
| BMI median (IQR) kg/m2 | 79 | 29.3 (26.4–32) | 29.3 (26.3–31.9), n = 63 | 29.6 (28–32.3), n = 16 |
| Comorbidity, n (%) | ||||
| None | 100 | 25 (25%) | 24 (31%) | 1 (5%) |
| Asthma | 100 | 15 (15%) | 13 (17%) | 2 (9%) |
| Obstructive sleep apnoea | 100 | 4 (4%) | 4 (5%) | 0 |
| Chronic obstructive pulmonary disease | 100 | 2 (2%) | 1 (1%) | 1 (5%) |
| Neurologic/neuromuscular disease | 100 | 8 (8%) | 6 (8%) | 2 (9%) |
| Cerebrovascular disease | 100 | 5 (5%) | 3 (4%) | 2 (9%) |
| Diabetes | 100 | 29 (29%) | 22 (28%) | 7 (31%) |
| Ischemic heart disease | 100 | 18 (18%) | 11 (14%) | 7 (31%) |
| Congestive heart failure | 100 | 4 (4%) | 2 (3%) | 2 (9%) |
| Hypertension | 100 | 53 (53%) | 38 (49%) | 15 (68%) |
| Liver disease/cirrhosis | 100 | 2 (2%) | 2 (3%) | 0 |
| Cancer | 100 | 5 (5%) | 4 (5%) | 1 (5%) |
| Chronic renal failure | 100 | 11 (11%) | 8 (10%) | 3 (14%) |
| Total no. Comorbidities, median (IQR) | 100 | 2 (1–2) | 1 (0–2) | 2 (1–2) |
| Medications upon admission n (%) | ||||
| ACEI/ARB | 100 | 44 (44%) | 31 (31%) | 13 (59%) |
| Warfarin | 100 | 5 (5%) | 5 (6%) | 0 |
| DOACs | 100 | 5 (5%) | 3 (4%) | 2 (9%) |
| Clopidogrel | 100 | 6 (6%) | 3 (4%) | 3 (14%) |
| ASA | 100 | 10 (10%) | 5 (6%) | 5 (23%) |
| Immunosuppressants | 100 | 8 (8%) | 6 (8%) | 2 (9%) |
| Statins | 99 | 33 (33%) | 26 (33%) n = 78 | 7 (33%) n = 21 |
| Expected mortality rate | 96 | 11.6% (7.3–20.8) | 10% (6.5–19.2) n = 75 | 17.6% (8.7–31.9) n = 21 |
Data are presented as n (%), or median (IQR).
3.2. Complications and outcomes
Mortality rates at 30, 60 and 90 days after admission to ICU were 19% (95% CI: 11%–27%), 22% (95% CI: 14%–30%) and 23% (95% CI: 15%–31%) respectively. The 60‐day mortality rate was 4% among the 25 patients with no underlying comorbidity compared to 28% among patients with comorbidity (p < .05). Fifteen patients were immediately transferred from the emergency room to the ICU and their 60‐day mortality was 40% (6/15), that is, twice the mortality rate observed among the patients not in need of immediate ICU treatment (19%, 16/85, p < .05) (Table 2). At 60 days after ICU admission, 6% of patients were still in the ICU. The 22 non‐survivors were significantly older (median age 70 vs. 61 years, p < .05, Table 1) and suffered more ICU complications (95% versus 67%, p < .05). Septic shock and acute kidney injury/failure were significantly more frequent among non‐survivors than survivors (59% vs. 18% and 82% vs. 42% respectively p < .05) (Figure 2). Ventilator‐associated pneumonia was confirmed in 18% of mechanically ventilated patients (16/88) and suspected in another 32% (28/88). Blood types A or AB were present in 42% of survivors and in 53% of non‐survivors. Among non‐survivors, the decision to withhold or withdraw treatment was made in 59% (13/22); in more than half (7/13) the decision to withdraw was made due to prolonged invasive mechanical ventilation (median 20.5 days in ICU) with poor predicted prognosis.
TABLE 2.
Duration of symptoms and hospital stay, stratified by survivors and non‐survivors (60 days after ICU admission)
| N | All (n = 100) | Survivors (n = 78) | Deceased (n = 22) | |
|---|---|---|---|---|
| Days from symptom debut to hospital admission, median (IQR) | 98 | 7 (5–10) | 7 (6–10), n = 76 | 7 (3–8), n = 22 |
| Days from symptom debut to ICU admission, median (IQR) | 98 | 9 (8–13) | 10 (8–13), n = 76 | 8 (6–15), n = 22 |
| Admission to ICU directly from ER, n (%) | 100 | 15 (15%) | 9 (12%) | 6 (27%) |
| Total days in hospital, median (IQR) | 99 | 27 (18–47) | 30 (19–55), n = 77 | 19 (7.25–24), n = 22 |
| Total days in ICU, median (IQR) | 99 | 16 (9–25) | 17 (10–29), n = 77 | 13.5 (5–23), n = 22 |
| Hospital free days from ICU admission and 60 days forward, median (IQR) | 99 | 39 (0–50) | 43 (31–50) | 0 (0–0) |
| Days on mechanical ventilation | 97 | 14 (7.0–23.0) | 14.5 (8.0–25.5), n = 76 | 11 (5.0–23.0), n = 21 |
| Limitation of level of care | 100 | 13 (13%) | 3 (4%) | 10 (45%) |
Data are presented as n (%), or median (IQR).
FIGURE 2.
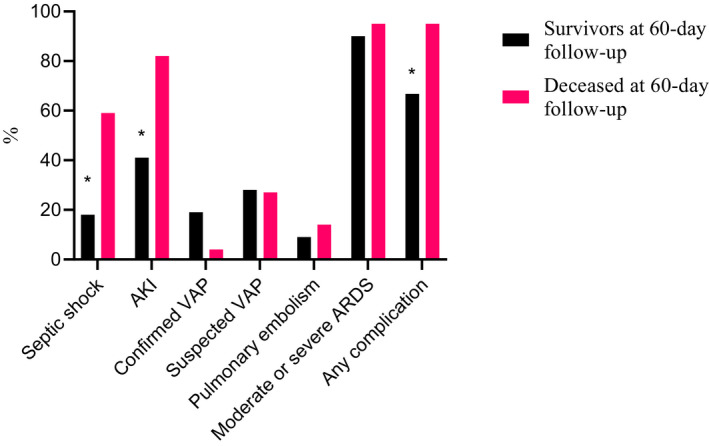
Complications during ICU stay stratified by survivors and non‐survivors (60‐day follow‐up), *p = significant, n = 100
At four months post‐discharge, 39% (24/62) rated their general health as good or very good, compared to a rating of 85% prior to COVID‐19 as recalled (Figure 3). Median time from ICU‐discharge to follow‐up was 130 days (IQR 114–149). Thirty‐four percent (21/62) of followed‐up survivors declined two or more levels down in self‐rated general health, and 29% (18/62) one level (Figure 3). The subgroup that did not deteriorate had a median age of 61 years, the majority were males (20/23), the median days in the hospital were 26 whereof 10 days in mechanical ventilation, 21/23 had moderate or severe ARDS and 10/23 had no comorbidity. In the subgroup whose general health had declined at least two points, the median age was 59 years, 14/21 were male and median time in hospital was 37 days of which 14 days were on mechanical ventilation, all had moderate or severe ARDS, and 7/21 had no comorbidity. At four months post‐discharge, 39% (25/64) of the survivors experienced limitations related to breathing (≥2 points on mMRC scale, Figure 4).
FIGURE 3.
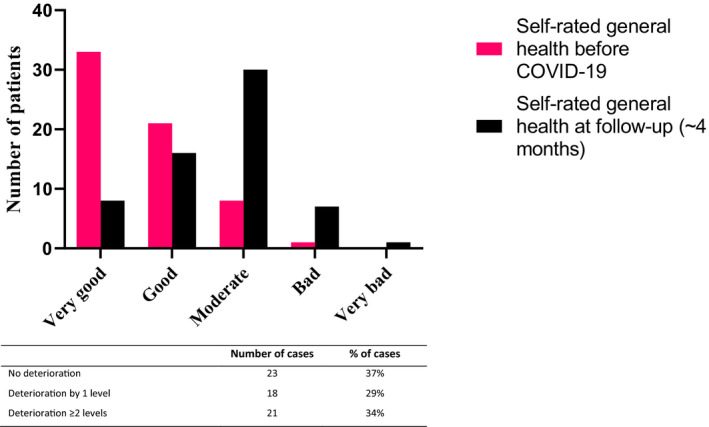
Self‐rated general health according to the question ‘Would you say your health was/is very good, good, moderate, bad or very bad’ before COVID‐19 and at time of follow‐up, respectively, n = 62
FIGURE 4.
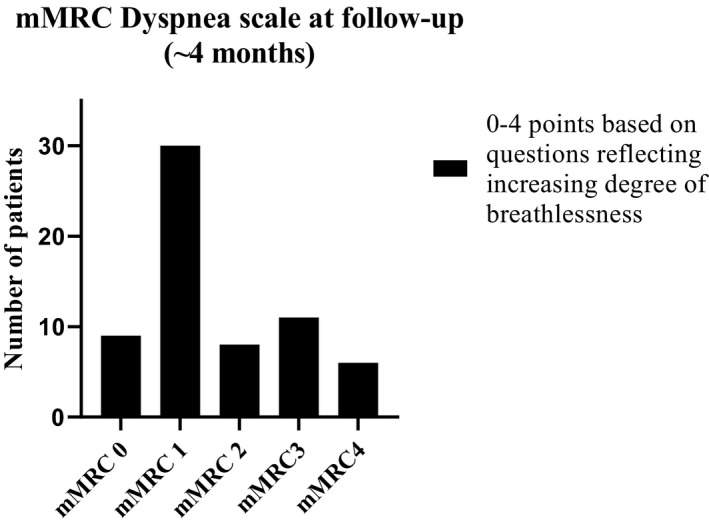
Survivors report at follow‐up of any persisting dyspnoea according to the modified Medical Research Council (mMRC) dyspnoea scale. Point‐based questions included in the mMRC Dyspnoea Scale are; 0p:’I only get breathless with strenuous exercise’, 1p: ‘ I get short of breath when hurrying on the level or walking up a slight hill’, 2p: ‘ I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level’, 3p: ‘I stop for breath after walking about 100 yards or after a few minutes on the level’, 4p:’I am too breathless to leave the house’ or ‘I am breathless when dressing’, n = 64
3.3. Treatments and biomarkers
In addition to antibiotics, the specific treatments given in the ICU were high‐dose LMWH (83%), corticosteroid equivalent to 6 mg dexamethasone (17%), and hydroxychloroquine (19%) (Table 3). Twenty‐seven (27%) patients received continuous renal replacement therapy. On admission to hospital, non‐survivors had significantly higher median procalcitonin levels compared to survivors (0.8 [IQR 0.3–2.4] vs. 0.2 [IQR 0.1–0.5] mg/L, p < .05). The median initial D‐dimer value after admission to hospital was significantly higher in non‐survivors (0.7 [IQR 0.3–2.2] vs. 0.3 mg/L [IQR 0.2–0.6], p < .05) as was the creatinine level on admission to hospital (118 [IQR 78–175] vs. 87 [71–107] µmol/L, p < .05).
TABLE 3.
Treatment received by patients during hospital and ICU stay, stratified by survivors and non‐survivors (60 days after ICU admission)
| N | All (n = 100) | Survivors (n = 78) | Deceased (n = 22) | |
|---|---|---|---|---|
| Days to start of antibiotic treatment, median (IQR) | 100 | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| Ongoing broadspectrum antibiotics at time of admission to ICU, n (%) | 100 | 92 (92%) | 70 (90%) | 22 (100%) |
| Corticosteroid treatment during ICU‐stay, n (%) | 100 | 28 (28%) | 15 (19%) | 13 (59%) |
| Dexamethasone | 100 | 3 (3%) | 2 (3%) | 1 (4%) |
| Hydrocortisone | 100 | 21 (21%) | 12 (15%) | 9 (41%) |
| Methylprednisolone | 100 | 8 (8%) | 4 (5%) | 4 (18%) |
| High‐dose corticosteroids | 100 | 17 (17%) | 9 (12%) | 8 (36%) |
| Chloroquine treatment | 100 | 19 (19%) | 13 (17%) | 6 (27%) |
| Antifungal treatment, n (%) | 100 | 21 (21%) | 14 (18%) | 7 (32%) |
| Thromboprophylaxis, distribution | n=77 | n=21 | ||
| LMWH low‐dose regime only | 98 | 8 (8%) | 6 (8%) | 2 (10%) |
| LMWH high‐dose regime | 98 | 81 (83%) | 64 (83%) | 17 (81%) |
| ASA | 100 | 17 (17%) | 15 (19%) | 2 (10%) |
| Thrombotic therapy, distribution n (%) | ||||
| Full‐dose LMWH | 100 | 15 (15%) | 12 (15%) | 3 (14%) |
| Heparin | 100 | 18 (18%) | 11 (14%) | 7 (32%) |
| Prone position | 100 | 71 (71%) | 58 (74%) | 13 (59%) |
| Highest level of respiratory support n (%) | ||||
| HFNO | 99 | 5 (5%) | 4 (5%) | 1 (5%) |
| NIV | 99 | 7 (7%) | 7 (9%) | 0 |
| Ventilator | 100 | 85 (85%) | 65 (83%) | 20 (90%) |
| ECMO | 100 | 3 (3%) | 2 (3%) | 1 (4%) |
| CRRT/HD | 100 | 27 (27%) | 18 (23%) | 9 (41%) |
| On HD/PD before admission | 100 | 3 (3%) | 3 (4%) | 0 |
Data are presented as n (%), or median (IQR).
3.4. Time trends
A decline in 60‐day mortality was observed over the three tertiles from 33% (95% CI: 24%–42%) to 15% (95% CI: 8%–22%), and 18% (95% CI: 11%–26%) respectively (p=NS) (Table 4). Thirty‐day mortality showed a similar pattern (30%, 15% and 12%, Figure 5). Median EMR was 14% in the first tertile, 10% in the second and 12% in the last tertile (Figure 5). The number of staffed ICU beds within the region expanded from 30 to 52 during the first wave. The most rapid increase in the number of treated patients in the ICU due to COVID‐19 occurred during the first tertile from the first admitted patient in mid‐March to 29 ICU patients treated simultaneously in the beginning of April (Figure 6). The highest daily number of COVID‐19 ICU‐patients, 39, was noted on the 20th of April, declining down to 6 patients by the end of June. The mean number of ICU patients treated per day was for the whole period March to June 2020 35.7, compared to 17.1 during the same period in 2019 and 22.7 in 2018. The corresponding number during tertiles was 46.4, 50.9 and 28.9 respectively.
TABLE 4.
Tertile subgroups characteristics and treatments
| N | All (n = 100) | Phase 1 | Phase 2 | Phase 3 | |
|---|---|---|---|---|---|
| Patients | 100 | 100 | 33 | 34 | 33 |
| Age | 100 | 63 (55–70) | 67 (56–73) | 62 (49–71) | 62 (56–69) |
| Male sex | 100 | 75 (75%) | 24 (73%) | 23 (68%) | 28 (85%) |
| No comorbidities | 100 | 23 (23%) | 8 (24%) | 9 (26%) | 6 (18%) |
| BMI | 79 | 29.3 (26.4–32.0) | 28.8 (27.0–31.7) | 29.0 (26.3–32.0) | 29.6 (25.8–32.7) |
| 30‐day mortality | 100 | 19 (19%) | 10 (30.3%) | 5 (14.7%) | 4 (12.1%) |
| 60‐day mortality | 100 | 22 (22%) | 11 (33%) | 5 (14.7%) | 6 (18.1%) |
| 30‐day EMR (%, IQR) | 96 | 11.7 (7.3–20.8) | 13.5 (7.3–22.5) | 10.0 (5.8–19.2) | 12.2 (7.7–20.8) |
| SMR individual | 98 | 0.0 (0.0–0.0) | 0.0 (0.0–3.8) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| SAPS 3 upon admission to ICU | 94 | 53 (49–60) | 53 (48–60) | 53 (48–60) | 55 (50–61) |
| SOFA upon admission to ICU | 97 | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (4–5) |
| Direct admission to ICU from ER | 100 | 15 (15%) | 6 (18%) | 4 (12%) | 5 (15%) |
| PaO2/FiO2 ratio on ICU admission | 98 | 13.5 (10.6–17.7) | 14.3 (11.6–23.3) | 13.0 (10.9–16.0) | 11.7 (10.0–14.4) |
| NEWS2 score in ER | 83 | 8 (5–9) | 8 (5–10) | 8 (5–10) | 7 (5–9) |
| Saturation in ER | 95 | 89 (86–94) | 88 (80–92) | 90 (86–94) | 91 (87–94) |
| CRP (mg/L) upon admission | 96 | 91 (60–149) | 90 (68–133) | 97 (54–180) | 90 (51–139) |
| PCT (µg/L) upon admission | 47 | 0.3 (0.1–0.8) | 0.2 (0.1–1.45) | 0.3 (0.2–1.1) | 0.3 (0.1–0.7) |
| D‐dimer (mg/L) | 93 | 0.4 (0.2–0.8) | 0.625 (0.2–2.05) | 0.5 (0.2–1.5) | 0.2 (1.2–0.4) |
| Creatinine upon admission (µmol/ml) | 96 | 90 (72–122) | 86 (68–108) | 98 (75–118) | 90 (76–135) |
| Moderate or severe ARDS | 100 | 91 (91%) | 30 (91%) | 30 (88%) | 31 (94%) |
| Mechanical ventilation | 100 | 88 (88%) | 30 (91%) | 29 (85%) | 29 (88%) |
| Days from symptom debut to ICU admission | 98 | 9 (8–13) | 8 (7–11) | 11 (8–15) | 12 (8–14) |
| Days from symptom debut to intubation | 86 | 10 (8–14) | 8 (7–11) | 12 (8–15) | 11 (8–15) |
| Days in mechanical ventilation | 97 | 14 (7–24) | 16 (7–23) | 14 (7–26) | 11 (9–27) |
| Total days in hospital | 99 | 27 (18–47) | 24 (16–43) | 27 (16–50) | 29 (18–48) |
| Hospital‐free days (of 0–60) | 99 | 19 (0–37) | 0 (0–39) | 28 (0–37) | 26 (0–37) |
| Limitation of level of care, withdrawal | 100 | 9 (9%) | 4 (12.1%) | 0 | 5 (15.2%) |
| Limitation of level of care, withhold | 100 | 9 (9%) | 5 (15.2%) | 3 (8.8%) | 1 (3.0%) |
| Chloroquine treatment | 100 | 19 (19%) | 19 (58%) | 0 | 0 |
| Low dose thromboprophylaxis | 98 | 8 (8%) | 7 (22%) | 1 (3%) | 0 (0%) |
| High‐dose thromboprophylaxis | 98 | 81 (83%) | 19/32 (59%) | 31/33 (94%) | 31/33 (94%) |
| High‐dose corticosteroid treatment | 100 | 19 (19%) | 6 (18%) | 4 (12%) | 9 (27%) |
| Complications during ICU stay | 100 | 73% (73%) | 24 (73%) | 25 (74%) | 24 (73%) |
| Septic shock | 100 | 27 (27%) | 10 (30%) | 8 (24%) | 9 (27%) |
| AKI, stage 2 | 100 | 12 (12%) | 3 (9%) | 6 (18%) | 4 (12%) |
| AKI, stage 3 | 100 | 38 (38%) | 15 (45%) | 9 (26%) | 14 (42%) |
| AKI, any | 100 | 49 (49%) | 18 (55%) | 15 (44%) | 18 (55%) |
Data are presented as n (%), or median (IQR).
FIGURE 5.
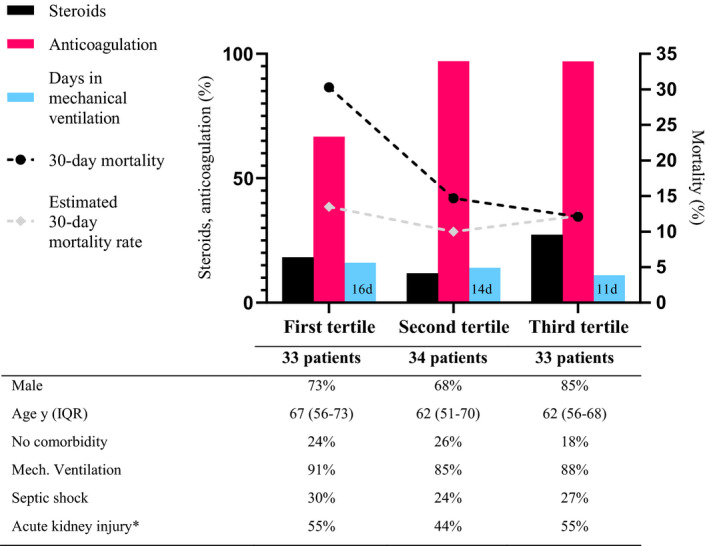
Timeline of observed and expected 30‐day mortality (dotted black and white line, right y‐axis) stratified by tertiles of patients admitted to ICU due to COVID‐19 during the first wave in Region Östergötland. Selected treatments used during hospital and ICU stay stratified by tertiles refer to the left y‐axis. The median time (days) in mechanical ventilation is displayed by the light blue bars. The proportion of high‐dose thromboprophylaxis (red bars) used in the first tertile compared with the second and third tertile, differed significantly. *=stage 2 and 3 AKI, n = 98
FIGURE 6.
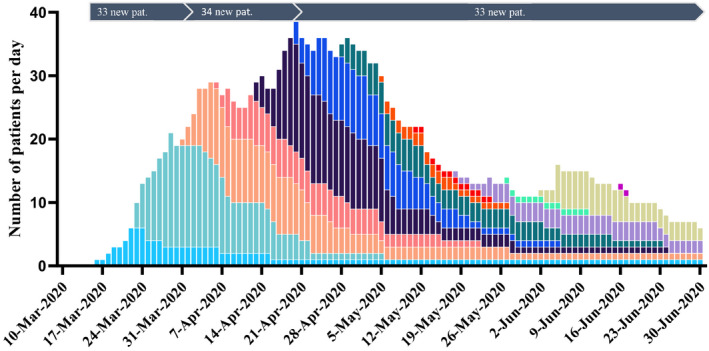
Number per day of ICU‐treated patients with confirmed COVID‐19 in Region Östergötland, one of 21 healthcare regions in Sweden, during the early COVID‐19 pandemic 2020. Each color correlates to the patients admitted to ICU during the same week and the color curve follows their total stay in ICU. Mean increase in the number of COVID‐19 patients treated per day in the ICU was 1.5 in the first tertile, 0.6 in the second tertile and negative (declining) in the third tertile, n = 100
Treatment with hydroxychloroquine was only used during the first tertile (19/33, 58%) with a median treatment duration of 4 days (Table 3). The use of high‐dose LMWH increased significantly in the second and third tertile compared to the first (from 59% to 94% in the last two tertiles, p < .05) (Figure 5, Table 4), but did not differ between survivors and non‐survivors (Table 3). Median D‐dimer on admission to hospital among patients subsequently admitted to the ICU in the first tertile was significantly higher than in the second and third tertiles (0.63 vs. 0.48 and 0.19, p < .05), but there was no significant difference in thromboembolic events between the three groups.
4. DISCUSSION
This ambidirectional population‐based study presents an in‐depth description of ICU‐treated COVID‐19 patients during the first pandemic wave in one of 21 healthcare regions in Sweden. Despite the high frequency of moderate or severe ARDS (91%) the overall 60‐day mortality rate was only 22%, declining rapidly as the first wave progressed. The decline was in parallel with significantly increased use of high‐dose thromboprophylaxis and expanded ICU resources. By four months post‐discharge a large proportion of the survivors experienced a retrospectively self‐assessed decline in general health and difficulties in breathing.
Mortality during the initial phase of COVID‐19 shows both geographical and temporal differences. A similar group of patients requiring mechanical ventilation in Lombardy, Italy during the first pandemic wave had an in‐hospital mortality rate of 53%. 6 Among 217 COVID‐19 patients admitted to ICU in Norway, 86% were intubated and the overall mortality rate was 20.7% at follow‐up approximately 90 days after ICU admission. 19 Britain had a lower rate (72%) of mechanical ventilated COVID‐19 patients with higher in‐hospital mortality (42%). 20 In another Swedish ICU‐cohort of 260 patients from the first pandemic wave, of which 82.3% mechanically ventilated, ICU‐mortality was 30.3%. 21 The overall 90‐day mortality was 23% in our study in comparison with 37% in Denmark and 31% reported from France, Belgium, and Switzerland. 22 , 23 Comparison between ICU studies of different country origin is difficult considering the wide varieties in case‐mix and level of care defined as intensive care. However, our mortality rate was lower than the 27% reported from a nationwide Swedish ICU registry study on 90‐day outcome of more than 4000 patients critically ill with COVID‐19. 24
In the present study there was a reduction in 60‐day mortality from a high level of 33% during the first tertile falling to 15% and 18% in the following two, with similar 30‐day mortality at 30%, 15% and 12% respectively. Although not statistically significant, this reflects the declines in mortality rate recently reported from other centres. In a large observational study, there was a gradual decline in 60‐day mortality for all ICU‐treated COVID‐19 patients in Sweden from 36% in March to 21% post‐wave (July‐September) 2020. 25 Western reports showed similar falls in mortality among critically ill patients during the first wave, from initial 42%–44% to 19%–25% in May 2020. 23 , 26 The reason for this decline in mortality is still unknown but likely multifactorial. There was no apparent difference in patient characteristics between the three tertiles of consecutively admitted ICU patients (Table 4). SAPS 3 scores and 30‐day EMR were similar during each of the tertiles, speaking against significant differences in patient population and disease severity. This indicates that changes in mortality were not due to a change in case mix, but rather changes in workload and case management over time. ICU admissions of patients with the highest observed mortality coincided with the steepest rise in the number of patients with COVID‐19 in need of intensive care (Figure 6). The increase in workload was substantial with nearly doubling of the patients treated per day compared to the same period the two previous years. The dramatic increase in COVID‐19 patients demanding intensive care during the first tertile phase was met by reorganizing and expanding facilities and recruiting new personnel. This increased ICU demand was subsequently met during the second tertile phase. The steep increase in resource strain during the first phase, together with limited knowledge of the new disease entity likely affected the quality of care and mortality negatively. Overload of intensive care resources is a well‐known risk factor for increased mortality. 27
No novel effective drugs were introduced for COVID‐19 treatment during the first wave. The RECOVERY study report on reduction of mortality by steroid treatment was not released until mid‐June 2020. 28 The risk for thromboembolic events was quickly recognized with aggressive thromboprophylaxis being instituted later in the first wave. In a meta‐analysis by Malas and co‐workers, 29 the overall incidence of venous thromboembolism amongst COVID‐19 ICU treated patients was 31% (95% CI: 23%–39%) compared to 14% (95% CI: 7%–21%) in the present study. Limited use of high‐ dose thromboprophylaxis as well as a trend towards more frequent decisions to limit level of care on the ICU (Table 4) may have contributed to the higher mortality observed in the beginning of the first wave.
Studies have suggested that blood types A and AB are associated with increased COVID‐19 mortality. 30 In this ICU cohort, blood type distribution was comparable to the general population in Sweden 31 with no significant difference between survivors and non‐survivors.
Poor self‐rated general health was seen in most ICU survivors four months after discharge. Despite 85% of followed‐up survivors recalling their health as being good or very good prior to COVID‐19, as few as 39% rated their health at that level four months after discharge. One third reported a decrease of at least two points compared to that recalled prior to infection, and all patients in this subgroup had pre‐existing comorbidity. Similar drops in self‐rated general health have been reported previously both in ICU and non‐ICU COVID‐19 patients, with the former group reporting considerably worse outcomes as compared to the latter. 18 , 32 In a previous Swedish study, pre‐existing comorbidity was the most important factor for poor health‐related quality‐of‐life (HRQoL) after critical care. 33 The aforementioned subgroup in our study, with decline in self‐rated general health, all had ARDS, more ventilator days, and longer hospital stay. This is consistent with a study by Taboada and co‐workers, reporting an association between deterioration in HRQoL and duration of mechanical ventilation and length of hospital stay. 34 While HRQoL and self‐rated general health are not equals, the latter can be seen as part of the former as evident from the short‐form health survey (SF‐36), where the first question addresses self‐rated general health. 35 Given that self‐rated general health has been found to be a strong predictor of healthcare demand, 36 our findings indicate that a considerable subgroup of ICU COVID‐19 survivors require long‐term support regarding both general health as well as dyspnoea, as a large proportion of this cohort's survivors also experienced limitations in breathing at follow‐up. Dyspnoea after COVID‐19 is described in several reports. 37 , 38 There are however reports indicating that the degree of dyspnoea post hospitalization related to COVID‐19 is independent of whether the patient was admitted to the ICU or not. 39 , 40
A limitation of the present study is the small sample size. Patient recollection of pre‐COVID‐19 general health assessment entails a risk of bias in overestimating the level of health before ICU and is regarded as a major limitation. Another limitation is that only survivors were possible to include for follow‐up, with lack of pre‐morbid data on self‐rated health for deceased patients. Furthermore, in addition to the 22 deceased patients another group of 14 patients in the study cohort did not participate in the telephone interview 4‐month post‐discharge. Study strengths are found in the rigorous data extraction.
5. CONCLUSIONS
In the cohort of all ICU‐treated COVID‐19 patients in the county during the first months of the pandemic, the initially high 60‐day mortality quickly declined, despite a continuing admittance of critically ill patients. This fall in mortality, without any change in case mix, was in parallel with successful adaptation to increased workload, less steep increase in patients treated in the ICU and implementation of gained knowledge of this new disease entity, where thromboembolic complications is a major pathogenetic mechanism. Four months post‐discharge a large proportion of surviving patients reported a decline in retrospectively self‐assessed general health and symptoms of impaired respiratory function.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
6. AUTHOR CONTRIBUTIONS
All authors contributed to the study design and had access to the data. GF entered and organized data, KN, and SB conducted all statistical analysis. All authors contributed to data acquisition, interpretation and manuscript writing. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Swedish Intensive Care Registry. This work was supported by ALF grants from the Region of Östergötland, Sweden.
Forsberg G, Berg S, Divanoglou A, et al. Improved 60‐day survival but impaired general health in Swedish ICU‐COVID patients: An ambidirectional population‐based study. Acta Anaesthesiol Scand. 2022;66:569–579. doi: 10.1111/aas.14054
Gustaf Forsberg, Åse Östholm Balkhed and Katarina Niward are equally contributed to this work.
REFERENCES
- 1. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Ma X. Acute respiratory failure in COVID‐19: is it "typical" ARDS? Critical Care (London, England). 2020;24(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293‐1304. [DOI] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Resp Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the lombardy region. Italy. Jama. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metnitz PGH, Moreno RP, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engerström L, Kramer AA, Nolin T, et al. Comparing Time‐fixed mortality prediction models and their effect on ICU performance metrics using the simplified acute physiology score 3. Crit Care Med. 2016;44(11):e1038‐e1044. [DOI] [PubMed] [Google Scholar]
- 10. ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526‐2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 11. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 12. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315(8):762‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 14. Swedish ICU Registry, Risk adjustment guideline (Only in Swedish) . Version 17.0. Jan 2021. 2021. Available from: https://www.icuregswe.org/globalassets/riktlinjer/riskjustering.pdf Accessed 15 June 2021
- 15. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580‐586. [DOI] [PubMed] [Google Scholar]
- 16. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . World Health Survey 2002 B – Individual Questionnaire Rotation ‐ A. Available from: https://www.who.int/healthinfo/survey/whslongindividuala.pdf
- 18. Divanoglou A, Samuelsson APK, Sjödahl PER, Andersson C, Levi PR. Rehabilitation needs and mortality associated with the Covid‐19 pandemic: a population‐based study of all hospitalised and home‐healthcare individuals in a Swedish healthcare region. EClinicalMedicine. 2021;36:100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laake JH, Buanes EA, Småstuen MC, et al. Characteristics, management and survival of ICU patients with coronavirus disease‐19 in Norway, March‐June 2020. A prospective observational study. Acta Anaesthesiol Scand. 2021;65(5):618‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richards‐Belle A, Orzechowska I, Gould DW, et al. COVID‐19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035‐2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson E, Brattström O, Agvald‐Öhman C, et al. Characteristics and outcomes of patients with COVID‐19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol Scand. 2021;65(1):76‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions, and longer term outcomes of COVID‐19 ICU patients in Denmark—A nationwide, observational study. Acta Anaesthesiol Scand. 2021;65(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 23. COVID‐ICU Group on behalf of the REVA Network and the COVID‐ICU Investigators . Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47(1):60‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zettersten E, Engerström L, Bell M, et al. Long‐term outcome after intensive care for COVID‐19: differences between men and women—a nationwide cohort study. Crit Care. 2021;25(1). 10.1186/s13054-021-03511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strålin K, Wahlström E, Walther S, et al. Mortality trends among hospitalised COVID‐19 patients in Sweden: a nationwide observational cohort study. Lancet Reg Health‐Europe. 2021;4:100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with COVID‐19‐related critical illness at a learning health system in the United States. Ann Intern Med. 2021;174(5):613‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kashiouris MG, Sessler CN, Qayyum R, et al. Near‐simultaneous intensive care unit (ICU) admissions and all‐cause mortality: a cohort study. Intensive Care Med. 2019;45(11):1559‐1569. [DOI] [PubMed] [Google Scholar]
- 28. RECOVERY Trial . Low‐cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID‐19. Oxford University News Release, 16 June 2020. Available from: https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf
- 29. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29–30:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hultström M, Persson B, Eriksson O, Lipcsey M, Frithiof R, Nilsson B. Blood type A associates with critical COVID‐19 and death in a Swedish cohort. Crit Care. 2020;24(1). 10.1186/s13054-020-03223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frequency of major blood groups in the Swedish population (Only in Swedish) . Geblod.nu. 2007‐10‐02. Available from: https://web.archive.org/web/20101124064308/http://www.geblod.nu/general.aspx?PageId=10 Accessed 17 June 2021
- 32. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 33. Orwelius L, Nordlund A, Nordlund P, et al. Pre‐existing disease: the most important factor for health related quality of life long‐term after critical illness: a prospective, longitudinal, multicentre trial. Crit Care. 2010;14(2):R67. doi: 10.1186/cc8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID‐19 patients. Br J Anaesth. 2021;126(3):e110‐e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nilsson E, Orwelius L, Kristenson M. Patient‐reported outcomes in the Swedish National Quality Registers. J Intern Med. 2016;279(2):141‐153. [DOI] [PubMed] [Google Scholar]
- 36. Cislaghi B, Cislaghi C. Self‐rated health as a valid indicator for health‐equity analyses: evidence from the Italian health interview survey. BMC Public Health. 2019;19(1). 10.1186/s12889-019-6839-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Sar ‐ van der Brugge S, Talman S, Boonman ‐ de Winter LJM, et al. Pulmonary function and health‐related quality of life after COVID‐19 pneumonia. Respir Med. 2021;176:106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santus P, Tursi F, Croce G, et al. Changes in quality of life and dyspnoea after hospitalization in COVID‐19 patients discharged at home. Multidiscip Respir Med. 2020;15(1):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID‐19. Eur Res J. 2021;57(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


