Abstract
Background
Olfactory dysfunction is common during SARS‐CoV‐2 infection. The pathophysiology of the persistence of this symptom and the potential relationship with central nervous system involvement is unknown.
Aim of the study
To evaluate the neural correlates of persistent olfactory dysfunction in a series of patients with post‐COVID syndrome.
Methods
Eighty‐two patients with post‐COVID syndrome were assessed with the Brief Smell Identification Test and a multimodal MRI study including 3D‐T1, T2‐FLAIR, diffusion‐tensor imaging, and arterial spin labeling. Olfactory and neuroimaging examinations were performed 11.18 ± 3.78 months after the acute infection. Voxel‐based brain mapping analyses were conducted to correlate the olfactory test with brain volumes, white matter microstructure, and brain perfusion.
Results
Olfactory dysfunction was associated with lower tissue perfusion in the orbital and medial frontal regions in the arterial spin labeling sequence. Conversely, no statistically significant findings were detected in brain volumes and diffusion‐tensor imaging. Mild changes in paranasal sinuses and nasal cavities were detected in 9.75% of cases, with no association with olfactory deficits.
Conclusions
We provide new insights regarding the pathophysiology of persistent olfactory dysfunction after COVID‐19, involving the main brain regions associated with the olfactory system.
Keywords: anosmia, COVID‐19, MRI, neuroimaging, olfactory, post‐COVID syndrome
1. INTRODUCTION
Olfactory dysfunction is a common symptom among patients with coronavirus disease 2019 (COVID‐19). The pathophysiology of this symptom is not well understood. The olfactory cleft mucosal thickening and obstruction of the olfactory cleft seem to be the main mechanisms in the acute phase, although findings are heterogeneous, and most of the studies were conducted in the first weeks of the infection. 1 , 2 , 3 In some studies, olfactory bulb hyperintensity, atrophy, or hypometabolism has been detected, suggesting the central nervous system (CNS) involvement as part of the pathogenesis of olfactory dysfunction. 4 , 5 In this regard, the olfactory bulb could be a route for the SARS‐CoV‐2 to enter into the CNS. 6 Conversely, one study has failed to find the evidence of infection of the olfactory sensory neurons and olfactory bulb. 7
We aimed to evaluate the neural correlates of persistent olfactory dysfunction in a series of patients with the post‐COVID syndrome (PCS). To this end, patients were assessed with an objective olfactory test and a multimodal magnetic resonance imaging (MRI) study, including 3DT1, diffusion‐tensor imaging (DTI), and arterial spin labeling (ASL). This neuroimaging protocol was chosen to capture both structural (gray and white matter) information and functional (cerebral blood flow) information potentially associated with olfactory function.
2. METHODS
2.1. Patients
We conducted a cross‐sectional study involving 82 patients with post‐COVID symptoms. Patients were evaluated in our center and met the diagnostic criteria proposed by the WHO for PCS. 8 The diagnosis of PCS was performed after a comprehensive study (clinical evaluation, assessment of fatigue, cognitive function, anxiety, depression, and sleep quality), laboratory testing, and other tests when required to exclude alternative diagnoses. Patients attended the first consultation at the general neurology consultations (derived from primary physicians) or at a specific post‐COVID consultation derived from internal medicine or other hospital specialties. All patients were examined from February to September 2021 at the post‐COVID consultations, where the diagnosis was performed. The diagnosis was conducted by a multidisciplinary team involving internal medicine and neurology. Other specialists (e.g., pulmonologists) were consulted in selected cases. Exclusion criteria included the following: any cognitive, neurological, or olfactory complaint before COVID‐19; history of stroke, traumatic brain injury, or any neurological or psychiatric disorder potentially associated with cognitive impairment or any neurological deficit; history of alcohol abuse or other toxics; drugs or uncontrolled medical condition; sensory disorder potentially biasing assessments; any neuroimaging artifact or quality issues. Patients with contraindications to MRI, claustrophobia, or potential artifacts (orthodontic material) were also excluded. In all cases, SARS‐CoV‐2 infection was confirmed by reverse transcription PCR. The 12‐item version of the Brief Smell Identification Test (BSIT) 9 was used for the assessment of olfactory dysfunction. In this test, lower scores are indicative of worse olfactory function.
2.2. Neuroimaging acquisition, preprocessing, and analysis
Patients were scanned using a 3.0T Magnet (GE Architect, system release 27) and a 48‐channel head coil. The following sequences were acquired:
A) Sagittal MPRAGE sequence with the following parameters: number of slices =200, slice thickness =1 mm, field of view =256 mm, matrix =256 × 256, flip angle =8, preparation time =974 ms, recovery time =700 ms, TR =7.7 ms, TE =3.1 ms, NEX =1, and acquisition time =9:27;
B) Sagittal 3D T2 FLAIR sequence with the following parameters: number of slices =186, slice thickness =1.2 mm, field of view =256 mm, matrix =256 × 256, inversion time =1793 ms, TR =6300 ms, TE =105 ms, NEX =1, and acquisition time =6:53;
C) Axial multishell diffusion 1 shot echo‐planar sequence, with 3 b values (500, 1000, 2000) and 125 diffusion directions, and the following parameters: number of slices =64, slice thickness =2.2 mm, field of view =256 mm, matrix =116 × 166, TR =6780 ms, TE =3.1 ms, NEX =1, acquisition time =14:35. An additional opposing gradients sequence was acquired, for geometrical distortions correction purpose;
D) ASL was acquired with the following parameters: number of slices =36, slice thickness =4 mm, field of view =240 mm, resolution =3.73 mm, flip angle =111, labeling time =1.5 s, post‐labeling delay =2025 ms, TR =4854 ms, TE =53.52 ms, NEX =3, acquisition time =4:25. Head holders and restraints were used to prevent motion artifacts. In addition, images were reviewed by trained neuroradiologists and acquired again if needed. Statistical Parametric Mapping v12 and FSL were used for preprocessing and analysis. Further details about neuroimaging preprocessing and analysis are provided in Appendix S1. Visual analysis of the paranasal sinuses and nasal cavities was performed from the structural images.
2.3. Statistical analysis
Descriptive data are shown as mean ± standard deviation and number (percentage). We used the two‐sample U Mann–Whitney test to compare BSIT scores between two groups (patients with and with no olfactory changes in paranasal sinuses and nasal cavities). A p‐value <.05 was considered statistically significant.
For neuroimaging, a voxel‐wise statistical analysis was conducted. A p‐value <.01 and a cluster‐based family‐wise error correction p < .05 were applied as the statistical threshold to correct for multiple comparisons in T1‐weighted (voxel‐based morphometry) and ASL. Regarding DTI, the randomise tool was used applying the Threshold‐Free Cluster Enhancement option with 5000 permutations and using a FWE‐corrected p‐value <.05 as the statistical threshold. Finally, we calculated Pearson's correlation between the BSIT and the brain perfusion in the cluster of voxels obtained with voxel‐based analysis.
2.4. Standard protocol approvals, registrations, and patient consents
The study protocol was approved by the Institutional Ethics Committee. We obtained written informed consent for research from all participants in this study.
2.5. Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
3. RESULTS
The mean age of the patients was 51.74 ± 10.85 years, and 58 (70.7%) were women. The mean time since the onset of COVID‐19 was 11.18 ± 3.78 months. Clinical characteristics of the sample are shown in Table 1. The distribution of BFIS scores in the sample is shown in Figure 1A. According to a cutoff of BSIT ≤9, 10 43 patients (52.4%) were regarded as having some degree of olfactory dysfunction.
TABLE 1.
Main clinical and demographic characteristics
| Age (years) [minimum, maximum] | 51.74 ± 10.85 [27, 77] |
| Sex (N, % of women) | 58 (70.7%) |
| Anosmia or ageusia reported at the onset of COVID−19 | 54 (65.85%) |
| BSIT (maximum score: 12) | 9.02 ± 2.42 [1–12] |
| Hyposmia (BSIT ≤9) | 43 (52.4%) |
| Hyposmia during the acute disease | 54 (65.85%) |
| Arterial hypertension | 24 (29.3%) |
| Diabetes | 12 (14.6%) |
| Active smoker | 14 (17.1%) |
| Hospitalization | 27 (32.9%) |
| ICU admission | 8 (9.8%) |
FIGURE 1.
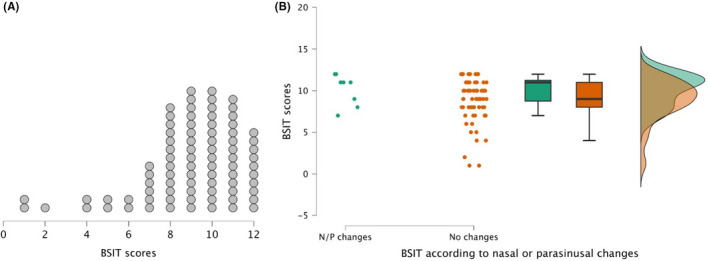
(A) BSIT scores distribution. Each dot represents one patient. (B) Raincloud plot showing the distribution and scores of the BSIT according to the presence (green) or not (orange) of nasal and/or parasinusal changes (N/P). Left plot represents BSIT data from each patient; middle graph represents mean and standard error using a box plot; right graph shows a split‐half violin plot to represent the data distribution using the probability density function of observations
Visual assessment of paranasal sinuses and nasal cavities revealed mild changes in 8 patients (9.75%), consisting in partial occupation of sinuses and mucosal thickening. There were no differences in olfactory assessment between patients with or without changes at this level (10.13 ± 1.88 vs. 8.89 ± 2.45, U = 380, p = .159) (Figure 1B).
In ASL, BSIT was positively correlated with a large cluster in the bilateral frontal lobes involving the bilateral superior medial frontal and medial orbital gyri, rectus and olfactory gyri, anterior cingulate, and caudate (Table 2 and Figure 2). The correlation between brain perfusion in this region and BSIT was r = .261 (p = .009). The opposite contrast (i.e. regions negatively correlated with BSIT scores) did not report significant findings. There were no statistically significant clusters in the correlation between brain volumes and DTI analysis.
TABLE 2.
Voxel‐based brain mapping analysis results
| Brain region | MNI coordinates | T value | Z score | K (number of voxels) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Bilateral superior medial frontal, medial orbital, rectus, anterior cingulate and olfactory gyri, and caudate. | −8 | 30 | −18 | 5.00 | 4.64 | 1753 |
| 6 | 52 | 34 | 4.37 | 4.12 | ||
| 6 | 44 | 46 | 4.27 | 4.04 | ||
Correlation between brain perfusion (ASL) and olfactory assessment (BSIT), using a FWE cluster‐based corrected p < .05.
FIGURE 2.
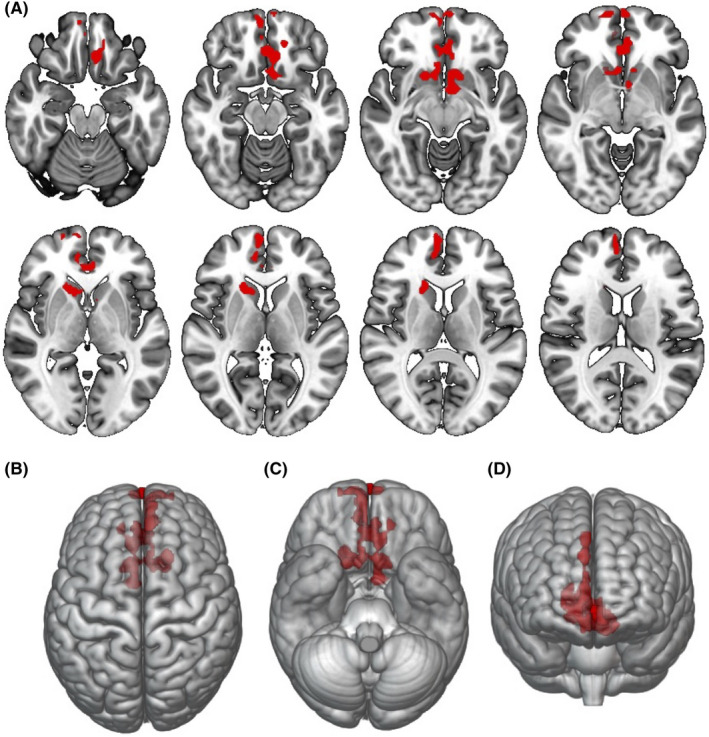
ASL voxel‐based analysis. Multiple regression showing brain regions associated with olfactory test score in red, using age and sex as nuisance covariates (p < .01, FWE cluster‐based corrected p < .05). (A) axial view. (B–D) surface‐rendered projections. Images are shown in radiological orientation. FWE, family‐wise error
4. DISCUSSION
This study evaluated a cohort of patients with PCS using a multimodal neuroimaging protocol. To our knowledge, this is the largest study evaluating the neuroimaging correlates of olfactory dysfunction after the acute phase of the disease and is the first to report ASL findings in PCS.
Our findings suggest reduced tissue perfusion in the orbital and medial frontal lobes. Conversely, there was no statistically significant correlation with brain volumes. These results could be interpreted from at least two points of view. On the one hand, these findings could be a functional consequence due to reduced input signaling from the peripheral olfactory system and are consistent with recent findings showing brain connectivity changes in the anterior piriform cortex in a cohort of 27 patients with post‐COVID hyposmia using functional MRI. 11 On the other hand, we could not exclude that these findings could be the evidence of CNS damage. In this regard, in a recent study using longitudinal structural neuroimaging from the UK Biobank, a reduction in gray matter thickness was detected in the orbitofrontal cortex and primary olfactory cortex in patients that tested positive for SARS‐CoV‐2 in comparison with the neuroimaging performed before the infection. 12 Because these regions are also involved in cognitive deficits and behavioral changes, these results could represent an indirect marker of CNS involvement. In addition, one of the main hypotheses of SARS‐CoV‐2 neurological effects is based on the effects on the cerebral vascular system. 13 In this regard, the impairment of tissue perfusion in the absence of other involvement (FLAIR, T1) could support the presence of endothelial dysfunction as a mechanism associated with COVID‐19 neurological complications. The absence of a significant correlation with DTI parameters is consistent with previous studies about hyposmia in neurodegenerative such as Parkinson's disease, in which the small tracts arising from the olfactory cortex could be a plausible explanation for the absence of findings in this sequence. 14
Our study has some limitations. First, we have the limitations of a cross‐sectional design, and it is not possible to establish a cause–effect relationship between brain perfusion findings and olfactory dysfunction. Although this design is often used to evaluate brain–behavior relationships with neuroimaging, longitudinal studies are necessary to confirm our findings. Second, we did not include a control group. Thus, the relationship between olfactory function and frontal lobe perfusion could not be specific to PCS. Further studies applying the same methodology to other causes of olfactory dysfunction (e.g., sinonasal disease) could be of interest to evaluate whether these findings are only a consequence of low olfactory input or represent a brain disturbance associated with COVID‐19.
In conclusion, persistent hyposmia in patients with PCS was associated with reduced regional blood flow in the orbital and medial frontal lobe. These findings contribute to the pathophysiology of persistent olfactory dysfunction after COVID‐19, which involves the main brain regions associated with the olfactory system.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
This study was approved by our center's Ethics and Research Committee (code 21/062‐E).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13627.
Supporting information
App S1
ACKNOWLEDGEMENTS
The authors would like to thank Pablo García‐Polo, GE Healthcare, Spain, for his help developing the protocol of MR imaging used in the study. We acknowledge Dr Andrea Valcárcel, Dr Mariam Farid, and Dr Ernesto Botella, from the Department of Internal Medicine of our centre, for the help in the recruitment and care of these patients.
Yus M, Matias‐Guiu JA, Gil‐Martínez L, et al. Persistent olfactory dysfunction after COVID‐19 is associated with reduced perfusion in the frontal lobe. Acta Neurol Scand. 2022;00:1–5. doi: 10.1111/ane.13627
Funding information
This research was supported by the Department of Health of the Community of Madrid (grant number FIBHCSC 2020 COVID‐19). Jordi A. Matias‐Guiu is supported by Instituto de Salud Carlos III through the project no. INT20/00079, co‐funded by European Regional Development Fund “A way to make Europe”
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Keshavarz P, Haseli S, Yazdanpanah F, Bagheri F, Raygani N, Karimi‐Galougahi M. A systematic review of imaging studies in olfactory dysfunction secondary to COVID‐19. Acta Radiol. 2021;28:1530‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, Naeini AS, Haseli S. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol. 2020;27:892‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID‐19) and anosmia. JAMA Neurol. 2020;77:1028‐1029. [DOI] [PubMed] [Google Scholar]
- 4. Niesen M, Trotta N, Noel A, et al. Structural and metabolic brain abnormalities in COVID‐19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging. 2021;48:1890‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donegani MI, Miceli A, Pardini M, et al. Brain metabolic correlates of persistent olfactory dysfunction after SARS‐CoV2 infection. Biomedicines. 2021;9(3):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toniolo S, Di Lorenzo F, Scarioni M, Frederiksen KS, Nobili F. Is the frontal lobe the primary target of SARS‐CoV‐2? J Alzheimers Dis. 2021;81:75‐81. [DOI] [PubMed] [Google Scholar]
- 7. Khan M, Yoo SY, Clijsters M, et al. Visualizing in deceased COVID‐19 patients how SARS‐CoV‐2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932‐5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . A Clinical Case Definition of Post COVID‐19 Condition by a Delphi Consensus, 6 October 2021. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Post_COVID‐19_condition‐Clinical_case_definition‐2021.1. Accessed February 8, 2022.
- 9. Doty RL, Marcus A, Lee WW. Development of the 12‐item cross‐cultural smell identification test (CC‐SIT). Laryngoscope. 1996;6:353‐356. [DOI] [PubMed] [Google Scholar]
- 10. El Rassi E, Mace JC, Steele TO, et al. Sensitivity analysis and diagnostic accuracy of the brief smell identification test in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito F, Cirillo M, De Micco R, et al. Olfactory loss and brain connectivity after COVID‐19. Hum Brain Mapp. 2022;43(5):1548‐1560. doi: 10.1002/hbm.25741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douaud G, Lee S, Alfaro‐Almagro F, et al. SARS‐CoV‐2 is associated with changes in brain structure in UK Biobank. Nature. 2022. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kakarla V, Kaneko N, Nour M, et al. Pathophysiologic mechanisms of cerebral endoteliopathy and stroke due to SARS‐CoV‐2. J Cereb Blood Flow Metab. 2021;41:1179‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Georgiopoulos C, Warntjes M, Dizdar N, et al. Olfactory impairment in Parkinson’s disease studied with diffusion tensor and magnetization transfer imaging. J Parkinsons Dis. 2017;2017(7):301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.
The data that support the findings of this study are available from the corresponding author upon reasonable request.


