CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
To the Editor,
Fatal coronavirus disease 2019 (COVID‐19) features inflammatory changes affecting the whole lung with profound parenchymal destruction. Severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) RNA is enriched in mononuclear phagocytes, endothelial cells, B lymphocytes, and plasma cells, and it remains unclear which cells and soluble mediators determine respiratory failure. Patients with chronic obstructive pulmonary disease (COPD) and lung fibrosis are thought to be at higher risk for unfavorable outcomes, but patients with asthma who have hyperplasia of muscularis mucosae mast cells 1 have not presented with asthma exacerbations during SARS‐CoV‐2 infection and do not have increased mortality due to lung damage. 2 Mast cells from the bone marrow do not express the angiotensin‐converting enzyme 2 (ACE‐2) receptor, and patients with mast cell activation disorders such as systemic mastocytosis do not show mast cell activation during COVID‐19. 3 Because mast cells can have a prolonged tissue impact through the release of potent inflammatory mediators, we wanted to investigate the role of mast cells in fatal COVID‐19 lung manifestation.
We evaluated lung tissue from 29 patients who died of COVID‐19 (detailed methods in Appendix S1). Mast cells were identified by KIT (CD117), tryptase, and chymase immunohistochemistry in lung tissue sections with diffuse alveolar damage (DAD). RNA Scope was used to co‐localize SARS‐CoV‐2 and KIT expressing mast cells, double staining with immunohistochemistry for KIT and ISH for TNF alpha and IL‐6 (Figure S2) and mass‐spectrometry proteomics to identify mast cell proteases. Twenty‐six cases with normal lung (NL), non‐COVID‐DAD (NCOVID‐19DAD), interstitial lung disease (ILD), organizing pneumonia (OP), and influenza served as controls.
Basic clinic‐pathological data are summarized in the Table S1. At autopsy, lesions of acute (occurrence of hyaline membranes) and fibrotic (lung destruction by fibroblast proliferation) DAD in different stages in both lungs were identified with widespread alterations of the lung parenchyma causing death as reported previously. 4
All mast cells (MC) in COVID‐19 lung autopsies were positive for tryptase and chymase and were defined as MCtc, indicating a connective tissue phenotype (Figure 1) in contrast to the physiologic situation. 5
FIGURE 1.
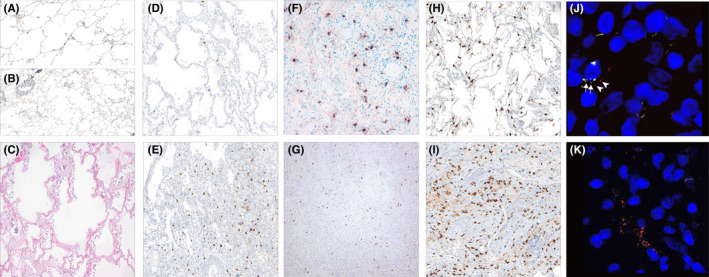
Number of mast cells in lung tissue in COVID‐19 and other conditions. (A) (100×, CD117) NL tissue, (B) higher numbers of MC in perivascular and peribronchiolar areas (40×, CD117). (C) (H.E., 40×): In very early DAD with hyaline membranes but lack of inflammatory infiltrate; (D) (100×, CD117): decrease of the number of MC in acute DAD; (E) (100×) high numbers in the organizing DAD. (F) (Tryptase, 100×) and (G) (chymase 40×) showing same number of MC as CD117 in organizing DAD. (H) (CD117, 100×): comparison with influenza; (I) (CD117, 100×): UIP. (J) (200×): RNA Scope with SARS‐CoV‐2 (red) ‐ signals) adjacent to CD117 (green) signals; (K) (100×): RNA Scope with red signals in SARS‐CoV‐2
Mast cells were counted in low‐ and high‐density areas (Figure S1). While other forms of non‐COVID‐19‐related lung injury showed a 3–5‐fold increase in mast cell numbers between low‐ and high‐density areas, acute COVID‐19 DAD showed a <2‐fold increase (Figure 2), indicating a suppression of mast cell proliferation which might be caused by high interferon levels in the early phase of COVID‐19. In contrast, organizing DAD in COVID‐19 lungs showed a 3‐fold increase in mast cells between low‐ and high‐density areas. In general, the mast cell density varied considerably between COVID‐19 lungs and the controls, as shown in Figure 1B. High density of mast cells has been associated with increased activation and release of proteases such as tryptase and chymase, which were abundant in COVID‐19 lung damage on the proteome level (Figure 2). Double labeling using RNA in situ hybridization (KIT and SARS‐CoV‐2) did not identify mast cell infection in a relevant number. Although we had shown previously an inverse correlation between viral load and DAD‐stage, 6 no association with viral load and mast cells were found (data not shown). Activation of mast cells during SARS‐CoV‐2 infection may occur without viral entry through soluble factors released by T cells and other neighboring cells through KIT/SCF (Stem Cell Factor) and other interactions in the later phase of COVID‐19‐pneumonia. Thus, mast cell proliferation and activation might be one mechanism that paves the way for the later stage of severe lung damage. This study provides the first preliminary evidence that mast cells are part of the inflammatory cellular changes in severe and lethal SARS‐CoV‐2 infection.
FIGURE 2.
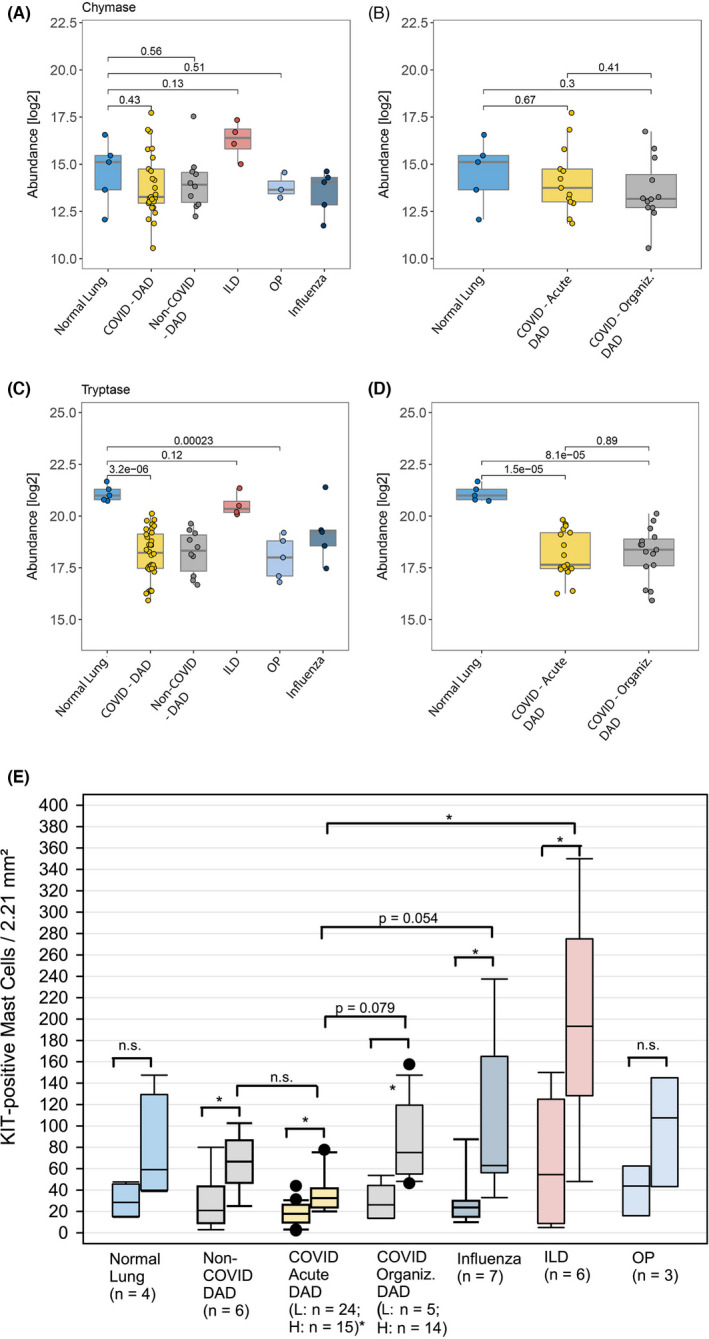
Tryptase and chymase protein expression (abundance) in COVID‐19 lungs compared with other conditions: (A and B) Chymase protein expression compared in NL, DAD and other pneumonias; (B) with division of acute and organizing phase of DAD; (C and D) Tryptase expression; (E) Mean MC counts stratified by different conditions and diseases. The left box plots represent the values from low and the right plots the high‐density areas. N.s., non‐significant; L, low; H, high; p‐values (two‐sided, unpaired t‐test) indicate the significance of a differential abundance (in A–D)
Supporting information
App S1
Fig S1
Fig S2
Tab S1
ACKNOWLEDGEMENTS
We would like to thank for the technical support by Alexandra Martin, Jenny Müller, Juliane Torpier, Christian Beul, Stefanie Weber, and Eva Sipos (for establishing the RNA‐ISH) of the Institute of General Pathology and Molecular Diagnostics, Medical Faculty, University of Augsburg, Augsburg, Germany. Further, we thank Mei Zheng of the Immunohistochemistry Laboratory in the Department of Pathology of the Brigham and Women’s Hospital, Boston, MA, USA. The authors are particularly thankful to all relatives who gave their consent for the postmortem examination and thus made an invaluable contribution to the research of this new disease.
REFERENCES
- 1. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast‐cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699‐1705. [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Cao Y, Du T, Zhi Y. Prevalence of comorbid asthma and related outcomes in COVID‐19: a systematic review and meta‐analysis. J Allergy Clin Immunol Pract. 2021;9(2):693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giannetti MP, Weller E, Alvarez‐Twose I, et al. COVID‐19 infection in patients with mast cell disorders including mastocytosis does not impact mast cell activation symptoms. J Allergy Clin Immunol Pract. 2021;9(5):2083‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID‐19. JAMA. 2020;323(24):2518‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren Y, Lyu Y, Mereness JA, Wang S, Pang J, Mariani TJ. Rare pulmonary connective tissue type mast cells regulate lung endothelial cell angiogenesis. Am J Pathol. 2020;190(8):1763‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirschbühl K, Dintner S, Schaller T, et al. Viral mapping in COVID‐19 deceased in the Augsburg autopsy series of the first wave: a multiorgan and multimethodological approach. Plosone. 2021;16(7):e0254872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
Fig S1
Fig S2
Tab S1


