Abstract
Nirmatrelvir/ritonavir (NR) use has not yet been described in solid organ transplant recipients (SOTRs) with mild COVID‐19. The objective was to evaluate outcomes among SOTR and describe the drug–drug interaction of NR. This is an IRB‐approved, retrospective study of all adult SOTR on a calcineurin inhibitor (CNI) or mammalian target of rapamycin inhibitor who were prescribed NR between December 28, 2021 and January 6, 2022. A total of 25 adult SOTR were included (n = 21 tacrolimus, n = 4 cyclosporine, n = 3 everolimus, n = 1 sirolimus). All patients were instructed to follow the following standardized protocol during treatment with 5 days of NR: hold tacrolimus or mTOR inhibitor or reduce cyclosporine dose to 20% of baseline daily dose. Four patients (16%) were hospitalized by day 30; one for infectious diarrhea and three for symptoms related to COVID‐19. No patients died within 30 days of receipt of NR. Median tacrolimus level pre‐ and post‐NR were 7.4 ng/ml (IQR, 6.6–8.6) and 5.2 (IQR, 3.6–8.7), respectively. Four patients experienced a supratherapeutic tacrolimus concentration after restarting tacrolimus post‐NR. Our results show the clinically significant interaction between NR and immunosuppressive agents can be reasonably managed with a standardized dosing protocol. Prescribers should carefully re‐introduce CNI after the NR course is complete.
Keywords: clinical research/practice, drug interaction, immunosuppressant, immunosuppression/immune modulation, infection and infectious agents—viral: SARS‐CoV‐2/COVID‐19, pharmacology, solid organ transplantation
Abbreviations
- CNI
calcineurin inhibitor
- CYP
cytochrome P450
- DAA
direct acting antiviral
- EUA
emergency use authorization
- IQR
interquartile range
- mTOR
mammalian target of rapamycin
- SOTRs
solid organ transplant recipients
1. INTRODUCTION
Compared with the general population, solid organ transplant recipients (SOTR) are at significantly higher risk of morbidity and mortality due to COVID‐19. 1 , 2 With decreased rates of response to SARS‐CoV‐2 vaccination and the need for ongoing immunosuppression, this high‐risk population can potentially benefit from early therapies to mitigate hospital admission and mortality. Unfortunately, the spread of the Omicron variant has decreased the number of effective drug therapies, as both bamlanivimab/etesevimab and casirivimab/imdevimab have diminished efficacy against this variant and were eventually removed from treatment guidelines. 3
Sotrovimab, a monoclonal antibody product with a unique mechanism of action, is believed to retain efficacy against the original Omicron variant. 3 However, supplies of sotrovimab have been insufficient to meet the high demand and coordinating ambulatory intravenous infusions can often be difficult. Similar logistical issues complicate the outpatient use of IV Remdesivir, despite supportive data. 4 Fortunately, two new oral options were granted emergency use authorization (EUA) by the FDA, and both became available in December of 2021.
One of these oral options, nirmatrelvir co‐administered with ritonavir, was shown to reduce the risk of hospitalization or death compared with placebo by 89% in 2,246 unvaccinated patients infected with SARS‐CoV‐2. 5 However, ritonavir is a potent cytochrome P450 (CYP) 3A and P‐glycoprotein inhibitor that complicates the use of commonly used immunosuppressive medications. Following the FDA EUA of nirmatrelvir/ritonavir (NR), we convened a group of multidisciplinary experts at our center to provide recommendations for managing SOTR on calcineurin inhibitors (CNIs) or mammalian target of rapamycin inhibitors (mTOR) who are to begin treatment with nirmatrelvir/ritonavir. 6 To date, limited data exist describing outcomes of nirmatrelvir/ritonavir in SOTR with COVID‐19 with respect to disease progression or the pharmacokinetic interaction with immunosuppressive agents. The objective of this study was to evaluate the impact of nirmatrelvir/ritonavir use in SOTR on key clinical outcomes and to assess the impact of the drug–drug interaction with CNIs and mTOR inhibitors. Here, we report on the first 25 SOTR treated with NR for mild COVID‐19 and describe 30‐day outcomes as well as the drug–drug interactions with common immunosuppressant medications.
2. METHODS
In this IRB‐approved, retrospective study, all adult SOTR receiving a new prescription for nirmatrelvir/ritonavir between December 28, 2021 and January 6, 2022 were included. Key demographics and background histories were obtained from review of the electronic medical record. The outcomes of interest were the incidence of hospital admission or mortality within 30 days of nirmatrelvir/ritonavir completion as well as immunosuppressant drug levels and dose adjustments. The baseline values represent the last known immunosuppressant trough concentration and dose. Patients were excluded if they did not complete 5 days of nirmatrelvir/ritonavir. Patient follow‐up was at least 30 days for all patients with day 1 being the start of nirmatrelvir/ritonavir. All patients were instructed to adhere to the following previously published dosing guideline from our clinical group 6 : hold tacrolimus or mTOR inhibitor or reduce cyclosporine dose to 20% of baseline daily dose. Patients were also routinely instructed to call the transplant clinic if they were to experience any signs/symptoms associated with CNI or mTOR inhibitor toxicity. A follow‐up level was suggested on day 3 of nirmatrelvir/ritonavir (if feasible) and strongly recommended within 1 to 2 days of completing nirmatrelvir/ritonavir. Follow‐up laboratory work were obtained via a facility capable of servicing patients with COVID‐19.
3. RESULTS
A total of 25 SOTR were included: kidney = 5 (20%), liver = 2 (8%), heart = 9 (36%), and lung = 9 (36%) (see Table 1). All patients completed 5 days of therapy. The median age was 57.7 years (IQR, 49.8–65.3), and the majority were male (56%). The median time from transplant to COVID‐19 was 3.6 years (IQR, 1.2–9.4). Time from COVID‐19 symptom onset to the receipt of nirmatrelvir/ritonavir was 2 days (IQR, 1–3). All patients followed the published protocol. The majority of patients were vaccinated (92%) with an approved mRNA vaccine with 69.6% of patients having received three doses. There were two patients with an unknown vaccination status.
TABLE 1.
Baseline characteristics
| Age, years | 57.7 (49.8–65.3) |
| Male sex | 14 (56) |
| Race/ethnicity | |
| Asian | 2 (8) |
| Black | 4 (16) |
| Caucasian | 12 (48) |
| Hispanic | 5 (20) |
| Unknown | 2 (8) |
| Organ transplant | |
| Kidney | 5 (20) |
| Liver | 2 (8) |
| Lung | 9 (36) |
| Heart | 9 (36) |
| Time from txp to COVID‐19, years | 3.6 (1.2–9.4) |
| Serum creatinine before, mg/dl | 1.2 (0.94–1.3) |
| Serum creatinine after, mg/dl | 1.2 (1.0–1.5) |
| Concomitant drug–drug interactions | 6 (24) |
| Azole antifungals* | 5 (20) |
| Primidone | 1 (4) |
| HMG‐CoA reductase inhibitors | 8 (32) |
| Ticagrelor | 1 (4) |
| Vaccinated | 23 (92) |
| mRNA | 21 (84) |
| Two doses | 5 (21.7) |
| Three doses | 16 (70) |
| Adenovirus vector | 2 (8) |
All values are n (%) or median (IQR, 25–75) unless otherwise specified.
Two patients on voriconazole, two patients on clotrimazole, and one patient on posaconazole.
Two patients of the 25 patients (8%) were hospitalized within 14 days of NR initiation. The first patient was a lung transplant recipient presenting with fever, diarrhea, tachycardia, and hypotension requiring 2 L of oxygen via nasal cannula intermittently that received no additional COVID‐19‐directed therapy. The patient's length of stay was prolonged (25 days) in the setting of severe diarrhea secondary to C. difficile and antibiotic treatment for bacteria pneumonia. The second patient was a liver transplant recipient admitted for recurrent C. difficile associated diarrhea, who was hospitalized for three days and discharged home. By 30 days, there were two additional patients that required hospitalization. One patient was a renal transplant recipient admitted on day 27 for fevers, hypoxia and productive cough requiring 2 L of oxygen via nasal cannula. This patient received a 3‐day course of remdesivir and corticosteroids and was discharged home on room air. The other patient was a lung transplant recipient admitted on day 28 with non‐productive cough, shortness of breath and intermittent fevers that required 3 L of oxygen via nasal cannula. This patient was treated with remdesivir/dexamethasone and piperacillin/tazobactam for ongoing COVID‐19 (cycle threshold value was 23) with a superimposed bacterial pneumonia. The patient was admitted for 11 days and subsequently discharged home on room air. No patients died within 30 days of receipt of nirmatrelvir/ritonavir.
The median time from beginning treatment with nirmatrelvir/ritonavir to the first measured CNI trough concentration was 6 days (IQR, 6–7). Four patients received a follow‐up CNI level during the 5‐day treatment with nirmatrelvir/ritonavir. No patients were diagnosed with acute rejection during the 30 day follow‐up period.
Of the 25 patients, 21 were receiving tacrolimus at the time of COVID‐19. Pre‐ and post‐ tacrolimus trough concentrations were available for assessment in 19 patients. Of the two patients excluded, one patient did not have a follow‐up level, and the other patient was for mistakenly taking a dose of tacrolimus prior to obtaining their first post‐NR trough. The median tacrolimus trough concentration before the receipt of nirmatrelvir/ritonavir was 7.4 ng/ml (IQR, 6.6–8.6), and the time from the last known trough concentration prior to receipt of nirmatrelvir/ritonavir was 27 days (IQR, 6–74). The median first trough concentration post‐receipt of nirmatrelvir/ritonavir was 5.2 ng/ml (IQR, 3.6–8.7) (see Figure 1A). No patients had a supratherapeutic trough concentration at first assessment after completing nirmatrelvir/ritonavir. One heart transplant recipient had an undetectable level on day three of nirmatrelvir/ritonavir therapy, which prompted an endomyocardial biopsy that was negative for rejection. Patients re‐initiated tacrolimus at 82% (IQR, 71–100) of their baseline total daily dose at a median of 8 days (IQR, 7–9) from the start of nirmatrelvir/ritonavir. After restarting tacrolimus, four patients experienced a supratherapeutic tacrolimus trough concentration (see Table S1). One heart transplant recipient did experience a rise in serum creatinine >0.3 mg/dl in the setting of an elevated tacrolimus trough concentration of 24.6 ng/ml (on day 10) after resumption of the full pre‐dose on day 7.
FIGURE 1.
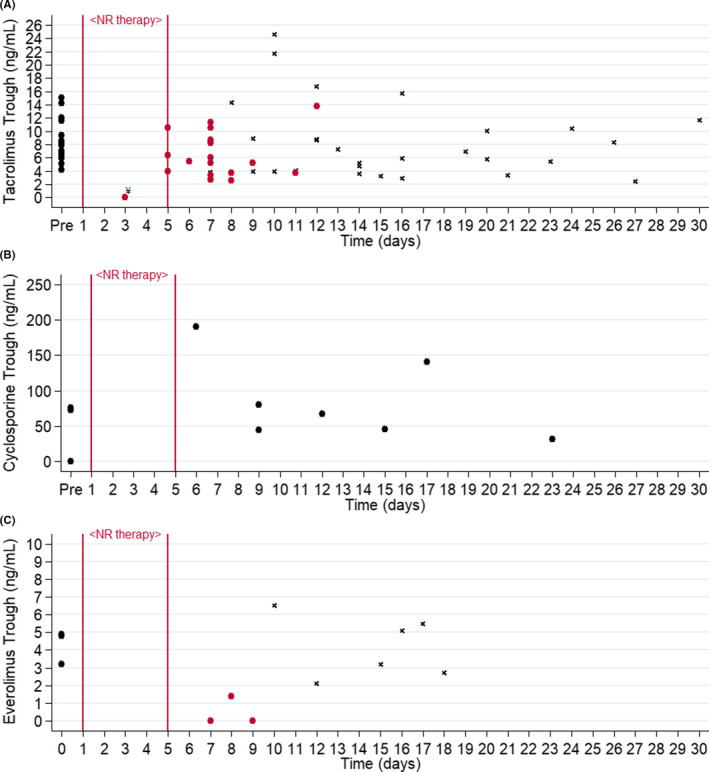
Immunosuppression trough concentrations. Each point represents a trough concentration. “Pre” refers to most recent trough concentration before receipt of nirmatrelvir/ritonavir (black circles). (A) Tacrolimus trough concentrations. All patients received nirmatrelvir/ritonavir on days 1–5, and tacrolimus was held during this time. The first tacrolimus trough concentrations while tacrolimus was withheld are represented as cranberry colored circles. All tacrolimus trough concentrations drawn after restarting tacrolimus within 30 days are represented as a small black x. (B) Cyclosporine trough concentrations. All patients reduced cyclosporine dose to 20% of total daily dose while on NR. (C) Everolimus trough concentrations. All patients received nirmatrelvir/ritonavir on days 1–5 and everolimus was held during this time. The first everolimus trough concentrations while everolimus was withheld are represented as cranberry colored circles. All everolimus trough concentrations drawn after restarting everolimus within 30 days are represented as a small black x. ¥Patient with an undetectable tacrolimus trough concentration on day 3 of NR was instructed to resume taking tacrolimus at a reduced dose
Of the 25 patients, 4 were receiving cyclosporine at the time of COVID‐19 and pre‐ and post‐ cyclosporine trough concentrations were available for 3 patients. All patients had their cyclosporine dose reduced to approximately 20% of their total daily dose. One patient had a cyclosporine trough concentration of 73 ng/ml, checked 58 days prior to receipt of nirmatrelvir/ritonavir. This patient had a measured cyclosporine trough of 45 ng/ml on day 9. The second patient had a cyclosporine trough of 75.9 ng/ml checked 70 days prior to receipt of nirmatrelvir/ritonavir. This patient had two cyclosporine trough concentrations (while taking the same reduced‐dose) checked on day 6 and day 9 of 190 ng/ml and 80 ng/ml, respectively. Patient three had a cyclosporine trough that was undetectable drawn 5 days prior to receipt of nirmatrelvir/ritonavir. This patient had a follow‐up cyclosporine trough concentration of 31.9 ng/ml on day 23, having resumed the pre‐nirmatrelvir/ritonavir dose on day 8 (see Figure 1B).
Additionally, three patients were receiving everolimus, and one patient was receiving sirolimus at the time of COVID‐19. All four patients were instructed to hold everolimus or sirolimus at the time of initiating a 5‐day course of nirmatrelvir/ritonavir. The median everolimus trough concentration prior to receipt of nirmatrelvir/ritonavir was 4.8 ng/ml (IQR, 3–4.9) and the time from the last known trough concentration until receipt of nirmatrelvir/ritonavir was 77 days (IQR, 58–140). Everolimus trough concentrations post‐nirmatrelvir/ritonavir were undetectable in two patients on day 7 and day 9, and 1.4 ng/ml on day 8 in the third patient. In the one patient taking sirolimus, there was a trough concentration of 5 ng/ml at baseline, drawn 13 days prior to the start of nirmatrelvir/ritonavir. The first measured sirolimus trough concentration following completion of nirmatrelvir/ritonavir was 9.5 ng/ml on day 14.
There were several concomitant drug–drug interactions that were managed: five patients receiving concomitant azole antifungal agents, eight patients on HMG‐CoA reductase inhibitors, one patient on ticagrelor, and one patient on primidone. No azole antifungal agents were dose adjusted/held, 7/8 patients were instructed to hold HMG‐CoA reductase inhibitors, the one patient on ticagrelor was instructed to hold their dose, and the single patient on primidone continued therapy during the nirmatrelvir/ritonavir course. Serum creatinine values were numerically similar, with only one patient experiencing a rise in serum creatinine greater than 0.3 mg/dl between NR start and NR completion. There were also an additional two patients with a similar rise within the 30 day follow‐up period. Liver enzymes and total bilirubin were not routinely checked, but no patients experienced a documented rise of AST/ALT or bilirubin greater than 3x the upper limit of normal.
4. DISCUSSION
Pooled studies across a variety of variant‐dominated COVID‐19 waves indicate significant morbidity and mortality among SOTR compared with that of the non‐immunocompromised population. 7 , 8 , 9 These findings underscore the need to identify safe and effective drug therapies for this vulnerable patient population. While nirmatrelvir/ritonavir was previously shown to be safe and effective in non‐transplant patients, SOTR carry a heightened risk of experiencing drug‐related harm with this agent (e.g., neurotoxicity, nephrotoxicity, etc.) as a result of the significant interaction with key immunosuppressive drug therapies. 10
This case series reports a low rate of COVID‐19‐related hospitalization and death in a broad cohort of both abdominal and cardiothoracic SOTR. In our cohort, only one patient was admitted with complications related to COVID‐19 within 14 days and two additional patients were admitted with late presentation of COVID‐19 pneumonia, requiring additional therapy while hospitalized. Nevertheless, we cannot conclusively associate this finding with NR given the uncontrolled nature of this study. The FDA EUA for nirmatrelvir/ritonavir is based on the EPIC‐HR phase 2/3, randomized, double‐blind, placebo‐controlled study in which patients that received nirmatrelvir/ritonavir were shown to have a significant reduction COVID‐19 related hospitalization or death. 5 Notably, this study included patients with immunosuppressive disease or immunosuppressive treatment but excluded individuals with a history of prior COVID‐19 or vaccination. The incidence of hospitalization or death in our study was higher than that reported in the nirmatrelvir/ritonavir EUA. This is in line with higher rates of hospitalization for COVID‐19 in SOTR compared with the general population. 9 This highlights the potential contribution of immunosuppression or comorbidities on COVID‐19 outcomes in SOTR. 11
Our findings in this case series, although preliminary, highlight the potential feasibility of managing the drug interaction between NR and key transplant medications using a standardized dosing protocol. While following the guidance of our previously published protocol, no patients experienced a supratherapeutic trough concentration at first assessment, but there were four patients who had a supratherapeutic concentration upon re‐initiation of tacrolimus. Importantly, there were no patients who experienced diagnosed acute rejection during the 30‐day follow‐up period. Since this was a retrospective review, it was difficult to ascertain how post‐COVID‐19 trough goals differs from pre‐COVID‐19 goals. Although the first follow‐up CNI/mTOR inhibitor trough concentrations were lower relative to baseline trough concentrations, this protocol sought to avoid toxicity due to supratherapeutic concentrations.
Ritonavir‐boosted HIV and direct acting antiviral (DAA) regimens have been shown to have a clinically significant interaction with CNI. 10 , 12 In healthy volunteers receiving a ritonavir‐boosted DAA regimen co‐administered with a CNI, patients experienced a 57‐fold increase in tacrolimus exposure and a 5.8‐fold increase in cyclosporine exposure. 12 This required a dose‐adjustment of cyclosporine to 20% of the total daily dose and a reduction to tacrolimus 0.5 mg once weekly. 10 In light of the potentially severe nature of the interaction with frequently used immunosuppressant drugs, a recent statement by the American Society of Transplantation encouraged the use of outpatient sotrovimab or intravenous remdesivir over nirmatrelvir/ritonavir. 13
However, the results of our analysis demonstrate that the clinically significant interaction between nirmatrelvir/ritonavir and immunosuppressants can be reasonably managed with a standardized dosing protocol. 6 We consider there are two “phases” of this interaction. The first phase is initiating nirmatrelvir/ritonavir and making empiric adjustments to baseline CNI/mTOR inhibitor dosing. The second phase is the re‐initiation or dose adjustment of CNI/mTOR inhibitor following completion of nirmatrelvir/ritonavir. In the first phase, no patients experienced supratherapeutic tacrolimus or cyclosporine concentrations, and only one patient had an undetectable first CNI trough concentration in the 30‐day follow‐up period. This patient was a heart transplant recipient on tacrolimus 7.5 mg twice daily prior to starting nirmatrelvir/ritonavir. On day 3 of therapy his tacrolimus trough concentration was undetectable, and he was re‐initiated on tacrolimus 5 mg twice daily. This patient also underwent endomyocardial biopsy that was negative for acute rejection. On day 8 (3 days after stopping nirmatrelvir/ritonavir), this patient's tacrolimus trough concentration was 8.9 ng/ml.
A significant component of managing this drug–drug interaction is determining when and how to restart CNI/mTOR inhibitors (the second phase). Ritonavir is an irreversible inhibitor of cytochrome (CYP) 3A activity. 14 , 15 On discontinuation of ritonavir, recovery of CYP 3A activity may not be fully achieved for 2–5 days depending on patient‐specific factors. 15 Clinically this is an important point to address to avoid toxicity. In the current study, patients resumed taking tacrolimus 3 days (2–5 days) after the completion of nirmatrelvir/ritonavir. In 19 patients with at least two follow‐up tacrolimus trough concentrations, there were four instances in which patients resumed either a reduced dose or their full pre‐nirmatrelvir/ritonavir tacrolimus dose and experienced a subsequent supratherapeutic tacrolimus trough concentration above 15 ng/ml (Table S1). This highlights the individual patient variability of recovery of CYP 3A enzyme activity. Thus, treating clinicians must weigh the individual risk or rejection and ability to obtain follow‐up levels when considering dose titrations of CNI after completion of nirmatrelvir/ritonavir.
In terms of safety, there were no patient‐reported neurological sequelae in the setting of concomitant use of nirmatrelvir/ritonavir and CNIs. One patient did experience acute kidney injury in the setting of an elevated tacrolimus trough concentration of 24.6 ng/ml (on day 10) after resumption of the full pre‐dose on day 7. There were several drug–drug interactions between HMG‐CoA reductase inhibitors and antiplatelet agents that did require discontinuation. Consultation with a transplant pharmacist to help manage these drug interactions were helpful.
Based on the results herein, at the start of nirmatrelvir/ritonavir we propose to hold tacrolimus and mTOR inhibitors and for those patients on cyclosporine to reduce the total daily dose by 80%. Future immunosuppressant dose changes should be made according to the follow‐up level. 6 The overall trend in our cohort was for the tacrolimus levels to drop in the setting of holding the drug for the entire course of nirmatrelvir/ritonavir (Figure 1A). For the majority of stable, ambulatory SOTR who are currently infected with COVID‐19, we feel that this slight decrease would be unlikely to result in adverse immunologic sequelae. However, for patients with high immunologic risk factors, such as recent transplant or history of rejection, clinicians might strongly consider referring patients to obtain a trough concentration on day 3 to avoid early subtherapeutic levels and either re‐initiate a reduced tacrolimus dose after the completion of therapy or use the close re‐assessment and dosing by levels approach. The use of “supplemental” doses for tacrolimus (i.e., 0.5 mg once per week) has been proposed in other treatment protocols of a ritonavir‐boosted protease inhibitor regimen. 10 Although we are unable to comment about the benefit of a supplemental dose in the setting of 5 days of ritonavir as this was not part of our current protocol.
Our analysis has several important limitations. First, we took a convenience sample population with a relatively limited size, although this was due to a shortage of nirmatrelvir/ritonavir during the peak of Omnicron variant wave in New York City. Second, our analysis does not have a comparator group, which precludes us from determining the efficacy of nirmatrelvir/ritonavir in preventing COVID‐19‐related hospitalization and death. These limitations notwithstanding, our analysis shows that nirmatrelvir/ritonavir was associated with a relatively low number of COVID‐19‐related hospitalization and death in SOTR. Furthermore, the use of a standardized dosing protocol can assist clinicians in navigating the drug interaction between nirmatrelvir/ritonavir and immunosuppressive therapy. Although our study has described the short‐term safety of nirmatrelvir/ritonavir, future studies with a comparator group are needed to establish the efficacy of nirmatrelvir/ritonavir in SOTR.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Table S1
Salerno DM, Jennings DL, Lange NW, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID‐19 in solid organ transplant recipients. Am J Transplant. 2022;00:1–6. doi: 10.1111/ajt.17027
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. doi: 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maggiore U, Riella LV, Azzi J, Cravedi P. Mortality in solid organ transplant recipients with COVID‐19: more than meets the eye. Am J Transplant. 2022. doi: 10.1111/ajt.16942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS‐CoV‐2. Nature. 2022;602(7898):676‐681. doi: 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid‐19 in outpatients. N Engl J Med. 2022;386(4):305‐315. doi: 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS‐CoV‐2 M. Science. 2021;374(6575):1586‐1593. doi: 10.1126/science.abl4784 [DOI] [PubMed] [Google Scholar]
- 6. Lange NW, Salerno DM, Jennings DL, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug‐drug interactions with transplant immunosuppressants. Am J Transplant. 2022. doi: 10.1111/ajt.16955 [DOI] [PubMed] [Google Scholar]
- 7. Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID‐19 in solid organ transplant recipients: a propensity‐matched analysis of a large research network. Transplant. 2021;105(6):1365‐1371. doi: 10.1097/TP.0000000000003670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ao G, Wang Y, Qi X, et al. The association between severe or death COVID‐19 and solid organ transplantation: a systematic review and meta‐analysis. Transplant Rev (Orlando). 2021;35(3):100628. doi: 10.1016/j.trre.2021.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS‐CoV‐2 infection on solid organ transplantation recipients: a nationwide population‐based study. Am J Transplant. 2021;21(7):2509‐2521. doi: 10.1111/ajt.16428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwo PY, Mantry PS, Coakley E, et al. An interferon‐free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371(25):2375‐2382. doi: 10.1056/NEJMoa1408921 [DOI] [PubMed] [Google Scholar]
- 11. Ringer M, Azmy V, Kaman K, et al. A retrospective matched cohort single‐center study evaluating outcomes of COVID‐19 and the impact of immunomodulation on COVID‐19‐related cytokine release syndrome in solid organ transplant recipients. Transpl Infect Dis. 2021;23(2):e13556. doi: 10.1111/tid.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badri P, Dutta S, Coakley E, et al. Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT‐450, ombitasvir, and dasabuvir. Am J Transplant. 2015;15(5):1313‐1322. doi: 10.1111/ajt.13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar D, Humar A, Ison MG, et al. AST statement on oral antiviral therapy for COVID‐19 for organ transplant recipients. Accessed January 19, 2022. https://www.myast.org/sites/default/files/AST%20Statement%20on%20Oral%20Antiviral%20Therapy%20for%20COVID%20Jan%204%20%282%29.pdf
- 14. Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using A limited sampling strategy. Clin Pharmacol Ther. 2011;90(5):666‐673. doi: 10.1038/clpt.2011.164 [DOI] [PubMed] [Google Scholar]
- 15. Stader F, Khoo S, Stoeckle M, et al. Stopping lopinavir/ritonavir in COVID‐19 patients: duration of the drug interacting effect. J Antimicrob Chemother. 2020;75(10):3084‐3086. doi: 10.1093/jac/dkaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


