Abstract
Drug‐induced hypersensitivity syndrome (DiHS) is a severe drug eruption that can induce reactivation of herpesviruses such as human herpesvirus 6, resulting in symptom flare‐up and organ damage. DiHS is known as drug reaction with eosinophilia and systemic symptoms (DRESS) in Europe. We report three cases of DiHS that could have been triggered by mRNA‐based coronavirus disease 2019 (COVID‐19) vaccines. In these three patients, symptoms of DiHS developed 2–6 days after the first dose of an mRNA‐based COVID‐19 vaccine. Although there have been no reports of DiHS/DRESS induced by mRNA‐based COVID‐19 vaccines in domestic and international journals despite the progress in vaccination worldwide, we speculate that mRNA‐based COVID‐19 vaccines might have triggered the development of DiHS/DRESS in our patients. In the current coronavirus epidemic, it might be important to assess mRNA‐based COVID‐19 vaccination status and date of vaccination when evaluating a patient with DiHS/DRESS.
Keywords: coronavirus disease 2019, drug‐induced hypersensitivity syndrome, human herpesvirus 6, mRNA, vaccine
1. INTRODUCTION
Drug‐induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe drug eruption that can lead to the development of viral reactivation, especially human herpesvirus 6 (HHV‐6). Here, we report three cases of DiHS that could have been triggered by mRNA‐based coronavirus disease 2019 (COVID‐19) vaccines.
2. CASE REPORT
2.1. Case 1
A 68‐year‐old Japanese man was referred and hospitalized with fever, erythroderma, vomiting, and loss of appetite. He began taking carbamazepine for a diagnosis of trigeminal neuralgia 49 days before the hospitalization. He received the first dose of COVID‐19 vaccine (Pfizer‐BioNTech) 22 days before hospitalization. He developed a slight fever with generalized fatigue and widespread eruptions on the trunk and extremities 16 days before hospitalization. Despite persistence of these symptoms, he received the second COVID‐19 vaccine dose 3 days before hospitalization. He rapidly developed erythroderma with facial swelling, high fever, vomiting, and loss of appetite. His past medical history included hypertension, diabetes mellitus, and myocardial infarction.
Physical examination revealed diffuse redness of his entire body with many scratch marks and swelling of his face and lower legs (Figure 1a–d). Redness was seen on the hard palate, which was tender to palpation (Figure 1e). Cervical, axillary, and inguinal lymphadenopathy were present. He had high fever (39.2°C).
FIGURE 1.
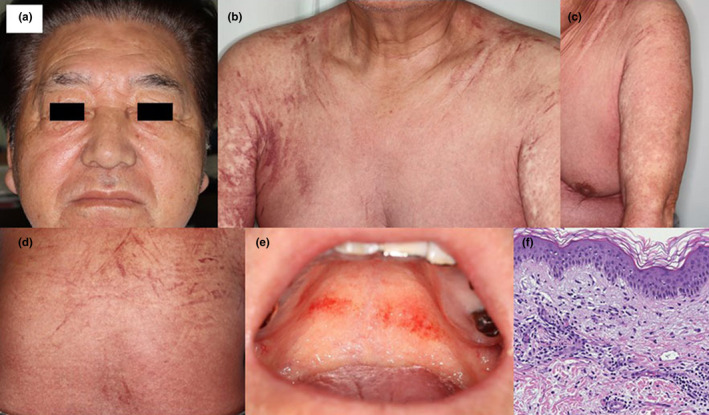
(a–d) Diffuse redness of the entire body with many scratch marks and swelling of the face in patient 1. (e) Redness on the hard palate in patient 1. (f) In patient 1, histopathological findings included an infiltration of lymphocytes surrounding the vessels in the dermis and mild vacuolar changes in the epidermis. There was no atypia of the infiltrating cells (hematoxylin–eosin, original magnification × 200)
Blood examination on the first day of hospitalization showed elevated leukocyte count (20 100/μL), eosinophil count (2312/μL), atypical lymphocyte sequestration (0.5%), and levels of creatinine (1.69 mg/dL), aspartate aminotransferase (AST; 35 U/L), aspartate aminotransferase (ALT; 94 U/L), and C‐reactive protein (CRP; 8.37 mg/dL). HHV‐6 reactivation was detected on hospital day 10. The serum level of thymus and activation‐regulated chemokine (TARC) on the second day of hospitalization was 73 200 pg/mL. Computed tomography did not reveal findings indicating an infectious lesion. Blood cultures did not show any bacteria or fungi.
We performed skin biopsy from an erythematous area. Histopathological findings included an infiltration of lymphocytes surrounding the vessels and hemorrhage in the dermis and mild vacuolar changes and lymphocytic infiltration in the epidermis (Figure 1f). A diagnosis of typical DiHS induced by carbamazepine was made.
Oral prednisolone (1 mg/kg/day) was administered. His skin rash, high fever, and laboratory test abnormalities decreased gradually over 5 weeks. The steroid dose is still being tapered.
2.2. Case 2
A 31‐year‐old Japanese man was transferred to our hospital with fever, erythroderma, and liver dysfunction. His past medical history was unremarkable. He started taking allopurinol for a diagnosis of gout 38 days before being transferred to our hospital. He received the first COVID‐19 vaccine dose (Moderna) 2 days before being rushed to the previous hospital because he developed a fever of 40°C and erythroderma. Before the transfer, he had received methylprednisolone sodium succinate 125 mg/day for 7 days, but erythroderma and liver dysfunction persisted.
Physical examination revealed diffuse redness of his entire body with many scratch marks and swelling of his face and lower legs (Figure 2a–d). Bilateral inguinal lymphadenopathy was present.
FIGURE 2.
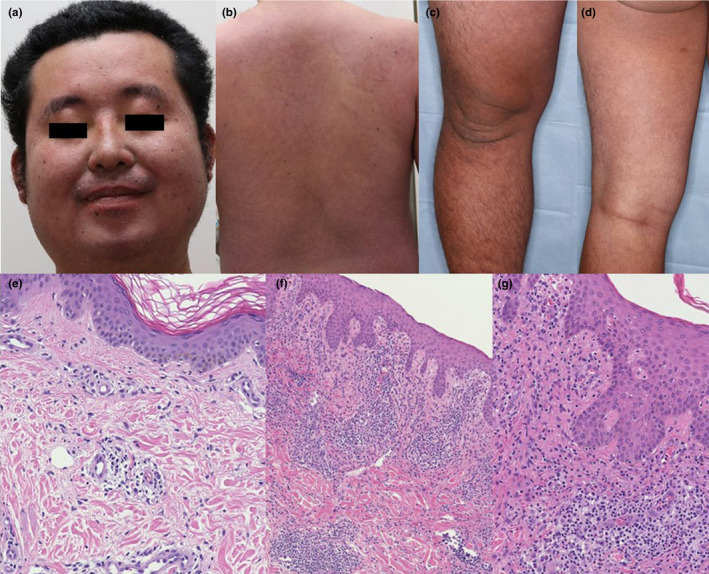
(a–d) Diffuse redness of the entire body with many scratch marks and swelling of the face and lower legs in patient 2. (e) In patient 2, histopathological findings included an infiltration of lymphocytes surrounding the vessels in the dermis (hematoxylin–eosin [HE], original magnification × 200.) (f,g) In patient 3, histopathological findings in the dermis included an infiltration of lymphocytes and eosinophils surrounding the vessels with hemorrhage. In the epidermis, there were mild vacuolar changes and lymphocytic infiltration (HE, [f] × 100, [g] × 200)
Blood examination after transfer to our hospital showed elevated leukocyte count (19 400/μL), eosinophil count (1212/μL), atypical lymphocyte sequestration (6%), and levels of AST (97 U/L), ALT (281 U/L), and CRP (6.07 mg/dl). HHV‐6 reactivation was detected on the 14th day after the transfer.
The TARC level on the fourth day after transfer was 33 800 pg/mL.
We performed skin biopsy from an erythematous area. Histopathological findings included an infiltration of lymphocytes surrounding the vessels in the dermis and mild vacuolar changes and lymphocytic infiltration in the epidermis (Figure 2e). A diagnosis of typical DiHS induced by allopurinol was made.
Oral prednisolone (1 mg/kg/day) was administered. His skin rash, high fever, and liver dysfunction decreased gradually over 6 weeks. The steroid dose is still being tapered.
2.3. Case 3
A 72‐year‐old Japanese man was hospitalized with fever and erythroderma. He started taking carbamazepine for a diagnosis of facial nerve paralysis 58 days before hospitalization. He received the first dose of COVID‐19 vaccine (Pfizer‐BioNTech) 8 days before hospitalization. He developed a fever of 38.5°C and erythroderma with facial swelling 3 days after vaccination. He had diabetes mellitus and pulmonary emphysema.
Physical examination revealed diffuse redness of his entire body and facial swelling. Mediastinal and inguinal lymphadenopathy were present.
Blood examination on the first day of hospitalization showed elevated leukocyte count (19 100/μL), eosinophil count (2197/μL), atypical lymphocyte sequestration (1%), levels of creatinine (2.62 mg/dL), AST (17 U/L), ALT (31 U/L), and CRP (7.52 mg/dl). HHV‐6 reactivation was not detected on hospital day 4.
We performed skin biopsy from an erythematous area. Histopathological findings in the dermis included an infiltration of lymphocytes and eosinophils surrounding the vessels with hemorrhage. In the epidermis, there were mild vacuolar changes and lymphocytic infiltration (Figure 2f,g). A diagnosis of atypical DiHS was made.
Oral prednisolone (30 mg/day) was administered. His skin rash and high fever decreased within 1 week. However, he had cough, respiratory discomfort, high fever (38°C), formation of new skin rashes, elevated blood eosinophil count, and oxygen desaturation on hospital day 9. We consulted a respiratory physician. The patient was diagnosed with acute exacerbation of interstitial pneumonia. Steroid pulse therapy was given. Respiratory symptoms, laboratory test abnormalities, fever, and skin rashes decreased within 3 days after the start of steroid pulse therapy. The steroid dose is still being tapered.
3. DISCUSSION
Drug‐induced hypersensitivity syndrome is a severe drug eruption associated with rash along with high fever, organ dysfunction (e.g., liver), and lymphadenopathy. 1 , 2 , 3 It is characterized by prolonged symptoms even after discontinuation of the causative drug. DiHS is known as DRESS in Europe. 1 , 2 , 3
A diagnosis of atypical DiHS or DRESS was made for case 3 because HHV‐6 reactivation was not detected. However, we thought that the timing of examination was inappropriate.
In these three patients, symptoms of DiHS developed 2–6 days after the first dose of an mRNA‐based COVID‐19 vaccine. There are few previous reports of DRESS triggered by vaccination. Two case reports of DRESS potentially triggered by influenza vaccination have been published. 4 , 5 There has also been a case report of DRESS or acute generalized exanthematous pustulosis‐like eruptions after receiving the Janssen COVID‐19 vaccine. 6
Fever, malaise, and rash may occur as side‐effects of mRNA‐based COVID‐19 vaccines. There are many similarities between the side‐effects of vaccines and symptoms of DiHS/DRESS. Therefore, like case 1, patients might not go to the hospital right away. Caution is advised for both patients and doctors.
The annual incidence of DiHS/DRESS is reported to be more than 10 per million. 1 Four patients with DiHS/DRESS have visited our hospital during the first 6 months of 2021, of whom three patients are described here. Our hospital is located in a community with a population of approximately 160 000. Up to 2020, our hospital saw approximately 2–3 patients with DiHS/DRESS per year; thus, we considered the number of patients seen in 2021 to be high. We believe that the incidence in 2021 is higher than the reported incidence. The period when the number of patients with DiHS/DRESS increased at our hospital coincided with the rapid increase in the rate of COVID‐19 vaccination.
There have been no reports of DiHS/DRESS induced by mRNA‐based COVID‐19 vaccines in domestic and international journals despite the progress in vaccination worldwide. The possible role of mRNA‐based COVID‐19 vaccines in inducing DiHS is difficult to prove. However, we speculate that the mechanism by which mRNA‐based COVID‐19 vaccines work might have activated drug‐specific T cells in DiHS, similar to adjuvants or Bacillus Calmette–Guérin vaccine. This might have led to the development of DiHS/DRESS in these three patients. All vaccines have powerful stimulatory effects on the immune system and could exacerbate pre‐existing skin eruptions or induce drug eruptions via non‐specific bystander activation of the immune system. 1 , 7
In conclusion, although accumulation of more cases is needed, we emphasize that it might be important to assess mRNA‐based COVID‐19 vaccination status and date of vaccination when evaluating a patient with DiHS/DRESS during the current coronavirus epidemic.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENT
We thank Zenis for English‐language editing.
Korekawa, A , Nakajima, K , Fukushi, K , Nakano, H , Sawamura, D . Three cases of drug‐induced hypersensitivity syndrome associated with mRNA‐based coronavirus disease 2019 vaccines. The Journal of Dermatology. 2022;49:652–655. 10.1111/1346-8138.16347
REFERENCES
- 1. Shiohara T, Mizukawa Y. Drug‐induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68:301–8. [DOI] [PubMed] [Google Scholar]
- 2. Mizukawa Y, Hirahara K, Kano Y, Shiohara T. Drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J Am Acad Dermatol. 2019;80:670–8. [DOI] [PubMed] [Google Scholar]
- 3. Mehrholz D, Urban AE, Herstowska M, Nowicki R, Cubała W, Barańska‐Rybak W. A retrospective study of DRESS ‐ drug reaction with eosinophilia and systemic symptoms. Psychiatr Pol. 2017;51:1079–93. [DOI] [PubMed] [Google Scholar]
- 4. Solak B, Dikicier BS, Kara RO, Erdem T. DRESS syndrome potentially induced by allopurinol and triggered by influenza vaccine. BMJ Case Rep. 2016;2016:bcr2016214563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hewitt N, Levinson M, Stephenson G. Drug reaction with eosinophilia and systemic symptoms associated with H1N1 vaccination. Intern Med J. 2012;42:1365–6. [DOI] [PubMed] [Google Scholar]
- 6. Lospinoso K, Nichols CS, Malachowski SJ, Mochel MC, Nutan F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID‐19 vaccine. JAAD Case Rep. 2021;13:134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiohara T, Mizukawa Y. Comment on 'Drug reaction with eosinophilia and systemic symptoms syndrome in a patient with COVID‐19′: involvement of herpesvirus reactivations and adverse drug reactions in diverse cutaneous manifestations and overall disease severity of COVID‐19. J Eur Acad Dermatol Venereol. 2021;35:e98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]


