CONFLICT OF INTEREST
LOM is a consultant to PrecisionBiotics and has received research funding from GSK and Chiesi. LOM has participated in speaker's bureau for Nestle, Nutricia, Reckitt, and Abbott. WCA has participated in advisory boards for Pfizer, MSD, and Sanofi, with reimbursements paid to his institution. None of the other authors report any conflict of interest.
AUTHOR CONTRIBUTIONS
CS, WCA, UN, NL, MH, PWOT, and LOM contributed to study design and securing funding. CS, WCA, and MH were responsible for patient care, sample, and data collection. UN, NL, and LOM generated the experimental data and performed the data analysis. NL, PWOT, and LOM wrote the manuscript. All authors reviewed and approved the manuscript.
To the Editor,
Infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) not only causes acute coronavirus 2019 (COVID‐19) disease but also can lead to significant long‐term effects that impact daily functioning and quality of life, termed postacute sequelae of COVID‐19 (PASC). 1 The most frequently reported symptoms include fatigue, cognitive dysfunction, and memory issues, but multisystem involvement and significant disability are also common. 2 Despite the rapid accumulation of knowledge on the acute phase of COVID‐19, a limited number of studies are currently available that examine the pathophysiology of PASC, particularly in relation to fatigue. Mechanisms are thought to include the direct consequences of viral infection, severe systemic inflammation, oxidative stress, neuroinflammation, microvascular thrombosis, and neurodegeneration processes. We previously showed that levels of multiple cytokines, including TH2 cytokines such as interleukin (IL)‐4, macrophage‐derived chemokine (MDC), and thymic stromal lymphopoietin (TSLP), remained elevated for an extended period of time following SARS‐CoV‐2 infection. 3 In this study, our aim was to identify additional potential novel mechanisms of disease, by investigating changes in metabolism that associate with PASC.
Serum was obtained from PASC patients (n = 20) at two time points (4–6 months following infection (T1) and 6–9 months following infection (T2)). All patients had been hospitalized for PCR‐proven SARS‐CoV‐2 infection (median in‐patient stay of 6.5 days, range 2 days to 25 days) during the first wave of the pandemic in Ireland (March–May 2020). The most common symptoms at follow‐up clinics were fatigue and/or dyspnea. Demographic and clinical details for this cohort are summarized in Table S1. Sera from healthy controls (n = 20), collected prior to the pandemic, were assayed in parallel. There were no statistically significant differences in age, gender, or BMI between the PASC patients and healthy volunteers, while PASC patients were more likely to have pre‐existing conditions such as hypertension or a respiratory disorder (i.e., asthma or COPD), compared to healthy volunteers (Table S1). All patients and healthy volunteers signed a patient informed consent, and the study was approved by local ethics committees (The Clinical Research Ethics Committee of the Cork Teaching Hospitals for Cork University Hospital). Untargeted metabolomic analysis on patient and control sera was performed by metabolon using the HD4 platform (see Supplementary Methods for details). Pairwise differential abundance analysis was performed between conditions using R package LIMMA, and the Benjamini‐Hochberg correction (BH) was applied for each comparison.
Distinct differences in circulating metabolite levels were evident in PASC patients compared to healthy volunteers (Figure 1). Of the 1086 metabolites quantified, levels of 253 metabolites or 266 metabolites were significantly different between patients and controls at T1 or T2, respectively (Tables S2 and S3). The metabolic profile of PASC patients observed at 6–9 months following SARS‐CoV‐2 infection (T2) was similar to the profile observed at 4–6 months (T1) following infection (Figure 1). Length of time stored in the freezer did not influence metabolite levels quantified in healthy volunteers or patient sera (Figure S1). Levels of multiple metabolites with immunomodulatory properties were elevated in PASC patients (examples include the sphingolipid sphingosine 1‐phosphate (S1P) and the eicosanoid 12‐HETE, Figure 2). In addition, significant disruption of central energy, fatty acid, and carbon metabolism was evident (examples include elevated mannose, glutamate, and succinate serum levels with reduced levels of the ketone body acetoacetate in PASC patients, Figure 2).
FIGURE 1.
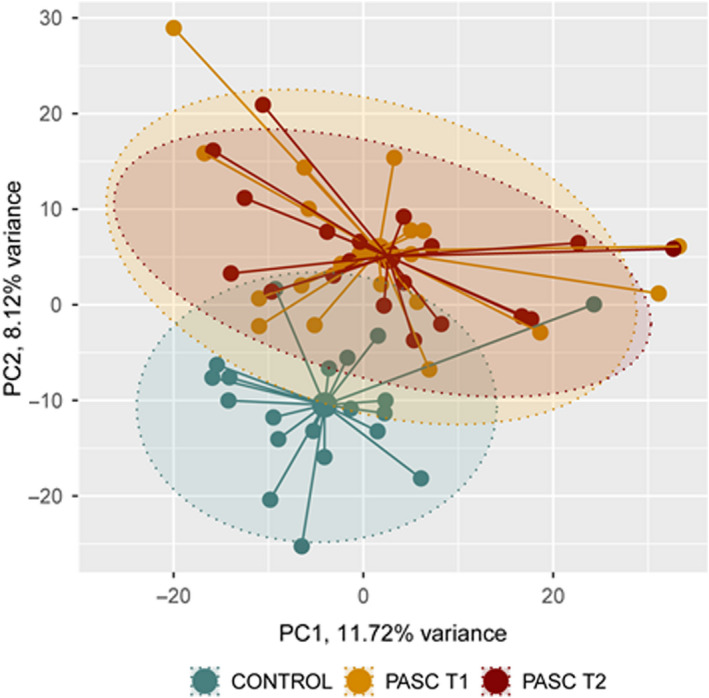
Principal component analysis of serum metabolite levels in healthy volunteers (Controls, n = 20) and PASC patients (n = 20) at 4–6 months (T1) and 6–9 months (T2) following hospital discharge
FIGURE 2.
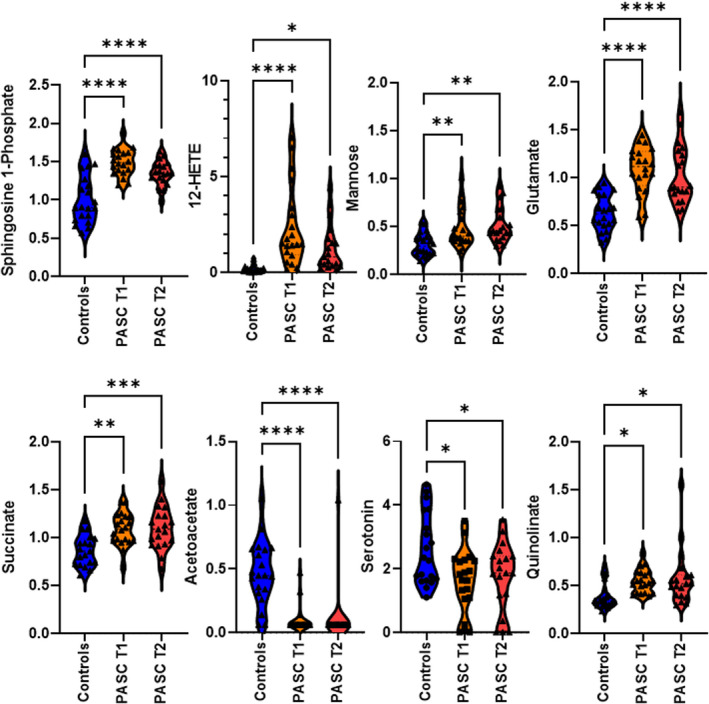
Violin plots illustrating the differences in specific serum metabolite levels in healthy volunteers (Controls, n = 20) and PASC patients (n = 20) at 4–6 months (T1) and 6–9 months (T2) following hospital discharge. Statistical significance was determined using one‐way ANOVA and Tukey’s multiple comparison test
Of particular interest was the dysregulated metabolism of tryptophan, characterized by a decrease in serotonin production coupled with the accumulation of quinolinate in PASC patients (Figure 2). We have previously shown that tryptophan metabolism was heavily disrupted in COVID‐19 patients during the acute phase of the infection, especially in those with the poorest outcomes. 4 Quinolinate is an excitotoxin that hinders neuronal function through multiple mechanisms including acting as an NMDA receptor agonist, and it indirectly drives accumulation of glutamate. In contrast, serotonin has essential modulatory effects on mood, anxiety, sleep, cognition, and memory. Recently, selective serotonin reuptake inhibitors (SSRIs) have been shown to have favorable results with respect to symptom resolution and hospitalizations in patients with COVID‐19. 5 , 6 Perhaps in situations where available serotonin levels are decreased (e.g., during and following SARS‐CoV‐2 infection), SSRIs may exert beneficial effects by helping to maintain adequate levels of serotonin signaling. The use of SSRIs in PASC patients should be further explored.
Our study contributes additional insights into underlying PASC mechanisms as we showed that infection‐induced metabolic reprogramming and compensatory responses were long‐lasting and did not substantially improve over the time course of the investigation (i.e., up to 9 months following the initial infection). These study findings identify novel mechanistic and diagnostic markers and potential therapeutic targets in PASC patients. These biomarkers should be included as integral components of future randomized controlled trials in order to better understand the pathobiology of SARS‐CoV‐2 infection and associated long‐term sequelae.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a Science Foundation Ireland research center grant 12/RC/2273_P2 and a Science Foundation Ireland project grant 20/COV/0158. Open access funding enabled and organized by IRel.
REFERENCES
- 1. World Health Organization . A clinical case definition of post COVID‐19 condition by a Delphi consensus. Published Online October 6, 2021. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Post_COVID‐19_condition‐Clinical_case_definition‐2021.1
- 2. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahearn‐Ford S, Lunjani N, McSharry B, et al. Long‐term disruption of cytokine signalling networks is evident in patients who required hospitalization for SARS‐CoV‐2 infection. Allergy. 2021;76(9):2910‐2913. doi: 10.1111/all.14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albrich WC, Ghosh TS, Ahearn‐Ford S, et al. A high‐risk gut microbiota configuration associates with hyperinflammatory immune and metabolic responses to acute SARS‐CoV‐2 infection. Preprint: https://biorxiv.org/cgi/content/short/2021.10.26.465865v1 [DOI] [PMC free article] [PubMed]
- 5. Reis G, dos Santos Moreira‐Silva EA, Medeiros‐Silva DC, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID‐19: The TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42‐e51. doi: 10.1016/S2214-109X(21)00448-4. (published online Oct 27.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oskotsky T, Maric I, Tang A, et al. Mortality risk among patients with COVID‐19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11):e2133090. doi: 10.1001/jamanetworkopen.2021.33090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


