Abstract
Background
The inactivated Sinopharm/BBIBP COVID‐19 vaccine has been widely used in the world and has joined the COVAX vaccine supply program for developing countries. It is also well adapted for usage in low‐ and middle‐income nations due to their low storage requirements.
Objective
This study aims to report on the kinetics, durability, and neutralizing ability of the induced immunity of the BBIBP vaccine, and the intensified antibody response elicited by the booster.
Methods
A total of 353 healthy adult participants, aged 20–74 years, were recruited in this multicenter study. A standard dose of the BBIBP vaccine was administered (Month 0), followed by a second standard dose (Month 1), and a booster dose (after Month 7). Vaccine‐induced virus‐specific antibody levels (SARS‐CoV‐2‐IgA/IgM/IgG), conventional virus neutralization test (cVNT), pseudovirus neutralization test (pVNT), and surrogate virus neutralization test (sVNT) were monitored over multiple time points.
Results
Neutralizing titers induced by the two doses of inactivated vaccine for COVID‐19 peaked at Month 2 and declined to 33.89% at Month 6. Following the booster dose, elevated levels of antibodies were induced for IgA, IgG, and neutralizing antibodies, with neutralizing titer reaching 13.2 times that of before the booster.
Conclusion
By monitoring the antibody titer levels postvaccination, this study has shown that serum antibody levels will decrease over time, but a notable spike in antibody levels postbooster highlights the anamnestic immune response. This signifies that the protection capability has increased following the injection of booster immunization.
Keywords: antibody persistence, booster, Coronavirus disease 2019, SARS‐CoV‐2, vaccine
This study monitored specific antibody levels (IgA/IgM/IgG), cVNT, pVNT, and sVNT tests in 353 healthy adult subjects over the course of vaccination. The first two injections of the vaccine induce humoral responses with a peak at 2 months. Following the booster dose, IgA, IgG, and neutralizing antibodies increase with a neutralizing titer 13.2 times higher than before the booster.

Abbreviations
- cVNT
conventional virus neutralization test
- Ig
immunoglobulin
- pVNT
pseudo virus neutralization test
- sVNT
surrogate virus neutralization test
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) global pandemic caused by the contagious virus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has dramatically accelerated vaccine development. 1 Vaccines can engage the body's immune system to guard against the disease, such as the production of protective neutralizing antibodies. The development of new vaccines has been speed up from 10–15 years to 1–2 years, 2 with numerous vaccines being tested quickly in phase I, II, and III clinical trials followed by application in different regions. This was made possible by the knowledge and experience gained during previous epidemics, the maturation of advanced technological platforms, and the timely and large‐scale investment of corporations, governments, and nonprofit organizations without regard for cost. 3 However, because these vaccinations are being developed and applied at such a quick speed, there are worries about not only their effectiveness in providing immunological protection to the community, but also the longevity of immunity in the population.
Inactivated vaccines are created by killing or deactivating the virus, preventing it from replicating. The development of inactivated vaccines is a mature technology with a well‐tested regulatory pipeline and a high safety profile, and it has been widely utilized for decades for the prevention and control of emerging infectious diseases such as hepatitis A, influenza virus, and poliovirus. Currently, there are two major inactivated COVID‐19 vaccines, both developed in China: the Sinovac CoronaVac COVID‐19 vaccine and the Sinopharm's Beijing Bio‐Institute of Biological Products Coronavirus Vaccine (BBIBP‐CorV). Both inactivated vaccines have been shown to be generally safe and have induced antibody responses in adults in multiple clinical trials. 4 , 5 , 6
Here, we report the immunogenicity, durability, and the effect of a booster dose of Sinopharm/BBIBP COVID‐19 vaccine, in healthy adults in China. In this study, we monitored the virus‐specific antibodies (SARS‐CoV‐2‐IgA/IgM/IgG) induced throughout the course of vaccination and performed virus neutralization experiments to test for the protective capability against virus invasion. This work tackles the critical question of how antibody levels, and the neutralizing capability of antibodies, vary during vaccination with the BBIBP vaccine.
2. METHODS
2.1. Sampling
Initially, 500 healthy adult participants from three authorized hospitals in Guangdong, China, have registered in this study: the First Affiliated Hospital of Guangzhou Medical University, the Fifth Affiliated Hospital of Zunyi Medical University, and the Dongguan Eighth People's Hospital. Following the initial injection, 479 individuals completed a questionnaire survey on adverse symptoms. However, a number of individuals dropped out of the study during follow‐up monitoring, resulting in a final count of 353 participants (106 male, 247 female), aged 20–74 years (median: 33), being included in the analysis. The sample size was not determined based on the statistical power calculation. None of the participants have been exposed to SARS‐CoV‐2 infection before or throughout the study, due to China's zero‐COVID approach and continual RT‐PCR screening during a small‐scale local outbreak in Guangzhou in June 2021. 7 Main inclusion criteria: participants must be healthy, aged 18 and above, willing to participate, able to understand, and sign the informed consent. Main exclusion criteria: 1. Those who are allergic to the active ingredient, any inactive ingredient, or substance used in the production process of the vaccine, or those who have previously been allergic to the same kind of vaccine; 2. those who have had severe allergic reactions to vaccines in the past (such as acute allergic reactions, angioneurotic edema, dyspnea, etc.); 3. those suffering from uncontrolled epilepsy and other serious neurological diseases (such as transverse myelitis, Guillain‐Barre syndrome, demyelinating diseases, etc.); 4. those who are febrile, or suffering from acute diseases, or acute episodes of chronic diseases, or patients with uncontrolled serious chronic diseases; 5. pregnant women; and 6. those who had had a positive PCR test, or previous history of COVID‐19 infection.
After providing written informed consent by themselves, participants were injected homologously with a two‐shot regiment (28 days apart) of 4‐μg dose of β‐propiolactone‐inactivated, aluminum hydroxide‐adjuvanted Sinopharm/BBIBP COVID‐19 vaccine, followed by a third booster injection 7 months following the initial injection. The vaccine was developed by the Beijing Institute of Biological Products (Beijing, China) and manufactured as previously described. 8 The sampling of blood was conducted on Month 0, Month 1 (second injection), Month 2, Month 3, Month 6, Month 0.5 after‐boost, and Month 1 after‐boost. Data were collected between 26 January 2021 and 6 December 2021.
We evaluated the vaccine‐elicited titers of SARS‐CoV‐2‐specific antibodies IgA/IgM/IgG, surrogate virus neutralization test (sVNT) using receptor‐binding domain– (RBD‐) neutralizing antibodies, pseudovirus neutralization test (pVNT), and conventional virus neutralization test (cVNT) of the participants. Due to research budget constraints, specific antibodies (IgA/IgM/IgG) and sVNT titers were detected for samples at all seven time points, whereas pVNT titers were detected only for the first three time points (Months 0, 1, and 2), and cVNT titers were detected for the first four time points (Months 0, 1, 2, and 3).
2.2. Serum antibody detection
SARS‐CoV‐2‐IgA/IgM/IgG‐specific antibodies in human serum samples in vitro were detected by the indirect chemiluminescence method, using the automated analytical instrument Axceed 260 for clinical chemiluminescence immunoassay (Tianjin Bioscience Diagnostic Technology Co., Ltd.) and accompanying immunoassay test kits. Axceed 260 automatically conducted the following tasks: adding reagents, adding samples, adding magnetic beads, incubation reaction and mixing, cleaning magnetic beads, adding substrate and mixing, and reading relative light unit (RLU). This RLU would then be converted to a final titer readout, in titer units of S/CO. The product name for the IgA test kit is “Diagnostic Kit for Novel Coronavirus (2019‐nCoV) IgA Antibody (Magnetic particle CLIA),” and similarly for IgM and IgG.
The test kits consisted of reagent 0 (magnetic particles‐anti‐FITC antibody), reagent 1 (FITC‐labeled SARS‐CoV‐2‐recombinant antigen), reagent 2 (alkaline phosphatase–labeled mouse anti‐human IgA/IgM/IgG monoclonal antibody), negative control, positive control, and other necessary auxiliary reagents.
During a test, reagent 0, reagent 1, and the sample were added to the reaction tube. If the sample contained an SARS‐CoV‐2 IgA/IgM/IgG antibody, it would form a complex with the recombinant antigen in the aforementioned reagent and bind to the magnetic particles at the same time. After that, the free components were rinsed away. Reagent 2 was then added to the reaction tube. The alkaline phosphatase‐labeled antibody served as a secondary antibody, binding to the IgA/IgM/IgG antibody in the sample to generate an alkaline phosphatase‐labeled antibody‐IgA/IgM/IgG antibody‐recombinant antigen‐magnetic particle complex. The RLU of each sample tube was determined by adding the substrate solution used in the automatic immunoassay system and catalyzing the luminescence of the substrate solution with alkaline phosphatase. The RLU of the sample was positively correlated with the IgA/IgM/IgG antibody concentration of the SARS‐CoV‐2, allowing for the detection of SARS‐CoV‐2 IgA/IgM/IgG antibody in human serum.
2.3. Surrogate virus neutralization test
Surrogate virus neutralization test titers in human serum samples were identified in vitro using RBD‐neutralizing antibodies (for wild‐type virus) utilizing the competitive chemiluminescence method, using the automated analytical apparatus Axceed 260 described above, with accompanying immunoassay test kits. The RLU obtained with Axceed 260 would be converted to a final titer readout, in titer units of AU/ml. Because the instrument has an upper detection titer limit of 30, if the final titer readout was greater than 30, the experiment was repeated with the sample diluted 10 times. If the readout remained above 30, the sample would be further diluted 2 times (total 20x dilution).
The test kit consisted of reagent 0 (magnetic particle receptor angiotensin‐converting enzyme (ACE2) antigen), reagent 1 (alkaline phosphatase‐labeled S protein RBD), calibrator 1, calibrator 2, and other necessary auxiliary reagents. During a test, reagent 0, reagent 1, and serum samples were added to the reaction tube. If the sample contained neutralizing antibody, it would compete with magnetic particle‐labeled ACE2 antigen to bind S protein RBD. Free components were then rinsed away. Substrate solution was added, substrate solution luminescence was catalyzed by alkaline phosphatase, and the RLU of each sample tube was quantified. The RLU of the sample negatively correlated with the concentration of SARS‐CoV‐2‐neutralizing antibody in the sample, allowing us to calculate the titer of sVNT (in units of AU/ml) in human serum. Titer levels of ≥2 AU/ml were regarded as positive (threshold designed by manufacturer).
2.4. Pseudovirus neutralization test
For pVNT, we use the pseudovirus system SARS‐CoV‐2‐Fluc AY.2 (Vazyme Biotech Co., Ltd). The virus system takes the firefly luciferase reporter gene (FLUC) carrying virus HIV‐1 as the host virus, and it expresses SARS‐CoV‐2 spike protein in the virus shell. The pseudovirus infects endogenous or exogenous cell lines expressing ACE2 (such as the HEK293‐ACE2 cell line, a transfected HEK293 cell line that overexpresses human ACE2), which closely mimics the SARS‐CoV‐2 invasion process of target cells via spike ACE2. When cells are infected with a pseudovirus, they express FLUC proteins, which can react with luminous substrate and emit light. This luminosity value would indicate the amount of active pseudovirus present. The degree to which the pseudovirus infects the target cell is positively correlated with FLUC luminescence and negatively correlated with the neutralizing activity of the antibody.
The experiments were carried out on 96‐well plates. After diluting the serum 1:30, we sequentially performed gradient dilutions of 1:3 each well for six wells. For reproducibility, the solutions and wells were produced twice.
The experimental protocol was as follows: first, 100 μl/well of serum was added, followed by 50 μl/well of virus, and the neutralization took place at 37°C for 1 h. 50 μl of HEK293‐ACE2 cells at a density of 2*104 cells/50μl were then added to each well, and the mixture was cultured at 37°C for 48 h. Bio‐Litetm Luciferase Assay System (Vazyme Biotech Co., Ltd) was then added directly to the cell culture to cause cell lysis, releasing luciferase, which emitted a stable light signal. The luciferase luminescence values of the samples in the 96‐well plate were measured using a full‐wavelength microplate analyzer. The 50 percent inhibitory dose (ID50) titer was calculated using the Reed‐Muench method (the dilution multiples when 50% of pseudoviruses are neutralized), indicating the neutralization ability of antibody against SARS‐CoV‐2. Titer values of ≥30 were considered positive (threshold designed by manufacturer).
2.5. Conventional virus neutralization test
During cVNT, an inactivated SARS‐CoV‐2 virus solution (wild‐type) with a titer of 100 TCID was combined with serum diluted at various levels and incubated under appropriate conditions before inoculating to Vero‐E6 cells. Cell cultures were examined to see whether antibodies could suppress viral cytopathic effect (CPE). The infectivity of the virus reveals the capacity of serum antibodies to neutralize the virus.
The cVNT experiments were carried out at Guangdong Center for Disease Control and Prevention (CDC). Serum samples were deactivated under 56°C for 30 min before being added to 96‐well plates. The samples were each diluted in the following ratios: 1:4, 1:16, 1:64, 1:256, and 1:1024. Solutions containing inactivated SARS‐CoV‐2 virus were diluted to 100 TCID50 per 50 μl before adding 120 μl into each well containing diluted serum. After thoroughly mixing, the solution was incubated at 36°C with 5% CO2 for 2 h and shook gently for 1.5 h. Primary cells, such as Vero‐E6 cells and human small airway epithelial cells (HPSAepiC), were well digested and grown into monolayer before being processed into cell suspension at a cell concentration of 1 × 104–2 × 104 cells/0.1 ml. Hundred milliliters of cell suspension was mixed with the serum‐virus solution and incubated at 36°C with 5% CO2. The results were observed every day, and CPE in each well was recorded on days 5–8 when the 100 TCID50 antigen control showed complete lesions. The reciprocal of the maximum serum dilution that could protect 50% cell wells from CPE was the neutralizing antibody titer of SARS‐CoV‐2 of the serum.
2.6. Statistical analysis
Geometric mean titer (GMT) of antibodies were calculated using the geometric mean of the titer levels, and their 95% confidence interval (CI) was calculated with the Student's t distribution on log‐transformed data and then back transformed. Participants were stratified according to age and sex. Comparison between titer‐level differences between two groups was performed using the Mann–Whitney Wilcoxon test. One‐way analysis of variance (one‐way ANOVA) was used to analyze the differences between mean values of different time points. Correlation analyses were performed using Pearson correlation analysis and the permutation test for Pearson's correlation coefficient. A value of p < .05 was considered to be significant. All calculations and figures were produced using MATLAB® R2021a (Natick, MA, USA).
3. RESULTS
The reported adverse reactions from the 479 recorded questionaries included muscle pain (injection site) 14/479 (2.9%), fatigue 4/479 (0.8%), muscle pain (noninjection site) 3/479 (0.6%), headache 2/479 (0.4%), dizziness 2/479 (0.4%), color change at injection site 2/479 (0.4%), nausea 1/479 (0.2%), and others 32/479 (6.7%). No serious adverse event was reported within 28 days postvaccination at all stages of injection.
Due to practical constraints during sample collection (fixed time of the week, working around weekends, national holidays, availability of personnel and participants, and varying coordination in the schedule amongst the three sites), there were modest variations in sampling time points. The sampling date (median, minimum, maximum) for sampling time point 1 (initial injection) was (0, 0, 0), for time point 2 (second injection) was (28, 20, 44), for time point 3 was (57, 41, 72), for time point 4 was (101, 56, 128), for time point 5 was (202, 119, 249), for time point 6 was (238, 224, 311), and for time point 7 was (266, 231, 290). The date of the booster dosage was (231, 200, 288). The number of dates separated from the injection of the booster dose for the two postbooster time points was (15, 7, 20) for time point 6 and (31, 28, 36) for time point 7.
The total positive rate and GMT of sVNT for each age group and time point are shown in Table 1. Additional results for SARS‐CoV‐2‐IgA/IgM/IgG antibody titer levels for each age group and time point are shown in Tables S1, S2 and S3. Figure 1 shows the specific antibody (SARS‐CoV‐2‐IgA/IgM/IgG) and sVNT titer levels at each monitoring point (with the two postbooster monitoring points combined). The results show that three antibodies and sVNT have distinct kinetics.
TABLE 1.
sVNT GMT results with 95% confidence intervals and positive rate by time point and age group
| Time point | Statistic | 18–30 y.o. | 31–50 y.o. | ≥51 y.o. | Total |
|---|---|---|---|---|---|
|
Month 0 (Dose I) |
n | 94 | 98 | 15 | 207 a |
| GMT | 0.18 | 0.39 | 0.36 | 0.28 | |
| 95% CI | 0.15–0.23 | 0.31–0.49 | 0.24–0.55 | 0.24–0.32 | |
| Positive rate | 1.06% | 6.12% | 0 | 3.38% | |
|
Month 1 (Dose II) |
n | 99 | 113 | 15 | 227 a |
| GMT | 2.04 | 1.53 | 1.65 | 1.74 | |
| 95% CI | 1.73–2.41 | 1.23–1.89 | 0.86–3.18 | 1.52–2.00 | |
| Positive rate | 61.62% | 39.82% | 53.33% | 50.22% | |
| Month 2 | n | 97 | 113 | 14 | 224 a |
| GMT | 7.67 | 6.57 | 3.89 | 6.80 | |
| 95% CI | 6.49–9.05 | 5.45–7.92 | 1.94–7.79 | 5.98–7.72 | |
| Positive rate | 91.75% | 91.15% | 78.57% | 90.63% | |
| Month 3 | n | 98 | 99 | 15 | 212 a |
| GMT | 3.87 | 3.26 | 2.24 | 3.44 | |
| 95% CI | 3.24–4.63 | 2.66–4.00 | 1.01–4.98 | 2.99–3.95 | |
| Positive rate | 80.61% | 67.68% | 66.67% | 73.58% | |
| Month 6 | n | 173 | 162 | 18 | 353 |
| GMT | 2.28 | 2.39 | 1.84 | 2.31 | |
| 95% CI | 1.95–2.67 | 1.99–2.89 | 1.27–2.68 | 2.05–2.59 | |
| Positive rate | 61.85% | 59.88% | 55.56% | 60.62% | |
| 0.5 Month postboost | n | 25 | 64 | 5 | 94 b |
| GMT | 35.85 | 27.52 | 24.34 | 29.33 | |
| 95% CI | 22.92–56.07 | 20.67–36.65 | 11.05–53.63 | 23.27–36.98 | |
| Positive rate | 100% | 96.88% | 100% | 97.87 | |
|
1 Month Postboost |
n | 9 | 33 | 5 | 47 b |
| GMT | 46.69 | 30.76 | 27.62 | 32.94 | |
| 95% CI | 23.37–93.29 | 21.74–43.52 | 7.03–108.48 | 24.22–44.81 | |
| Positive rate | 100% | 100% | 100% | 100% |
Due to budget limitations, tests were performed for randomly selected subgroups.
Not all participants received booster injections. Some participants were excluded from the postbooster time points monitoring if they receive a booster from other vaccines.
FIGURE 1.
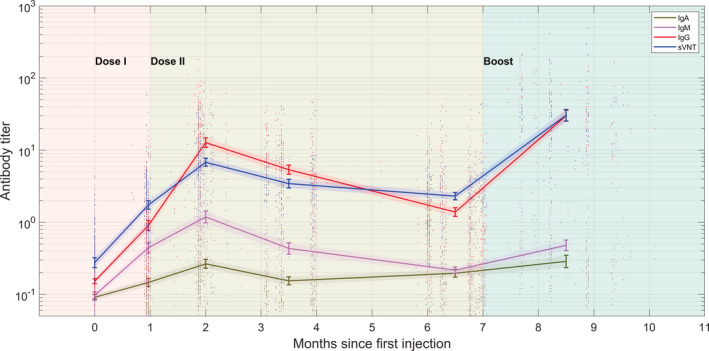
The antibody titer of IgA (dark green), IgM (magenta), IgG (red), and sVNT (blue). The curves connect the geometric mean end‐point titers (GMTs) at each monitoring point, with the two postbooster monitoring points combined. Shaded regions denote the 95% CI range of the GMT
Antibody titer‐level distributions amongst the tested population are shown in the violin plot in Figure 2. Throughout the course of vaccination, SARS‐CoV‐2‐IgA remained relatively constant at a low level compared with other antibodies (Figure 2A), with GMT remaining below the positive threshold for all stages of the vaccination. After the initial GMT peak of 0.26 S/CO (0.23–0.31), it diminished to 0.20 (0.17–0.22) at Month 6. The injection of a booster dose stimulated it back up to 0.32 (0.25–0.40) at Month 0.5 postbooster, a 1.6‐fold increase from the lowest point, but then, it rapidly diminishes back down to 0.24 (0.16–0.34) at Month 1 postbooster. This difference in titer level between the two postbooster points is significant (p = .042).
FIGURE 2.
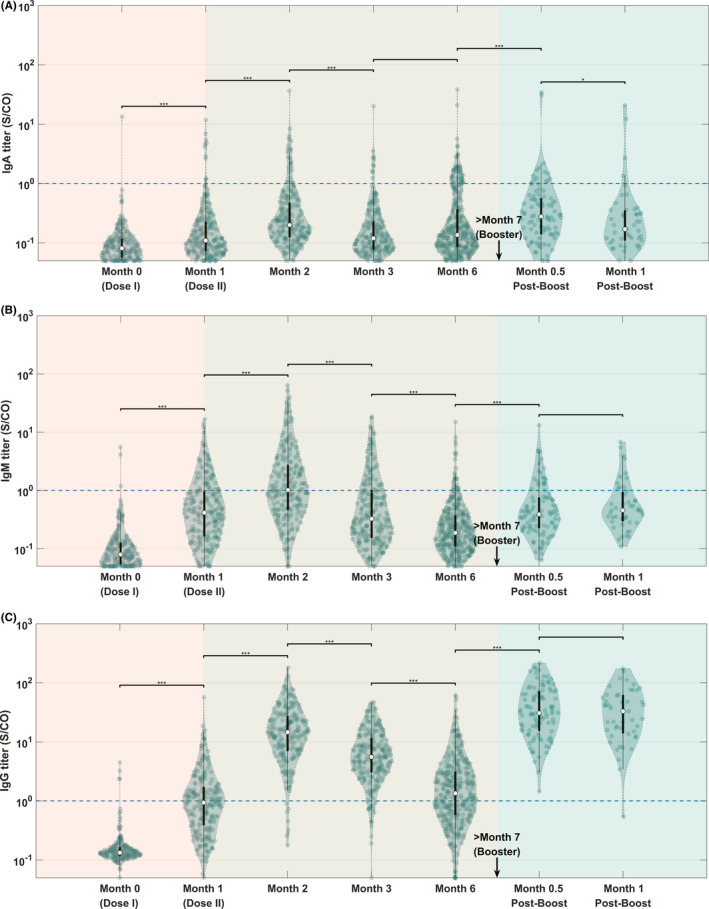
Violin plot showing distributions of antibody titer at each monitoring point. (A) IgA, P ANOVA = 0.008. (B) IgM, P ANOVA < 0.001. (C) IgG, P ANOVA < 0.001. The black lines in the center of each violin show the median (circle), and the 25th and 75th percentiles. The blue dashed line shows the manufacturer‐defined cutoff for positive results. Significance star for the Mann–Whitney Wilcoxon test: * represents p ≤ .05, ** represents p ≤ .01, *** represents p ≤ .001
At Month 2, IgM, IgG, and neutralizing antibodies (sVNT) reached the first peak level (Figures 2B,C and 3C), after which point, they slowly diminished over time, until a booster injection activated the anamnestic immunity, which is consistent with the conventional understanding of antibody kinetics following vaccination. In particular, for SARS‐CoV‐2‐IgM (Figure 2B), after the initial GMT peak of 1.19 S/CO (0.99–1.43), it diminished to 0.22 (0.20–0.24) at Month 6. With the injection of a booster dose, the titer level increased to 0.48 (0.40–0.57, two postbooster time points combined). No significant difference was observed in titer levels between Month 0.5 postbooster and Month 1 postbooster (p = .22). Interestingly, the postbooster GMT was lower than the peak at Month 2 and was consistent with the level at Month 1 after the initial injection.
FIGURE 3.

Virus neutralization test results at different time points. (A) Conventional virus neutralization test, P ANOVA < 0.001. (B) Pseudovirus neutralization test, P ANOVA < 0.001. (C) Surrogate neutralization test, P ANOVA < 0.001. Horizontal bars show GMT level for each time point. The blue dashed line shows the manufacturer‐defined cutoff for positive results. Significance star for the Mann–Whitney Wilcoxon test: * represents p ≤ .05, ** represents p ≤ .01, *** represents p ≤ .001
Figure 2C, on the other hand, shows that the SARS‐CoV‐2‐IgG GMT level peaked at 12.7 S/CO (10.9–14.9), before gradually decreasing to 1.4 (1.2–1.6) at Month 6, representing 10.9% of the peak value. After a booster dose, the SARS‐CoV‐2‐IgG GMT level increased by a factor of 21.4 to 30.0 (25.2–35.8, two postbooster time points combined) postbooster, up from the level at Month 6. No significant difference was observed in titer levels between Month 0.5 postbooster and Month 1 postbooster (p = .59).
Figure 3 depicts the results of three neutralization tests (cVNT, pVNT, and sVNT). After the first two injections, a similar and increasing trend of neutralization titer was observed. Figure 3C shows that the GMT of sVNT peaked at 6.8 AU/ml (95% CI, 6.0–7.7), and at Month 6, it dropped to 2.3 (2.1–2.6), representing 33.9% of the peak value, with the total positive rate dropping from 90.6% to 60.6%. After a booster injection, the GMT increased to 30.5 (22.1–42.0, two postbooster time points combined), a factor of 13.2 increase from Month 6, or 4.5 times the first peak. There was no significant difference in titer levels between Month 0.5 postbooster and Month 1 postbooster (p = .39).
Finally, levels of antibodies grouped by age (i.e., ≤40 y.o. and >40 y.o.) at each monitoring point are presented in Figure 4. Similarly, levels of antibodies grouped by gender at each monitoring point are presented in Figure 5. The results showed no statistically significant differences in antibody titer levels between different age groups and genders. Figure 6 shows that the measured SARS‐CoV‐2‐IgG correlates with sVNT titer levels (r = .647, p = 3 * 10−162).
FIGURE 4.

The antibody titer time profile for the two age groups (≤40 and >40 years old). The curves connect the geometric mean end‐point titers (GMTs) at each monitoring point, with the two postbooster monitoring points combined. Shaded regions denote the 95% CI range of the GMT
FIGURE 5.

The antibody titer time profile for the two genders. The curves connect the geometric mean end‐point titers (GMTs) at each monitoring point, with the two postbooster monitoring points combined. Shaded regions denote the 95% CI range of the GMT
FIGURE 6.
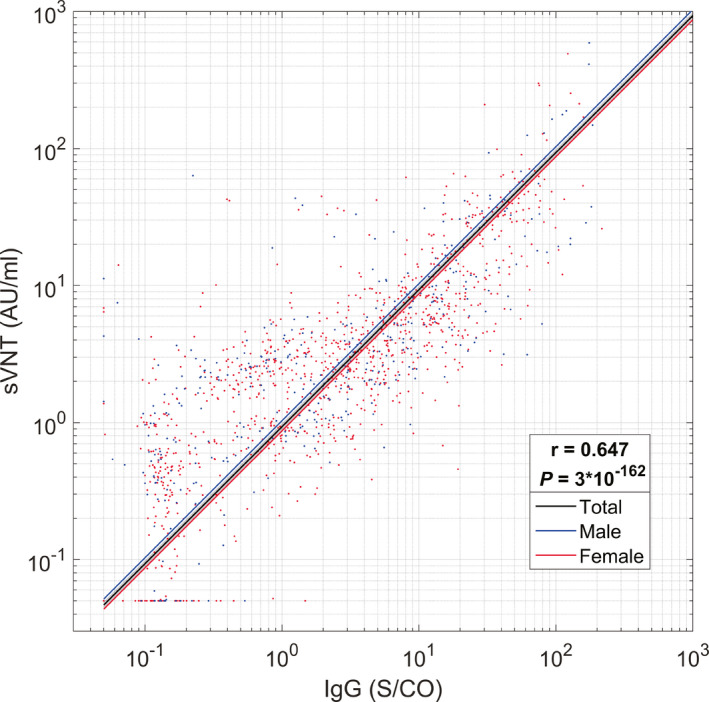
SARS‐CoV‐2‐IgG vs. surrogate virus neutralization test results. A high correlation could be observed for both male population and female population. r: Pearson correlation coefficient. p: p‐value of permutation test for Pearson's correlation coefficient. Lines represent 1‐degree polynomial (linear) fits of the results
4. DISCUSSION
The most effective regimen for COVID‐19 vaccination is still unknown as data continue to evolve following the waning of immunity over time and the emergence of new variants. 9 Israel's study on the real‐world protection of the boosters of vaccination showed that the third dose of BNT162b2 mRNA vaccination significantly reduced the COVID‐19 infection rate, severe illness rate, and mortality rate of vaccinators. 10 COVID‐19 vaccine boosting might ultimately be required in the general population due to diminishing immunity or the emergence of new variants. 11 However, the data are scarce on the antibody level and protective efficacy of inactivated vaccines after booster injection.
Currently, the Sinopharm/BBIBP COVID‐19 vaccine has been certified by the World Health Organization for emergency use, 12 and it is approved for use in more than 45 countries worldwide. 13 Interim results from phase 3 trials of Sinopharm vaccines indicated 78.1% efficacy in preventing COVID‐19. 12 Despite being one of the most widely used vaccines in the world, the vaccine's long‐term effectiveness is unknown. Several key questions remain unanswered: how durable is the immunity induced by the inactivated vaccine Sinopharm/BBIBP, and do people need a booster dose? To what extent does the booster dose elicit an anamnestic antibody response? How do different specific antibodies responsible for the protection of the body against viral invasion?
Through monitoring the humoral responses induced throughout the course of vaccination, such as the virus‐specific antibodies (SARS‐CoV‐2‐IgA/IgM/IgG), and performing virus neutralization tests, we demonstrated the durability of the vaccine‐induced immunity and the decline in neutralizing titer over time. The effect of booster dose was also demonstrated, as a strong anamnestic antibody response was recorded. The SARS‐CoV‐2‐IgG was found to be primarily responsible for the neutralizing titer, indicating its critical role in protecting the human body against virus invasion. Interestingly, SARS‐CoV‐2‐IgM behaved much unlike the other two types of antibodies, as a booster injection only eliciting a titer similar to the first injection, indicating characteristics consistent with those seen in the absence of anamnestic immunity.
After 6 months from the initial injection, the antibody titers, and neutralizing titers, have shown a decline. This would imply a decrease in vaccine protection. 14 This finding is consistent with a recent report on the waning of BNT162b2 vaccine protection against infection in Qatar, where the effectiveness of the vaccine decays rapidly after injection, but protection against severe COVID‐19 cases remains high after 6 months. 15 In the case of this study, the actual efficacy of the vaccine remains unclear, as the threshold of protection for antibody titers against COVID‐19 remains unknown. Another study discovered that the occurrence of COVID‐19 breakthrough infections is correlated with the time from injecting the BNT162b2 vaccine. 16 Together, all the evidence points to a decrease in the long‐term protection of vaccines. However, in addition to inducing neutralizing antibodies, another aspect of the protective power of vaccines is dependent on the formation of immune memory. The findings of this study indicate that immunity against SARS‐COV‐2 induced by the third dose of inactivated vaccine helps to provide better immune protection and enhance neutralization titer.
Cao et al. evaluated the humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine, the results showed that the third dose of either CoronaVac or ZF2001 vaccine rapidly induced a significantly high degree of humoral immunogenicity. 17 The recently published clinical trials also showed that the third dose of CoronaVac in adults administered 8 months after a second dose effectively resulted in a remarkable increase in the concentration of antibodies. 18 Both studies have shown that booster vaccination can significantly increase antibody levels and is safe. Different from these researches, we not only tested the level of neutralizing antibodies after the booster injection of the inactivated vaccine, but also we further explained the role of booster injections from different types of antibody levels and virus neutralization tests. This lays the groundwork for the clinical application of the inactivated vaccine booster.
According to a study on the Moderna mRNA‐1273 vaccine, antibody activity remained high in all age groups at 180 days after the second dose, with antibodies detected among all participants. 19 For BNT162b2 (Pfizer–BioNTech) and ChAdOx1 nCoV‐19 (Oxford–AstraZeneca), 21–41 days and 70 days or longer after the second dose, RBD‐antibody levels declined about twofold and fivefold, respectively. 20 This trend remained consistent when the results were stratified by gender, age, and clinical vulnerability. However, it is difficult to directly compare these estimates with the results of this study due to the heterogeneity of the antibody neutralization analysis.
Our study has several limitations. First, we did not evaluate B‐ and T‐cell responses after vaccination. Second, we did not include people with a higher risk of infection, more severe prognosis, or other comorbidities. Finally, more real‐world studies based on large‐scale outbreaks, and data from preclinical trials, are needed in future to comprehensively assess the immune persistence of inactivated vaccines and determine neutralizing antibody thresholds associated with preventive clinical outcomes.
5. CONCLUSION
We found that the inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV, is safe and well‐tolerated in healthy adults. Humoral responses were induced after the first two injections of the vaccine and peaked at 2 months following the initial injection before gradually declining over time. A booster dose considerably elicited the anamnestic immunity, as the neutralizing titer reaches 13.2 folds a month after the booster immunization.
CONFLICT OF INTEREST
None to report.
AUTHOR CONTRIBUTIONS
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
ROLE OF THE FUNDING SOURCE
The funders of the study took no role in data collection, analysis, interpretation, or writing of the article.
Supporting information
Tab S1
Tab S2
Tab S3
Acknowledgments
The authors would like to acknowledge funding supports by Zhongnanshan Medical Foundation of Guangdong Province (ZNSA‐2021005, ZNSA‐2020001, ZNSA‐2021016), Guangzhou Institute of Respiratory Health Open Project (Funds provided by China Evergrande Group) (2020GIRHHMS04), State Key Laboratory of Respiratory Disease, Guangdong‐Hong Kong‐Macao Joint Laboratory of Respiratory Infectious Disease (GHMJLRID‐Z‐202102), Emergency key project of Guangzhou Laboratory (EKPG21‐30‐2), and Cultivation Project of the First Affiliated Hospital of Guangzhou Medical University (ZH202105).
Cheng ZJ, Huang H, Zheng P, et al. Humoral immune response of BBIBP COVID‐19 vaccination before and after the booster immunization. Allergy. 2022;00:1–11. doi: 10.1111/all.15271
Zhangkai J. Cheng, Huimin Huang, Peiyan Zheng and Mingshan Xue contributed equally.
Funding information
This study was supported by Zhongnanshan Medical Foundation of Guangdong Province (ZNSA‐2021005, ZNSA‐2020001, ZNSA‐2021016), Guangzhou Institute of Respiratory Health Open Project (Funds provided by China Evergrande Group) (2020GIRHHMS04), State Key Laboratory of Respiratory Disease, Guangdong‐Hong Kong‐Macao Joint Laboratory of Respiratory Infectious Disease (GHMJLRID‐Z‐202102), Emergency key project of Guangzhou Laboratory (EKPG21‐30‐2), and Cultivation Project of the First Affiliated Hospital of Guangzhou Medical University (ZH202105).
Contributor Information
Siping Li, Email: lsp020@163.com.
Hongman Wang, Email: 2496453591@qq.com.
Baoqing Sun, Email: sunbaoqing@vip.163.com.
REFERENCES
- 1. Cheng ZJ, Shan J. 2019 novel coronavirus: where we are and what we know. Infection. 2020;48(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586(7830):516‐527. [DOI] [PubMed] [Google Scholar]
- 3. Cheng ZJ, Xue M, Zheng P, et al. Factors affecting the antibody immunogenicity of vaccines against SARS‐CoV‐2: a focused review. Vaccines (Basel). 2021;9(8):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng ZJ, Zhan Z, Xue M, et al. Public health measures and the control of COVID‐19 in China. Clin Rev Allergy Immunol. 2021:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Zhang Y, Huang B, et al. Development of an inactivated vaccine candidate, BBIBP‐CorV, with potent protection against SARS‐CoV‐2. Cell. 2020;182(3):713‐721 e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kherabi Y, Fiolet T, Rozencwajg S, Salaün J‐P, Peiffer‐Smadja N. COVID‐19 vaccine boosters: what do we know so far? Anaesth Crit Care Pain Med. 2021;40(6):100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection against Covid‐19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385(26):2421‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID‐19 vaccine immune responses. Lancet. 2021;398(10308):1377‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO validates Sinopharm COVID‐19 vaccine for emergency use and issues interim policy recommendations. 2021; Accessed June 01, 2021. https://www.who.int/news/item/07‐05‐2021‐who‐lists‐additional‐covid‐19‐vaccine‐for‐emergency‐use‐and‐issues‐interim‐policy‐recommendations
- 13. WHO Evidence assessment: Sinopharm/BBIBP COVID‐19 vaccine. 2021; Accessed June 01, 2021. https://cdn.who.int/media/docs/default‐source/immunization/sage/2021/april/2_sage29apr2021_critical‐evidence_sinopharm.pdf.
- 14. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 15. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS‐CoV‐2 infection in Qatar. N Engl J Med. 2021;385(24):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS‐CoV‐2‐breakthrough infections to time‐from‐vaccine. Nat Commun. 2021;12:6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Y, Hao X, Wang XI, et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res. 2021;32:107‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng G, Qianhui WU, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two‐dose schedule, in healthy adults: interim results from two single‐centre, double‐blind, randomised, placebo‐controlled phase 2 clinical trials. Lancet Infect Dis. 2021. doi: 10.1016/S1473-3099(21)00681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doria‐Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA‐1273 vaccine for Covid‐19. N Engl J Med. 2021;384(23):2259‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike‐antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Tab S2
Tab S3


