Dear Editor,
A 41‐year‐old woman with a 12‐year history of systemic lupus erythematosus (SLE) was previously treated with prednisolone for fever, rash, Raynaud’s phenomenon, arthritis, and leukopenia. Since 2017, she had maintained a stable condition, including blood tests, and had been receiving hydroxychloroquine (200 mg/day). She experienced a slight facial erythema symptom in February 2021, which persisted. On 16 April, she received her first dose of the mRNA‐1273 coronavirus disease 2019 (COVID‐19) vaccine (Moderna) and developed fever and worsening erythema 2 weeks later. A butterfly rash (Figure 1a) was observed upon physical examination on 7 May, and the anti‐dsDNA titer was 4.1 IU/ml (normal, ≤12). Anti‐RNP and anti‐Sm were positive, but anti‐Ro/SSA and antiphospholipid antibodies were negative. The complement levels were as follows: CH50, 18.5 U/ml (normal, 30–46); C3, 39 mg/dl (normal, 73–138); and C4, 10 mg/dl (normal, 11–31). She was administered 0.3 mg/kg/day prednisone. Following her second vaccine dose on 28 May, she developed high fever, muscle pain, epistaxis, stomatitis, and facial and arm skin rash exacerbation. At 6 days after the second vaccination, she developed widespread facial erythema (Figure 1b) with digital ulcers and gangrene (Figure 1c). Additionally, she experienced chest pain and hair loss. Pleural effusion was observed on chest X‐ray, indicating pleurisy. Laboratory tests showed white blood cell and platelet counts of 1880/μl and 82 000/μl, respectively. The examination findings were as follows: serum level of creatine kinase, 1072 IU/L; aspartate aminotransferase, 708 IU/L; alanine aminotransferase, 282 IU/L; lactic acid dehydrogenase, 1724 IU/L; ferritin, 9609 ng/ml; triglycerides, 109 mg/dl; fibrinogen, 282 mg/dl (normal, 200–400); dsDNA titer, 5.2 IU/ml; CH50, 23.2 U/ml; C3, 46 mg/dl; and C4, 11 mg/dl. She was diagnosed with severe SLE exacerbation and was suspected of having hemophagocytic syndrome. However, no bone marrow examination was performed. She was hospitalized, and after receiving 1000 mg of methylprednisolone infusion for 3 days, prednisone was administered (1.0 mg/kg/day). Subsequently, her condition improved, and she was discharged after 2 weeks. At 1 week after discharge, her fingers were healed. Prednisolone was gradually reduced to 10 mg at 3 months after discharge, at which point no disease activity was observed, including hypocomplementemia. Hydroxychloroquine was the only concomitant drug used for SLE during this period.
FIGURE 1.
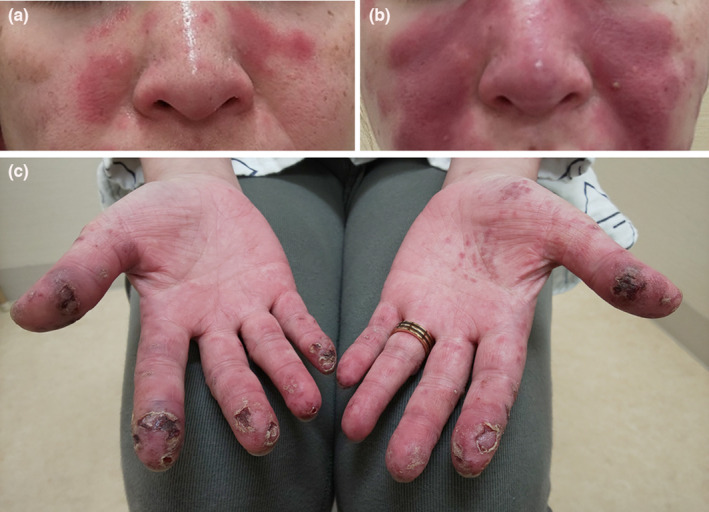
Picture of the patient’s face and hands. (a) The face at 3 weeks after the first dose of the mRNA‐1273 vaccine. (b) The face at 6 days after the second vaccine dose. (c) The hands at 6 days after the second vaccine dose
Externally injected mRNA induces type I interferon (IFN) production, especially IFN‐α and IFN‐β. 1 The IFN signature in patients with SLE is thought to involve many IFN types, but type I IFN is considered to be the most important. 2
To our knowledge, this is the first report of SLE exacerbation induced by the mRNA‐1273 vaccine. There were some cases where other COVID‐19‐related mRNA vaccines might have exacerbated skin symptoms in patients with cutaneous lupus erythematosus. 3 , 4 No patient, including ours, was receiving corticosteroids and immunosuppressants at the time of the first vaccination, and three patients were receiving hydroxychloroquine. Treatment at the time of vaccination may affect the outcome, but patients with lupus with cutaneous symptoms may be at risk of disease activity exacerbation after mRNA vaccination.
According to the instructions of the Ethical Committee of Hiroshima University Hospital, ethics board approval is not required for case reports. However, informed consent is necessary. We obtained written informed consent from the patient for the publication of this report. The Ethical Committee of Hiroshima University Hospital follows the guidelines published by the Ministry of Health, Labor, and Welfare, Japan, for ethical regulations on case reports.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI grants (no. 19K07940 to S.H.).
REFERENCES
- 1. Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. [Google Scholar]
- 2. Rönnblom L, Leonard D. Interferon pathway in SLE: One key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niebel D, Ralser‐Isselstein V, Jaschke K, Braegelmann C, Bieber T, Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol Ther. 2021;34:e15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kreuter A, Licciardi‐Fernandez MJ, Burmann SN, Burkert B, Oellig F, Michalowitz AL. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA‐based or adenoviral vector‐based SARS‐CoV‐2 vaccination. Clin Exp Dermatol. 2022;47:161–3. 10.1111/ced.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]


