Abstract
Increased COVID‐19 vaccine hesitancy presents a major hurdle in global efforts to contain the COVID‐19 pandemic. This study was designed to estimate the prevalence of adverse events after the first dose of the Covishield (AstraZeneca) vaccine among physicians in Bangladesh. A cross‐sectional study was conducted using an online questionnaire for physicians (n = 916) in Bangladesh. Physicians who received at least one dose of the COVID‐19 vaccine were included. The study was carried out from April 12 to May 31, 2021. More than 58% of respondents (n = 533) reported one or more adverse events. Soreness of the injected arm (71.9%), tiredness (56.1%), fever (54.4%), soreness of muscles (48.4%), headache (41.5%) and sleeping more than usual (26.8%) were the most commonly reported adverse events. Most vaccine‐related reactogenicities were reported by the younger cohorts (<45 years). The majority of respondents reported severity of reactogenicity as “mild,” experienced on the day of vaccination, and lasting for 1–3 days. The most common reactogenicity was pain at the injection site; the second most common was tiredness. Almost half (49.2%) of the physicians took acetaminophen (paracetamol) to minimize the effects of vaccine reactogenicity. Multivariate logistic regression analyses showed that physicians with diabetes and hypertension (OR = 2.729 95% CI: 1.282–5.089) and asthma with other comorbidities (OR = 1.885 95% CI: 1.001–3.551) had a significantly higher risk of vaccine‐related reactogenicities than physicians without comorbidities. Further safety studies with larger cohorts are required to monitor vaccine safety and provide assurance to potential vaccine recipients.
Keywords: AstraZeneca vaccine, Bangladesh, COVID‐19, physicians, reactogenicity
1. INTRODUCTION
Recent studies have demonstrated that high rates of COVID‐19 vaccine hesitancy among both the general population and healthcare professionals (HCPs) present a major hurdle in global efforts to contain the COVID‐19 pandemic. 1 , 2 , 3 , 4 , 5 Public dissemination of evidence for the safety and efficacy of vaccines may encourage vaccine acceptance. 2 In the absence of sufficient vaccine acceptance, universal access to vaccination may not achieve immunization coverage essential to control the ongoing pandemic. 6 In fact, global herd immunity (population vaccine coverage of 60%–80%) is becoming unachievable due to stark disparities in vaccination rates among different countries. 7 , 8 As of 23 February 2022, more than 4.9 billion vaccine doses have been administered worldwide, which is equal to 63.9% of the world population. 7
Several countries have temporarily discontinued the Oxford‐AstraZeneca vaccine over concerns that the vaccine may be linked to an increased risk of blood clots. 9 Although blood clots have been reported as an infrequent side effect in some populations, the risk of clotting due to COVID‐19 infection appears to be greater than that posed by the vaccine. Nonetheless, these concerns may contribute to vaccine hesitancy. 10 , 11 , 12 In addition to these rare, serious complications, more commonly reported symptoms associated with reactogenicity may also contribute to vaccine reluctance.
The reactogenicity of COVID‐19 vaccines is emerging. 13 Thus far, data on vaccine safety and adverse events has been obtained primarily from manufacturer‐sponsored studies. 14 A few clinical trials have published short‐term findings of the efficacy and safety of COVID‐19 vaccines. 13 Various government agencies monitor vaccine reactogenicity to rapidly detect safety ranges and rare adverse events, as well as provide real‐time data for risk analysis and decision‐making. 15 , 16 , 17 For example, the U.S. Centers for Disease Control (CDC) 16 , 17 and the U.K. Medicines & Healthcare products Regulatory Agency (MHRA) 18 collect self‐reported data from vaccine recipients via online tools. 19 Despite the recognized challenges of self‐reporting symptoms, including inconsistency of data, reporting biases and lack of control groups, 20 health authorities frequently use this approach to make inferences about the wider population of vaccine recipients. 16 , 17 , 18 Bangladesh started vaccination for COVID‐19 from 8 February 2021. To our knowledge, this study is the first to report the prevalence and severity of COVID‐19‐vaccine associated reactogenicity among physicians in Bangladesh.
The current study aimed to estimate the prevalence of the AstraZeneca vaccine reactogenicity among physicians who received vaccinations in the initial phase of vaccine roll‐out in Bangladesh. We surveyed only physicians and excluded other contemporary vaccine recipients to document reactogenicity in professionals with training to identify and clearly articulate symptoms. Monitoring the reactogenicity of COVID‐19 vaccines has the potential to identify uncommon adverse responses particular to Bangladeshi cohorts. Documenting reactogenicity is crucial for planning necessary clinical supports following COVID‐19 vaccination in Bangladesh and establishing safety data to promote vaccine acceptance.
2. MATERIALS AND METHODS
2.1. Study design and participants
A cross‐sectional survey was conducted among physicians working in different government and private sector academic institutes and hospitals in Bangladesh. Inclusion criteria were physicians who received at least one dose of the AstraZenica COVID‐19 vaccine. The study was conducted from 12 April 2021 to 31 May 2021.
2.2. Data collection
We asked physicians to complete a self‐administered online survey (via the Google Docs® platform) adapted for Bangladesh from an instrument developed by researchers working in Barbados (Hinkson‐Lacorbiniere and team). The questionnaire was validated by a multinational panel of public health specialists and amended as per their suggestions. A pilot study was conducted among 29 respondents who were excluded from the formal evaluation, and further adjustment was done based on their inputs.
The modified questionnaire included demographic information, vaccination status (single dose or both required doses), history of COVID‐19 infection and presence of comorbidities (including diabetes, hypertension, lung disease, kidney disease and cancer). Vaccine reactogenicity was recorded in terms of time of symptom onset (same day, 1–3 days post‐vaccination, 4–7 post‐vaccination), severity (Severe—I had to seek medical attention; Moderate—I had to stop my daily activities; Mild—I was still able to do most daily activities), duration (1 day, 2–3 days, 4–7 days, still present) and whether treatment measures were taken (yes, no). Additionally, the questionnaire elicited physicians’ awareness of thromboembolic events and thrombocytopenia following vaccination.
The survey was conducted and reported based on the checklist for reporting results of internet e‐surveys (CHERRIES). 21 Because the survey was time sensitive, we recruited participants using convenience sampling by sharing the survey link via social networks (Facebook, Messenger, WhatsApp and Viber) and e‐mail. Investigators took the advantage of social media groups, professional associations and healthcare organizations to promote the survey.
Participation in the survey was voluntary and anonymous. All the participants gave consent before participation. No identifiable personal information was collected or stored.
2.3. Ethical approval
Prior ethical approval was granted by the Research Ethics Committee of Shaheed Suhrawardy Medical College, Dhaka, Bangladesh (No: ShSMCH/Ethical/2021/09).
2.4. Statistical analysis
We calculated the reported prevalence of reactogenic events and their relationship with recorded demographic information. The primary outcome variable of interest was the presence of reactogenicity following COVID‐19 vaccination. Further, bivariate analyses were performed to examine the link between existing comorbidities, demographic characteristics and reported adverse events. Multivariate logistic regression was performed to investigate the individual effects of predictor variables on reactogenic symptoms. All statistical analysis was performed using IBM SPSS 22.
3. RESULTS
3.1. Responders’ characteristics
The demographic characteristics of the participants are shown in Table 1. A total of 916 physicians completed the questionnaire. The majority of respondents were male (52.8%) and were employed in the public/government sector (60.6%). Many of the respondents (35%) were those between 31‐40 years. More than half of the respondents (52.2%) reported no history of chronic diseases. More than a quarter of respondents (28.5%) had tested positive for COVID‐19 infection, and about three‐quarters (78.3%) had received both first and second doses of COVID‐19 vaccination at the time of the survey. All participants received the Covishield (AstraZeneca) vaccine, which was the only available vaccine in Bangladesh during the study period.
TABLE 1.
Demographic and background information of study respondents (n = 916)
| Variables | Number of observations | Percentages |
|---|---|---|
| Gender of respondent | ||
| Male | 484 | 52.8 |
| Female | 432 | 47.2 |
| Age of respondents (in years) | ||
| 21–30 | 142 | 15.5 |
| 31–40 | 321 | 35.0 |
| 41–50 | 233 | 25.4 |
| 51–60 | 161 | 17.6 |
| 61–70 | 52 | 5.7 |
| 71–80 | 1 | 0.1 |
| Workplace of respondent | ||
| Private | 344 | 37.6 |
| Public/government | 555 | 60.6 |
| Other research institutions | 14 | 1.5 |
| Work type of respondents (detailed) | ||
| Medical colleges/universities and affiliated hospitals | 491 | 53.6 |
| Government Hospitals | 210 | 22.9 |
| Private hospitals | 119 | 13.0 |
| Others | 96 | 10.5 |
| Vaccination status | ||
| First dose only | 193 | 21.1 |
| Both first and second doses | 717 | 78.3 |
| COVID−19 test status | ||
| Tested positive (RT‐PCR) | 261 | 28.5 |
| Never tested | 58 | 6.3 |
| No | 591 | 64.5 |
| Timing of getting infected with COVID−19 | ||
| Before the 1st dose | 200 | 21.8 |
| Between 1st dose and 2nd dose | 68 | 7.4 |
| After the 2nd dose | 5 | 0.5 |
| Prior presence of any chronic illness a | ||
| No illness | 478 | 52.2 |
| Diabetes | 31 | 3.4 |
| Diabetes; Hypertension | 45 | 4.9 |
| Diabetes; Hypertension and other comorbidities | 24 | 2.6 |
| Diabetes and other comorbidities | 15 | 1.6 |
| Hypertension and other comorbidities | 164 | 17.9 |
| Obesity and other comorbidities | 39 | 4.3 |
| Asthma and other comorbidities | 63 | 6.9 |
| Other comorbidities | 32 | 3.5 |
| Measures take to alleviate adverse effects a | ||
| Drug taken: Paracetamol | 451 | 49.2 |
| Drug taken: Ibuprofen | 10 | 1.1 |
| Drug taken: Other pain killer | 20 | 2.2 |
| Cold bath/shower/sponge | 51 | 5.6 |
| Sleep | 212 | 23.3 |
| Drinking more water | 205 | 22.4 |
| Nothing worked | 24 | 2.6 |
| Nothing taken | 42 | 4.6 |
| Other actions | 13 | 1.4 |
| Experienced similar adverse effects from other vaccines (e.g. BCG, HPV) | ||
| Yes | 104 | 11.4 |
| No | 390 | 42.5 |
| Don't remember | 422 | 46.1 |
| Awareness: Risk of blood clotting after vaccination | ||
| Yes | 690 | 75.3 |
| No | 145 | 15.8 |
| Don't know | 81 | 8.8 |
| Awareness: Risk of low platelets (thrombocytopenia) after vaccination | ||
| Yes | 506 | 55.2 |
| No | 278 | 30.3 |
| Don't know | 132 | 14.4 |
Multiple answers.
3.2. Prevalence of vaccine reactogenicity
The prevalence of vaccine reactogenicity among respondents is shown in Figure 1. More than 58% (n = 533) respondents reported one or more reactogenic symptoms. The six most commonly reported adverse events were “soreness of the injected arm” (71.9%), “tiredness” (56.1%), “fever” (54.4%), soreness of muscles” (48.4%), “headache” (41.5%) and “sleeping more than usual” (26.8%). Most respondents characterized the severity of symptoms as mild. However, some respondents did rate their experience of symptoms as severe. The most common severe symptoms were fever (9.4%) and tiredness (20.1%). Only 11.5% of the respondents recalled similar adverse events from previous vaccinations for other diseases (e.g., BCG, HPV). Approximately half (49.2%) of the respondents took acetaminophen to treat reactogenic symptoms. Other actions taken to treat symptoms were sleep (23.1%) and drinking water (22.4%). More than 75% of the respondents were aware of the risk of thromboembolic events, and more than half (55.5%) were mindful of thrombocytopenia.
FIGURE 1.
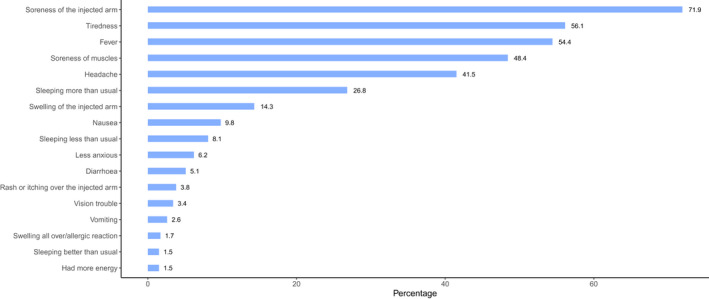
Prevalence of reactogenicity among respondents after receiving the first dose of Covishield (AstraZeneca) vaccine
The observed types of reactogenicity, including onset and duration, are summarized in Table 2. For most respondents, these adverse events appeared on the same day of vaccination, except for tiredness (24%), which appeared 2–3 days post vaccination. For 46.5% of participants, soreness in the arm occurred on the same day of vaccination; same‐day fever was reported by 34.3% of respondents. However, most respondents reported duration of 1–3 days for these frequently observed reactogenicities. For 45.8% of participants, soreness in the arm lasted for 1–3 days, followed by fever (31.5%). Tiredness persisted for 7 days for 7.7% of participants and beyond 7 days for 3.9%.
TABLE 2.
Summary of six most commonly reported reactogenic symptoms (n = 533)
| Adverse effect | The severity of adverse events | Time of appearance | Duration adverse events last | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe | Moderate | Mild | Total | That same day | Between 2–3 days | Between 4–7 days | Total | Same day | 1–3 days | 4‐7days | >7 days | Total | |
| Soreness of the injected arm | 30 (5.6%) | 74 (13.9%) | 279 (52.3%) | 383 (71.9%) | 248 (46.5%) | 128 (24.0%) | 6 (1.1%) | 382 (71.7%) | 57 (10.7%) | 244 (45.8%) | 65 (12.2%) | 6 (1.1%) | 366 (68.7%) |
| Soreness of muscles | 27 (5.1%) | 84 (15.8%) | 147 (27.6%) | 258 (48.4%) | 132 (24.8%) | 106 (19.9%) | 3 (0.6%) | 241 (45.2%) | 39 (7.3%) | 160 (30.0%) | 30 (5.6%) | 6 (1.1%) | 229 (43.0%) |
| Fever | 50 (9.4%) | 87 (16.3%) | 153 (28.7%) | 290 (54.4%) | 183 (34.3%) | 96 (18.0%) | 2 (0.4%) | 281 (52.7%) | 87 (16.3%) | 168 (31.5%) | 15 (2.8%) | 6 (1.1%) | 276 (51.8%) |
| Headache | 34 (6.4%) | 63 (11.8%) | 124 (23.3%) | 221 (41.5%) | 125 (23.5%) | 74 (13.9%) | 4 (0.8%) | 203 (38.1%) | 49 (9.2%) | 124 (23.3%) | 23 (4.3%) | 15 (2.8%) | 211 (39.6%) |
| Tiredness | 35 (6.6%) | 107 (20.1%) | 157 (29.5%) | 299 (56.1%) | 107 (20.1%) | 128 (24.0%) | 11 (2.1%) | 246 (46.2%) | 42 (7.9%) | 127 (23.8%) | 41 (7.7%) | 21 (3.9%) | 231 (43.3%) |
| Sleeping more than usual | 16 (3.0%) | 57 (10.7%) | 70 (13.1%) | 143 26.8%) | 64 (12.0%) | 54 (10.1%) | 4 (0.8%) | 122 (22.9%) | 36 (6.8%) | 56 (10.5%) | 18 (3.4%) | 19 (3.6%) | 129 (24.2%) |
The prevalence of reactogenicity among physicians stratified by gender and age is shown in Table 3. Females reported a higher incidence of reactogenicity compared to males. Fever, vision trouble, sleeping more than usual, rash/itching over the injected arm, and nausea were significantly more common among females (p < 0.05). Most of the adverse events were reported by respondents <45 years, irrespective of gender. Adverse events classified as “other” are shown in Appendix 1. Four case studies describing these reports are contained in Appendix 2.
TABLE 3.
Prevalence of reactogenic symptoms among physicians stratified by gender and age
| Adverse events | Gender | Age | ||||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 269) | Female (n = 264) | Total | p‐value | 21–44 years old a (n = 370) | 45+ years old b (n = 160) | Total | p‐value | |
| Soreness of the injected arm | 200 (74.3%) | 199 (75.4%) | 399 | 0.518 | 303 (81.9%) | 94 (58.8%) | 397 | 0.092 |
| Soreness of muscles | 136 (50.1%) | 127 (48.1%) | 263 | 0.678 | 197 (53.2%) | 64 (40.0%) | 261 | 0.486 |
| Fever | 147 (54.7%) | 147 (55.7%) | 294 | 0.007* | 218 (58.9%) | 74 (46.3%) | 292 | 0.101 |
| Headache | 114 (42.4%) | 110 (41.7%) | 224 | 0.257 | 168 (45.4%) | 56 (35.0%) | 224 | 0.398 |
| Vision trouble | 6 (2.2%) | 13 (4.9%) | 19 | 0.039* | 16 (4.3%) | 3 (1.9%) | 19 | 0.729 |
| Tiredness | 148 (55.0%) | 159 (60.2%) | 307 | 0.090 | 236 (63.8%) | 70 (43.8%) | 306 | 0.919 |
| Sleeping more than usual | 66 (24.5%) | 83 (31.4%) | 149 | 0.076 | 118 (31.9%) | 30 (18.8%) | 148 | 0.407 |
| Sleeping less than usual | 22 (8.2%) | 22 (8.3%) | 44 | 0.925 | 31 (8.4%) | 13 (8.1%) | 44 | 0.039* |
| Sleeping more than usual | 3 (1.1%) | 8 (3.0%) | 11 | 0.038* | 7 (1.9%) | 4 (2.5%) | 11 | 0.750 |
| Had more energy | 6 (2.2%) | 4 (1.5%) | 10 | 0.422 | 9 (2.4%) | 1 (0.6%) | 10 | 0.558 |
| Less anxious | 21 (7.8%) | 14 (5.3%) | 35 | 0.103 | 25 (6.8%) | 10 (6.3%) | 35 | 0.241 |
| Swelling of the injected arm | 31 (11.5%) | 48 (18.2%) | 79 | 0.023 | 69 (18.6%) | 10 (6.3%) | 79 | 0.164 |
| Swelling all over/allergic reaction | 3 (1.1%) | 6 (2.3%) | 9 | 0.407 | 7 (1.9%) | 2 (1.3%) | 9 | 0.912 |
| Rash/itching over the injected arm | 4 (1.4%) | 16 (6.0%) | 20 | 0.003* | 15 (4.0%) | 5 (3.2%) | 20 | 0.768 |
| Diarrhea | 12 (4.4%) | 16 (6.0%) | 28 | 0.499 | 19 (51.4%) | 8 (5.0%) | 27 | 0.464 |
| Nausea | 20 (7.43%) | 33 (12.5%) | 53 | 0.038* | 44 (11.9%) | 9 (5.6%) | 53 | 0.586 |
| Vomiting | 6 (2.2%) | 9 (3.4%) | 15 | 0.426 | 13 (3.5%) | 2 (1.3%) | 15 | 0.915 |
21‐44 years: Younger participants.
45+ years: Older participants.
Significance: p < 0.05.
3.3. Determinants of adverse events
Findings from binary logistic regression analyses are presented in Table 4. All age groups had a significant impact on having adverse events than the physicians with younger age group. Physicians aged 61–70 years were almost 96% less likely to have an adverse event than physicians in their twenties (OR = 0.041 with 95% CI lies between 0.016 and 0.105). Existing comorbidity has an impact on having adverse events as well. Physicians with diabetes and hypertension were 2.72 times more likely to have an adverse event than physicians without prior conditions. Asthma and other comorbidities (OR = 1.885 95% CI: 1.001–3.551) also significantly increased the risk of reactogenicities than physicians without comorbidities.
TABLE 4.
Logistic regression coefficients and odds ratios (95% CI) for determinants reactogenic symptoms
| Variables | β | SE (β) | Exp(β) with 95% CI |
|---|---|---|---|
| Gender of respondent | |||
| Male (ref) | |||
| Female | −0.007 | 0.158 | 0.993 (0.729, 1.353) |
| Age of respondents (in years) | |||
| 21–30 (ref) | |||
| 31–40 | −0.762** | 0.264 | 0.467 (0.278, 0.783) |
| 41–50 | −1.243*** | 0.280 | 0.289 (0.167, 0.500) |
| 51–60 | −1.842*** | 0.321 | 0.159 (0.084, 0.298) |
| 61–70 | −3.205*** | 0.484 | 0.041 (0.016, 0.105) |
| Work type of respondents (detailed) | |||
| Medical college/hospital (ref) | |||
| Medical university/hospital | −0.077 | 0.294 | 0.926 (0.521, 1.647) |
| Private hospital | 0.223 | 0.245 | 1.250 (0.773, 2.021) |
| District hospital | 0.802* | 0.418 | 2.231 (0.984, 5.058) |
| Government specialized hospital | −0.306 | 0.249 | 0.737 (0.452, 1.200) |
| Upazilla health complex | 0.571 | 0.372 | 1.771 (0.855, 3.669) |
| Institute of health technology | 0.924 | 1.121 | 2.520 (0.280, 22.677) |
| Dental college | −1.492 | 1.218 | 0.225 (0.021, 2.446) |
| Others | −0.039 | 0.276 | 0.962 (0.560, 1.651) |
| Prior presence of any chronic illness | |||
| No illness (ref) | |||
| Diabetes | 0.130 | 0.434 | 1.139 (0.486, 2.667) |
| Diabetes; Hypertension | 1.004** | 0.385 | 2.729 (1.282, 5.089) |
| Diabetes; Hypertension and other diseases | 0.304 | 0.457 | 1.356 (0.554, 3.319) |
| Diabetes and other diseases | 0.726 | 0.614 | 2.066 (0.620, 6.880) |
| Hypertension and other diseases | 0.194 | 0.213 | 1.214 (0.799, 1.842) |
| Obesity and other diseases | 0.707* | 0.422 | 2.027 (0.886, 4.636) |
| Asthma and other diseases | 0.634* | 0.323 | 1.885 (1.001, 3.551) |
| Other diseases | 0.483 | 0.435 | 1.621 (0.691, 3.802) |
Reference category is denoted by (ref). Significance: ***p < 0.01, **p < 0.05, *p < 0.1.
4. DISCUSSION
The study estimated the prevalence of reactogenicity after the first dose of the AstraZeneca vaccine among Bangladeshi physicians. To the best of our knowledge, this is the first study of its type in Bangladesh. A key strength of this survey is the accuracy and reliability of symptom reporting by medical professionals. 22 , 23 We found that over half (58.2%) of respondents reported at least one reactogenic side effect after the first dose of vaccine. Two studies of the general population in Bangladesh at approximately the same time as the current study reported similar prevalence of adverse events: 50.9% in February‐June 2021 24 and 54.1% in May 2021. 25 Compared to Bangladesh, higher vaccine reactogenicity has been reported in studies of HCPs in India (65.9% 26 and 69.7% 27 ), South Korea (99.8%, 28 98.1%, 29 90.9%, 30 and 93% 31 ), Germany, Czech Republic (94.6%), 32 Togo (71.6%), 33 Nepal (85%), 34 Saudi Arabia (96.1%), 35 Ethiopia (68.4%) 36 and Ghana (80.7%). 37 However, lower rates were found among HCPs in two studies from India—40% 38 and 56.9%. 22 These disparities may be due to greater representation of elderly participants (≥65 years), as older adults generally exhibit milder symptoms. 29 Jeon et al. 29 noted the higher incidence (0% vs. 8.9%) and greater severity of reactogenic events in a younger age group compared to a study conducted by Voysey et al. 39 with participants ≥65 years. In the present study, 5.8% of respondents were ≥60 years old, which may be one of the reasons for lower reported adverse events. Further, our study found that physicians aged 61–70 years were almost 96% less likely to have adverse events than physicians in their twenties. Similar age‐related findings were reported in other studies of Covisheild, 32 , 36 Pfizer‐BioNTech and Moderna vaccine recipients. 40
Reactogenicity is usually induced by innate and adaptive immune responses leading to the release of chemokine and cytokines. Reactogenic symptoms are the result of chemokines and cytokines that mimic systemic immune response and include fever, tiredness, fatigue, pain and headache. Similarly, the release of inflammatory mediators due to immune response at the injection site leads to local reactions. These symptoms are evidence of effective vaccination. 41 The most commonly reported reactogenicity in our study was pain at the injection site, which was more prevalent among females and younger respondents. These findings are consistent with those of previous studies on the vaccination of HCPs. The most common reactogenicity reported in our study coincides with other studies conducted among HCPs 26 , 29 , 33 , 34 , 42 and studies conducted among the general population. 19 , 43 As in other studies, 26 , 27 , 29 , 33 , 34 , 42 , 44 most of our respondents experienced mild symptoms that were self‐limiting and resolved within a few days (1–3 days). Approximately half of the respondents took acetaminophen to treat symptoms, which is more than reported in other recent studies. One‐quarter of respondents in a general‐population Bangladeshi study used acetaminophen to minimize vaccine‐associated discomfort, 25 as did 33.3% of HCPs in an Ethiopian study. 36
We found that fever, vision trouble, sleeping more than usual, rash/itching over the injected arm and nausea were more commonly reported by females (p < 0.05). From the detailed frequency distribution, we found that female physicians experienced vaccine reactogenicity earlier than their male counterparts and that symptoms usually disappeared within 1–3 days in female physicians (not shown in tables of result section). Studies demonstrated that increased experience of adverse vaccination‐related events in women is related to estradiol, which can induce a more robust immune responses following vaccination. 45 , 46 Females typically exhibit higher innate, humoral and cellular immune responses to viral infections as well as in response to vaccines. 47 Specific manifestations of gender differences in immune response have been documented in several studies. Females tend to have more robust immune responses due to greater generation of antibodies and a more robust T‐cell response. 48 Further, females exhibit higher levels of antibody response, humoral response and cell‐mediated immune response to antigenic stimulation, vaccination and infection. 49 This higher vaccine reactogenicity is associated with higher basal and post‐vaccination IgG levels and increased B cell numbers and functions compared to men. 47 , 50 Finally, higher body fat content in females may reduce the distribution and clearance of medications. 51
We found that existing comorbidities increased the likelihood of adverse reactogenic events. Physicians with “diabetes and hypertension” and “obesity and other complications” had a double risk of reactogenicity. A recent general‐population Bangladeshi study reported similar findings with an odds ratio of reactogenic symptoms after the first vaccine dose of 1.8 for participants with comorbidities. An Ethiopian study of HCPs also found that the presence of comorbidities doubled the risk of reactogenicity. 36 Despite an increased risk of adverse vaccine reactions, people with underlying medical conditions are also at increased risk of COVID‐19 infections. 52 The World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization clinical trials with the Oxford‐AstraZeneca (Covishield) vaccine (AZD1222) concluded that people with comorbidities (obesity, cardiovascular disease, respiratory disease and diabetes) had an increased risk of severe COVID‐19. 53 For most people with comorbidities, the benefits of COVID‐19 vaccination outweigh the risks of adverse events.
In Appendix 1, we list the adverse events reported by respondents in the ‘other’ categories. Earlier studies have not revealed some of these infrequent adverse events (e.g., cracked teeth, meningismus, severe eye pain, menstrual irregularities including spotting, excessive menstrual bleeding, decreased urine output and hematuria). Because these are idiosyncratic events, their clinical significance is unclear. We present four case studies of clinically significant adverse events (Appendix 2). One of the surveyed physicians complained of sudden vertigo and lost consciousness for a few seconds which occurred 2.5 h following vaccination. After regaining consciousness, ECG suggested acute myocardial infraction, and the physician required surgical intervention for blockage in the left anterior descending artery. Another physician reported menstrual irregularities with spotting lasting for 15 days. Severe neck pain and severe pain while walking (spasm of bilateral quadriceps muscles) were experienced by another physician. The last case study describes the experience of vertigo and orthostatic hypotension starting immediately after vaccination and persisting for 3 days. These unusual complications should be carefully documented, but their relationship to immunization is not established. Similarly, a UK‐based phase 2/3 trial identified 13 serious adverse events (SAEs), but none were established to be related to vaccination. 54 Voysey et al. 39 reported 175 SAEs occurring in 168 of 11,636 participants, of which only three events were shown to be related to vaccination.
4.1. Study limitations
Because of sampling limitations, there is a possibility that survey results might not generalize to the entire HCP population of Bangladesh. However, since all participants were physicians, we believe that their reporting of reactogenicity is exceptionally accurate. This study design explicitly does not address the general population. Broader multicentric studies are required to obtain a true picture of reactogenicity in the general population after both or booster doses of vaccination. Additionally, we evaluated only short‐term reactogenicity, and surveillance will be needed to determine possible long‐term effects of vaccination. More robust probability sampling will provide better understanding of prevalence and underlying causes of reactogenic and other adverse vaccination‐related events.
5. CONCLUSION
The majority of vaccine recipients in our study reported reactogenicity, but symptoms were mild and of short duration. The most common reactogenic symptoms were pain at the injection site and tiredness. Reactogenicity was reported more frequently among females and younger age groups. Vaccine recipients and healthcare staff should be aware of possible reactogenicity and management protocols to ensure that vaccination benefits are maximized relative to risks. Further studies on vaccine safety are required for monitoring and to assure the public regarding safety of available vaccines.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Majumder MAA, Lutfor AB, Razzaque MS, Alam ABMM: planned & designed the study. Lutfor AB, Alam ABMM, Majumder MAA, Siddiqui MTH, Nessa K, Khondoker MU, Rahman M, Saha N, Jahan F, Ivy R, Islam R, Haider Y, Haque M, Omar E, Ahmed SMM, Reza AMS, Daud AKM, Choudhury MUA, Hossain MA, Rahman S, Pappu AM: actively collected the data. Majumder MAA, Lutfor AB, Razzaque MS, Mashreky SR, Rahman S, Rabbi AMF, Wahab A: wrote the manuscript. Rabbi AMF, Mashreky SR, Wahab A, Majumder MAA: analyzed the data. Hinkson‐Lacorbiniere K: developed the original questionnaire and edited the manuscript. Majumder MAA, Lutfor AB, Razzaque MS, Mashreky SR, Rahman S, Rabbi AMF & Wahab A: Modified the questionnaire. Campbell MH: critically read the manuscript, edited the manuscript & provided useful suggestions on data analysis. All authors: critically reviewed the manuscript and approved the final draft. Majumder MAA, Lutfor AB, MuAlam ABMM have full access to all the data and take responsibility for the integrity of the data.
ACKNOWLEDGMENTS
The authors thank the physicians who consented to participate in this study and completed the questionnaire.
APPENDIX 1. Adverse events reported in the “other” category
|
|
APPENDIX 2. Four case studies related to severe adverse events
|
Case study 1 Age: 63 years Gender: Male Co‐morbidity: Diabetes Vaccine status: First dose only The participant received the vaccine at about 11 a.m. on 13.02.2021. About 2.5 h later, he had sudden vertigo with the tingling whole of the left upper extremity and immediately became unconscious for a few seconds. After gaining consciousness, he had severe bouts of vomiting. The participant had no chest pain, no compression chest, no dyspnoea, but exhibited profuse sweating. Tingling left upper extremity was persisting. He had an ECG with very high ST elevations suggesting acute myocardial infarction (AMI). He took Ecosprin 4, clopidogrel 4, Emistat 1 & anti‐ulcerent. He then was rushed to Dhaka (capital city). On his way, initially, he felt chest compression and took Nitroglycerin sublingually. When he arrived in Dhaka, all of his discomforts disappeared, and he started to feel quite better. He had an angiogram which revealed two long segment blocks in the left anterior descending artery. Two days later, two stentings were done, and the patient returned home 2 days after surgery. Past history: He never had any dyspnoea, chest pain, compression, any sudden sweating or discomfort on going up (4 to 7/8 stairs at stress). He was a chain smoker of 20+ sticks/day. He was taking no medicine to control diabetes, which was always 10–14 mmol/L since 2006. He was not on exercise for diabetes mellitus. |
|
Case study 2 Age: 28 years Gender: Female Co‐morbidity: No illness Vaccine status: First dose only Her menstrual cycle changed. The menstrual cycle started 15 days after vaccination and lasted for 15 days. She had not experienced this previously. She was also experiencing spotting. |
|
Case study 3 Age: 30 years Gender: Male Co‐morbidity: Nonalcoholic fatty liver disease Vaccine status: Taken both dosages The participant experienced severe neck pain and had severe pain and spasms of the bilateral quadriceps muscles, causing unbearable pain while walking. The pain was relieved by hot compression, analgesics and topical Vollygel (diclofenac gel) application on affected muscles. |
|
Case study 4 Age: 34 years Gender: Male Co‐morbidity: No illness Vaccine status: Taken both dosages The respondent experienced vertigo and orthostatic hypotension, which started immediately after vaccination and lasted for 3 days. |
Majumder MAA, Lutfor AB, Rabbi AMF, et al. Prevalence of COVID‐19 vaccine reactogenicity among Bangladeshi physicians. FASEB BioAdvances. 2022;4:379–390. doi: 10.1096/fba.2021-00158
REFERENCES
- 1. Alam ABMM, Azim Majumder MA, Haque M, et al. Disproportionate COVID‐19 vaccine acceptance rate among healthcare professionals on the eve of nationwide vaccine distribution in Bangladesh. Expert Rev Vaccines. 2021;20(9):1167‐1175. [DOI] [PubMed] [Google Scholar]
- 2. Solís Arce JS, Warren SS, Meriggi NF, et al. COVID‐19 vaccine acceptance and hesitancy in low‐ and middle‐income countries. Nat Med. 2021;27:1385‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishnamurthy K, Sobers N, Kumar A, et al. COVID‐19 Vaccine intent among Health Care Professionals of Queen Elizabeth Hospital, Barbados. J Multidiscip Healthc. 2021;14:3309‐3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashok N, Krishnamurthy K, Singh K, Rahman S, Majumder MAA. High COVID‐19 vaccine hesitancy among healthcare workers: should such a trend require closer attention by policymakers? Cureus. 2021;13(9):e17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kabir R, Mahmud I, Chowdhury MTH, et al. COVID‐19 vaccination intent and willingness to pay in Bangladesh: a cross‐sectional study. Vaccines (Basel). 2021;9(5):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan RM, Milstein A. Influence of a COVID‐19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021;118(10):e2021726118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracking Coronavirus Vaccinations Around the World. The New York Times; 2021. https://www.nytimes.com/interactive/2021/world/covid‐vaccinations‐tracker.html
- 8. Mandavilli A. Reaching ‘Herd Immunity’ is unlikely in the U.S., experts now believe. The New York Times 2021.
- 9. France24 . Covid‐19: Why countries are suspending AstraZeneca vaccinations. France24 2021.
- 10. World Health Organization . Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID‐19 subcommittee on safety signals related to the AstraZeneca COVID‐19 vaccine. 2021; https://www.who.int/news/item/19‐03‐2021‐statement‐of‐the‐who‐global‐advisory‐committee‐on‐vaccine‐safety‐(gacvs)‐covid‐19‐subcommittee‐on‐safety‐signals‐related‐to‐the‐astrazeneca‐covid‐19‐vaccine
- 11. European Medicines Agency . COVID‐19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. 2021; https://www.ema.europa.eu/en/news/covid‐19‐vaccine‐astrazeneca‐benefits‐still‐outweigh‐risks‐despite‐possible‐link‐rare‐blood‐clots
- 12. GOV.UK . UK regulator confirms that people should continue to receive the COVID‐19 vaccine. AstraZeneca; 2021. https://www.gov.uk/government/news/uk‐regulator‐confirms‐that‐people‐should‐continue‐to‐receive‐the‐covid‐19‐vaccine‐astrazeneca#full‐publication‐update‐history
- 13. Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID‐19 vaccines: a systematic review and meta‐analysis of randomized clinical trials. Vaccines. 2021;9(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID‐19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahman S, Montero MTV, Rowe K, Kirton R, Kunik F Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID‐19: a review of current evidence. Expert Rev Clin Pharmacol. 2021;14(5):601‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CDC . Ensuring COVID‐19 Vaccine Safety in the US. 2021; https://www.CDC.gov/coronavirus/2019‐ncov/vaccines/safety.html
- 17. CDC . V‐safe After Vaccination Health Checker. 2021; https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/safety/vsafe.html
- 18. MHRA . Yellow Card Vaccine Monitor. 2021; https://vaccinemonitor‐yellowcard.mhra.gov.uk/
- 19. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith B, Chu LK, Smith TC, et al. Challenges of self‐reported medical conditions and electronic medical records among members of a large military cohort. BMC Med Res Methodol. 2008;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E‐Surveys (CHERRIES). J Med Internet Res. 2004;6:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamal D, Thakur V, Nath N, Malhotra T, Gupta A, Batlish R. Adverse events following ChAdOx1 nCoV‐19 Vaccine (COVISHIELD) amongst health care workers: a prospective observational study. Med J Armed Forces India. 2021;77(Suppl 2):S283‐s288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuschieri S, Borg M, Agius S, Souness J, Brincat A, Grech V. Adverse reactions to Pfizer‐BioNTech vaccination of healthcare workers at Malta's state hospital. Int J Clin Pract. 2021;75(10):e14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sultana A, Shahriar S, Tahsin MR, et al. A retrospective cross‐sectional study assessing self‐reported Adverse Events following Immunization (AEFI) of the COVID‐19 vaccine in Bangladesh. Vaccines (Basel). 2021;9(10):1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalil MM, Mahbub‐Uz‐Zaman K, Hossain A‐S, et al. Adverse events following COVISHIELD vaccination among adult population in Bangladesh. SN Compr Clin Med. 2021;3(11):2207‐2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayadevan R, Shenoy R, Ts A. Survey of symptoms following COVID‐19 vaccination in India. medRxiv. 2021:2021.2002.2008.21251366.
- 27. Inbaraj LR, George CE, Franklyn NN. How safe is Covishield (ChAdOx1nCoV‐19) vaccine? Experience from a tertiary care hospital in South India. medRxiv. 2021:2021.2003.2016.21253744.
- 28. Song JY, Cheong HJ, Kim SR, et al. Early safety monitoring of COVID‐19 vaccines in healthcare workers. J Korean Med Sci. 2021;36(15):e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeon M, Kim J, Oh CE, Lee JY. Adverse events following immunization associated with Coronavirus Disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci. 2021;36(17):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim S‐H, Wi YM, Yun SY, et al. Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV‐19 or BNT162b2 mRNA COVID‐19 Vaccination: a Single Center Experience. J Korean Med Sci. 2021;36(14):e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bae S, Lee YW, Lim SY, et al. Adverse reactions following the first dose of ChAdOx1 nCoV‐19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riad A, Pokorná A, Mekhemar M, et al. Safety of ChAdOx1 nCoV‐19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021;9(6):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Konu YR, Gbeasor‐Komlanvi FA, Yerima M, et al. Prevalence of severe adverse events in health professionals after receiving the first dose of the COVID‐19 vaccination in Togo, March 2021. medRxiv. 2021:2021.2004.2020.21254863. [DOI] [PMC free article] [PubMed]
- 34. Shrestha S, Devbhandari RP, Shrestha A, et al. Adverse events following the first dose of ChAdOx1 nCoV‐19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. J Patan Acad Health Sci. 2021;8(1):9‐17. [Google Scholar]
- 35. Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel‐Moneim AS. BNT162b2 and ChAdOx1 SARS‐CoV‐2 post‐vaccination side‐effects among Saudi vaccines. Front Med (Lausanne). 2021;8:760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tequare MH, Abraha HE, Adhana MT, et al. Adverse Events of Oxford/AstraZeneca's COVID‐19 Vaccine Among Health Workers of Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia. IJID Regions. 2021. [DOI] [PMC free article] [PubMed]
- 37. Serwaa D, Osei‐Boakye F, Nkansah C, et al. Non‐life‐threatening adverse reactions from COVID‐19 vaccine; a cross‐sectional study with self‐reported symptoms among Ghanaian healthcare workers. Hum Vaccin Immunother. 2021;1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaur U, Ojha B, Pathak BK, et al. A prospective observational safety study on ChAdOx1 nCoV‐19 corona virus vaccine (recombinant) use in healthcare workers – first results from India. EClinicalMedicine. 2021;38:101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chapin‐Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA‐based COVID‐1s9 vaccines. JAMA. 2021;325(21):2201‐2202. [DOI] [PubMed] [Google Scholar]
- 41. Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahapatra S, Nagpal R, Marya CM, Taneja P, Kataria S. Adverse events occurring post‐covid‐19 vaccination among healthcare professionals ‐ a mixed method study. Int Immunopharmacol. 2021;100:108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gautam A, Dangol N, Bhattarai U, et al. ChAdOx1 nCoV‐19 vaccine and its self‐reported adverse events: a cross‐sectional study from Western Nepal. J Glob Health Rep. 2021;5:e2021069. [Google Scholar]
- 44. Solomon Y, Eshete T, Mekasha B, Assefa W. COVID‐19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in low‐income country: Ethiopia. J Multidiscip Healthc. 2021;14:2577‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potluri T, Fink AL, Sylvia KE, et al. Age‐associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine‐induced humoral immunity. Semin Immunopathol. 2019;41(2):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruggieri A, Anticoli S, D'Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016;52(2):198‐204. [DOI] [PubMed] [Google Scholar]
- 48. Fathi A, Addo MM, Dahlke C. Sex differences in immunity: implications for the development of novel vaccines against emerging pathogens. Front Immunol. 2020;11:601170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577‐599. [DOI] [PubMed] [Google Scholar]
- 51. Lewis PJ, Catanzano TM, Davis LP, Jordan SG. Web‐based conferencing: what radiology educators need to know. Acad Radiol. 2020;27(3):447‐454. [DOI] [PubMed] [Google Scholar]
- 52. CDC . Underlying Medical Conditions Associated with Higher Risk for Severe COVID‐19: Information for Healthcare Providers. 2021; https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐care/underlyingconditions.html Accessed Dec 2021
- 53. World Health Organization . Interim recommendations for use of the ChAdOx1‐S [recombinant] vaccine against COVID‐19 (AstraZeneca COVID‐19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™). Geneva 2021.
- 54. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


