Abstract
Aim
We focused on the clinical picture, severity and prognosis of children who experienced long‐term respiratory issues after COVID‐19.
Methods
This was a national Czech multicentre study of paediatric post‐COVID syndrome, which used a standard protocol to evaluate structural and functional anomalies and exclude alternative diagnoses. From 6 January to 30 June 2021, 11 paediatric pulmonologists enrolled all paediatric referrals aged 2–18 years with persistent respiratory symptoms more than 12 weeks after COVID‐19, namely cough, dyspnoea and chest pain. Medical histories were taken, and physical examinations, lung function testing, chest X‐ray and blood tests were performed.
Results
The dominant symptoms in the 39 children (56.4% girls) were exertional dyspnoea (76.9%) and a chronic cough (48.7%), while dyspnoea at rest (30.8%) and chest pain (17.9%) were less prevalent. More than half (53.8%) reported more than 1 symptom, and 38.5% had abnormal results for 1 of the following tests: lung function, chest X‐ray or D‐dimers. The median age of the children was 13.5 years (interquartile range ±4.8 years), and the median recovery time was 4 months (range 1.5–8 months).
Conclusion
Our initial data suggest that the long‐term respiratory impact of COVID‐19 was relatively mild in our cohort, with a favourable prognosis.
Keywords: chronic cough, coronavirus, COVID‐19, dyspnoea, post‐COVID syndrome
Abbreviations
- CT
computed tomography
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Key Notes.
This Czech multicentre study focused on 39 children aged 2–18 who experienced long‐term respiratory issues after COVID‐19.
The dominant symptoms were exertional dyspnoea (76.9%) and a chronic cough (48.7%), while dyspnoea at rest (30.8%) and chest pain (17.9%) were less prevalent.
The long‐term respiratory impact of COVID‐19 was relatively mild in our cohort, who had a median age of 13.5 years and a median recovery time of 4 months.
1. INTRODUCTION
The Czech Republic faced a critical situation as the result of the COVID‐19 pandemic at the beginning of 2021. By the end of that year, the country had recorded 2.3 million infections and nearly 35,000 deaths since the start of this global health emergency. 1 Children and adolescents represented 10–15% of those cases, and there were 1500–2000 confirmed cases per day among this age group in January 2021, which was the start date for this study. 1 This was a significant number for a country with a population of 10.7 million residents. 2 Epidemiological evidence has indicated that children are less likely to develop severe COVID‐19 than adults. One explanation for this has been that they have more trained immunity than adults, which makes them less susceptible to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 3
Long‐term sequelae following COVID‐19 have been extensively described in the adult population. Diagnoses of post‐COVID syndrome have usually referred to clinical features that have persisted for more than 12 weeks after acute COVID‐19 and could not be explained by any alternative diagnosis. 4 A meta‐analysis by Fernandez et al showed that more than 60% of patients infected by SARS‐CoV‑2 presented with post‐COVID‐19 symptoms. 5 Fatigue and dyspnoea were the most prevalent symptoms, particularly 60 days and at least 90 days after COVID‐19, with a pooled prevalence that ranged from 35% to 60%, depending on the follow‐up period. Other post‐COVID‐19 symptoms included a cough (20–25%), loss of smell (10–20%), loss of taste (15–20%) and joint pain (15–20%). 5 Another study, by Shah et al, stated that common long‐term COVID‐19 symptoms were fever, pain and neurological, cardiovascular, gastrointestinal, dermatological and musculoskeletal symptoms. 6
Although a meta‐analysis by Behnood et al indicated a lower incidence and severity of acute paediatric COVID‐19, 7 greater attention is now being paid to severe complications following infections in paediatric populations. These include multisystem inflammatory syndrome in children, which is also known as paediatric inflammatory multisystem syndrome temporarily associated with SARS‐CoV‐2. 8 This syndrome develops within four weeks of exposure to the SARS‐CoV‐2 virus, regardless of whether COVID‐19 takes a symptomatic or asymptomatic course. It has been associated with a high risk of multiorgan failure, especially with severe cardiac involvement. Only limited data regarding the incidence, clinical picture, diagnosis, therapy or prognosis of paediatric post‐COVID are available. These have included several case series that focused on parents who contacted a physician about their children as a result of discussions on a social media forum 9 and surveys carried out on patients by paediatric departments. 10 They have also included cohorts of randomly selected school classes with population‐based seronegative control groups, 11 single‐centre cohorts examined by phone calls and outpatient assessments 12 and a prospective cohort study based on self‐data reporting by a mobile application. 13
The aim of our study was to explore the clinical picture, severity and prognosis of post‐COVID syndrome in children, with a particular focus on the respiratory system. Children were referred to, and followed up by, the national network for Czech paediatric pulmonologists. In addition, we suggested a uniform panel of screening tests to maintain an identical procedure between all of the centres. The goal of this diagnostic battery was to use currently available techniques to explore possible structural and functional anomalies of the respiratory tract in paediatric patients with post‐COVID syndrome.
2. METHODS
This prospective, national observational study focused on centres for paediatric pulmonology that covered all the regions of the Czech Republic. Four centres of paediatric pulmonology in Prague participated in this study, together with one centre in each of the 7 regions outside the capital. However, it is probably that these centres did not cover the whole Czech paediatric population, because limited capacity in larger remote regions means that fewer children are referred to specialists. From 6 January to 30 June 2021, 11 paediatric pulmonologists enrolled all children aged 2–18 years with persistent respiratory symptoms after COVID‐19. The patients had all been referred by paediatricians working in inpatient or outpatient settings (Figure 1). The inclusion criteria comprised three key elements. First, the presence of dyspnoea, at rest or on exertion, a cough or chest pain for 12 weeks or more after being infected with SARS‐CoV‐2. Second, documentation of a previous SARS‐CoV‐2 infection, confirmed by a positive polymerase chain reaction test and/or the detection of a significant elevation of antibodies. Third, the absence of any pre‐existing chronic respiratory disease.
FIGURE 1.
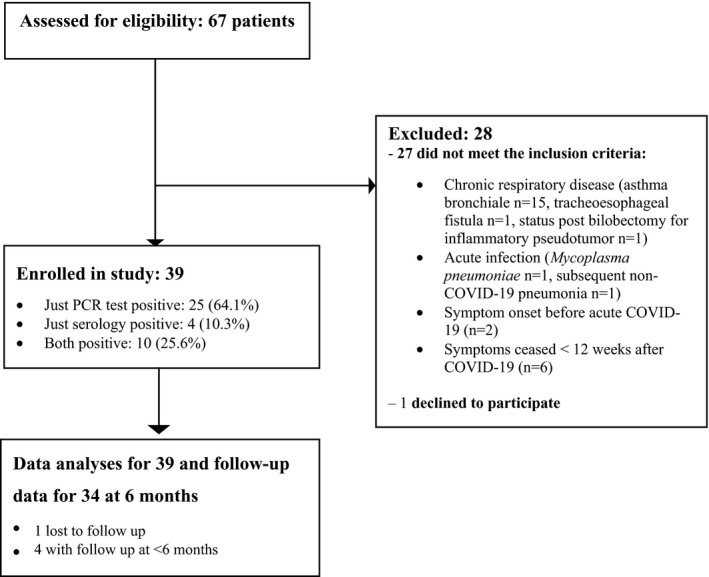
Flow chart—recruitment of children eligible for post‐COVID syndrome assessment
A standard protocol was put in place to evaluate structural and functional anomalies and exclude alternative diagnoses. The medical histories of the children were taken during the first consultation with the patient and their parents, in accordance with current best practice. Standardised questionnaires were not used. Symptoms were both self‐reported and reported by the child's parents. The patients then underwent a physical examination, plain chest radiograph, blood tests and lung function tests, which comprised spirometry, diffusing capacity and the 6‐minute walk test. The laboratory tests comprised a blood count, basic biochemistry and allergy panel, D‐dimer levels and SARS‐CoV‐2 antibodies. If the initial panel showed that the children had abnormal results, we considered an extended spectrum of investigative methods. This included the following: a chest ultrasound, a spiral chest computed tomography (CT) scan with high‐resolution reconstruction, CT pulmonary angiograms and a ventilation/perfusion lung scan. The subjects were invited to attend at least two outpatient visits over a 6‐month period.
There were possible risks during the data collection that post‐COVID symptoms could be confused with, or overlap with, bronchial asthma, even if the child had not been diagnosed with this disease. Children with suspected bronchial asthma usually had a positive family history, atopic history, positive allergy panel and an excellent response to anti‐asthmatic treatment. We placed these in an asthmatic subgroup. However, such patients would have needed a longer follow‐up to reach a final diagnosis of asthma.
During the data analysis, we observed other striking differences in the post‐COVID cohort, in relation to their medical histories, whether they played sport and the presence of concomitant non‐respiratory symptoms. In addition to the asthmatic subgroup, we decided to establish three more possible post‐COVID subgroups: standard, polymorphous and sporty. The standard subgroup was the dominant category and it comprised children with the expected respiratory symptomatology of cough, dyspnoea and fatigue. The subjects in the polymorphous subgroup described a wide variety of symptoms, in addition to the respiratory symptomatology. These included headaches, difficulties concentrating, anxiety, hair loss, chest pain, a cough, dyspnoea and tachycardia. We also noticed that the symptoms showed different characteristics in the sporty subgroup. These children were used to high‐intensity physical activities and the key issue that they reported was worsening of their physical performance.
We compared numerical variables using one‐way analysis of variance and categorical variables with Fisher's exact test. All analyses were performed using SPSS Statistics, version 21 (IBM, New York, USA). The study was approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine and the Thomayer University Hospital (ref A‐21‐01).
3. RESULTS
We examined 67 children and 40 fulfilled the inclusion criteria. One refused to participate and the other 39 were included in the study (Figure 1). All the children had a mild acute course of COVID‐19. One 18‐year‐old girl with haemoptysis, which was an unusual symptom, was hospitalised for 5 days, but she did not require ventilatory support or oxygen therapy (Table 1). None of the subjects had been diagnosed with multisystem inflammatory syndrome in children. Thirty‐nine children (56.4% girls) had a median age of 13.5, with an interquartile range of 8–15 years. SARS‐CoV‐2 was confirmed by just a positive polymerase chain reaction test in 25 (64.1%) children, by just a serology test in 4 (10.3%) and by both tests in 10 (25.6%).
TABLE 1.
Details and clinical data of 3 unusual cases who underwent chest CTs or ventilation/perfusion scan imaging
|
Gender age |
Symptom | Details of assessment | Chest CT/VP scan | Time to remission | |
|---|---|---|---|---|---|
| Case 1 |
Male patient 15 years |
Pleural pain | Pleural effusion on chest ultrasound, negative D‐dimers | Normal VP scan | 7 months |
| Case 2 |
Female patient 18 years |
Haemoptysis during acute COVID−19 | Negative D‐dimers, abnormal 6MWT | Normal chest CTA |
6 months The only subject who was hospitalised. |
| Case 3 |
Female patient 14 years |
Syncope |
Elevated D‐dimers (750 µg/l, normal value<500) Desaturation during 6MWT |
Normal VP scan | 4 months |
Abbreviations: CT, computed tomography, VP ventilation/perfusion lung scan, CTA, CT pulmonary angiogram, 6MWT, 6‐minute walk test.
The dominant symptoms were exertional dyspnoea (76.9%) and a chronic cough (48.7%), while dyspnoea at rest (30.8%) and chest pain (17.9%) were less prevalent (Table 2). More than half of the children (53.8%) reported more than 1 symptom. We found that 38.5% had abnormal results for 1 of the following tests, lung function, chest X‐ray or D‐dimers, and 7.7% had abnormal results for 2 of those tests. There were no associations between any symptoms and abnormal results. We suspected that 9 of the subjects had incipient bronchial asthma, which was related to COVID‐19, based on their personal and family histories, spirometry, laboratory results and the therapeutic effects of asthma treatment. However, no statistically significant differences in symptoms or laboratory and functional abnormalities were found between the standard, asthmatic, sporty and polymorphous subgroups. This was despite their different post‐COVID clinical manifestations.
TABLE 2.
Symptoms and abnormal results for all 39 study subjects
| All subjects (n=39), 56.4% females, median age 13.5 years (range 2–18, interquartile range 8–15) | Functional or laboratory results in detail | ||
|---|---|---|---|
| Symptoms | Cough | 19 (48.7%) | |
| Dyspnoea at rest | 12 (30.8%) | ||
| Exertional dyspnoea | 30 (76.9%) | ||
| Chest pain | 7 (17.9%) | ||
| Abnormal functional or laboratory results | Spirometry | 5 (13.2%) |
1 with mild reduction and 1 with moderate reduction of vital capacity 2 with mild obstruction on F‐V loop, with positive BDT, and 1 with moderate obstruction on F‐V loop, with negative BDT |
| 6MWT | 3 (8.8%) | Decline in oxygen saturation during 6MWT to 83%, 88% and 92% respectively | |
| DLCO | 3 (11.5%) | Decline to 68%, 66% and 60% of predictive value, respectively | |
| Chest X‐ray | 7 (18.9%) | 2 with basal limited infiltration, 5 with perihilar opacities | |
| D‐dimers | 2 (8%) |
Girl with polymorphous symptoms without other abnormities and physiological result of repeated D‐dimers. Another girl (case 3 in Table 1). |
|
Data presented as numbers (percentages). F‐V loop, flow‐volume loop, BDT, standardised bronchodilatation test with 4 puffs of salbutamol, 6MWT, 6‐minute walk test, DLCO, diffusing capacity for carbon monoxide.
We followed up 34 of the 39 subjects for the whole duration of our 6‐month study: 1 was lost to follow‐up and the other 4 were followed up for less than 6 months. These 34 patients experienced complete remission of their tracked symptoms within 1.5 to 8 months and the median recovery time was 4 months (Table 3). Three of the children displayed unusual diseases courses. A 15‐year‐old boy showed complete remission within 7 months of his first post‐COVID symptoms, after experiencing pleural pain, pleural effusion, which was confirmed by lung ultrasound, a normal ventilation/perfusion lung scan and normal D‐dimers. The 18‐year‐old girl, who was hospitalised with haemoptysis during her acute COVID‐19 phase, had an abnormal 6‐minute walk test, physiological CT chest scan and D‐dimers and achieved complete remission within 6 months. A 14‐year‐old girl with 1 episode of syncope, desaturation to 83% during her 6‐minute walk test and positive D‐dimers, had a normal ventilation/perfusion lung scan and spontaneously recovered within 4 months (Table 2).
TABLE 3.
Months of persistent reported post‐COVID symptoms until remission
| Post‐COVID subgroups with complete follow‐up data | Duration in months | One‐way analysis of variance |
|---|---|---|
| Standard (16/20) | 5.0 (±1.5) | p=0.7 |
| Asthmatic (8/9) | 4.1 (±1.9) | |
| Sporty (5/5) | 4.9 (±1.8) | |
| Polymorphous (5/5) | 4.5 (±2.0) |
Data expressed as means and standard deviations.
4. DISCUSSION
This study shows that the 39 children in our cohort had relatively mild and limited respiratory consequences as a result of COVID‐19. There are several results from our research that are particularly worth highlighting.
First, we found a relatively low risk of structural and/or functional abnormalities of the respiratory tract and clinical remission usually occurred within a few months. The main temporary radiological anomalies included perihilar opacities and limited pleural basal adhesions. Moreover, we observed both obstructive or restrictive transient ventilatory patterns of mild to moderate severity in 5 subjects and a mild decline of diffusing lung capacity in 3 children.
Second, we perceived the heterogeneity of the subjects during the data collection and analysis and decided that we needed to cluster them into more homogeneous subgroups of respiratory post‐COVID syndrome. These include an asthmatic subgroup that comprised children with probable, incipient bronchial asthma. There is currently no evidence whether SARS‐CoV‐2 may act as a potential trigger for bronchial asthma. However, there is a high incidence of asthma in the paediatric population, and this increases the risk of this disease being confused with, or occurring at the same time as post‐COVID syndrome. Furthermore, parents may have paid more attention to their children's respiratory symptoms after COVID‐19, which could have accelerated their referral to a pulmonologist. The asthmatic group usually had a positive family history, atopic history, positive allergy panel and an excellent response to asthma treatment. It is possible that the children in this group could have been over‐reported in our cohort, due to detection bias by pulmonologists. This is because longer follow‐ups are usually necessary to reach a final diagnosis of asthma. The dominant subgroup of patients who were referred to paediatric pulmonologists had the expected respiratory symptoms and they formed the standard subgroup. The subjects in the polymorphous subgroup described a wide variety of symptoms, in addition to the respiratory symptoms that have been thoroughly described in previous studies of paediatric post‐COVID symptoms. 9 , 10 , 12 , 13 These included headaches, difficulties concentrating, anxiety, hair loss, chest pain, a cough, dyspnoea, anxiety and tachycardia. This group was probably under‐reported, due to selection bias in this study. In addition, a number of non‐respiratory problems may lead to patients being referred to other specialists rather than pulmonologists. We also noticed that the symptoms had different characteristics in the sporty subgroup. These children were used to regular high‐intensity physical activities, and they said that their worsening physical performance was the dominant symptom. Children who do not engage in regular sporting activities could have missed discrete symptoms of exertional dyspnoea.
Our results were in accordance with other published case series. 9 , 10 , 11 , 12 , 13 These studies confirmed the existence of paediatric post‐COVID syndrome and reported diverse symptoms and infrequent occurrences. As far as we know, the first paper on post‐COVID syndrome was published by Ludgvisson in November 2020. 10 The author collected data from the parents of 5 Swedish children with persistent symptoms that lasted 2 months or more after acute COVID‐19. The parents contacted the author after discussing their children's symptoms on an Internet‐based social media forum. These children's symptoms lasted for 6–8 months after their clinical diagnoses of COVID‐19 and included fatigue, dyspnoea, cardiac palpitations, chest pains, headaches, muscle weakness, dizziness and sore throats. 9 Buonsenso et al. subsequently published a cross‐sectional study of 129 children aged 6–16 years who were diagnosed with COVID‐19 between March and November 2020. The subjects were interviewed by phone or in an outpatient clinic. More than a third reported 1 or 2 lingering symptoms 4 months or more after COVID‐19 and a further quarter had 3 or more symptoms. Insomnia, fatigue, muscle pain and persistent cold‐like complaints were common. 12 A prospective cohort study by Molteni et al. found that about 1.8% of 1379 children experienced symptoms for at least 56 days after acute COVID‐19, according to data reported by their parents using a mobile application. 13 Meanwhile, a longitudinal cohort study by Radtke et al. found that 4% of seropositive children and 2% of seronegative children reported at least 1 symptom that lasted for longer than 12 weeks. 11 Their parents reported the symptoms via an online questionnaire. The most common complaints included fatigue, difficulty concentrating and increased sleepiness. Brackel et al. conducted a national Dutch survey on 89 children with long COVID, who were identified by paediatric departments. The most frequent symptoms were fatigue, dyspnoea and difficulties concentrating and the authors described 6 case studies in detail. 10
To our knowledge, this was the first prospectively planned study that focused on the respiratory aspects of paediatric post‐COVID symptoms. Our data suggest a lower risk of functional lung impairment in children, compared to the published data for adults. 14 , 15
4.1. Strengths and limitations
The strengths of our study were that it was carried out following a peak in the country's pandemic in the early months of 2021 and the centres who took part collected data from all regions of the Czech Republic. In fact, the country had one of the highest COVID‐19‐related mortality rates worldwide in March 2021. 16 Our study was based on 2.3 million confirmed SARS‐CoV‐2 infections by mid‐December 2021 and children and adolescents represented 10–15% of those cases. 2 This provided an adequately large sample for monitoring paediatric post‐COVID syndrome.
The main limitations of this study were the differences in the laboratory equipment and software used by the centres and the rather short follow‐up period. However, this was necessary so that we could publish these results in a timely manner. A major limitation of the study was the relatively small number of patients that were included, even though this was a national study.
5. CONCLUSION
Our initial data suggest that the long‐term respiratory impact of COVID‐19 appeared to be relatively mild in our cohort, with a favourable prognosis. Further studies with more subjects and longer follow‐up periods are needed. The test panel that we designed seemed to be effective for evaluating the structural and functional abnormalities of the respiratory tract in paediatric post‐COVID syndrome.
CONFLICT OF INTEREST
The authors have no conflicts of interests to declare.
ACKNOWLEDGEMENT
We thank the colleagues who assisted us with patient recruitment and data sharing: Vendula Látalová, (Palacký University and University Hospital Olomouc), Renata Říhová (University Hospital Bulovka, Prague), Veronika Mohylová, (University of Ostrava and University Hospital Ostrava), Vendula Martinů, (Charles University and University Hospital Motol, Prague), Marcela Kreslová, (University Hospital and Faculty of Medicine, Pilsen), Stanislav Červíček (Hospital České Budějovice), Adam Cipra, (Krajská zdravotní, a.s. Ústí nad Labem).
Doležalová K, Tuková J, Pohunek P. The respiratory consequences of COVID‐19 lasted for a median of 4 months in a cohort of children aged 2–18 years of age. Acta Paediatr. 2022;111:1201–1206. doi: 10.1111/apa.16297
Doležalová Karolína and Tuková Jana contributed equally to this work.
Funding information
This study received funding from the Charles University, Prague (UNCE 204064)
REFERENCES
- 1. COVID‐19 in the Czech Republic according to age CoVdata [Internet]. [cited 2022 Jan 16]. Available from: https://www.covdata.cz/cesko‐vek.php
- 2. Population | CZSO [Internet]. [cited 2022 Jan 13]. Available from: https://www.czso.cz/csu/czso/population
- 3. Netea MG, Giamarellos‐Bourboulis EJ, Domínguez‐Andrés J, et al. Trained Immunity: a Tool for Reducing Susceptibility to and the Severity of SARS‐CoV‐2 Infection. Cell Press. 2020;181:969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yong SJ. Long COVID or post‐COVID‐19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53(10):737‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernández‐de‐las‐Peñas C, Palacios‐Ceña D, Gómez‐Mayordomo V, et al. Prevalence of post‐COVID‐19 symptoms in hospitalized and non‐hospitalized COVID‐19 survivors: A systematic review and meta‐analysis. Eur J Intern Med. 2021;92:55‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid‐19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:136. [DOI] [PubMed] [Google Scholar]
- 7. Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS‐CoV‐2 infection amongst children and young people: A meta‐analysis of controlled and uncontrolled studies. J Infect. 2022;84(2):158‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos MO, Gonçalves LC, Silva PAN, et al. Multisystem inflammatory syndrome (MIS‐C): a systematic review and meta‐analysis of clinical characteristics, treatment, and outcomes. Jornal De Pediatria. 2021;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ludvigsson JF. Case report and systematic review suggest that children may experience similar long‐term effects to adults after clinical COVID‐19. Acta Paediatr. 2021;110:914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brackel CLH, Lap CR, Buddingh EP, et al. Pediatric long‐COVID: An overlooked phenomenon? Pediatr Pulmonol. 2021;56:2495‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long‐term Symptoms After SARS‐CoV‐2 Infection in Children and Adolescents. JAMA. 2021;326(9):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buonsenso D, Munblit D, de Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school‐aged children tested for SARS‐CoV‐2. The Lancet Child & Adolescent. Health. 2021;5(10):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres‐Castro R, Vasconcello‐Castillo L, Alsina‐Restoy X, et al. Respiratory function in patients post‐infection by COVID‐19: a systematic review and meta‐analysis. Pulmonology. 2021;27(4):328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J. 2021;63:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Czechia: Coronavirus Pandemic Country Profile ‐ Our World in Data [Internet]. [cited 2022 Jan 16]. Available from: https://ourworldindata.org/coronavirus/country/czech‐republic


