Abstract
Objectives
To describe the placental pathology, fetal autopsy findings and clinical characteristics of pregnancies that resulted in stillbirth owing to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) placentitis, and to identify potential risk factors.
Methods
This was a prospective multicenter study of non‐vaccinated pregnant women affected by coronavirus disease 2019 (COVID‐19) in Greece from April 2020 to August 2021. A total of 165 placentas were examined histologically and six cases of stillbirth associated with SARS‐CoV‐2 placentitis were retrieved. Complete fetal autopsy was performed in three of these cases. Gross, histopathological, immunohistochemical, molecular and electron microscopy examinations were carried out in the stillbirth placentas and fetal organs. The histological findings of cases with SARS‐CoV‐2 placentitis were compared with those in 159 cases with maternal COVID‐19 which resulted in a live birth. Regression analysis was used to identify predisposing risk factors for SARS‐CoV‐2 placentitis.
Results
The placentas of all six stillborn cases showed severe and extensive histological changes typical of SARS‐CoV‐2 placentitis, characterized by a combination of marked intervillositis with a mixed inflammatory infiltrate and massive perivillous fibrinoid deposition with trophoblast damage, associated with intensely positive immunostaining for SARS‐CoV‐2 spike protein, the presence of virions on electron microscopy and positive reverse‐transcription polymerase chain reaction test of placental tissues. The histological lesions obliterated over 75% of the maternal intervillous space, accounting for intrauterine fetal death. Similar histological lesions affecting less than 25% of the placenta were observed in seven liveborn neonates, while the remaining 152 placentas of COVID‐19‐affected pregnancies with a live birth did not show these findings. Complete fetal autopsy showed evidence of an asphyctic mode of death without evidence of viral transmission to the fetus. The mothers had mild clinical symptoms or were asymptomatic, and the interval between maternal COVID‐19 diagnosis and fetal death ranged from 3 to 15 days. Statistically significant predisposing factors for SARS‐CoV‐2 placentitis included thrombophilia and prenatally diagnosed fetal growth restriction (FGR). Multiple sclerosis was seen in one case.
Conclusions
SARS‐CoV‐2 placentitis occurred uncommonly in COVID‐19‐affected pregnancies of non‐vaccinated mothers and, when extensive, caused fetal demise, with no evidence of transplacental fetal infection. Thrombophilia and prenatally detected FGR emerged as independent predisposing factors for the potentially lethal SARS‐CoV‐2 placentitis. © 2022 International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: COVID‐19, intrauterine fetal death, multiple sclerosis, placenta, pregnancy, risk factors, SARS‐CoV‐2 placentitis, thrombophilia
CONTRIBUTION —
What are the novel findings of this work?
This study provides novel insight into the histology and pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) placentitis, a condition that is potentially lethal for the fetus. Maternal thrombophilia and prenatally diagnosed fetal growth restriction (FGR) were identified as independent predisposing factors for this condition. We hypothesize that there may be a synergistic effect between SARS‐CoV‐2 and underlying risk factors which increase the likelihood of occurrence of potentially lethal SARS‐CoV‐2 placentitis.
What are the clinical implications of this work?
In rare cases, coronavirus disease 2019 (COVID‐19) may cause intrauterine fetal death due to a specific type of placental injury, SARS‐CoV‐2 placentitis, which is potentially associated with predisposing factors. The lesion evolves rapidly and, in its initial stages, appears to be undetectable by ultrasound. Risk groups may include mothers with thrombophilia and prenatally detected FGR.
Introduction
The coronavirus disease 2019 (COVID‐19) global pandemic has raised major concerns regarding, among many others, the impact of infection on pregnancy course, resulting in regular updates of guidance and recommendations based on emerging evidence and epidemiological studies.
It is thought that intrauterine transplacental transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from the mother to the fetus can occur, typically via a hematogenous route, but is uncommon 1 , 2 . Most placentas studied had no evidence of infection, and of those tested, a minority were positive for SARS‐CoV‐2 genome (7.7–21%) 3 , 4 . As the COVID‐19 pandemic evolved during 2020, a small number of international reports began to emerge describing a particular pattern of inflammation in placentas of COVID‐19‐positive women, which was termed SARS‐CoV‐2 placentitis 5 in January 2021. SARS‐CoV‐2 placentitis seems to be uncommon, but has the potential to cause significant placental injury, potentially resulting in fetal compromise 5 .
In this study, we report on six cases of stillbirth attributed to SARS‐CoV‐2 placentitis from a cohort of 165 COVID‐19‐affected pregnancies in Greece. We describe the specific placental pathology, fetal autopsy findings and ancillary tests in the stillborn cases, compare histological findings with those in SARS‐CoV‐2‐affected pregnancies that resulted in a live birth and identify potential risk factors.
METHODS
This was a prospective multicenter study, designed and supervised by the Third Department of Pediatrics, National and Kapodistrian University of Athens, School of Medicine, University General Hospital Attikon, Athens and the First Department of Pathology, Perinatal Pathology Unit, School of Medicine, National and Kapodistrian University of Athens (NKUA), Athens, Greece. In the context of this study, which was conducted from 18 April 2020 to 31 August 2021, a total of 165 placentas of COVID‐19‐affected non‐vaccinated pregnant women were sent for histological examination at the Units of Perinatal Pathology, University Laboratories of Pathology, Medical School NKUA, Athens, and the Department of Pathology, Hippokration Hospital, Thessaloniki, both of which are referral centers for perinatal and placental pathology in Greece. All women provided written informed consent for the examination of their placentas, and the study was approved by the Ethics Committee of Attikon University Hospital, Medical School, NKUA (ID 166/7‐4‐2020).
During the study period, six COVID‐19‐affected pregnancies were complicated by stillbirth, and the parents opted for full fetal autopsy in three. The remaining 159 pregnancies resulted in a live birth. Clinical characteristics of the pregnancies, including maternal history, were available in 69 cases, including the six with stillbirth.
Placental investigations
On admission to the pathology laboratory, the placentas of four of the six stillbirths were sampled fresh for real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) testing using protective equipment. The fetal surface was cleaned with 75% alcohol solution to minimize the possibility of viral RNA contamination from traces of maternal blood, amniotic fluid or other maternal fluids, and a 1‐cm section was cut with a sterile blade from the placental parenchyma. The other two placentas were received in formalin and were not sampled. Fresh tissue samples were also obtained from the lungs, liver and spleen of the three fetuses that underwent autopsy. Samples were delivered to the University Department of Microbiology, School of Medicine, NKUA, Athens, Greece and were tested for the presence of viral RNA using the genesig Real‐Time PCR COVID‐19 assay (2019‐nCoV; Primerdesign Ltd, Chandler's Ford, UK), following RNA extraction using the Promega Maxwell viral nucleic acid extraction system with magnetic beads (Promega Corp., Madison, WI, USA). The same samples were also tested for cytomegalovirus (CMV), herpes simplex viruses (HSV‐1 and ‐2), parvovirus (ParvoB19), enterovirus and Epstein–Barr virus (EBV). Additional placental samples obtained from 16 formalin‐fixed paraffin‐embedded placentas showing parenchymal inflammatory lesions were tested retrospectively for DNA viruses (CMV, HSV‐1 and ‐2, ParvoB19, EBV).
Pathological examination of all placentas was carried out with appropriate precautions following a standardized protocol after fixation in 10% buffered formalin for 10–14 days before gross examination. Slices of the fixed placentas were photographed for the evaluation of gross pathological changes. Ten tissue blocks of each placenta, including umbilical cord sections and membrane rolls, were embedded in paraffin and stained with Harris hematoxylin and eosin stain for histological evaluation.
Immunohistochemical stains were performed on selected sections from the placentas of six stillbirths using the following antibodies: SARS‐CoV‐2 (COVID‐19) spike antibody (GeneTex Inc., Irvine, CA, USA; 1A9, 1:100), and CD68 ((KP1) Agilent DAKO, Santa Clara, CA, USA; M0814, 1:300); CD3 (Agilent DAKO; A0452, 1:200); CD4 (Agilent DAKO; M7310, 1:100); CD8 (Agilent DAKO; M7103, 1:100); CD15 (Agilent DAKO; M0733 (C3D‐1), 1:50); CD20 (Agilent DAKO; M0755, 1:100), to identify leukocyte subpopulations, according to standard procedures. Negative controls for SARS‐CoV‐2 immunohistochemistry were six age‐matched placental specimens of SARS‐CoV‐2‐negative mothers, as well as sections from the six index placentas in which the primary antibody was not applied.
The placentas of four stillborn cases and fetal organs (lungs, liver and spleen) of the three cases that underwent postmortem examination were processed for electron microscopy after previous fixation in formalin. Small tissue fragments (1–2 mm3) from each placenta were cut and washed with distilled H2O for 5 min (×3) and refixed in 2.5% glutaraldehyde in phosphate buffered saline (PBS) for 2 h at room temperature and then overnight at 4°C. After washing with PBS for 5 min (×3), the specimens were postfixed in 1% aqueous osmium tetroxide for 1 h at 4°C, then dehydrated in a graded series of ethyl alcohol, followed by propylene oxide (PO), infiltrated gradually in a mixture of Epon/Araldite resins diluted in PO (1:2, 1:1, 2:1; 1 h each) and finally embedded in fresh epoxy resin mixture. Ultrathin epoxy sections (70–80 nm thickness) were cut on a Leica Ultracut R ultramicrotome (Leica Microsystems, Wetzlar, Germany), equipped with a Diatome diamond knife (Diatome, Hatfield, PA, USA) and observed with a FEI Morgagni™ 268 transmission electron microscope equipped with Olympus Morada digital camera.
Statistical analysis
Continuous variables were presented as median and interquartile range and categorical variables as percentages. Mann–Whitney U‐test was used for comparisons of continuous variables between the groups. Chi‐square or Fisher's exact test were used for pairwise comparisons of proportions, as appropriate. Logistic regression analysis (backward, by likelihood ratios) was performed to identify factors associated with the incidence of SARS‐CoV‐2 placentitis. Potential predictors included thrombophilia, asthma, smoking, hypothyroidism, gestational diabetes mellitus, gestational hypertension, pre‐eclampsia, fetal growth restriction (FGR, defined as estimated fetal weight (EFW) or abdominal circumference (AC) < 3rd centile; or EFW or AC > 3rd and < 10th centile combined with uterine artery pulsatility index > 95th centile and/or umbilical artery pulsatility index > 95th centile or cerebroplacental ratio < 5th centile) 6 , maternal age, gestational age at SARS‐CoV‐2 infection, asymptomatic COVID‐19, mild COVID‐19, moderate COVID‐19 and severe COVID‐19. Adjusted odds ratios (aOR), along with their 95% CIs, were calculated. Statistical analysis was performed using SPSS (IBM SPSS for Windows, version 24.0; IBM Corp., Armonk, NY, USA) and open‐source software R 2.15.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Characteristics of stillbirths
All six stillbirths occurred in six different towns across Greece, between December 2020 and August 2021. Clinical information is shown in Table 1. The gestational age at death ranged from 21 + 1 to 39 + 6 weeks. Stillbirth occurred 3–15 days after maternal diagnosis of COVID‐19, determined as the date of positive PCR result of maternal nasopharyngeal swab or the onset of symptoms. Four mothers had mild clinical symptoms, according to the National Institutes of Health (NIH) criteria 7 for clinical severity of COVID‐19, including fever, cough, malaise and myalgias, without need for hospitalization. Two mothers were asymptomatic and were found to be SARS‐CoV‐2‐positive at routine PCR testing before delivery. Four mothers had a history of underlying conditions associated potentially with pregnancy complications (Table 1). In Case 3 (gravida 2, para 1), the mother had a history of multiple sclerosis and had stopped treatment during gestation. In Case 4 (primigravida), the mother was investigated after the event and was found to bear a homozygous pathogenic variant (4G/4G) for the mild thrombophilia factor plasminogen activator inhibitor‐1 (PAI‐1). In Case 5 (gravida 2, para 0) the mother had a previous second‐trimester miscarriage; in this pregnancy she was diagnosed with gestational diabetes, which was managed by diet. In Case 6 (gravida 2, para 0), the mother had a previous first‐trimester miscarriage and was diagnosed with combined heterozygous methylenetetrahydrofolate reductase (MTHFR) variants (C677T and A1298C) at thrombophilia testing; during this pregnancy she had received anticoagulant treatment. In Case 1 (primigravida), the mother was investigated thoroughly after stillbirth and no comorbidities or other contributing risk factors were identified. Case 2 was lost to follow‐up. In Case 2 (gravida 2, para 1), prenatal ultrasound monitoring before maternal COVID‐19 had shown FGR, oligohydramnios and abnormal Doppler, while prenatal findings were normal in the remaining five cases (Table 1). Decreased fetal movements were recorded in three cases postinfection.
Table 1.
Maternal characteristics, prenatal findings, fetoplacental pathology and ancillary investigations in six pregnancies that resulted in stillbirth following SARS‐CoV‐2 infection of the mother
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Date of stillbirth | December 2020 | February 2021 | March 2021 | May 2021 | May 2021 | August 2021 |
| GA at stillbirth (weeks) | 39 + 6 | 24 + 4 | 35 + 0 | 27 + 2 | 37 + 0 | 21 + 1 |
| Maternal age (years) | 39 | 40 | 36 | 26 | 38 | 36 |
| Clinical severity of COVID‐19 | Asymptomatic | Asymptomatic | Mild | Mild | Mild | Mild |
| COVID‐19‐to‐stillbirth interval (days) | 4 | 3 | 13 | 7 | 15 | 10 |
| Maternal history | Unremarkable | Unknown | Multiple sclerosis | Thrombophilia (PAI‐1; 4G/4G) | Second‐trimester miscarriage, GDM | First‐trimester miscarriage, thrombophilia (MTHFR; C677T and A1298C) |
| Prenatal findings | ||||||
| Fetal anatomy | Normal | Normal | Normal | Normal | Normal | Normal |
| Fetal growth | Normal | FGR | Normal | Normal | Normal | Normal |
| Risk for FGR/SGA* | Low | N/A | N/A | Low | Low | Low |
| Uterine Doppler | Normal | Abnormal | N/A | Normal | Normal | N/A |
| Amniotic fluid | Normal | Oligohydramnios | N/A | Normal | Polyhydramnios | Normal |
| Placenta | Normal | N/A | N/A | Normal | Normal | N/A |
| Fetoplacental pathology | ||||||
| Fetal body weight (g, centile) | 3700, 75th | 280, < 1st | 2480, 42nd | 1090, 44th | 3200, 75th | 397, 54th |
| Placental weight (g, centile) | 460, 25–50th | 114, < 10th | 391, 25–50th | 262, 50–75th | 430, 25–50th | 126, 25th |
| Extent of SARS‐CoV‐2 placentitis (%) | 90 | 75 | 90 | 75 | 90 | 90 |
| Other findings | True umbilical cord knot | Placental abruption, MMP | None | None | None | None |
| Fetal autopsy | No | No | Yes, asphyxia | Yes, asphyxia | No | Yes, asphyxia |
| SARS‐CoV‐2 RT‐PCR | ||||||
| Placenta | N/A | N/A | Positive, Alpha variant | Positive, Alpha variant | Positive, Alpha variant | Positive, Alpha variant |
| Fetal organs | N/A | N/A | Negative | Negative | N/A | Negative |
| Electron microscopy | ||||||
| Placenta | N/A | N/A | Virions | Virions | Virions | Virions |
| Fetal organs | N/A | N/A | No virions | No virions | N/A | No virions |
Risk for small‐for‐gestational age (SGA) or fetal growth restriction (FGR) based on pregnancy‐associated plasma protein‐A and placental growth factor.
GA, gestational age; GDM, gestational diabetes mellitus; MMP, maternal malperfusion; MTHFR, methylenetetrahydrofolate reductase; N/A, not available; PAI‐1, plasminogen activator inhibitor‐1; RT‐PCR, real‐time reverse‐transcription polymerase chain reaction.
Placental pathology, histology and immunohistochemistry
The placenta of Case 2 was small (weight below the 10th centile), but the remaining five placentas were appropriately grown for gestational age. On gross examination and cut sections, all six placentas were compact and stiff, with a mosaic pattern of whitish streaks running through the parenchyma and intervening hemorrhagic lesions (Figure 1). This indicated massive perivillous fibrinoid deposition (MPFD) and involved over 75% of the placental parenchyma in all cases. Histologically, these lesions corresponded to a combination of MPFD with severe diffuse intervillositis and intervillous thrombosis (Figure 2). The inflammatory infiltrate was mixed and consisted of abundant macrophages and mononuclear cells, variable numbers of immature granulocytes and neutrophils, along with T‐lymphocytes and sparse B‐lymphocytes (Figure 3). The inflammatory cells appeared entrapped in a meshwork of interwoven fibers (Figure 2d), while intervillous thrombosis was also conspicuous (Figure 2a). Trophoblast damage was evident as necrosis, erosion and blebbing of the perivillous trophoblast (Figure 3a, inset). Viral immunostaining with anti‐SARS‐CoV‐2‐spike protein antibody showed areas of intensely positive cytoplasmic staining in the perivillous and extravillous trophoblast, as well as in scattered cells of the villous stroma (Figure 3a). Interestingly, areas with MPFD and destruction of the trophoblast showed weak or absent perivillous positivity.
Figure 1.
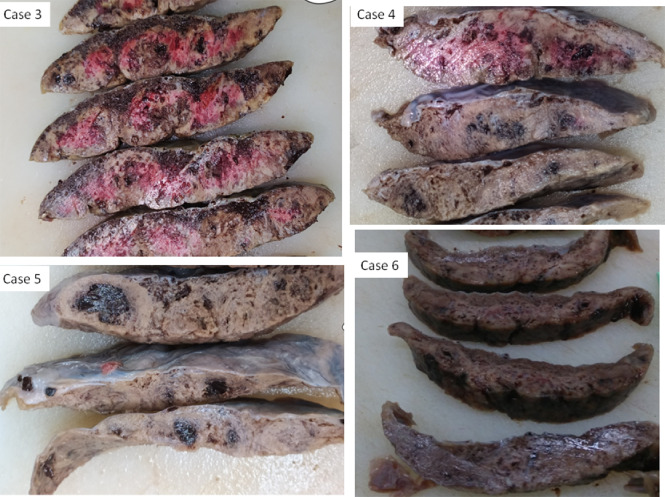
Gross pathology of placental sections after formalin fixation in Cases 3, 4, 5 and 6, which resulted in stillbirth following SARS‐CoV‐2 infection of the mother. The placentas were compact and stiff, with a mosaic pattern of whitish streaks running through the parenchyma and intervening hemorrhagic lesions. Variable coloration is due to variable degree of fixation.
Figure 2.
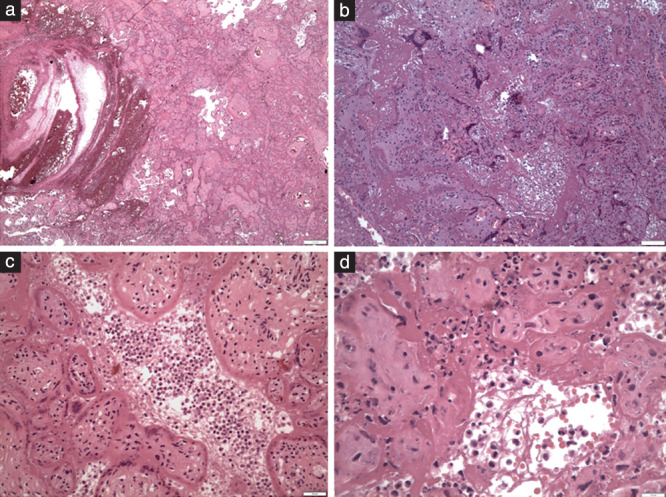
Hematoxylin‐and‐eosin‐stained placental sections showing histopathology of SARS‐CoV‐2 placentitis: (a) fresh intervillous thrombosis (×45); (b) massive perivillous fibrinoid deposition (×100); (c) inflammation in the intervillous space (intervillositis) and damage of the perivillous trophoblast (×200); and (d) inflammatory cells of mixed type within a meshwork of fibers in the intervillous space (×400).
Figure 3.
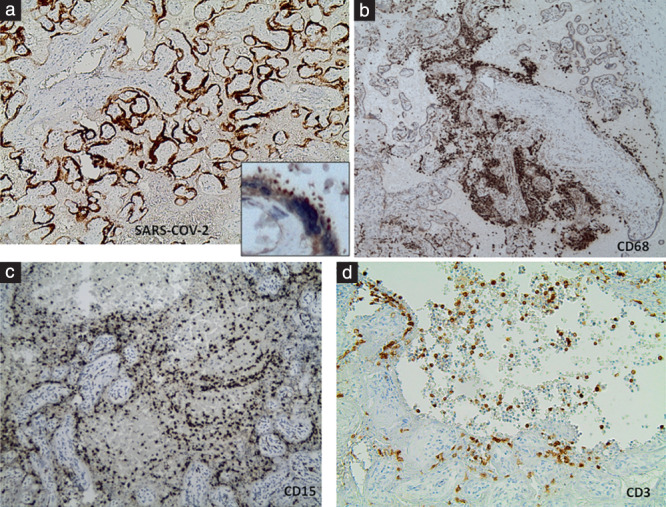
Immunohistochemistry of SARS‐CoV‐2 placentitis. (a) Staining with anti‐SARS‐CoV‐2‐spike protein antibody showed intense positivity of the perivillous trophoblast (×45); inset: magnification (×400) of positively stained perivillous trophoblast area shows cytoplasmic blebbing. (b) CD68/Kp1‐stained section showing prevailing macrophages and monocytes among the intervillous inflammatory population (×45); (c) CD15‐stained section showing a large number of granulocytes among the polymorphous intervillous inflammatory populations (×100); (d) CD3‐stained section showing scattered T‐lymphocytes among the mixed inflammatory cells (×200).
Of the 159 placentas of SARS‐CoV‐2‐affected pregnancies with liveborn neonates, seven showed evidence of focal/segmental SARS‐CoV‐2 placentitis involving less than 25% of the placenta. These placentas showed positive immunostaining for SARS‐CoV‐2, while PCR testing for other viruses (CMV, HSV‐1 and ‐2, EBV and ParvoB19) was negative (Table 2). Extensive placental involvement by SARS‐CoV‐2 placentitis was associated with stillbirth, as compared to the seven placentas of liveborn neonates with similar histological lesions of a lesser extent (Fisher's exact test, P = 0.0006). Prenatal ultrasound failed to detect SARS‐CoV‐2 placentitis in cases that resulted in stillbirth as well as in those that resulted in a live birth (Figure 4). The remaining 152 placentas of liveborn neonates did not show histological lesions of SARS‐CoV‐2 placentitis. Comparative clinical and pathological data in the six stillbirth cases and 24 cases with matched risk factors that resulted in live birth are shown in Table 2.
Table 2.
Maternal risk factors, placental pathology and outcome of 165 COVID‐19‐affected pregnancies
| Maternal history | Cases | Placental histopathology | Outcome | ||||
|---|---|---|---|---|---|---|---|
| SARS‐CoV‐2 placentitis (extent) | Increase in fibrinoid deposition* | Inflammation (villitis, intervillositis)* | Other findings* | Live birth | Stillbirth | ||
| Maternal risk factors | |||||||
| GDM | 13 | 1 (90%); 2† (< 25%) | 4 | 5† | 11 (RVM, IV thrombi, ST) | 12 | 1 (Case 5) |
| Thrombophilia | |||||||
| PAI‐1 (4G/4G) | 1 | 1 (75%) | — | — | — | — | 1 (Case 4) |
| MTHFR (C677T, A1298C) | 1 | 1 (90%) | — | — | — | — | 1 (Case 6) |
| FV‐R2 heterozygosity | 1 | No | 1 (mild) | 1† | 1 (MMP, DV, RVM) | 1 | — |
| APS | 1 | 1† (< 25%) | — | — | — | 1 | — |
| Autoimmune disease | |||||||
| Multiple sclerosis | 1 | 1 (90%) | — | — | — | — | 1 (Case 3) |
| Hypothyroidism/thyroiditis | 4 | No | 2 (mild) | 2† | 2 (MMP, RVM) | 4 | — |
| Essential hypertension | 1 | 1† (< 25%) | — | — | 1 (MMP, DV) | 1 | — |
| Miscellaneous | 5 | No | 2 (mild) | 1† | 4 (MMP, DV, RVM) | 5 | — |
| Total cases with known risk factors | 28 | — | — | — | — | 24 | 4 |
| Maternal history unremarkable | 41 | 1 (90%); 1† (< 25%) | 23 | 11 | 34 (MMP, DV, RVM, FMP, FIR, chorionitis, deciduitis, IV thrombi, ST) | 40 | 1 (Case 1) |
| Maternal history not available | 96 | 1 (75%); 2† (< 25%) | N/A | N/A | N/A | 95 | 1 (Case 2) |
| Total cases examined histologically | 165 | 13 | — | — | — | 159 | 6 |
Data are given as n except where indicated otherwise.
Non‐specific histological changes not covering the full spectrum of SARS‐CoV‐2 placentitis or completely irrelevant to it.
In 16 cases with parenchymal inflammatory placental lesions that resulted in live birth, reverse‐transcription polymerase chain reaction was negative for cytomegalovirus, herpes simplex virus, Epstein–Barr and parvovirus.
APS, antiphospholipid syndrome; DV, decidual vasculopathy; FIR, fetal inflammatory response; FMP, fetal malperfusion; FV‐R2, factor V Leiden 4070G > A (H1299R) mutation; GDM, gestational diabetes mellitus; IV, intervillous; MMP, maternal malperfusion; MTHFR, methylenetetrahydrofolate reductase; N/A, data not available or not included in this study; PAI‐1, plasminogen activator inhibitor‐1; RVM, retarded villous maturation; ST, subchorionic thrombosis.
Figure 4.
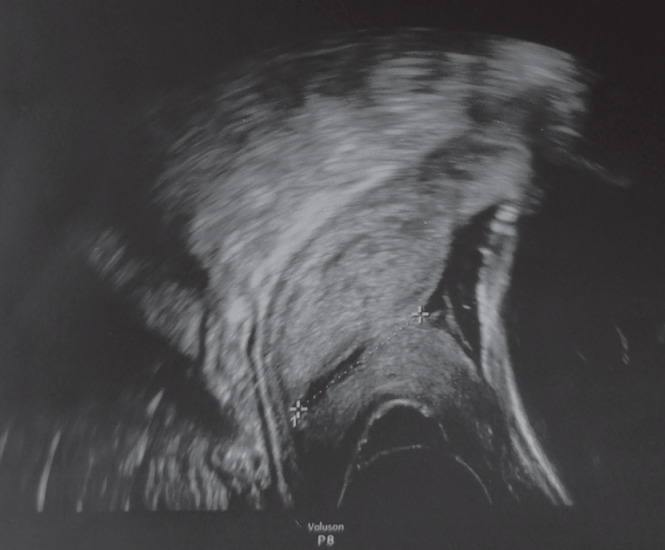
Transabdominal ultrasound image of placenta at 35 + 3 weeks' gestation, after SARS‐CoV‐2 infection, obtained 4 days before delivery of a liveborn neonate. The placenta showed histological features of SARS‐CoV‐2 placentitis involving < 25% of the parenchyma. The lesions were undetectable on ultrasound.
RT‐PCR
The Alpha B117 variant of SARS‐CoV‐2 was detected in the four tested stillbirth placentas with low cycle threshold (Ct < 20), in favor of high levels of genomic RNA. Samples from the fetal organs, lungs, liver and spleen obtained from three autopsied fetuses were negative for SARS‐CoV‐2 genome. RT‐PCR tests on fresh placental and fetal samples were negative for all other viruses tested (CMV, HSV‐1 and ‐2, ParvoB19, enterovirus and EBV). RT‐PCR tests of formalin‐fixed samples obtained from the placentas of 16 liveborn cases with parenchymal inflammatory lesions were negative for other viruses (CMV, HSV‐1 and ‐2, ParvoB19, EBV).
Electron microscopy
Electron microscopy of the placental tissues of four stillborn cases revealed viral particles consistent morphologically with coronavirus. They were mostly observed within the cytoplasm of syncytiotrophoblast cells, either grouped within enlarged vesicles (Figure 5a,b) or as single particles (Figure 5c). On higher magnification, the virions showed faint surface projections, with the helical nucleocapsid appearing as internal black dots (Figure 5c). Occasional virions were also identified within the basement membrane of the syncytiotrophoblasts bordering the intravillous space (Figure 5d). No virions were found on electron microscopy in samples obtained from the lungs, liver and spleen of the three autopsied fetuses.
Figure 5.
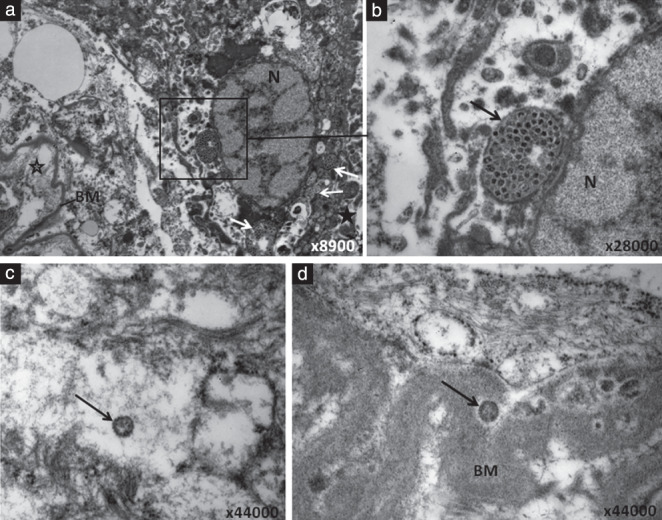
Electron microscopy of SARS‐CoV‐2‐infected trophoblast from pregnancy that resulted in stillbirth. (a) Low magnification of an infected syncytiotrophoblast showing virion‐containing vesicles (white arrows and square frame) in the cytoplasm. Intervillous space is indicated by  and intravillous space by
and intravillous space by  . (b) Higher magnification of area marked by square frame in (a), depicting the vesicular structure (arrow) containing virions with either electron‐lucent or dark center. (c) High magnification of a syncytiotrophoblast showing an intracellular SARS‐CoV‐2 particle (arrow) with visible surface projections and the helical nucleocapsid appearing as black dots inside. (d) High magnification of a syncytiotrophoblast showing a SARS‐CoV‐2 particle (arrow) located in the basement membrane (BM) of the syncytiotrophoblast. N, nucleus.
. (b) Higher magnification of area marked by square frame in (a), depicting the vesicular structure (arrow) containing virions with either electron‐lucent or dark center. (c) High magnification of a syncytiotrophoblast showing an intracellular SARS‐CoV‐2 particle (arrow) with visible surface projections and the helical nucleocapsid appearing as black dots inside. (d) High magnification of a syncytiotrophoblast showing a SARS‐CoV‐2 particle (arrow) located in the basement membrane (BM) of the syncytiotrophoblast. N, nucleus.
Fetal autopsy
Full postmortem examination was performed in Case 3 (35 + 0 weeks' gestation), Case 4 (27 + 2 weeks) and Case 6 (21 + 1 weeks) (Table 1). The fetuses were appropriately grown for gestational age, weighing 2480 g (42nd centile), 1090 g (44th centile) and 397 g (54th centile), respectively. There was evidence of an asphyctic mode of death, with pulmonary petechiae, meconium aspiration and visceral hemorrhages, without any histological evidence suggestive of infection. The brain of Case 3 showed focal subarachnoid hemorrhage with no evidence of hypoxic/ischemic neuronal injury.
Logistic regression analysis
Of 165 histologically examined placentas, clinical data for statistical analysis were available in 69 cases. Potential risk factors are presented in Table 3. Regression analysis revealed thrombophilia (aOR, 29.014; 95% CI, 1.672–503.482), FGR (aOR, 24.250; 95% CI, 1.072–544.081) and mild COVID‐19 infection (rather than asymptomatic COVID‐19 infection) (aOR, 17.366; 95% CI, 1.955–154.228) to be significant independent predictors of SARS‐CoV‐2 placentitis (Nagelkerke R 2 = 0.593).
Table 3.
Clinical characteristics of 69 pregnancies affected by COVID‐19, according to whether they showed evidence of SARS‐CoV‐2 placentitis on histological examination of the placenta
| Characteristic | Placentitis (n = 10) | No placentitis (n = 59) | P |
|---|---|---|---|
| Thrombophilia | 3 (30) | 1 (1.7) | 0.008 |
| Asthma | 0 (0) | 2 (3.4) | 0.729 |
| Smoker | 0 (0) | 2 (3.4) | 0.729 |
| Hypothyroidism | 0 (0) | 6 (10.2) | 0.376 |
| GDM | 3 (30) | 10 (16.9) | 0.280 |
| Gestational hypertension | 1 (10) | 0 (0) | 0.145 |
| Pre‐eclampsia | 0 (0) | 2 (3.4) | 0.729 |
| FGR* (before infection) | 1 (10) | 2 (3.4) | 0.380 |
| COVID‐19 severity | |||
| Asymptomatic | 5 (50) | 52 (88.1) | 0.007 |
| Mild | 4 (40) | 3 (5.1) | 0.007 |
| Moderate | 0 (0) | 2 (3.4) | 0.729 |
| Severe | 1 (10) | 2 (3.4) | 0.327 |
| Maternal age (years) | 35 (34–37.5) | 30 (25–33.5) | 0.007 |
| Gestational age at infection (weeks) | 35.5 (27.3–37.8) | 38 (35–39) | 0.041 |
Data are given as n (%) or median (interquartile range).
Before SARS‐CoV‐2 infection.
FGR, fetal growth restriction; GDM, gestational diabetes mellitus.
DISCUSSION
Evidence obtained from meta‐analyses covering the first phases of the COVID‐19 pandemic was controversial with regard to the association of SARS‐CoV‐2 infection with adverse pregnancy outcome 8 , 9 . Data obtained at subsequent phases of the pandemic, however, point towards an increase in the rates of fetal death, preterm birth, pre‐eclampsia and emergency Cesarean delivery 10 , 11 , 12 , 13 .
SARS‐CoV‐2 placentitis and stillbirth
The spectrum of placental histopathology in COVID‐19‐affected pregnancies is reportedly wide, with variable and overall non‐specific findings, related generally to uninfected neonates and a favorable pregnancy outcome 3 , 14 . The term SARS‐CoV‐2 placentitis, used to describe a particular pattern of placental pathology, first emerged in 2020 in relation to PCR‐positive placentas and/or infected neonates 15 , 16 , 17 , 18 , was subsequently attributed to the Alpha B117 variant 5 in 2021, and is currently becoming more widely adopted in association with stillbirth 19 .
Our observations in the six cases of stillbirth reported herein, which occurred between December 2020 and August 2021 when the Alpha B117 variant was prevalent in Greece, confirm the detrimental potential on the placenta of (at least) the Alpha SARS‐CoV‐2 variant, which can cause SARS‐CoV‐2 placentitis, characterized by a constellation of severe histological placental lesions, and is potentially lethal when the placenta is affected diffusely. The lesions of SARS‐CoV‐2 placentitis were identical in all six cases of stillbirth and were rapidly progressive (as indicated by the short interval between maternal infection and fetal death), producing extensive occlusion of the intervillous space and compromising the maternal‐fetal exchange. In this series, SARS‐CoV‐2 placentitis consisted of a combination of MPFD, severe intervillositis with a mixed inflammatory infiltrate, trophoblast damage and intervillous thrombosis. This histology appears to differentiate SARS‐CoV‐2 placentitis from the so far described COVID‐associated chronic histiocytic intervillositis 5 , 15 , 16 , 19 , due to the mixed
phenotype (histiocytic, lymphocytic and granulocytic) of the inflammatory populations, which is more consistent with an infectious etiology. Nevertheless, the combination of a predominantly mononuclear inflammation with MPFD in the placenta is not unique to SARS‐CoV‐2, having been reported also in the context of other infectious etiology, such as CMV and malaria 20 , 21 .
Electron microscopy and fetal autopsy findings
The detection of virions within the cytoplasm and the basal membrane of perivillous syncytiotrophoblasts, suggests that the virus proceeded halfway within the placental barrier, but failed to enter the fetoplacental circulation. These observations underscore the crucial role of the perivillous syncytiotrophoblast as a key component of the placental barrier, but also demonstrate that at least the Alpha variant could cause fetal death by placental injury, without being transmitted to the fetus. This is further supported by the absence of any evidence of viral transmission in our three fetuses that underwent autopsy, confirming that in these cases the virus failed to cross the placental barrier, but caused fetal death by asphyxia due to the diffuse occlusion of the intervillous space and the hypermetabolic inflammatory condition of the placenta, both compromising fetomaternal exchange. This experience is not confined to Greece; internationally, a number of stillbirths have been attributed to SARS‐CoV‐2 placentitis, occurring during the period of prevalence of the Delta variant 19 , 22 , 23 .
Potential predisposing factors
As, in accordance with previous work 24 , the incidence of SARS‐CoV‐2 placentitis was low among COVID‐19‐affected pregnancies in this study, with no obvious correlation between its occurrence and the severity of maternal disease, the question was raised regarding the potential contribution of predisposing factors. Four of six mothers of the cases of stillbirth with SARS‐CoV‐2 placentitis, and four of five mothers with live birth and less extensive SARS‐CoV‐2 placentitis (and with available clinical information) had underlying conditions associated with pregnancy complications, namely multiple sclerosis, an autoimmune disease associated with an increased risk of infection 25 , gestational diabetes (which is commonly associated with adverse pregnancy outcome), thrombophilia factors, hypertension and prenatally detected FGR. Logistic regression analysis failed to confirm gestational diabetes to be a significant predisposing factor for SARS‐CoV‐2‐placentitis, but showed a significant association for thrombophilia and FGR. Two mothers had homozygous or compound heterozygous polymorphisms for the PAI‐1 and MTHFR genes, associated with abnormal coagulation or impaired fibrinolysis, considered as mild thrombophilias of uncertain clinical significance with regard to adverse pregnancy outcome. Of note, immune‐mediated diseases and thrombophilias have been associated with MPFD, a rare and poorly understood placental disorder 26 , 27 , which was one of the main components of SARS‐CoV‐2 placentitis seen in all stillborn cases, and to a lesser extent in seven liveborn cases. In this context, given that coagulation and fibrinolysis abnormalities are common among COVID‐19 patients 28 , we suggest the hypothesis that certain maternal risk factors may act synergistically with SARS‐CoV‐2 and result in the specific combination of placental lesions that characterize SARS‐CoV‐2 placentitis. Maternal history in Case 2 was unknown, however, the findings of FGR and placental changes of maternal malperfusion suggest underlying uteroplacental insufficiency, related potentially to some predisposing maternal risk factor. The occurrence of these stillbirths in Greece and internationally during the periods when the Alpha and Delta SARS‐CoV‐2 variants prevailed suggests a potential deleterious effect of these particular variants in pregnant women with certain comorbidities or predisposing factors. The number of cases is too small to draw robust conclusions, yet the synergistic action of SARS‐CoV‐2 with particular predisposing factors may be assumed plausibly.
Strengths and limitations
The strengths of this study lie in the novelty of our observations, the broad spectrum of analyses and the assessment of potential predisposing factors for SARS‐CoV‐2 placentitis, supporting the hypothesis of a synergistic effect between these factors and COVID‐19. Limitations include the small number of placentas with SARS‐CoV‐2 RT‐PCR results and the lack of placental ultrasound images postinfection and before fetal demise, to correlate ultrasound imaging with placental histopathology.
Future perspectives
At the time of writing, the Alpha and Delta variants have been replaced widely by the Omicron variant, and as this disease and our experience continue to evolve, it is essential to highlight the importance of the pathological investigation of fetal death and its provision of information critical for the management of pregnancy during the COVID‐19 pandemic. Further pathological and epidemiological investigations appear even more important, as the impact of novel globally spreading SARS‐CoV‐2 variants is still unknown, this challenge remaining to be confronted in the immediate future.
Conclusions
SARS‐CoV‐2 placentitis occurred uncommonly in COVID‐19‐affected pregnancies of non‐vaccinated mothers, appeared to evolve rapidly and, when extensive, caused fetal demise, with no evidence of transplacental fetal infection. Thrombophilia and prenatally detected FGR emerged as independent predisposing factors for the potentially lethal SARS‐CoV‐2 placentitis.
ACKNOWLEDGMENTS
We thank Dr Y. Koskosas, Dr D. Koutsoulis, Dr G. Stathoudakis, Dr K. Soumakis and Prof. M. Lambropoulou for contributing their cases and Mr A. Pergaris for assisting with placenta sampling. Prof. A. Konstantinidou is funded by REA Maternity Clinic, Athens, Greece, to conduct research on Perinatal Pathology of Stillbirth (NKUA SARG 11191).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, Taylor HS, Tal R. Vertical transmission of coronavirus disease 2019: a systematic review and meta‐analysis. Am J Obstet Gynecol 2021; 224: 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huntley BJF, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS‐Co‐V‐2) infection. Obstet Gynecol 2020; 136: 303–312. [DOI] [PubMed] [Google Scholar]
- 3. Sharps MC, Hayes DJL, Lee S, Zou Z, Brady CA, Almoghrabi Y, Kerby A, Tamber KK, Jones CJ, Adams Waldorf KM, Heazell AEP. A structured review of 343 placental morphology and histopathological lesions associated with SARS‐COV‐2 infection. Placenta 2020; 101: 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goh XL, Low YF, Ng CH, Amin Z, Ng YPM. Incidence of SARS‐COV‐2 vertical transmission: a meta‐analysis. Arch Dis Child Fetal Neonatal Ed 2021; 106: 112–113. [DOI] [PubMed] [Google Scholar]
- 5. Linehan L, O'Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS‐COV‐2 placentitis: An uncommon complication of maternal COVID‐19. Placenta 2021; 104: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC, Salomon LJ, Unterscheider J. ISUOG Practice Guidelines: diagnosis and management of small‐for‐gestational‐age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020; 56: 298–312. [DOI] [PubMed] [Google Scholar]
- 7. National Institutes of Health . COVID‐19 Treatment Guidelines. Clinical Spectrum of SARS‐CoV‐2 Infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum.
- 8. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol‐Urganci I, O'Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P , Magee L, Khalil A. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health 2021; 9: e759–e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang J, D'Souza R, Kharrat A, Fell DB, Snelgrove JW, Murphy KE, Shah PS. COVID‐19 pandemic and population‐level pregnancy and neonatal outcomes: a living systematic review and meta‐analysis. Acta Obstet Gynecol Scand 2021; 100: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gurol‐Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, Harris T, Hawdon J, Morris E, Muller P, Waite L, Webster K, van der Meulen J, Khalil A. Maternal and perinatal outcomes of pregnant women with SARS‐COV‐2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol 2021; 225: 522.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papageorghiou AT, et al. Preeclampsia and COVID‐19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol 2021; 225: 289.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, do Vale MS, Cardona‐Perez JA, Maiz N, Cetin I, Savasi V, Deruelle P, Easter SR, Sichitiu J, Soto Conti CP, Ernawati E, Mhatre M, Teji JS, Liu B, Capelli C, Oberto M, Salazar L, Gravett MG, Cavoretto PI, Nachinab VB, Galadanci H, Oros D, Ayede AI, Sentilhes L, Bako B, Savorani M, Cena H, García‐May PK, Etuk S, Casale R, Abd‐Elsalam S, Ikenoue S, Aminu MB, Vecciarelli C, Duro EA, Usman MA, John‐Akinola Y, Nieto R, Ferrazi E, Bhutta ZA, Langer A, Kennedy SH, Papageorghiou AT. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID‐19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021; 175: 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, Playle R, Perry A, Bourne T, Lees CC, PAN‐COVID investigators and the National Perinatal COVID‐19 Registry Study Group . Pregnancy and neonatal outcomes of COVID‐19: coreporting of common outcomes from PAN‐COVID and AAP‐SONPM registries. Ultrasound Obstet Gynecol 2021; 57: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briana DD, Papaevangelou V, Syridou G, Paparizou K, Siafakas N, Konstantinidou AE, Malamitsi‐Puchner A. Clinical symptoms associated with laboratory findings and placental histopathology in full‐term, non‐infected neonates born to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) positive mothers. J Matern Fetal Neonatal Med 2021; 11: 1–4. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz DA, Baldewijns M, Benachi A, Bugatti M, Collins RRJ, De Luca D, Facchetti F, Linn RL, Marcelis L, Morotti D, Morotti R, Parks WT, Patanè L, Prevot S, Pulinx B, Rajaram V, Strybol D, Thomas K, Vivanti AJ. Chronic Histiocytic Intervillositis With Trophoblast Necrosis Is a Risk Factor Associated With Placental Infection From Coronavirus Disease 2019 (COVID‐19) and Intrauterine Maternal–Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) Transmission in Live‐Born and Stillborn Infants. Arch Pathol Lab Med 2021; 145: 517–528. [DOI] [PubMed] [Google Scholar]
- 16. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS‐COV‐2 infection. Nat Commun 2020; 11: 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, Gismondo MR, Perotti F, Callegari C, Mancon A, Cammarata S, Beretta I, Nebuloni M, Trabattoni D, Clerici M, Savasi V. Analysis of SARS‐CoV‐2 vertical transmission during pregnancy. Nat Commun 2020; 11: 5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Debelenko L, Katsyv I, Chong AM, Peruyero L, Szabolcs M, Uhlemann AC. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum Pathol 2021; 109: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz DA, Avvad‐Portari E, Babál P, Baldewijns M, Blomberg M, Bouachba A, Camacho J, Collardeau‐Frachon S, Colson A, Dehaene I, Ferreres JC, Fitzgerald B, Garrido‐Pontnou M, Gerges H, Hargitai B, Helguera‐Repetto AC, Holmström S, Irles CL, Leijonhfvud Å, Libbrecht S, Marton T, McEntagart N, Molina JT, Morotti R, Nadal A, Navarro A, Nelander M, Oviedo A, Oyamada Otani AR, Papadogiannakis N, Petersen AC, Roberts DJ, Saad AG, Sand A, Schoenmakers S, Sehn JK, Simpson PR, Thomas K, Valdespino‐Vázquez MY, van der Meeren LE, Van Dorpe J, Verdijk RM, Watkins JC, Zaigham M. Placental Tissue Destruction and Insufficiency from COVID‐19 Causes Stillbirth and Neonatal Death from Hypoxic‐Ischemic Injury: A Study of 68 Cases with SARS‐CoV‐2 Placentitis from 12 Countries. Arch Pathol Lab Med 2022. DOI: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 20. Taweevisit M, Tansatit M, Sutthiruangwong P, Siranart N, Thorner PS. Combined Placental Maternal Floor Infarction and Cytomegalovirus Placentitis: A Case Report. Fetal Pediatr Pathol 2020; 9: 1–6. doi: 10.1080/15513815.2020.1857487. [DOI] [PubMed] [Google Scholar]
- 21. Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol 1998; 22: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 22. Naranjo Ruiz‐Atienza L, Navarro Jiménez A, Camacho Soriano J, Hernández Losa J, Cerro L, Strohecker Santos I, Moliné Marimon T, Ramón y Cajal‐Agüeras S, Garrido‐Pontnou M. Impact of SARS‐COV‐2 on foetus and placenta of pregnant women affected by COVID‐19. Study of 177 cases received during the pandemic. OFP‐21‐002, 33rd European Congress of Pathology, Gothenburg (online) 29–31 August 2021. Virchows Archiv (suppl 1) 2021; 479: S48. [Google Scholar]
- 23. Martinovic J: Stillbirth & SARS‐CoV2. Symposium: Covid experience. Hargitai D, Kramer Z, Mozes E, Barbai T, Timar J, Schaff Z, Kiss A. SARS‐CoV‐2 placentitis in Semmelweis University, Hungary since the outbreak of the coronavirus pandemic. OFP‐05: 67th Annual Meeting of Paediatric and Perinatal Pathology Society, Belgrade (online) 30th September–1st October 2021.
- 24. Thomas J, Sun Y, Debelenko L. Infrequent Placental and Fetal Involvement in SARS‐CoV‐2 Infection: Pathology Data from a Large Medical Center. J Dev Biol 2021; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacDonald SC, McElrath TF, Hernández‐Díaz S. Pregnancy Outcomes in Women with Multiple Sclerosis. Am J Epidemiol 2019; 188: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gogia N, Machin GA. Maternal thrombophilias are associated with specific placental lesions. Pediatr Dev Pathol 2008; 11: 424–429. [DOI] [PubMed] [Google Scholar]
- 27. Kim EN, Lee JY, Shim JY, Hwang D, Kim KC, Kim SR, Kim CJ. Clinicopathological characteristics of miscarriages featuring placental massive perivillous fibrin deposition. Placenta 2019; 86: 45–51. [DOI] [PubMed] [Google Scholar]
- 28. Wei L, Jie Y, Zhengwei L, Jinpeng L, Sichao C, Danyang C, Shipei W, Qianqian L, Di H, Jianglong H, Wen Z, Liang G, Xiaohui W. Abnormal Fibrinogen Level as a Prognostic Indicator in Coronavirus Disease Patients: A Retrospective Cohort Study. Front Med (Lausanne) 2021; 8: 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


