Abstract
The aim of this systematic review and network meta‐analysis is to evaluate the comparative effectiveness of N95, surgical/medical and non‐medical facemasks as personal protective equipment against respiratory virus infection. The study incorporated 35 published and unpublished randomized controlled trials and observational studies investigating specific mask effectiveness against influenza virus, SARS‐CoV, MERS‐CoV and SARS‐CoV‐2. We searched PubMed, Google Scholar and medRxiv databases for studies published up to 5 February 2021 (PROSPERO registration: CRD42020214729). The primary outcome of interest was the rate of respiratory viral infection. The quality of evidence was estimated using the GRADE approach. High compliance to mask‐wearing conferred a significantly better protection (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.23–0.82) than low compliance. N95 or equivalent masks were the most effective in providing protection against coronavirus infections (OR, 0.30; CI, 0.20–0.44) consistently across subgroup analyses of causative viruses and clinical settings. Evidence supporting the use of medical or surgical masks against influenza or coronavirus infections (SARS, MERS and COVID‐19) was weak. Our study confirmed that the use of facemasks provides protection against respiratory viral infections in general; however, the effectiveness may vary according to the type of facemask used. Our findings encourage the use of N95 respirators or their equivalents (e.g., P2) for best personal protection in healthcare settings until more evidence on surgical and medical masks is accrued. This study highlights a substantial lack of evidence on the comparative effectiveness of mask types in community settings.
Keywords: coronavirus, COVID‐19, facemask, influenza virus, network meta‐analysis
Abbreviations
- AGP
aerosol generating procedure
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CoV
coronavirus
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ILI
influenza‐like illness
- MERS
Middle East respiratory syndrome
- NMA
network meta‐analysis
- OR
odds ratio
- PICOS
population, intervention, comparator, outcomes, and setting
- PPE
personal protective equipment
- RCTs
randomized controlled trials
- SARS
Severe acute respiratory syndrome
- WHO
World Health Organization
1. INTRODUCTION
The coronavirus disease (COVID‐19) pandemic has led to an unprecedented increase in the demand for facemasks globally. The types of facemasks currently in use include N95 respirators, surgical masks, medical masks and non‐medical masks (e.g., cloth or cotton masks). 1 , 2 , 3 , 4 However, there is no established evidence or consensus on which type of facemask is superior in preventing respiratory viral infection either by the wearer or those they encounter. Different facemask guidelines recommend the use of different facemasks against COVID‐19, 1 , 2 , 3 , 4 and this is an area of concern as certain mask types may not be as capable as others in preventing respiratory viral infections. Previous systematic reviews exclusively performed pairwise comparisons of mask types, 5 , 6 , 7 and did not evaluate the capacities of all existing mask types simultaneously, leading to the unconsolidated information on the comparative effectiveness of different facemask types.
Therefore, we conducted the first network meta‐analysis (NMA) to evaluate the comparative prevention effectiveness of the most common types of facemasks (N95 respirators, surgical or medical masks, and non‐medical masks) that have been used as personal protective equipment (PPE). NMA is an analytical tool that enables a single coherent ranking of multiple interventions; thus, it can provide information that helps policy‐makers and healthcare workers choose appropriate equipment from an array of protective equipment. 8 , 9 To inform optimized protective strategies for different causative viruses and clinical settings, we separately analysed comparative mask effects in various respiratory viral infections, including influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS) and COVID‐19, in both community and healthcare settings.
2. METHODS
2.1. Search strategy and selection criteria
We conducted a meta‐analysis following a pre‐registered protocol in PROSPERO (CRD42020214729). Two researchers (Min Seo Kim and Dawon Seong) independently searched the PubMed, Google Scholar and medRxiv databases from inception to 5 February 2021 using the search strategy detailed in the Supplementary Appendix (p. 2). The manual research and screening of reference lists of review articles were also conducted to include additional relevant studies that have not been retrieved through the primary search. Any conflicts were resolved by consensus, with the mediation of a third independent investigator (Jae Il Shin).
Our research question could be summarized in PICOS (Population, Intervention, Comparator, Outcomes and Setting) as follows: people at risk of respiratory virus infection (P), adhered to facemask wearing (I), compared with either no mask‐wearing or little mask‐wearing (C), reduction in the risk of laboratory‐confirmed viral infection (O), in health care or community settings (S). Eligible studies met the following criteria: (1) randomized controlled trials (RCTs), cluster RCTs, prospective cohort studies, retrospective cohort studies, case–control studies and cross‐sectional studies; (2) studies comparing the effectiveness of N95 respirators or their equivalent (e.g., P2), surgical masks, medical masks or non‐medical (e.g., cloth or cotton) masks with each other or with not wearing masks/very low compliance to wearing masks. Studies were excluded if they did not specify the types of mask used, and did not present isolated outcomes for individual mask types. There was no limitation regarding the type of mask, compliance to wearing masks, and the fitting of the mask; however, we preferentially used results from high compliance and better mask fitting when stratified results were presented within a study. Pre‐prints have been used relatively frequently in meta‐analyses for the urgent topic of COVID‐19 10 , 11 , 12 , 13 , 14 as a large amount of relevant data is still unpublished. We included pre‐prints to reduce the risk of selection and publication bias and increase network density, as done elsewhere. 15 We included both RCTs and observational studies in our NMA; inclusion of real‐world data from non‐randomized studies has the potential to improve precision of findings from RCTs if appropriately integrated 16 , 17 and many previous NMAs have increased the density of network and enhanced the statistical power of findings using the approach. 18 , 19 , 20 , 21
2.2. Data extraction
Two investigators (Dawon Seong and Min Seo Kim) extracted data on the PICOS for each study. Moreover, information on the following was collected: first author, publication year, study design, estimated effect sizes or number of events, population information, type of respiratory virus, details of interventions and comparisons (mask type and compliance, if applicable), and outcome of interest. The intervention group included participants wearing a specific type of mask for protection, and the control group consisted of participants not wearing a mask or those who had a very low compliance to wearing a mask. For studies involving facemask and other non‐pharmaceutical interventions (i.e., hand hygiene), we extracted data from selective groups to make the facemask the only difference. The primary outcome of the current NMA was laboratory‐confirmed infection of various respiratory viruses—influenza virus, SARS‐CoV, MERS‐CoV and SARS‐CoV‐2. Disagreements were resolved by consensus, with any persistent conflict resolved by a third independent investigator (Jae Il Shin).
2.3. Quality assessment
Two investigators (Dawon Seong and Min Seo Kim) evaluated the risk of bias for all included studies according to meta‐analysis guidelines. The risk of bias of RCTs was assessed using the ROB2 tool. 22 The risk of bias of observational studies was assessed using the ROBINS‐I tool. 23 The certainty of evidence for primary outcomes was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach specifically designed for NMA. 24 , 25 , 26 , 27 Using the GRADE approach, outcomes were classified as high, moderate, low or very low certainty of evidence.
2.4. Data synthesis
This NMA assessed the effectiveness of facemasks in preventing respiratory viral infection by presenting binary outcomes as odds ratio (OR) with 95% confidence interval (CI). The frequentist framework was used to perform the NMA using STATA (Stata Corp, version 15.0) and R software (version 3.6.0) 28 ; self‐programmed routines of STATA 29 , 30 and the ‘netmeta’ package in R 31 were used as described in the previous studies. 15 , 32 The ‘netmeta’ package utilises graph theoretical approach, which constructs the Moore‐Penrose pseudoinverse matrix and calculates the fitted values of the network model using a weighted least squares approach. 33 Review Manager (REVMAN version 5.3; Nordic Cochrane Centre) was used for pairwise meta‐analysis using inverse variance random‐effects model. We applied random‐effects model as we deemed that the expected heterogeneity between studies is likely to be due to real differences between studies rather than by chance.
In this NMA, the rank hierarchy for each mask type was investigated using the surface under the cumulative rank curve (SUCRA) of the P rank score of R. 34 We assessed the consistency of evidence between direct and indirect comparisons where p < 0.05 under the design‐by‐treatment interaction random‐effects model or inconsistency factors with 95% credible intervals containing 0 was deemed a lack of consistency. 35 As consistency could be considered as statistical measure of transitivity, 36 transitivity assumption was estimated along with consistency test. The net heat plot was constructed to visualise the inconsistency matrix. 35 Heterogeneity was measured using the I 2 value, with I 2 > 50% indicating moderate‐to‐high heterogeneity. Publication bias was assessed using comparison‐adjusted funnel plots and Egger's test. 29 A two‐sided p‐value of <0.05 was considered statistically significant.
2.5. Subgroup analysis
Subgroup analyses were performed for virus types (influenza virus, SARS‐CoV, MERS‐CoV and SARS‐CoV‐2), clinical settings (healthcare setting and community setting), and study design (RCT and observational study) as planned in priori. Post‐hoc subgroup analysis for usual healthcare setting (patient contact) versus aerosol‐generating procedure (AGP) was further conducted given that increasing evidence has supported the difference in the risk of infection in those settings. 37 , 38
3. RESULTS
3.1. Study characteristics
A total of 5892 articles were identified through an initial search, and an additional 54 articles were identified from other sources after reviewing references (Figure 1). Duplicates and irrelevant studies were excluded; hence, a total of 185 articles were selected. After screening the full text of the articles to identify studies meeting the prespecified inclusion and exclusion criteria, 35 articles were included in the final meta‐analysis. Among them, 8 studies were conducted in non‐healthcare settings, and 27 studies investigated mask effectiveness in healthcare settings. Twelve studies were randomized or cluster‐randomized controlled trials and 23 studies were observational studies. The PICOS data of individual studies and reference list of included studies are described in Supplementary Tables S1 and S2 (pp. 4–14). The risk of bias in the included studies was generally low to moderate (Supplementary Appendix, pp. 46–80).
FIGURE 1.
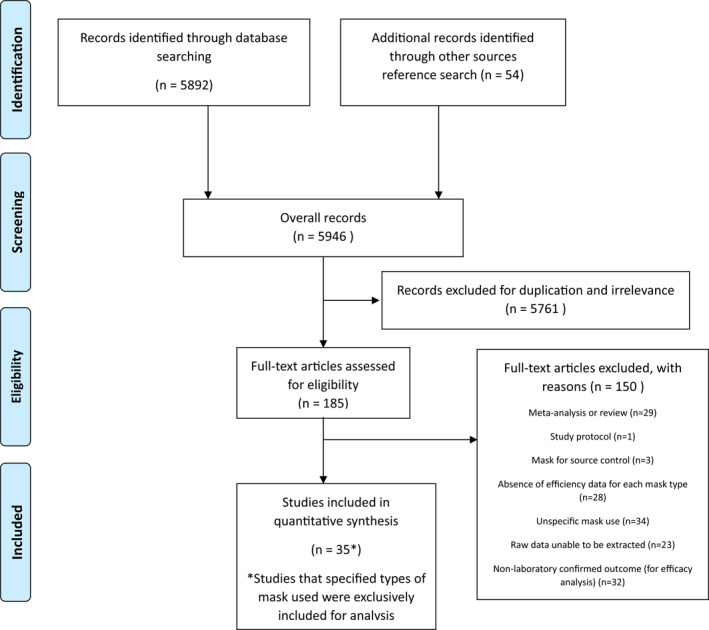
PRISMA diagram showing selection of articles for pairwise and network meta‐analysis
In pairwise meta‐analysis and NMA, heterogeneity (I 2) ranged from 0% to 53.7% (Supplementary Appendix, pp. 15–45). Inconsistencies in NMA outcomes were evaluated to identify disagreement between direct and indirect assessments; global inconsistency was found in results of coronavirus (overall), coronavirus (healthcare setting) and COVID‐19. Networks of eligible comparisons are shown in Figure 2. The certainty of evidence (GRADE) for the primary outcomes is depicted in Table 1.
FIGURE 2.
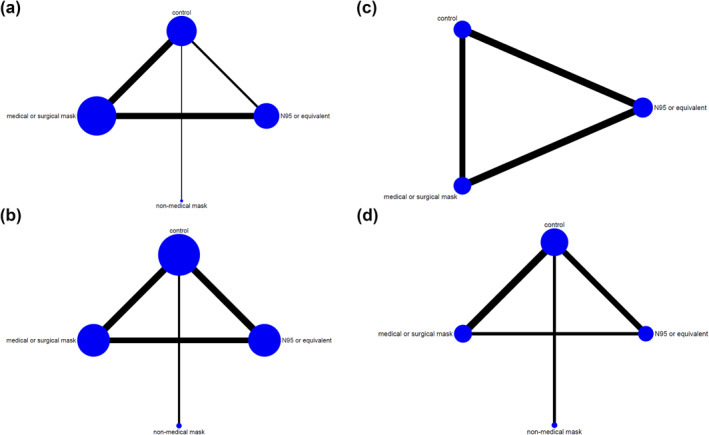
Network of eligible comparisons for respiratory viruses. (a) Influenza virus. (b) Coronavirus (including SARS, MERS and COVID‐19). (c) SARS (SARS‐CoV) and MERS (MERS‐CoV). (d) COVID‐19 (SARS‐CoV‐2). Control includes no mask wearing, or mask wearing at very low frequencies. Non‐medical masks include clothes or cotton masks. Lines indicate direct comparisons of agents, and the thickness of line corresponds to the number of trials in the comparison. The size of node corresponds to the number of studies that involve the intervention. COVID‐19, coronavirus disease‐19; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome
TABLE 1.
Certainty of evidence evaluated with Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework for primary outcomes
| Comparisons (vs. control) | Comparison no. | OR (95% CI), p‐value | Study design a | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| Overall mask effect | |||||||||
| Preventive effect of wearing mask (any type) on respiratory viral infection | |||||||||
| Overall respiratory viral infection | 22 | 0.50 (0.37, 0.68), p < 0.001 | Observational study | Not serious | Not serious | Not serious | Not serious | Not serious | Low |
| Influenza | 8 | 0.71 (0.42, 1.21), p = 0.208 | RCT | Not serious | Not serious | Not serious | Serious | Not serious | Moderate |
| SARS/MERS | 6 | 0.30 (0.14, 0.63), p = 0.001 | Observational study | Serious | Not serious | Not serious | Not serious | Not serious | Low b |
| COVID‐19 | 8 | 0.49 (0.31, 0.78), p = 0.003 | Observational study | Not serious | Not serious | Not serious | Not serious | Not serious | Low |
| Compliance (vs. low compliance) | |||||||||
| High adherence to mask behaviour | 6 | 0.43 (0.23, 0.82), p = 0.010 | Observational study | Not serious | Serious | Not serious | Not serious | Not serious | Very low |
| Per specific mask type | |||||||||
| Influenza virus infection | |||||||||
| Medical and surgical mask | 17 | 0.75 (0.51, 1.09), p = 0.132 | RCT | Not serious | Not serious | Not serious | Serious | Not serious | Moderate |
| N95 or equivalent | 11 | 0.84 (0.56, 1.28), p = 0.417 | RCT | Not serious | Not serious | Not serious | Serious | Not serious | Moderate |
| Non‐medical mask | 1 | 1.29 (0.24, 6.94), p = 0.767 | Observational study | Not serious | Not serious | Not serious | Very serious | Not serious | Very low |
| Coronavirus infection, overall (SARS, MERS, and COVID‐19) | |||||||||
| N95 or equivalent | 14 | 0.30 (0.20, 0.44), p < 0.001 | Observational study | Not serious | Not serious | Not serious | Not serious | Serious | Low b |
| Medical or surgical mask | 14 | 0.72 (0.51, 1.01), p = 0.057 | Observational study | Not serious | Not serious | Not serious | Serious | Serious | Very low |
| Non‐medical mask | 2 | 0.77 (0.29, 2.07), p = 0.605 | Observational study | Not serious | Not serious | Not serious | Serious | Serious | Very low |
| SARS/MERS infection | |||||||||
| N95 or equivalent | 8 | 0.24 (0.13, 0.46), p < 0.001 | Observational study | Not serious | Not serious | Not serious | Not serious | Serious | Low b |
| Medical and surgical mask | 7 | 0.70 (0.38, 1.30), p = 0.259 | Observational study | Not serious | Not serious | Not serious | Serious | Serious | Very low |
| COVID‐19 infection | |||||||||
| N95 or equivalent | 6 | 0.30 (0.17, 0.55), p < 0.001 | Observational study | Not serious | Not serious | Not serious | Not serious | Serious | Low b |
| Medical or surgical mask | 7 | 0.71 (0.44, 1.14), p = 0.156 | Observational study | Serious | Not serious | Not serious | Serious | Serious | Very low |
| Non‐medical mask | 2 | 0.73 (0.25, 2.14), p = 0.566 | Observational study | Not serious | Not serious | Not serious | Serious | Serious | Very low |
| Health care settings | |||||||||
| Influenza virus infection | |||||||||
| Medical or surgical mask | 10 | 0.65 (0.28, 1.49), p = 0.309 | RCT | Not serious | Not serious | Not serious | Serious | Not serious | Moderate |
| N95 or equivalent | 9 | 0.72 (0.31, 1.69), p = 0.451 | RCT | Not serious | Not serious | Not serious | Serious | Not serious | Moderate |
| Non‐medical mask | 1 | 1.29 (0.24, 6.94), p = 0.767 | Observational study | Not serious | Not serious | Not serious | Very serious | Not serious | Very low |
| Coronavirus infection, overall (SARS, MERS, and COVID‐19) | |||||||||
| N95 or equivalent | 14 | 0.29 (0.19, 0.44), p < 0.001 | Observational study | Not serious | Not serious | Not serious | Not serious | Serious | Low b |
| Medical or surgical mask | 12 | 0.69 (0.44, 1.07), p = 0.097 | Observational study | Serious | Not serious | Not serious | Serious | Serious | Very low |
| Community settings | |||||||||
| Influenza virus infection | |||||||||
| Medical or surgical mask | 7 | 0.76 (0.47, 1.20), p = 0.239 | RCT | Serious | Not serious | Not serious | Serious | Not serious | Low |
| N95 or equivalent | 2 | 3.50 (0.44, 27.97), p = 0.237 | RCT | Not serious | Not serious | Not serious | Very serious | Not serious | Low |
| Coronavirus infection, overall (SARS, MERS, and COVID‐19) | |||||||||
| Medical or surgical mask | 2 | 0.78 (0.53, 1.12), p = 0.150 | Observational study | Serious | Not serious | Not serious | Serious | Not serious | Very low |
| Non‐medical mask | 1 | 1.29 (0.48, 3.45), p = 0.612 | Observational study | Not serious | Not serious | Not serious | Serious | Not serious | Very low |
Note: Statistically significant results are marked in bold.
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MERS, Middle East respiratory syndrome; OR, odds ratio; RCT, randomized controlled trial; SARS, severe acute respiratory syndrome.
Dominant study design.
Upgraded by one for a large magnitude of effect.
Rationale:
Study design: If randomized trials form the majority of evidence base, the quality rating starts at ‘high’. If observational studies form the majority of evidence, base the quality rating starts at ‘low’.
Risk of bias: Downgraded for failure to conceal random allocation or blind participants in randomized controlled trials or failure to adequately control for confounding in observational studies.
Inconsistency: Downgraded if direct and indirect evidence are not coherence as demonstrated by the difference in point estimates and the lack of overlap in the 95% confidential intervals (CIs) between direct and indirect evidence (Global incoherence tests such as Q statistic to assess consistency under the assumption of a full design‐by‐treatment interaction random effects model were used as supplementary information for judgement).
Indirectness. Downgraded if there present substantial differences in study characteristics (PICO) that may modify treatment effect in the direct comparisons (such as A v C and B v C) that form the basis for the indirect estimate of effect of the comparison of interest (A v B), or the result is solely derived from indirect comparisons.
Imprecision: Downgraded when cases are small; or 95% CIs are wide and include or are close to null effect.
Publication bias: Downgraded when substantial asymmetry is observed in funnel plot or p < 0.10 in egger's test.
GRADE Definition (suggested by Puhan et al. in ‘A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis’):
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate that is the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited that is the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate that is the true effect is likely to be substantially different from the estimate of effect.
3.2. Overall effect of wearing masks against respiratory viral infections
Wearing masks, regardless of the type, was associated with a reduced risk of infection from all respiratory viruses (OR, 0.50; 95% CI, 0.37–0.68; GRADE, low), SARS‐CoV/MERS‐CoV (OR, 0.30; 95% CI, 0.14–0.63; GRADE, low), and SARS‐CoV‐2 (OR, 0.49; 95% CI, 0.31–0.78; GRADE, low), but not with the risk of infection from influenza virus (OR, 0.71; 95% CI, 0.42–1.21; GRADE, moderate; Figure 3). High adherence to wearing masks was associated with a lower risk of respiratory viral infection relative to low adherence (Figure 3).
FIGURE 3.
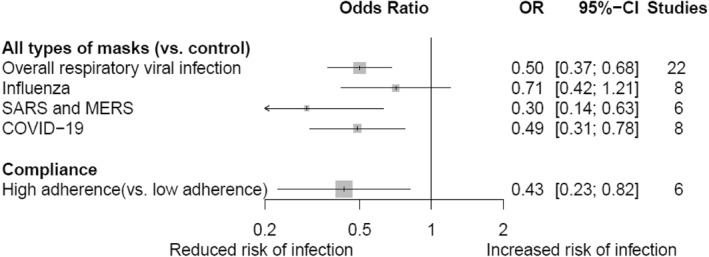
Pairwise meta‐analysis for the impact of wearing masks and adhering to mask behaviour on the risk of infection to respiratory viral diseases. Control includes no mask wearing, or mask wearing at very low frequencies. COVID‐19, coronavirus disease‐19; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome
3.3. Comparative effectiveness of facemasks against influenza
The use of facemask, including medical/surgical masks (OR, 0.75; 95% CI, 0.51–1.09; GRADE, moderate), N95 or equivalent masks (OR, 0.84; 95% CI, 0.56–1.28; GRADE, moderate), and non‐medical masks (OR, 1.29; 95% CI, 0.24–6.94; GRADE, very low), was not associated with reduced infection from influenza virus, similar to the non‐use of facemasks or a very low compliance to wearing masks in all studies (Figure 4a). The results were consistent in subgroup analyses of RCTs (Figure 4b) and observational studies (Figure 4c).
FIGURE 4.
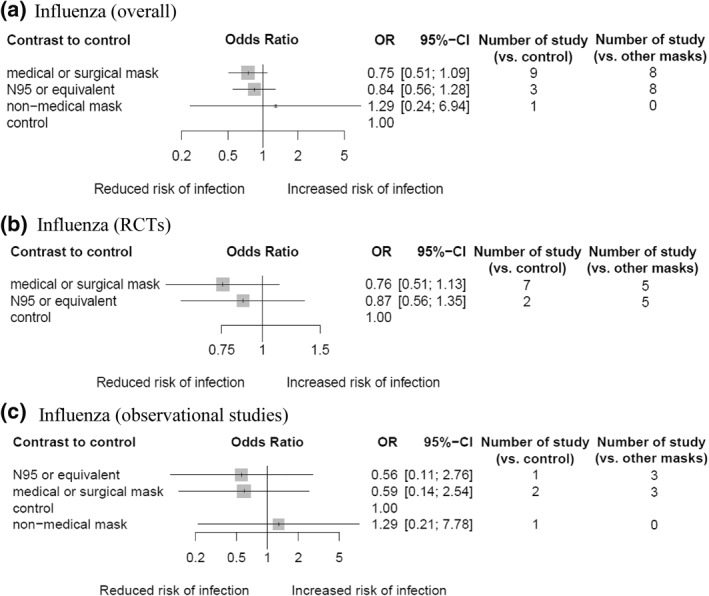
Network meta‐analysis of different types of facemask compared with control (no mask or very low frequencies) for influenza virus infections. Risk of laboratory‐confirmed infection by influenza virus in (a) overall, (b) RCTs and (c) observational studies. Effect estimates are presented in odds ratios with 95% confidence interval. Facemasks are ranked by surface under the cumulative ranking curve value. RCT, randomized controlled trial
3.4. Comparative effectiveness of facemasks against coronaviruses
Only wearing N95 or equivalent masks (OR, 0.30; 95% CI, 0.20–0.44; GRADE, low) was associated with a decreased risk infection from all coronaviruses (SARS‐CoV, MERS‐CoV and SARS‐CoV‐2). The results were similar for assessment of the comparative effectiveness of masks against SARS and MERS (Figure 5b) and COVID‐19 (Figure 5c).
FIGURE 5.
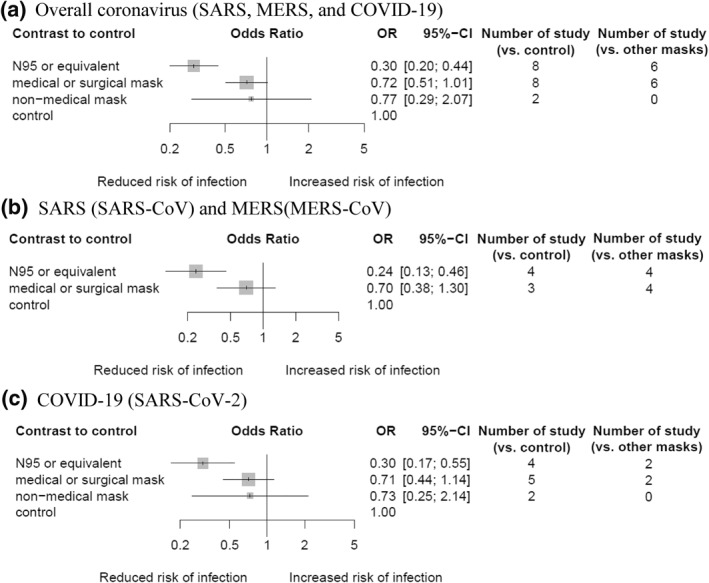
Network meta‐analysis of different types of facemask compared with control (no mask or very low frequencies) for coronavirus infections. Rate of diagnosed with coronavirus infection. (a) Risk of overall coronavirus infection (SARS, MERS, and COVID‐19), (b) SARS (SARS‐CoV) and MERS (MERS‐CoV), and (c) COVID‐19 (SARS‐CoV‐2). Effect estimates are presented in odds ratios with 95% confidence interval. Facemasks are ranked by surface under the cumulative ranking curve value. COVID‐19, coronavirus disease‐19; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome
3.5. Comparative effectiveness of facemasks in healthcare and community settings
No facemask type was associated with a reduced influenza infection rate in healthcare settings (Figure 6a) and community settings (Figure 6b). For all coronavirus infections, including SARS, MERS and COVID‐19, in healthcare settings, the use of N95 or equivalent mask was associated with a lower infection rate (OR, 0.29; 95% CI, 0.19–0.44; GRADE, low), but not the use of medical/surgical masks (Figure 6c); the results were consistent in subgroup analysis particularly limited to mask effectiveness during AGP (Figure S1). Insufficient data were collected on the effectiveness of N95 or equivalent masks against coronavirus infection in community settings (Figure 6d).
FIGURE 6.
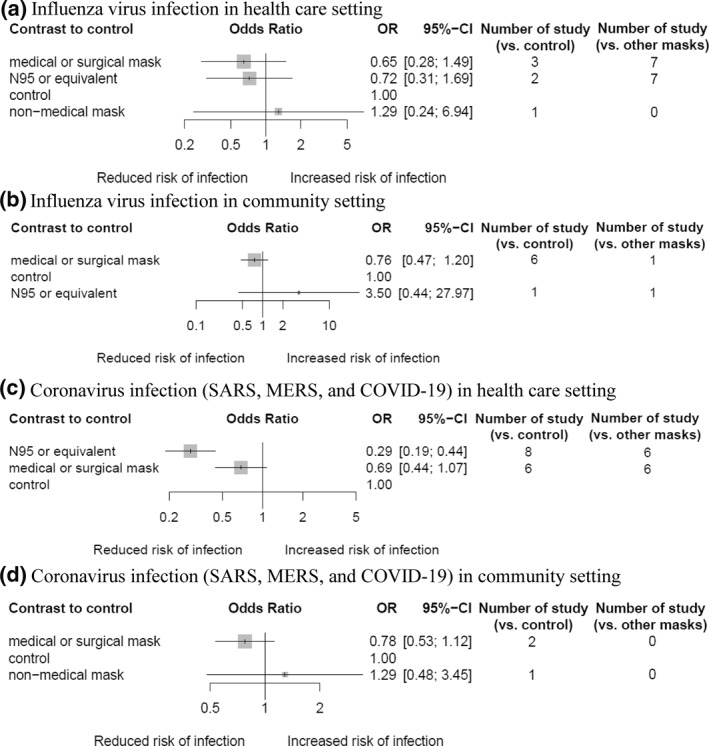
Network meta‐analysis of different types of facemask compared with control (no mask or very low frequencies) for respiratory viral infections in health care and non‐health care settings. (a) Risk of influenza virus infection in health care setting, (b) risk of influenza virus infection in community setting, (c) risk of coronavirus infection (SARS, MERS, and COVID‐19) in healthcare setting, and (d) risk of coronavirus infection (SARS, MERS, and COVID‐19) in community setting. For studies that investigated mask effectiveness separately for usual care and aerosol‐generating procedure within the healthcare setting, results from usual care were preferentially used for the analysis. Effect estimates are presented in odds ratios with 95% confidence interval. Facemasks are ranked by surface under the cumulative ranking curve value
4. DISCUSSION
We conducted the first NMA to evaluate the comparative effectiveness of facemasks against various respiratory viral infections (influenza, MERS, SARS and COVID‐19) in both community and healthcare settings. This NMA mainly focussed on using facemask as PPE (i.e., to protect the uninfected wearer) rather than as source control or transmission prevention, and as such, the interpretation of the results was confined to this regard. Our study revealed that the use of facemasks provides protection against respiratory viral infections in general, but the effectiveness may vary according to the type of facemask used. The N95 respirator or its equivalent was the most effective mask type, while evidence supporting the use of medical or surgical masks against influenza or coronavirus infections (SARS, MERS and COVID‐19) was weak.
The current facemask guidelines for COVID‐19 vary from one organization to another. 6 The World Health Organization (WHO) recommends non‐medical masks for the general population; medical/surgical masks for individuals aged >60 years, those with underlying medical conditions, the frail, and/or those attending the ill; and respirator masks including N95 masks for healthcare workers in settings where procedures that may aerosolize the virus are performed. 1 While our findings agree with the use of N95 or equivalent in the healthcare setting for both usual patient contact and AGP, this study highlights insufficient evidence on the effectiveness of medical or surgical masks in community settings. The Centers for Disease Control and Prevention (CDC) advises the use of non‐medical masks with multiple layers for community dwellers and advocates the reservation of medical/surgical masks or N95 respirators for healthcare workers. 2 Although we acknowledge that identifying the optimal mask distribution strategy based on mask effectiveness and supply is complicated, our finding raises the concern that non‐medical masks may not provide sufficient protection against respiratory viral infections as our results show very large CIs and even an increased OR towards infection in community settings (Figure 6d), which leads to the belief that non‐medical masks are less likely to be shown to be effective even after accumulation of more evidence. The findings of this study support that N95 or equivalent (e.g., P2) masks should be the primary choice, and further investigations on N95 or equivalent masks, including effects of reusing N95 masks or extending their use period, 39 , 40 , 41 would be useful in mitigating the demand and supply imbalance and protecting the globe against current and future respiratory infection pandemics.
Although N95 or equivalent masks were effective against coronavirus infections (e.g., SARS, MERS and COVID‐19), they did not show effectiveness in preventing influenza virus infections (Figure 4). Four potential explanations are provided for this discrepancy. First, we investigated laboratory‐confirmed influenza infection, rather than clinically diagnosed influenza (i.e., standard CDC classification of fever ≥37.8°C plus cough or sore throat) or influenza‐like illness (ILI); this is because the clinical diagnosis cannot guarantee if the person was indeed infected by influenza virus given the numerous respiratory viruses (i.e., respiratory syncytial virus, adenovirus and rhinovirus) can induce similar symptoms. This different focus of outcome may in part explain our counterintuitive results on mask effectiveness against influenza infection, considering previous studies have made conclusions for mask effectiveness in light of ILI. 42 , 43 Second, there was a consistent trend towards reduced influenza infection with facemasks (Figures 3 and 4); given the imprecision of the effect estimates for wearing masks against influenza according to GRADE (Table 1), we cannot yet discount facemasks' effectiveness in prevention of influenza infection. Third, the poor effectiveness of masks against influenza may be attributable to the higher aerosol transmission potency of influenza virus compared to that of coronaviruses. 44 , 45 The higher aerosol potency of influenza virus may allow more particles to be penetrated through unfitted masks. Lastly, the difference in the findings can be possibly explained by a higher adherence to wearing masks in pandemic settings than during the seasonal spread of influenza. 6 The global effect of SARS, MERS and COVID‐19 led to unprecedentedly high standards, regulations and education regarding facemask usage, and this may have contributed to a significant reduction in the numbers of coronavirus infections. This is also supported by our result that higher compliance to masks significantly reduced respiratory viral infection (Figure 3).
This study does not claim the ineffectiveness of surgical or medical masks nor does it oppose their use. Their effect directions were consistently towards lower risk for infection but with substantial imprecision according to GRADE, which may reflect a lack of statistical power rather than absence of actual effectiveness. Moreover, facemasks can be used to block the spread of droplets by an infected person (source control), as well as PPE. 46 , 47 Since the present study mainly focussed on the protection of uninfected wearer but not the source control or transmission, the interpretation of the results on surgical and medical masks should be limited to protection. Wearing medical or surgical masks can still be meaningful in preventing transmissions of influenza virus and coronavirus as they can serve as shields to prevent the spreading of droplets carrying the infectious viruses from infected persons. 48 , 49 , 50 Laboratory findings insisted that wearing of surgical masks or KN95 respirators reduced the number of particles emitted from breath and coughing, 51 even without proper fit testing. 52
This study has several limitations. First, in contrast to the wealth of RCTs investigating mask potencies for preventing influenza virus infection, there is one RCT investigating mask effectiveness against COVID‐19. Thus, analysis of mask usage against coronaviruses was performed primarily based on observational studies, which may be prone to reporting, selection and confounding biases. To account for such biases, we evaluated the certainty of evidence using the GRADE framework 24 and downgraded the evidence level for limited study design and any detection of bias. Second, individual studies were heterogeneous in terms of causative viruses, settings, protocols for wearing facemasks and participants' compliance. We conducted various subgroup analyses to address these issues and reached relatively low heterogeneity, ranged from I 2 0% to 53.7%, compared to previous meta‐analysis investigating facemask effectiveness (I 2 ranging from 48% to 87%). 5 Lastly, it is observed in the GRADE framework that certainty of evidence for medical or surgical masks are generally lower than that for N95 or equivalent (Table 1). This may support the necessity for reappraisal of surgical/medical masks after more studies are published. Although the certainty of evidence is yet suboptimal, this study presents the highest level of evidence to date.
Coronaviruses are a serious public health threat, as demonstrated during the previous SARS and MERS epidemics and the current COVID‐19 pandemic. Our study demonstrated that the use of facemasks provides protection against respiratory viral infections in general. Among various types of facemasks, it is likely safer to use N95 or equivalent in healthcare settings as PPE for the moment until more evidence on other types of masks are realized.
CONFLICT OF INTEREST
No conflict of interest declared. This manuscript has been reviewed and is approved by all authors. Min Seo Kim, Dawon Seong, Han Li, Seo Kyoung Chung, Youngjoo Park, Minho Lee, Seung Won Lee, Dong Keon Yon, Jae Han Kim, Keum Hwa Lee, Marco Solmi, Elena Dragioti, Ai Koyanagi, Louis Jacob, Andreas Kronbichler, Kalthoum Tizaoui, Sarah Cargnin Phar, Salvatore Terrazzino, Sung Hwi Hong, Ramy Abou Ghayda, Joaquim Radua, Hans Oh, Karel Kostev, Shuji Ogino, I‐Min Lee, Edward Giovannucci, Yvonne Barnett, Laurie Butler, Daragh McDermott, Petre‐Cristian Ilie, Jae Il Shin and Lee Smith have no commercial associations that may present a conflict of interest in relation to this manuscript.
ETHICS STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
Min Seo Kim, Dawon Seong and Jae Il Shin contributed to the study concept and design. Min Seo Kim and Dawon Seong identified and acquired relevant trials and extracted data. Min Seo Kim and Dawon Seong drafted the protocol for this study. Min Seo Kim analysed the data. Min Seo Kim, Han Li and Jae Han Kim wrote the first draft of the manuscript. Min Seo Kim finalized the manuscript. Seo Kyoung Chung, Youngjoo Park and Minho Lee contributed to evaluating the Risk of Biases. Seung Won Lee, Dong Keon Yon, Keum Hwa Lee, Marco Solmi, Elena Dragioti, Ai Koyanagi, Louis Jacob, Andreas Kronbichler, Kalthoum Tizaoui, Sarah Cargnin, Salvatore Terrazzino, Sung Hwi Hong, Ramy Abou Ghayda, Joaquim Radua, Hans Oh, Shuji Lee, Karel Kostev, Shuji Ogino, I‐Min Lee, Edward Giovannucci, Yvonne Barnett, Laurie Butler, Daragh McDermott, Petre‐Cristian Ilie and Jae Il Shin contributed to the interpretation of data and critical revision of the manuscript. Jae Il Shin, Edward Dragioti, Ai Koyanagi, Andreas Kronbichler, Shuji Ogino and I‐Min Lee provided statistical advice or supervised the statistical interpretations. All authors saw and approved the final submitted version.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENT
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Kim MS, Seong D, Li H, et al. Comparative effectiveness of N95, surgical or medical, and non‐medical facemasks in protection against respiratory virus infection: a systematic review and network meta‐analysis. Rev Med Virol. 2022;32(5):e2336. 10.1002/rmv.2336
Min Seo Kim, Dawon Seong, and Han Li contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. WHO . Coronavirus Disease (COVID‐19) Advice for the Public: When and How to Use Masks. 2020. Accessed November 23, 2020. [Google Scholar]
- 2. CDC . Considerations for Wearing Masks. 2020. Accessed November 23, 2020. [Google Scholar]
- 3. Clase CM, Fu EL, Joseph M, et al. Cloth masks may prevent transmission of COVID‐19: an evidence‐based, risk‐based approach. Ann Intern Med. 2020;173(6):489‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javid B, Weekes MP, Matheson NJ. Covid‐19: should the public wear face masks? BMJ Clin Res Ed. 2020;369:m1442. [DOI] [PubMed] [Google Scholar]
- 5. Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet. 2020;395(10242):1973‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chou R, Dana T, Jungbauer R, Weeks C, McDonagh MS. Masks for prevention of respiratory virus infections, including SARS‐CoV‐2, in health care and community settings : a living rapid review. Ann Intern Med. 2020;173(7):542‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ Clin Res Ed. 2008;336(7635):77‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JP, Welton NJ. Network meta‐analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628‐630. [DOI] [PubMed] [Google Scholar]
- 9. Nikolakopoulou A, Mavridis D, Furukawa TA, et al. Living network meta‐analysis compared with pairwise meta‐analysis in comparative effectiveness research: empirical study. BMJ Clin Res Ed. 2018;360:k585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID‐19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4(5):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of Covid‐19: systematic review and critical appraisal. BMJ Clin Res Ed. 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li JW, Han TW, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta‐analysis. Prog Cardiovasc Dis. 2020;63(4):518‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultan S, Altayar O, Siddique SM, et al. AGA Institute rapid review of the gastrointestinal and liver manifestations of COVID‐19, meta‐analysis of international data, and recommendations for the consultative management of patients with COVID‐19. Gastroenterology. 2020;159(1):320‐334.e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA. 2021;325(12):1185‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID‐19: a systematic review and network meta‐analysis. PLoS Med. 2020;17(12):e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Efthimiou O, Mavridis D, Debray TP, et al. Combining randomized and non‐randomized evidence in network meta‐analysis. Statistics Med. 2017;36(8):1210‐1226. [DOI] [PubMed] [Google Scholar]
- 17. Riley RD, Jackson D, Salanti G, et al. Multivariate and network meta‐analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ Clin Res Ed. 2017;358:j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tricco AC, Zarin W, Cardoso R, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta‐analysis. BMJ Clin Res Ed. 2018;363:k4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta‐analysis of randomised and observational studies. BMJ Clin Res Ed. 2012;345:e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanters S, Socias ME, Paton NI, et al. Comparative efficacy and safety of second‐line antiretroviral therapy for treatment of HIV/AIDS: a systematic review and network meta‐analysis. Lancet HIV. 2017;4(10):e433‐e441. [DOI] [PubMed] [Google Scholar]
- 21. Kim MS, An MH, Kim WJ, Hwang T‐H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID‐19: a systematic review and network meta‐analysis of confounder‐adjusted 20212 hospitalized patients. medRxiv. 2020:20132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ Clin Res Ed. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ Clin Res Ed. 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 25. Brignardello‐Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta‐analysis. J Clin Epidemiol. 2018;93:36‐44. [DOI] [PubMed] [Google Scholar]
- 26. Brignardello‐Petersen R, Mustafa RA, Siemieniuk RAC, et al. GRADE approach to rate the certainty from a network meta‐analysis: addressing incoherence. J Clin Epidemiol. 2019;108:77‐85. [DOI] [PubMed] [Google Scholar]
- 27. Brignardello‐Petersen R, Murad MH, Walter SD, et al. GRADE approach to rate the certainty from a network meta‐analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60‐67. [DOI] [PubMed] [Google Scholar]
- 28. Xu C, Niu Y, Wu J, Gu H, Zhang C. Software and package applicating for network meta‐analysis: a usage‐based comparative study. J Evidence‐Based Med. 2018;11(3):176‐183. [DOI] [PubMed] [Google Scholar]
- 29. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shim S, Yoon BH, Shin IS, Bae JM. Network meta‐analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta‐analysis using R: a review of currently available automated packages. PLoS One. 2014;9(12):e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim MS, Rhim HC, Park A, et al. Comparative efficacy and acceptability of pharmacological interventions for the treatment and prevention of delirium: a systematic review and network meta‐analysis. J Psychiatr Res. 2020;125:164‐176. [DOI] [PubMed] [Google Scholar]
- 33. Rücker G. Network meta‐analysis, electrical networks and graph theory. Res Synth Methods. 2012;3(4):312‐324. [DOI] [PubMed] [Google Scholar]
- 34. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta‐analyses. BMC Med Res Methodol. 2013;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watt J, Tricco AC, Straus S, Veroniki AA, Naglie G, Drucker AM. Research techniques made simple: network meta‐analysis. J Investig Dermatol. 2019;139(1):4‐12.e11. [DOI] [PubMed] [Google Scholar]
- 37. Radonovich LJ, Jr. , Simberkoff MS, Bessesen MT, et al. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322(9):824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macintyre CR, Seale H, Yang P, et al. Quantifying the risk of respiratory infection in healthcare workers performing high‐risk procedures. Epidemiol Infect. 2014;142(9):1802‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ballard DH, Jammalamadaka U, Meacham KW, et al. Quantitative fit tested N95 respirator‐alternatives generated with CT imaging and 3D printing: a response to potential shortages during the COVID‐19 pandemic. Acad Radiol. 2021;28(2):158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhong H, Zhu Z, You P, et al. Plasmonic and superhydrophobic self‐decontaminating N95 respirators. ACS Nano. 2020;14(7):8846‐8854. [DOI] [PubMed] [Google Scholar]
- 41. Cai C, Floyd EL. Effects of sterilization with hydrogen peroxide and chlorine dioxide on the filtration efficiency of N95, KN95, and surgical face masks. JAMA Netw Open. 2020;3(6):e2012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15(2):233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacIntyre CR, Wang Q, Seale H, et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187(9):960‐966. [DOI] [PubMed] [Google Scholar]
- 44. Cowling BJ, Ip DK, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57(5):501‐508. [DOI] [PubMed] [Google Scholar]
- 46. Bundgaard H, Bundgaard JS, Raaschou‐Pedersen DET, et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS‐CoV‐2 infection in Danish mask wearers: a randomized controlled trial. Ann Intern Med. 2021;174(3):335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID‐19. Proc Natl Acad Sci U S A. 2021;118(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Howard J, Huang A, Li Z, et al. Face Masks Against COVID‐19: An Evidence Review. 2020. [Google Scholar]
- 49. Moussaoui A, Zerga EH, Hadi Zerga E. Transmission dynamics of COVID‐19 in Algeria: the impact of physical distancing and face masks. AIMS Public Health. 2020;7(4):816‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arumuru V, Pasa J, Samantaray SS. Experimental visualization of sneezing and efficacy of face masks and shields. Phys Fluids. 2020;32(11):115129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Asadi S, Cappa CD, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10(1):15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


