Abstract
An electronic survey canvassing current policies of transplant centers regarding a COVID-19 vaccine mandate for transplant candidates and living donors was distributed to clinicians at US solid organ transplant centers performing transplants from October 14, 2021–November 15, 2021. Responses were received from staff at 141 unique transplant centers. These respondents represented 56.4% of US transplant centers, and responding centers performed 78.5% of kidney transplants and 82.4% of liver transplants in the year prior to survey administration. Only 35.7% of centers reported implementing a vaccine mandate, while 60.7% reported that vaccination was not required. A minority (42%) of responding centers with a vaccine mandate for transplant candidates also mandated vaccination for living organ donors. Centers with a vaccine mandate most frequently cited clinical evidence supporting the efficacy of pre-transplant vaccination (82%) and stewardship obligations to ensure organs were transplanted into the lowest risk patients (64%). Centers without a vaccine mandate cited a variety of reasons including administrative, equity, and legal considerations for their decision. Transplant centers in the United States exhibit significant heterogeneity in COVID-19 vaccination mandate policies for transplant candidates. While all centers encourage vaccination, most centers have not mandated COVID-19 vaccination for candidates and living donors, citing administrative opposition, legal prohibitions, and concern about equity in access to transplants.
KEYWORDS: ethics, ethics and public policy, infection and infectious agents – viral, infectious disease, patient safety, SARS-CoV-2/COVID-19, solid organ transplantation, vaccine
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic profoundly impacted the care and health outcomes of transplant recipients, of patients on the waiting list for a transplant, as well as transplant center and organ procurement operations. Mohan et al. recently reported that 16% of all deaths among kidney transplant recipients and 11% of all deaths on the kidney transplant waiting list in 2020 were due to SARS-CoV-2 infection.1 An Italian survey reported a 32.7% mortality among liver transplant candidates during the public health emergency, increasing to 45% among those with a Model for End-Stage Liver Disease (MELD) score >15.2 It has been demonstrated that solid-organ transplant recipients on immunosuppression tend to be hyporesponsive to COVID-19 vaccines, particularly those taking antimetabolites.3 This observation has led to a general recommendation from the American Society of Transplantation (AST) and the American Society of Transplant Surgeons (ASTS) that candidates for solid-organ transplantation should receive two doses of the COVID-19 vaccine, and complete vaccination at least two weeks prior to transplantation.4 , 5
While recommendations for COVID-19 vaccination of all active transplant candidates are not generally disputed as a clinical matter, the issue of transplant centers adopting COVID-19 vaccine mandates as a requirement for active transplant candidacy has been the subject of public controversy.6 A recent review of the impact of vaccine hesitancy on solid organ transplantation illustrated both the conflict of moral appeals in considering a policy of vaccine mandates, as well as “downstream” decisions faced by centers considering a vaccine mandate.7 A prior survey by members of our team canvassing US transplant centers’ approaches to candidates with asymptomatic SARS-CoV-2 infection during Spring 2021 noted that only 7% of centers either inactivated listed candidates in the absence of COVID-19 vaccination or required a completed vaccine series before making candidates waitlist active, but notably, this survey was conducted soon after COVID-19 vaccines became available.8 The prior survey was focused on operational challenges during the pandemic and to date, there has not been a comprehensive query of solid organ transplant center practices and policies regarding COVID-19 vaccine mandates.
To better inform clinicians, policy makers, and the public about the prevalence, rationale, and scope of vaccine mandate policies instituted (or not) by solid organ transplant centers in the United States, we designed and conducted an electronic survey of clinical transplant staff. Our survey assessed the reasons cited by transplant providers for or against implementing a vaccine mandate. For centers with a mandate as a condition of active transplant candidacy, we queried whether or to what extent the mandate extended to medical contraindications to the vaccine (e.g., anaphylaxis), religious objections, pediatric candidates, support persons, co-habitants, and living donor candidates.
2. MATERIALS AND METHODS
2.1. Survey design
The survey instrument was developed by the study investigators (Table S1). The authors constructed several of the survey questions to address ethical concerns raised in a recent review.7 The final survey instrument asked for respondents’ center (for response rate computation and aggregation), role at the center, and vaccination policies for transplant candidates, caretakers, and co-habitants. For centers without a mandate, reasons for not instituting a mandate were examined using non-exclusive, non-ranked choices. For centers with a vaccine mandate, the scope of a mandate was assessed across several hypothetical scenarios. Centers with and without a mandate for active candidates were also queried regarding vaccination requirements for living donors. Informed consent was obtained through the online “cover page” of the survey, which the surveyed acknowledged by clicking a box at the bottom of the cover page. This survey study was approved by the Saint Louis University Institutional Review Board (IRB protocol #32292).
2.2. Survey administration
The target population was transplant providers at US transplant centers (n = 250), including nephrologists, surgeons, infectious disease specialists, hepatologists, cardiologists, pulmonologists, coordinators, administrators, and social workers. Potential participants were derived from the working group’s professional connections as well as solicitation through professional society listservs (e.g., AST Kidney-Pancreas Community of Practice [COP], Infectious Disease COP, Outstanding Questions in Transplantation [OQiT], and eNews). COP postings were approved by COP leadership, and the AST e-News posting was approved by the AST Education Committee. Data are analyzed from a distribution between October 14, 2021–November 15, 2021. The first page of the survey notes that the decision to proceed indicates consent to participate. Up to two reminders were provided for non-respondents.
2.3. Statistical analyses
Each transplant center was represented once in the primary analysis. Representative responses from centers with multiple respondents were selected using a hierarchical algorithm, prioritizing responses from transplant surgeons, transplant physicians, and other providers, similar to previous methods.9, 10, 11, 12, 13 For centers with more than one response from a respondent in the same role at the same transplant center, after the above two steps, we retained the earliest submitted survey (Figure S1). The volume of transplant practice represented by responding centers was computed by center-level linkages to Scientific Registry of Transplant Recipients (SRTR) data.
All responses from transplant providers were categorized by transplant center role and summarized in a secondary analysis. Responses to each survey question were reported with frequencies and percentages. To obtain percentages, we divided the number of center responses (i.e., row totals) by the total number of centers that responded to the question, such percentages reflect the proportions of respondents. For questions where participants were asked to “select all that apply,” the denominator for calculating percentages was the total number of participants responding to that question. For these questions, as one respondent could choose more than one option, column totals may exceed 100%. Respondents selecting “Other” were allowed to enter free-text responses. Analyses were performed using SAS version 9.4.
To assess whether there was a correlation between the presence or absence of a vaccine mandate with prevalent COVID-19 vaccination rates, we cross-referenced survey data with publicly available state-level data on point prevalent COVID-19 vaccination rates on October 27, 2021.14 To address the problem of dynamic changes in the rates of vaccination over time, we chose vaccination rates reported on a single date during the administration of the survey. We stratified US states into tertiles by percent of population vaccinated: Group 1 with a vaccination rate of 41%–50%; Group 2 with a vaccination rate of 51%–60%; Group 3 with a vaccination rate of 61%–71% (Table S2).
3. RESULTS
We received 298 survey responses from US solid organ transplant centers, of which 22.8% were from a center with only one survey respondent and 77.1% were from centers with more than one respondent. After limiting to unique center responses, 141 center responses were available for primary analyses (Figure S1). Respondents represented 56.4% of US transplant centers. Responding centers performed 78.5% of kidney transplants and 82.4% of liver transplants in the year prior to survey administration. Participants represented diverse geographies of practice across the country ( Table 1).
TABLE 1.
Survey participant characteristics
| Role in transplant center (n = 141) | n (%) |
|---|---|
| Surgeon | 51 (36.2) |
| Nephrologist | 26 (18.4) |
| Infectious Disease Physician | 17 (12.1) |
| Administrator | 16 (11.4) |
| Other | 15 (10.6) |
| Clinical Coordinator | 7 (5) |
| Hepatologist | 6 (4.3) |
| Cardiologist | 1 (0.7) |
| Pulmonologist | 1 (0.7) |
| Social Worker | 1 (0.7) |
| Which solid organ transplants are performed at your center? (check all that apply) (n = 141) | n (%) |
| Kidney | 136 (96.5) |
| Liver | 93 (66) |
| Pancreas | 88 (62.4) |
| Heart | 78 (55.3) |
| Lung | 55 (39) |
| Geographical Regiona (n = 141) | n (%) |
| Southeast | 24 (17) |
| Northeast | 23 (16.3) |
| Great Lakes | 22 (15.6) |
| Southwest | 19 (13.5) |
| North Midwest | 18 (12.8) |
| Mid Atlantic | 18 (12.8) |
| South Midwest | 11 (7.8) |
| Northwest | 6 (4.3) |
Geographical region defined by UNOS summary reports.30
3.1. Centers with no vaccine mandate
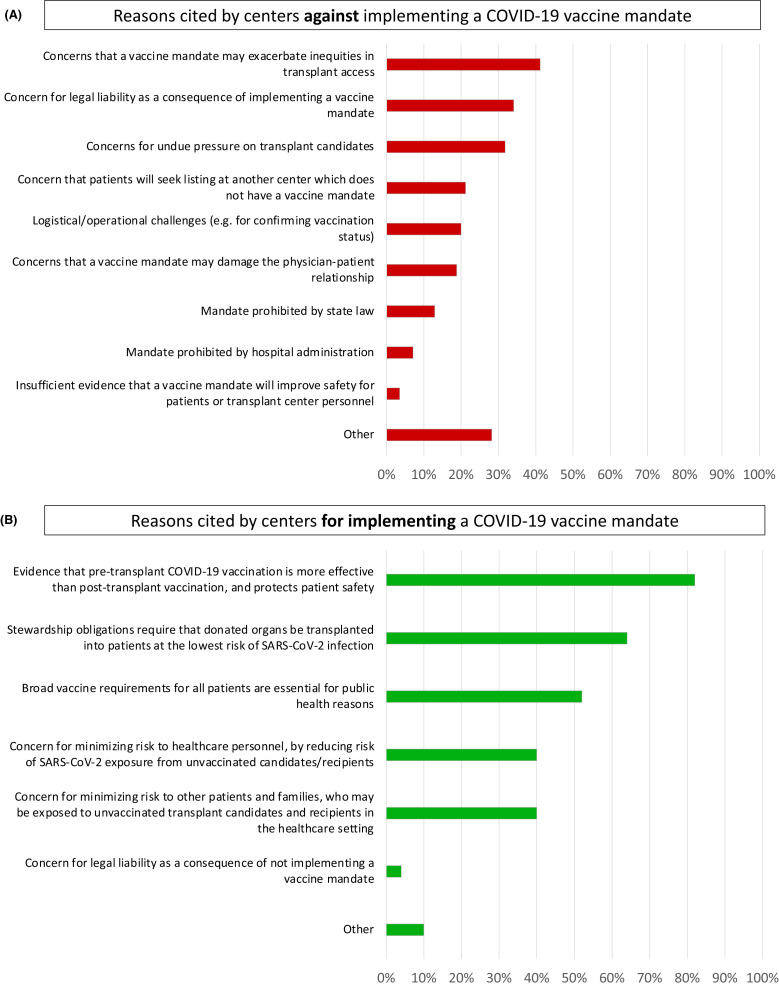
Among responding centers, 60.7% had not instituted a policy mandating vaccination for all patients as a requirement for active transplant listing ( Table 2). Among those centers that had not instituted a vaccine mandate policy, the most frequently cited reasons included: concerns that a mandate may exacerbate inequities in access to transplantation (41%), would result in undue pressure on transplant candidates (32%), and could result in legal consequences for the center (34%) ( Figure 1A). No center without a vaccine mandate for transplant candidates imposed a vaccine mandate for living donors.
TABLE 2.
Consideration and reported implementation of vaccine mandates at US transplant centers
| Has your center considered a COVID−19 vaccine mandate for any candidates for any solid-organ transplant? (n = 140) | n (%) |
| Yes | 123 (87.9) |
| No | 13 (9.3) |
| Unsure | 4 (2.9) |
| Has your center instituted a COVID−19 vaccine mandate for any candidates for any solid-organ transplant? (n = 140) | n (%) |
| Yes | 50 (35.7) |
| No | 85 (60.7) |
| Unsure | 5 (3.6) |
FIGURE 1.
Reasons cited by centers against (A) and for (B) implementing a vaccine mandate
3.2. Centers with a vaccine mandate
We found that 35.7% of centers had instituted a policy mandating vaccination as a requirement for active transplant candidacy (Table 2). Among those centers instituting a vaccine mandate policy, the most frequent justification included: public health-based obligations (52%), stewardship obligations to ensure organs are transplanted into patients at the lowest risk for SARS-CoV-2 infection (64%), and the need to reduce transmission risks to healthcare personnel (40%), other patients, and family members (40%) (Figure 1B). At the time of our survey, most centers with a vaccine mandate in place did not require booster doses for candidates. Nearly all centers (95%) with a mandate did not require clinical evidence demonstrating vaccine responsiveness (e.g., measurement of anti-SARS-CoV-2 spike protein antibodies). Few centers extended a vaccine requirement to a candidate’s support person (10%) or cohabitants (5%). In the case of religious objections to the vaccine, 45% of centers with a mandate reported they would decline these patients for active candidacy, while the remainder would either agree to an exemption (17%) or some version of a case-by-case consideration (38%) ( Table 3). Finally, only 13.6% of all responding centers and 42% of centers with a vaccine mandate for transplant candidates extended a vaccine requirement to living donors ( Table 4).
TABLE 3.
Scope of mandate requirements in centers implementing a vaccine mandate
| If your center has instituted a COVID−19 vaccine mandate AND a candidate has demonstrated an immediate allergic reaction to the vaccine or one of its essential components, what is your center’s practice regarding candidacy? (n = 42) | n (%) |
| Decline for active candidacy | 4 (9) |
| Exempt from the vaccine mandate | 26 (62) |
| Other | 12 (29) |
| If your center has instituted a COVID−19 vaccine mandate AND a candidate registers a religious objection to accepting the vaccine, what is your center’s practice regarding candidacy? (n = 42) | n (%) |
| Decline for active candidacy | 19 (45) |
| Exempt from the vaccine mandate | 7 (17) |
| Other | 16 (38) |
| Does your center’s COVID−19 vaccine mandate distinguish between the type of solid organ transplant? (n = 40) | n (%) |
| No: ALL actively listed solid organ transplant candidates at our center are subject to the vaccine mandate | 23 (57) |
| Yes: Only patients with non-life-threatening organ failure (e.g., renal failure requiring a kidney transplant) are subject to the vaccine mandate | 8 (20) |
| Yes: Only patients with life-threatening organ failure (e.g., decompensated cirrhosis, end-stage heart failure, end-stage lung disease) requiring a solid organ transplant are subject to the vaccine mandate | 0 (0) |
| Yes, other | 9 (22) |
| Does your center’s COVID−19 vaccine mandate distinguish between adult vs pediatric transplant candidates? (n = 41) | n (%) |
| No: The vaccine mandate applies equally to adult and all pediatric candidates (defined as age <18) | 4 (10) |
| No: The vaccine mandate applies to adult and pediatric candidates, but is limited to pediatric candidates for whom the COVID−19 vaccine is approved under EUA or full FDA approval | 5 (12) |
| Yes: The vaccine mandate applies to adult candidates, but pediatric candidates (defined as age<18) are excluded from the mandate | 11 (27) |
| N/A (Our center only performs transplants in adult candidates) | 16 (39) |
| Other | 5 (12) |
| If your center has instituted a COVID−19 vaccine mandate for (EUA-eligible) pediatric candidates, and the candidate’s parent and/or guardian refuses vaccination on behalf of the candidate, what is your center’s practice regarding candidacy? (n = 41) | n (%) |
| Decline active candidacy for transplants in all circumstances | 2 (5) |
| Waive the vaccine requirement in all circumstances | 2 (5) |
| Decline active candidacy if end-stage organ failure is life-threatening (e.g., decompensated cirrhosis) | 1 (2) |
| Decline active candidacy if end-stage organ failure is NOT life-threatening (e.g., kidney failure alone) | 5 (12) |
| N/A (Our center’s vaccine mandate does not include pediatric candidates OR our center does not transplant pediatric patients) | 27 (66) |
| Other | 4 (10) |
| If your center has instituted a COVID−19 vaccine mandate for adult or pediatric candidates, does your center require evidence of “vaccine responsiveness” as a condition of active candidacy? (n = 41) | n (%) |
| No: Documentation of COVID−19 vaccination is sufficient | 39 (95) |
| Yes: Vaccinated candidates must demonstrate spike-protein IgG titers above a designated threshold, or some other objectively defined measure of vaccine responsiveness | 1 (2) |
| Other | 1 (2) |
| If your center requires evidence of COVID−19 “vaccine responsiveness” as a condition of active candidacy, how does your center disposition a candidate who is “hypo-responsive”? (n = 39) | n (%) |
| N/A (documentation of COVID−19 vaccination alone is sufficient) | 38 (97) |
| Require serial vaccine (booster) doses in hypo-responsive candidates until sufficient responsiveness thresholds are achieved | 1 (3) |
| Other | 0 (0) |
| Does your center require solid organ transplant candidates to have a designated support person as a condition for receiving a transplant? (n = 40) | n (%) |
| Yes | 38 (95) |
| No | 1 (2) |
| Unsure | 1 (2) |
| If your center has instituted a COVID−19 vaccine mandate for adult or pediatric transplant candidates and ALSO requires candidates to have a designated support person, does your center also require the candidate’s support person to be vaccinated against COVID−19? (N = 40) | n (%) |
| Yes | 4 (10) |
| No | 30 (75) |
| N/A (Our center does not require a support person) | 3 (7) |
| Other (please specify) | 3 (7) |
| If your center has instituted a COVID−19 vaccine mandate for adult or pediatric transplant candidates, and your candidate regularly cohabitates with others (family or otherwise), does your center also require that the candidate’s cohabitants be vaccinated against COVID−19? (n = 40) | n (%) |
| Yes | 2 (5) |
| No | 36 (90) |
| Other (please specify) | 2 (5) |
| Does your center’s COVID−19 vaccine mandate distinguish candidates with a documented prior SARS-CoV−2 infection? (n = 40) | n (%) |
| No: The vaccine mandate applies to candidates regardless of prior SARS-CoV−2 infection status, and regardless of pre-vaccination spike-protein antibody titer or other markers or prior infection | 38 (95) |
| Yes: The vaccine mandate is not required for candidates with documented prior SARS-CoV−2 infection | 1 (2) |
| Yes: The vaccine mandate is not required for candidates with documented prior SARS-CoV−2 infection AND evidence of a threshold spike-protein antibody titer or another marker of prior infection | 1 (2) |
| Other | 0 (0) |
TABLE 4.
Center policies regarding vaccine mandates for living donor candidates
| Does your center require living kidney or liver donors to complete COVID−19 vaccination prior to donation surgery? (n = 125 all centers, n = 40 centers with mandate for transplant candidates) | Proportion of all centers, n (%) | Proportion of centers with Recipient Candidate Mandate, n (%) |
|---|---|---|
| YES – unvaccinated persons are not candidates for the donation of either organ | 17 (13.6) | 17 (42) |
| YES – unvaccinated persons are not candidates for liver donation but can donate a kidney | 0 (0) | 0 (0) |
| YES – unvaccinated persons are not candidates for kidney donation but can donate a liver segment | 2 (1.6) | 2 (5) |
| NO – Our center recommends COVID−19 vaccination but has no requirement for living donors | 98 (78.4) | N/A |
| Other (please specify) | 8 (6.4) |
Although limited by small sample size, the survey provides insights regarding the extension of vaccine mandates to pediatric transplant candidates. Among respondents with both a pediatric transplant center and a vaccine mandate for adults (n = 25), 44% of centers excluded pediatric candidates from the mandate. The survey was conducted before the FDA’s announcement of an Emergency Use Authorization for the COVID-19 vaccine for children aged 5–11, but it is unlikely that the timing of FDA’s announcement would have meaningfully changed the survey results.
COVID-19 vaccination rates have been shown to be correlated on the county level with political voting trends.15 We found no correlation between point prevalent rates of COVID-19 vaccination and whether a transplant center implemented a COVID-19 vaccine mandate. Rather, the rates of mandate versus no mandate were nearly identical across all tertiles (Table S2), although fear of legal consequences and damaging the doctor-patient relationship were cited more commonly in areas with low vaccine prevalence.
Some respondents included free-text responses to the survey questions where the respondents chose “Other.” The verbatim free-text responses by question are included in Table S3 and are grouped via thematic analysis in Table S4. Themes identified for centers without a vaccine mandate policy included a lack of institutional consensus for a mandate policy across all organs in a transplant center and ongoing planning or discussion of a future mandate policy.
4. DISCUSSION
The ethical concerns surrounding COVID-19 vaccine mandates for transplant candidates have been debated in the national press16 , 17 and in the peer-reviewed literature.7 , 18 To date, this debate has unfolded without information about the proportion and type of mandate policies implemented in transplant centers across the United States. To fill this gap, we conducted a comprehensive survey on center vaccination policies for candidates, living donors, and support persons. Our hope is that by understanding the overall prevalence of vaccine mandate policies (or lack thereof) among peer transplant centers, and the rationales cited for and against mandate policies, centers will be able to use this survey to guide their own deliberations in determining whether or not to institute a vaccine mandate.
Among the 35.7% of centers with a vaccine mandate, a large majority of respondents cited obligations to promote public health, to provide sound stewardship of transplantable organs, and to ensure the safety of healthcare professionals, other patients, and family members. Nearly half of responding centers with a vaccine mandate for candidates exempted patients from vaccination or considered exemptions on a case-by-case basis if a candidate raised a religious objection. Furthermore, despite evidence of hypo-responsiveness to COVID-19 vaccination in patients with end-stage kidney disease,3 , 19, 20, 21 very few centers with a vaccine mandate required additional testing to prove sufficient immune responsiveness to COVID-19 vaccination as a condition of active listing, few required vaccinations for frequent contacts (e.g., support persons and co-habitants) or living donors.
A significant majority of all respondents agreed that pretransplant vaccination was more effective than posttransplant vaccination (or no vaccination). Among the expressed justifications for not implementing a mandate, centers cited societal concerns (exacerbating existing inequities in access to transplantation22, 23, 24, 25, 26 and fear of patient coercion7) and perceived risk of legal liability.27, 28, 29 A high proportion of transplant centers (~88%) considered a vaccine mandate, suggesting that at the time of our survey the reasons against a mandate were convincing to a majority of respondent centers.
Despite widespread clinical consensus on the efficacy of COVID-19 vaccination to attenuate the risk of severe morbidity and death from SARS-CoV-2 infection in solid organ transplant recipients, our study confirms the challenges and complexity in deciding whether a transplant center should mandate COVID-19 vaccination for actively waitlisted candidates. Recent reviews of the ethical issues related to solid organ transplantation and vaccine hesitancy illustrate the multiple conflicts of competing obligations.7 , 18 To date, both the AST and the ASTS have issued position statements supporting a strong recommendation for administering COVID-19 vaccinations to transplant candidates, but neither professional society has articulated a position on centers implementing vaccine mandates.4 , 5 The proportion of respondents without a vaccine mandate citing “Other” and the accompanying free-text responses (Tables S3 and S4) suggest that discussions around instituting a mandate policy remain fluid in many transplant programs, with some programs without a mandate working through operational or administrative challenges prior to instituting a mandate.
This study has several limitations. The survey represents a snapshot in time of extant transplant center policies regarding a vaccine mandate. It is possible that the survey itself may have prompted some centers to reconsider their mandate policies, and it is probable that some centers have changed their mandate policies between the time the survey was conducted, and when this survey was published. A theme in the free text responses included ongoing plans to consider a mandate, supporting this possibility. While the survey returned responses from centers accounting for a high percentage of kidney and liver transplants in the United States, respondents may be over-represented by interested and engaged clinicians and may not generalize to centers that did not participate. Responses from individuals were assumed to be consonant with the positions of their transplant center which may not be true in all instances. While participants were assured of deidentification at the study’s outset, in some circumstances, the intense public interest in and contentiousness of the issue may have caused some respondents to adjust answers to avoid approbation. We did not ask respondents to rank-order their responses in order of importance, so the results do not illuminate which reasons for or against a vaccine mandate were weighted by individual center leaders. In some circumstances, sub-questions regarding vaccine mandates (e.g., pediatric versus adult, life-threatening versus life-saving transplant, vaccine hypo-responsiveness) may have raised questions not considered or not directly covered by a given center’s vaccine mandate policy. To that extent, answers provided to these questions may be a posteriori to a respondent seeking to clarify those points in their center’s mandate policy after taking the survey, or the respondents’ answers to these sub-questions may be more fulsome than the center’s mandate policy. The survey began before the FDA’s announcement of an Emergency Use Authorization for the COVID-19 vaccine for children ages 5–11, but it is unlikely that the timing of the FDA’s announcement would have meaningfully changed the survey results. We chose to focus on COVID-19 vaccine prevalence on a fixed date rather than primary SARS-CoV-2 infection prevalence in examining correlations with the presence or absence of a COVID-19 vaccine mandate. It is possible, despite widespread clinical consensus on the value of COVID-19 vaccination after recovery from primary SARS-CoV-2 infection, that higher SARS-CoV-2 infection rates may be associated with lower rates of COVID-19 vaccination, which in turn may confound our correlation.
In conclusion, at the time of our survey, transplant centers have taken a heterogeneous approach to vaccine mandate policies. Centers with a vaccine mandate did not typically extend vaccine requirements to candidates’ support persons or cohabitants, infrequently required additional testing to demonstrate vaccine efficacy, and less than half extended a vaccine requirement to candidates for living donation. Centers without a mandate cited a diverse array of reasons for justifying a no mandate policy. The absence of a center mandate was not predicated on a perceived lack of efficacy for pre-transplant COVID-19 vaccination in reducing and attenuating post-transplant SARS-CoV-2 infections. The presence or absence of a vaccine mandate was not correlated with point prevalent rates of COVID-19 vaccination by state, and consistent with that lack of correlation, centers without a mandate typically cited the risk of exacerbating inequities in access to transplant and concerns for patient coercion, rather than constraints by state law or hospital administrative leaders. There is some reason to suppose that at least some transplant centers without a mandate at the time of our survey are moving toward the implementation of a vaccine mandate. Professional transplant societies exploring consensus around COVID-19 vaccine guidelines will need to contend with extant heterogeneous practices and justifications for and against vaccine mandates evidenced by this survey, while transplant centers will need to weigh ethical, legal, and competitive concerns with an evolved consensus about best medical practices.
ACKNOWLEDGMENTS
The authors thank survey respondents, including members of the American Society of Transplantation (AST) Kidney Pancreas Community of Practice, Infectious Disease COP, and AST Outstanding Questions in Transplantation listservs. We also thank Saint Louis University Biostatisticians Ruixin Li, MS, and Huiling Xiao, MS, for assistance with manuscript preparation.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. B. Hippen: Employer: Fresenius Medical Care, Equity: InterWell Health; D. Axelrod: Consulting: Sanofi, CareDx, Talaris. Equity: CareDx; K. Maher: None; R. Li: None; D. Kumar: Consulting: Roche, GSK, Merck, Astellas, Exevir. Grant support: Roche, GSK; Y. Caliskan: None; T. Alhamad: Consulting and advisory board: CareDx, Veloxis, and Paladine. Speaker: Veloxis and Sanofi. Research grant support: CareDx, Angion, Natera, Europhines, Veloxis; M. Schnitzler: Consulting: CareDx, Optum; K. Lentine: Consulting fee: CareDx, Sanofi. Speaker: Sanofi. Equity: CareDx. Financial support from the Mid-America Transplant endowed chair in transplantation.
DATA AVAILABILITY STATEMENT
Data availability is limited to aggregate summaries as reported, based on IRB requirements.
Footnotes
Benjamin E. Hippen and David A. Axelrod are considered as co-first authors.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Figure S1
Table S1
Table S2
Table S3
Table S4
REFERENCES
- 1.Mohan SKK, Husain SA, Schold J. COVID-19-associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021;11(16):1695–1703. doi: 10.2215/CJN.02690221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belli LS, Duvoux C, Cortesi PA, et al. COVID-19 in liver transplant candidates: pretransplant and post-transplant outcomes - an ELITA/ELTR multicentre cohort study. Gut. 2021;70(10):1914–1924. doi: 10.1136/gutjnl-2021-324879. [DOI] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society of Transplantation (AST). Statement about Vaccine Efficacy in Organ Transplant Recipients. https://www.myast.org/sites/default/files/ast%20ishlt%20guidance%20vaccine%2008132021FINAL%20DRAFT2.pdf. Accessed November 18, 2021
- 5.American Society of Transplant Surgeons (ASTS). ASTS Position on COVID-19 Vaccine and Transplant Patients. https://asts.org/news-and-publications/asts-news/2021/08/25/asts-position-on-covid-19-vaccine-transplant-patients#.YZG-Zk7MJPZ. Accessed November 18, 2021.
- 6.NBCUniversal News Group. Organ Centers to Transplant Patients: Get a Covid Vaccine or Move down on Waitlist. https://www.nbcnews.com/health/health-news/organ-centers-transplant-patients-get-covid-vaccine-or-move-down-n1281084. Accessed November 21, 2021
- 7.Kates OS, Stohs EJ, Pergam SA, et al. The limits of refusal: an ethical review of solid organ transplantation and vaccine hesitancy. Am J Transplant. 2021;21(8):2637–2645. doi: 10.1111/ajt.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelrod DA, Ince D, Harhay MN, et al. Operational challenges in the COVID era: asymptomatic infections and vaccination timing. Clin Transplant. 2021;35(11):e14437. doi: 10.1111/ctr.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi MD, Singh N, Hippen BE, et al. Transplant clinician opinions on use of race in the estimation of glomerular filtration rate. Clin J Am Soc Nephrol. 2021;16(10):1552–1559. doi: 10.2215/CJN.05490421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentine KL, Motter JD, Henderson ML, et al. Care of international living kidney donor candidates in the United States: A survey of contemporary experience, practice, and challenges. Clin Transplant. 2020;34(11) doi: 10.1111/ctr.14064. doi: [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Peipert JD, Alhamad T, et al. Survey of clinician opinions on kidney transplantation from hepatitis C virus positive donors: identifying and overcoming barriers. Kidney360. 2020;1(11):1291–1299. doi: 10.34067/KID.0004592020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lentine KL, Vest LS, Schnitzler MA, et al. Survey of US living kidney donation and transplantation practices in the COVID-19 era. Kidney Int Rep. 2020;5(11):1894–1905. doi: 10.1016/j.ekir.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhamad T, Lubetzky M, Lentine KL, et al. Kidney recipients with allograft failure, transition of kidney care (KRAFT): a survey of contemporary practices of transplant providers. Am J Transplant. 2021;21(9):3034–3042. doi: 10.1111/ajt.16523. [DOI] [PubMed] [Google Scholar]
- 14.The New York Times. See How Vaccinations Are Going in Your County and State. https://www.nytimes.com/interactive/2020/us/covid-19-vaccine-doses.html. Accessed December 3, 2021
- 15.Kates J, Tolbert J & Orgera K. The Red/Blue Divide in COVID-19 Vaccination Rates. https://www.kff.org/policy-watch/the-red-blue-divide-in-covid-19-vaccination-rates/. Accessed November 29, 2021.
- 16.Aleccia J. More organ transplant centers require patients to get COVID-19 vaccine, or get bumped down waitlist. Kaiser Health News, October 8, 2021. https://khn.org/news/article/organ-centers-to-transplant-patients-get-a-covid-shot-or-move-down-on-waitlist/. Accessed January 9, 2022.
- 17.Knowles H & Anders C. Hospital system says it will deny trnasplants to the unvaccinated in ‘almost all situations.’ Washington Post, October 6, 2021. https://www.washingtonpost.com/health/2021/10/05/uchealth-transplant-unvaccinated/. Accessed January 9, 2022.
- 18.Hippen B. Mandating COVID-19 vaccination prior to kidney transplantation in the United States: no solutions, only decisions. Am J Transplant. 2022;22(2):381–385. doi: 10.1111/ajt.16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikizler TA, Coates PT, Rovin BH, Ronco P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021;99(6):1275–1279. doi: 10.1016/j.kint.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longlune N, Nogier MB, Miedouge M, et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704–1709. doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36(9):1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kricorian K, Turner K. COVID-19 vaccine acceptance and beliefs among black and hispanic Americans. PLoS One. 2021;16(8):e0256122. doi: 10.1371/journal.pone.0256122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni S, Ladin K, Haakinson D, Greene E, Li L, Deng Y. Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation System. JAMA Surg. 2019;154(7):618–625. doi: 10.1001/jamasurg.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purnell TS, Luo X, Cooper LA, et al. Association of race and ethnicity with live donor kidney transplantation in the United States From 1995 to 2014. JAMA. 2018;319(1):49–61. doi: 10.1001/jama.2017.19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Melanson TA, Plantinga LC, et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant. 2018;18(8):1936–1946. doi: 10.1111/ajt.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schold JD, Mohan S, Huml A, et al. Failure to advance access to kidney transplantation over two decades in the United States. J Am Soc Nephrol. 2021;32(4):913–926. doi: 10.1681/ASN.2020060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The National Law Review. Class Action Trends Report, Fall 2021: The Great Vaccination Dilemma. https://www.natlawreview.com/article/class-action-trends-report-fall-2021-great-vaccination-dilemma. Accessed November 21, 2021
- 28.Congressional Research Service. COVID-19 Liability: Tort, Workplace Safety, and Securities Law. https://crsreports.congress.gov/product/details?prodcode=R46540. Accessed November 23, 2021.
- 29.National Academy For State Health Policy. State Efforts to Ban or Enforce COVID-19 Vaccine Mandates and Passports. https://www.nashp.org/state-lawmakers-submit-bills-to-ban-employer-vaccine-mandates/. Accessed December 3, 2021.
- 30.United Network for Organ Sharing (UNOS). COVID-19 and solid organ transplant. https://unos.org/covid/. Accessed November 21, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Data availability is limited to aggregate summaries as reported, based on IRB requirements.