Abstract
Background and purpose
Fatigue and cognitive difficulties are reported as the most frequently persistent symptoms in patients after mild SARS‐CoV‐2 infection. An extensive neurophysiological and neuropsychological assessment of such patients was performed focusing on motor cortex physiology and executive cognitive functions.
Methods
Sixty‐seven patients complaining of fatigue and/or cognitive difficulties after resolution of mild SARS‐CoV‐2 infection were enrolled together with 22 healthy controls (HCs). Persistent clinical symptoms were investigated by means of a 16‐item questionnaire. Fatigue, exertion, cognitive difficulties, mood and ‘well‐being’ were evaluated through self‐administered tools. Utilizing transcranial magnetic stimulation of the primary motor cortex (M1) resting motor threshold, motor evoked potential amplitude, cortical silent period duration, short‐interval intracortical inhibition, intracortical facilitation, long‐interval intracortical inhibition and short‐latency afferent inhibition were evaluated. Global cognition and executive functions were assessed with screening tests. Attention was measured with computerized tasks.
Results
Post COVID‐19 patients reported a mean of 4.9 persistent symptoms, high levels of fatigue, exertion, cognitive difficulties, low levels of well‐being and reduced mental well‐being. Compared to HCs, patients presented higher resting motor thresholds, lower motor evoked potential amplitudes and longer cortical silent periods, concurring with reduced M1 excitability. Long‐interval intracortical inhibition and short‐latency afferent inhibition were also impaired, indicating altered GABAB‐ergic and cholinergic neurotransmission. Short‐interval intracortical inhibition and intracortical facilitation were not affected. Patients also showed poorer global cognition and executive functions compared to HCs and a clear impairment in sustained and executive attention.
Conclusions
Patients with fatigue and cognitive difficulties following mild COVID‐19 present altered excitability and neurotransmission within M1 and deficits in executive functions and attention.
Keywords: cognitive difficulties, executive functions, fatigue, mild COVID‐19, primary motor cortex, transcranial magnetic stimulation
Patients with fatigue and cognitive difficulties following mild COVID‐19 present altered excitability and neurotransmission within the primary motor cortex and deficits in sustained and executive attention.
INTRODUCTION
With an epochal research effort, the biology of SARS‐CoV‐2 has been unveiled and effective and safe vaccines have been developed. Despite these advancements, crucial issues remain unsolved, and many questions about the nature of some long‐term infection‐related symptoms have not yet been clarified.
In particular, little is known about two cardinal symptoms affecting many individuals who recovered from even mild COVID‐19 [1, 2]: perceived fatigue and cognitive difficulties [3, 4]. Perceived fatigue following SARS‐CoV‐2 infection is more pronounced than in the general population [5]. Indeed, these individuals generally report to not feeling fully recuperated despite being deemed medically recovered from the primary illness [5]. Most interestingly, fatigue has been not correlated with initial COVID‐19 severity [5]. The high rate of patients complaining of cognitive difficulties and fatigue raises the question whether these symptoms might represent a mild form of post COVID‐19 encephalopathy [6]. Dysfunction of executive attention in patients who suffered from severe COVID‐19 with neurological complications have been demonstrated previously [7]. In parallel, transcranial magnetic stimulation (TMS) studies showed lack of physiological inhibition of the corticospinal system expected after a fatiguing task, together with a strong impairment of GABAergic interneuronal activity within the primary motor cortex (M1) [7, 8]. A further study on hospitalized COVID‐19 patients with neurological manifestations confirmed an impairment of frontoparietal cognitive functions and frontoparietal hypometabolism by 18F‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography [9]. Notably, hypometabolism of frontal regions, including the olfactory gyrus, has also been identified as a hallmark of post COVID‐19 syndrome [10, 11].
In line with our previous findings on severe COVID‐19 patients, in this study the aim was to investigate whether abnormal motor cortex physiology and deficits in executive attention could be found also in patients complaining of persisting fatigue and cognitive difficulties following mild SARS‐CoV‐2 infection. For this purpose, the focus was on the neurophysiological evaluation of excitability and neurotransmission within M1 and on neuropsychological assessment of frontal lobe cognitive functions.
METHODS
Participants
The study was conducted at the ‘Post COVID’ outpatient clinic of the Department of Neurorehabilitation (Hospital of Vipiteno, SABES‐ASDAA) between January and March 2021.
Inclusion criteria were (a) a previous diagnosis of SARS‐CoV‐2 infection confirmed through detection of virus RNA by polymerase chain reaction (PCR) testing of a nasopharyngeal swab; (b) subsequent recovery from infection as defined by two consecutive negative PCR tests separated by at least a day; (c) mild form of COVID‐19 (symptoms may include fever, cough, sore throat, malaise, myalgia, anorexia, nausea, diarrhoea, anosmia and ageusia) without necessitating hospital admission; (d) complaints of cognitive difficulties and/or sense of fatigue, persisting after SARS‐CoV‐2 infection.
No restrictions about the interval between disease onset and study participation were considered.
The exclusion criteria were (a) prior or concurrent diagnosis of neurological, psychiatric, endocrine, metabolic or cardiopulmonary conditions; (b) clinical and/or radiological evidence of COVID‐19 related pneumonia during the active phase of the disease; (c) anaemia; (d) current pharmacological treatment with corticosteroids, antihistamines, antihypertensives, diuretics, antidepressants, anxiolytic or hypnotic drugs at the time of study.
Sixty‐seven patients (mean age 49.7 years; mean education 14.1 years; 97.0% right‐handed) fulfilling the criteria were enrolled. Twenty‐two healthy controls (HCs) who were similar in terms of age and gender (age 46.4 years; mean education 14.3 years; 95.4% right‐handed) were also recruited. HCs presented no evidence of SARS‐CoV‐2 infection. They were enrolled amongst hospital personnel who underwent weekly SARS‐CoV‐2 screening tests by nasopharyngeal swabs.
The study was approved by the local ethics committee (Comitato Etico del Comprensorio Sanitario di Bolzano, 65‐2020) and was in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki, 1967). All participants signed an informed written consent form for the use of their clinical data for scientific purposes.
Demographic data, medical history and previous PCR test results were collected. All patients and HCs underwent an extensive neuropsychological and neurophysiological evaluation.
Clinical assessment
Patients were requested to complete a 16‐item questionnaire, modified from a previous study [12], to explore whether and which symptoms were present at the time of evaluation. Furthermore, patients were specifically asked to report whether anosmia and/or ageusia had occurred at any time during the disease course, which could indicate central nervous system involvement. To evaluate the impact of post COVID‐19 syndrome on perceived well‐being, participants were asked to rate their ‘own health state in the last week’ on a Likert scale, ranging from 0 (worst health state) to 100 (best health state). Finally, to assess the affective condition, the Beck Depression Inventory (BDI‐II) [13], a 21‐item self‐report rating inventory, was administered: participants were asked to rate each item according to symptom severity.
Perceived fatigue
Perceived fatigue was assessed in patients and HCs with the Fatigue Severity Scale (FSS) and Fatigue Rating Scale (FRS), referring to the week preceding the evaluation. The FSS consists of nine items exploring the interference of fatigue with certain activities of daily living and rates the perceived severity on a 7‐point Likert scale (1, strongly disagree; 7, strongly agree) [14]. Concerning FRS, participants were asked to rate fatigue on a numerical rating scale (0, no fatigue; 10, extreme fatigue) [15].
Perceived exertion
Perceived exertion was assessed using a pinching task of 1 min duration, in which patients and HCs were asked to squeeze a dynamometer (Jamar, Patterson Medical) between the thumb and index finger of their dominant hand as strongly as possible. Participants were verbally encouraged to provide maximum contraction during the whole minute. During the task, participants sat comfortably on a chair with their arms adducted and elbow flexed at 90°. Maximum pinch strength (kg) obtained during 1 min was considered. At the end of the sustained pinching task, participants were asked to report their level of perceived exertion using the Borg Category Ratio (CR100) scale [16]. This scale ranges from 0 to 100 (0, nothing at all; 100, extremely strong). The number 100 implies an extremely strong perceived intensity, that is, the strongest exertion a person has ever experienced. Data obtained were also used to quantify the maximal force produced during the pinching task of both patients and HCs (measured in kilograms).
‘Perceived cognitive difficulties’ assessment
Patients were asked to describe their cognitive difficulties in daily living by referring to one or more of the following: forgetfulness, cloudiness, difficulty focusing, thinking and communicating. To evaluate the severity of them, patients were asked to rate their perceived cognitive difficulties, over the week preceding the evaluation, on a 4‐point Likert scale: 0, I have no cognitive difficulties; 1, I have slightly more cognitive difficulties than before COVID; 2, I have moderate cognitive difficulties most of the time; 3, I have persistent cognitive difficulties.
Neurophysiological evaluation
The detailed description of the neurophysiological evaluation is provided in Appendix S1.
Neuropsychological evaluation
The detailed description of the neuropsychological evaluation is provided in Appendix S1.
Statistics
The central tendency and dispersion of continuous variables are reported as mean and standard deviation (SD) for demographical, clinical and neuropsychological data; as mean and standard error (SE) for neurophysiological outcomes. Due to violations to the normality assumption (Shapiro–Wilk statistic), hypothesis testing was based on non‐parametric statistics. Descriptive statistics for categorical variables were reported as N (per cent frequency). Between‐group comparisons (patients vs. HCs) were carried out using the Mann–Whitney U test and the chi‐squared test for continuous and categorical variables, respectively. To assess whether reaction times (RTs) in computerized tasks carried out in different conditions had a different pattern between patients and HCs, a two‐factor analysis of variance (ANOVA) was carried out. The first factor was ‘group’ (post COVID‐19 vs. HC); the second factor, with repeated measures, was the ‘condition’ of each task (e.g., colour naming [CN], reading [R] and word‐colour naming [WCN] for the Stroop Task). A significant result was followed up by post hoc analysis (Tukey's honestly significant difference test).
Relevant associations between couples of variables were assessed by Spearman's correlation coefficient ρ. All statistical tests were two‐tailed and statistical significance was set at p < 0.05. To deal with multiple comparisons, the Benjamini–Hochberg method was used, controlling the false discovery rate at 5%. All analyses were carried out using the SAS/STAT statistical package, release 9.4 (SAS Institute Inc.).
RESULTS
Demographic and clinical data of post COVID‐19 patients are depicted in Table 1. A prevalence of women (74.6%) characterizes our sample. Eighty‐eight per cent of patients were less than 65 years old and 97.0% were right‐handed.
TABLE 1.
Comparison of demographic and clinical data between post COVID‐19 patients and healthy controls
| Patients | Healthy controls | p values | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 49.7 (13.3) | 46.4 (14.2) | 0.34 |
| Sex (female, N) | 50 (74.5%) | 11 (50%) | 0.08 |
| Education (years) | 14.1 (2.7) | 14.3 (2.7) | 0.78 |
| Clinical assessment | |||
| Time from onset (days) | 109.4 (77.5) | — | — |
| 16‐item questionnaire (post COVID‐19 symptoms) (prevalence, %) | |||
| Cognitive difficulties | 92.5 | — | — |
| Fatigue | 86.4 | — | — |
| Dyspnoea | 33.8 | — | — |
| Myalgia | 40.0 | — | — |
| Joint pain | 41.5 | — | — |
| Cough | 10.8 | — | — |
| Anosmia | 23.1 | — | — |
| Ageusia | 18.5 | — | — |
| Dry mouth | 32.3 | — | — |
| Rhinitis | 16.9 | — | — |
| Sleep disturbances | 69.2 | — | — |
| Headache | 43.1 | — | — |
| Red eyes | 24.6 | — | — |
| Lack of appetite | 12.3 | — | — |
| Vertigo | 32.3 | — | — |
| Diarrhoea | 9.2 | — | — |
| Mean number of post COVID‐19 symptoms per patient | 4.9 (2.5) | — | — |
|
Prevalence of anosmia (%) During and/or post SARS‐CoV‐2 infection |
48.0 (72.7) | — | — |
|
Prevalence of ageusia (%) During and/or post SARS‐CoV‐2 infection |
50.0 (75.8) | — | — |
|
Self‐perceived well‐being rating scale Range 0–100 |
65.7 (17.0) | 87.1 (8.9) | <0.001 |
|
Beck Depression Inventory‐II Range 0–63 |
1.86 (2.70) | 16.34 (8.26) | <0.001 |
| Fatigue and exertion evaluation | |||
|
Fatigue Rating Scale (FRS) Range 0–10 |
6.4 (1.9) | 1.0 (0.8) | <0.001 |
|
Fatigue Severity Scale (FSS) Range 0–63 (cut‐off 36) |
48.2 (11.5) | 15.5 (7) | <0.001 |
|
Perceived exertion (Borg CR100) (after motor task) |
80.0 (1.9) | 32.7 (3.4) | <0.001 |
| Maximum force in motor task (kg) | 8.0 (0.3) | 8.3 (0.3) | 0.76 |
| ‘Perceived cognitive difficulties‘ evaluation | |||
|
Cognitive difficulties severity Range 0–3 |
1.5 (0.6) | 0.2 (0.4) | <0.001 |
Unless otherwise specified, results are reported as mean (standard deviation). Significant differences (Mann–Whitney U test) are reported in bold.
Patients did not differ significantly from HCs in age, education and gender. The average time from onset (the elapsed interval between COVID‐19 infection and study enrolment) was 109.4 (77.5) days, with 10.4% of cases exceeding 6 months since the infection. No associations emerged between the time elapsed from disease onset and the neuropsychological and/or neurophysiological variables (see Table S1 in Appendix S1).
Clinical assessment
Results are shown in Table 1. Cognitive difficulties were reported by 92.5% of patients, 86.4% complained of fatigue and 77.0% reported both symptoms. During the entire disease course, 85.1% of patients suffered from anosmia (72.7%) and/or ageusia (75.8%). In 25.4% of cases one of these symptoms persisted until the study inclusion.
A mean of 4.9 (2.5) symptoms was reported in the post COVID‐19 patients' group. In the 100‐point Likert well‐being rating scale, patients reported significantly lower scores in comparison with HCs. BDI‐II showed significantly higher scores in patients than in HCs. To investigate the determinants of the observed values of the BDI‐II score in post COVID‐19 patients, the relative contribution of each item was assessed. ‘Loss of energy’, ‘changes in sleep’, ‘concentration difficulty’ and ‘tiredness or fatigue’ were the strongest determinants of total score in comparison with all the others: these items accounted for 42.3% ± 15.5% of total BDI‐II score, in comparison with an expected 23% (p < 0.0001).
Coherently, both self‐evaluation scales measuring perceived fatigue, FRS and FSS, revealed significantly higher scores in patients with post COVID‐19 syndrome than in HCs.
All patients and HCs completed the 1‐min pinching task. The mean produced maximum force in the pinching task was no different between patients and HCs. However, the perceived exertion, expressed as a CR100 score, was significantly higher in patients compared to HCs.
Amongst patients complaining of cognitive difficulties, 40.3% reported level 1 on the 4‐point Likert scale, 56.4% reported level 2 and 4.9% reported level 3.
Neurophysiological findings
All neurophysiological results are reported in Table 2 and in part illustrated in Figures 1 and 2.
TABLE 2.
Neurophysiological findings of post COVID‐19 patients and healthy controls
| Test | Patients | Healthy controls | p values |
|---|---|---|---|
| TMS studies | |||
| RMT (% MSO) | 53.4 (2.1) | 44.7 (1.3) | 0.014 |
| MEP amplitude (mV) | 1.2 (0.1) | 1.8 (0.2) | <0.001 |
| SP duration (ms) | 94.2 (4.5) | 73.9 (6.4) | 0.023 |
| CMCT (ms) | 5.3 (1.0) | 5.7 (0.4) | 0.08 |
| SICI 2 (% of test amplitude) | 63.5 (4.3) | 55.4 (6.3) | 0.31 |
| ICF 15 (% of test amplitude) | 124.7 (6.1) | 125.5 (8.1) | 0.94 |
| LICI 100 (% of test amplitude) | 52.2 (4.8) | 31.0 (5.8) | 0.010 |
| SAI 20 (% of test amplitude) | 78.7 (4.5) | 61.2 (4.6) | 0.035 |
| Ulnar nerve conduction studies | |||
| CMAP amplitude (mV) | 17.7 (0.4) | 19.1 (0.9) | 0.09 |
| CMAP onset latency (ms) | 3.0 (0.5) | 3.1 (0.2) | 0.42 |
| F‐wave minimum latency (ms) | 27.2 (0.3) | 27.6 (0.5) | 0.54 |
| F‐wave persistence (%) | 87.2 (2.0) | 90.9 (3.7) | 0.16 |
| SNAP amplitude D5 (µV) | 26.8 (1.5) | 25.9 (2.0) | 0.92 |
Results are reported as mean (standard error). Significant differences (Mann–Whitney U test) are indicated in bold. See text for further details.
Abbreviations: CMAP, compound muscle action potential; CMCT, central motor conduction time; ICF, intracortical facilitation; LICI, long‐interval intracortical inhibition; MEP, motor evoked potential; MSO, maximum stimulator output; RMT, resting motor threshold; SAI, short‐latency afferent inhibition; SICI, short‐interval intracortical inhibition; SNAP, sensory nerve action potential; SP, silent period; TMS, transcranial magnetic stimulation.
FIGURE 1.
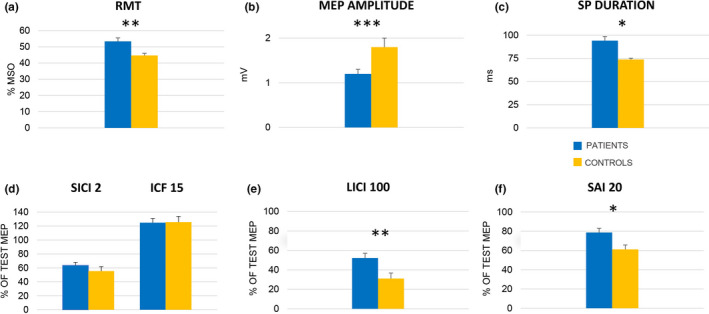
Neurophysiological results for patients (blue) and healthy controls (yellow). (a) Measures of M1 excitability. The columns represent group mean (n = 67) resting motor threshold (RMT) expressed as a percentage of maximum stimulator output (MSO). (b) The columns represent group means (n = 58) of the peak‐to‐peak amplitude of motor evoked potentials (MEP) at rest expressed in mV. (c) The columns represent group means (n = 58) of cortical silent period duration (SP) expressed in ms. (d)–(f) Results of paired‐pulse TMS protocols testing different intracortical circuits. In all sub‐sections, the columns represent the mean amplitude (n = 58) of conditioned MEPs expressed as a percentage of the corresponding mean unconditioned (test) response. (d) SICI 2, i.e., short‐interval intracortical inhibition at 2 ms interstimulus interval (ISI), and ICF 15, i.e., intracortical facilitation at ISI 15 ms; SICI and ICF are depicted in the same graph because they belong to the same test paradigm (see text). (e) LICI 100, i.e., long‐interval intracortical inhibition at ISI 100 ms. (f) SAI 20, i.e., short‐latency afferent inhibition at the latency of individual N20 + 0 ms. Whiskers represent standard error. *p < 0.05, **p < 0.01, ***p <0.001 [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
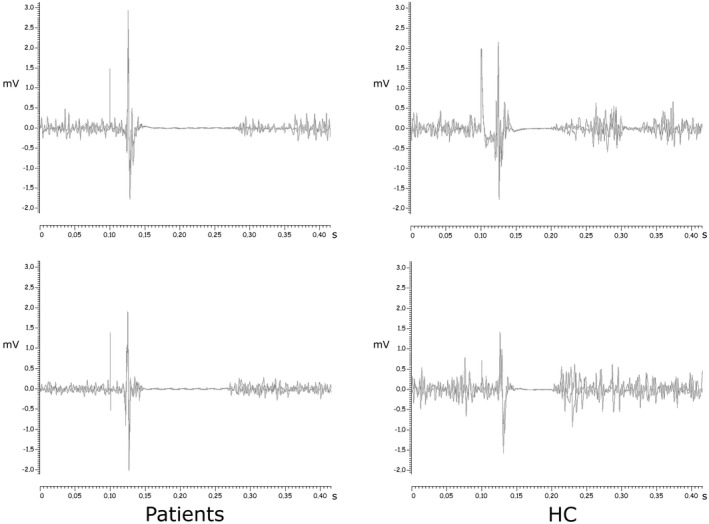
Examples of cortical silent period in patients and healthy controls. Representative traces of cortical silent period evoked in the first dorsal interosseous muscle of two patients and two HCs at 130% RMT intensity 30% during self‐estimated 50% maximum voluntary contraction. Two superimposed traces are presented for each subject
Compound muscle action potential onset latency, compound muscle action potential baseline‐to‐peak amplitude, minimum F‐wave latency, F‐wave persistence and baseline‐to‐peak amplitude of the sensory nerve action potential recorded from the digital branches of the fifth finger (D5) did not differ significantly between patients and HCs.
Resting motor threshold (RMT) was significantly higher in patients. Nine patients presented very high RMTs (between 86% and 100% maximum stimulator output, MSO), preventing further TMS studies that would have required stimulation intensities exceeding MSO. In the remaining 58 patients, mean motor evoked potential (MEP) amplitudes recorded at 130% RMT intensity were significantly smaller and mean silent period (SP) duration was significantly longer in patients than HCs. In contrast, central motor conduction time did not differ significantly between patients and HCs.
The amount of inhibition of the conditioned MEP recorded in the short‐interval intracortical inhibition test at 2 ms interstimulus interval (ISI)—henceforth SICI 2—and the amount of facilitation tested in the intracortical facilitation paradigm at ISI 15 ms (ICF 15) did not differ between patients and HCs.
Patients displayed a significantly reduced amount of inhibition in the long‐interval intracortical inhibition test at ISI 100 ms (LICI 100) compared with HCs. A similar reduction of inhibition was found for short‐latency afferent inhibition at ISI 20 ms (SAI 20) in the patient group.
Correlation analysis between LICI 100 and SAI 20 values in patients revealed a negative association (ρ = −0.27, p < 0.05).
Neuropsychological findings
The results are shown in Table 3. With respect to global cognition, the Montreal Cognitive Assessment (MoCA) revealed a significantly poorer performance in patients compared to HCs. Analysing the MoCA sub‐scores in each specific cognitive domain, significant differences between patients and HCs emerged for executive functions, language and attention. Notably, 65.7% of patients failed in the sub‐item ‘phonological fluency’. Memory, visuo‐spatial functions and orientation did not differ significantly. Significantly lower scores were obtained in patients compared to HCs in the frontal assessment battery.
TABLE 3.
Comparison of neuropsychological findings between post COVID‐19 patients and healthy controls
| Patients | Healthy controls | p values | |
|---|---|---|---|
| Cognitive assessment | |||
|
Montreal Cognitive Assessment (MoCA) Range 0–30, cut‐off 15.5 |
25.4 (2.9) | 27.6 (2.2) | 0.005 |
| Executive functions (sub‐scores) | 2.8 (1.0) | 3.6 (0.7) | <0.001 |
| Language (sub‐scores) | 4.7 (1.0) | 5.5 (0.7) | <0.001 |
| Memory (sub‐scores) | 3.2 (1.5) | 3.9 (1.8) | 0.21 |
| Visuo‐spatial (sub‐scores) | 3.6 (0.6) | 3.9 (0.4) | 0.065 |
| Orientation (sub‐scores) | 5.8 (0.7) | 6.0 (0.0) | 0.18 |
| Attention (sub‐scores) | 5.5 (0.9) | 6.0 (0.2) | 0.010 |
|
Frontal assessment battery (FAB) Range 0–18, cut‐off 13.4 |
16.4 (1.9) | 17.59 (0.7) | 0.004 |
| Reaction time (RT) in computerized attention tasks (ms) | |||
| Sustained attention (SA) | 421.2 (170.6) | 324.7 (41.7) | <0.001 |
| Stroop Task—word colour naming (WCN) | 1114.1 (314.6) | 886.3 (165.7) | 0.003 |
| Stroop Task—colour naming (CN) | 865.5 (155.0) | 760.8 (94.8) | 0.003 |
| Stroop Task—reading (R) | 1007.7 (214.1) | 878.9 (136.7) | 0.027 |
| Stroop Task—derived interference (I) | 177.5 (203.0) | 66.5 (110.0) | 0.040 |
| Navon Task—all conditions (NT‐AC) | 1055.9 (253.3) | 871.3 (185.3) | 0.002 |
| Navon Task—congruent conditions (NT‐C) | 994.6 (238.9) | 827.1 (157.5) | 0.003 |
| Navon Task—incongruent conditions (NT‐I) | 1092.7 (268.4) | 907.7 (211.7) | 0.003 |
Results are reported as mean (standard deviation). Significant differences (Mann–Whitney U test) are reported in bold.
With respect to the sustained attention task (SA), RTs were significantly longer and intra‐individual SD was higher in patients than in HCs (both p < 0.001). Correlation analysis revealed direct associations between SA RTs mean and FRS scores (ρ = 0.27, p = 0.029), as well as between SA RTs mean and FSS scores (ρ = 0.37, p = 0.003). Repeated‐measures ANOVA performed on RTs mean of the Stroop Task revealed a significant main effect of condition (p < 0.001), group (p = 0.002) and interaction (p = 0.006). Post hoc analysis revealed differences between patients and HCs in all conditions (WCN RTs mean, p = 0.002; CN RTs mean, p = 0.004; R RTs mean, p = 0.010) and differences between conditions in patients (WCN RTs mean vs. CN RTs mean, p < 0.0001; WCN RTs mean vs. R RTs mean, p < 0.0001; and CN RTs mean vs. R RTs mean, p < 0.0001) and HCs (WCN RTs mean vs. CN RTs mean, p = 0.009; and CN RTs mean vs. R RTs mean, p < 0.0001). Finally, Stroop Task Interference RTs were significantly higher in patients than in HCs (p = 0.040). Repeated‐measures ANOVA performed on the RTs mean of the Navon Task revealed a significant main effect of condition (p < 0.001) and group (p = 0.003), whilst interaction was not statistically significant (p = 0.45). Post hoc analysis revealed differences between patients and HCs in all conditions (Navon Task—all conditions [NT‐AC] RTs mean, p = 0.003; Navon Task—congruent conditions [NT‐C] RTs mean, p = 0.003; Navon Task—incongruent conditions (NT‐I) RTs mean, p = 0.005), and differences between conditions in patients and HCs (NT‐AC RTs mean vs. NT‐C RTs mean, p < 0.0001 in both patients and HCs; NT‐AC RTs mean vs. NT‐I RTs mean, p = 0.0002 and p < 0.0001 in patients and HCs, respectively; and NT‐C RTs mean vs. NT‐I RTs mean, p < 0.0001 in both patients and HCs).
DISCUSSION
This study provides evidence for abnormal motor cortex physiology in a cohort of patients reporting fatigue and cognitive difficulties following mild COVID‐19. Alongside, the neuropsychological evaluation showed signs of reduced cognitive efficiency, reduced executive functions and impaired sustained and executive attention (see Figure 3). These data are in line with previous findings, which showed a cognitive pattern of dysexecutive syndrome in patients who survived after COVID‐19, even months after the recovery [17, 18]. Patients showed reduced M1 excitability with lower motor output, as indicated by higher RMTs and lower MEP amplitudes. Moreover, the alterations found in the LICI 100 and SAI 20 protocols suggest alterations in intracortical GABAB‐ergic and cholinergic neurotransmission. TMS and nerve conduction studies excluded any evidence of subclinical dysfunction of the corticospinal tract, of spinal motor neurons, ventral roots or peripheral nerve to the target first dorsal interosseous (FDI) muscle. Higher RMTs and lower MEP amplitudes, in the absence of impairment of spinal and peripheral motor conduction, clearly indicate motor cortex hypo‐excitability.
FIGURE 3.
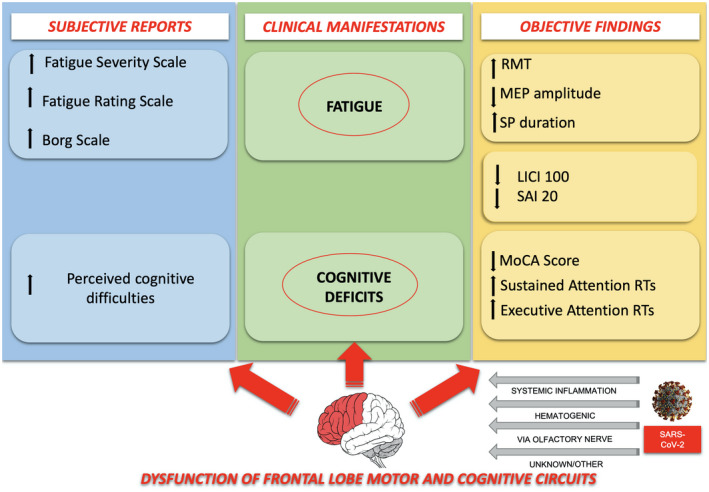
Pathophysiological interpretation of fatigue and perceived cognitive difficulties following mild COVID‐19. Link between personal subjective feelings, cortical involvement and objective clinical, neuropsychological and neurophysiological findings. See the text for details [Colour figure can be viewed at wileyonlinelibrary.com]
In these patients, both perceived fatigue and exertion were altered. FRS and FSS scores indicated that patients who recovered from mild COVID‐19 could still perceive clinically significant fatigue in daily life for a long time after the acute illness, with mean FSS scores extensively higher than normal cut‐off values [14]. Concurrently, CR100 showed exaggerated higher perception of physical exertion compared to HCs, despite no differences in exerted force. Mean RMTs (the minimum magnetic stimulation intensity required to transcranially activate M1) were significantly higher in post COVID‐19 patients than in HCs; remarkably, nine out of 67 patients presented such high RMTs that they did not undergo further TMS tests, which would have required stimulation intensities higher than MSO. RMTs can reflect excitability of both cortical M1 neurons and spinal motor neurons [19] and can be affected by pathology of the peripheral nervous system [20, 21]. Mean MEP amplitudes were also smaller in patients. Since they did not present corticospinal tract or neuromuscular dysfunction, as assessed in clinical and neurophysiological examination, the alteration of RMT and MEP must reflect a reduced baseline level of corticomotor excitability. The relationship between high RMTs and fatigue has previously been reported in other medical conditions with definite brain involvement, for example stroke and multiple sclerosis [22, 23]. A model to explain fatigue in neurological diseases suggests that suppression of the excitatory system and not over‐activity of the inhibitory system may lead to the development of fatigue [22, 24]. The motor cortex has strong anatomical connections with other cortical and subcortical regions such as the pre‐motor cortices, supplementary motor areas, cingulate motor areas, basal ganglia and the cerebellum [25, 26, 27] which can modulate motor cortex excitability.
Inadequate input from brain regions upstream of M1 or reduced excitability of the motor cortex could cause suboptimal neural drive to the α‐motor neurons and thus reduced activation of muscle fibres, thus contributing to the genesis of central fatigue.
The cortical SP, that is, the transient suppression of voluntary muscle activity following a TMS‐evoked motor response during tonic contraction of the target muscle [28], was longer in patients than in HCs. Although the initial part of the SP is due to spinal inhibitory mechanisms, it largely results from activation of GABAB inhibitory cortical interneurons projecting onto the pyramidal cells [28, 29]. As the SP duration is known to increase with higher stimulation intensities [30], it is possible that longer SPs are simply due to higher RMTs in patients, which consequently require higher stimulation intensities to elicit MEPs. However, prolonged SPs have been reported in patients with fatigue in multiple sclerosis and stroke [31, 32] and in healthy subjects after mentally fatiguing tasks [33]. In a recent study, patients with fatigue and dysexecutive syndrome after severe COVID‐19 presented with reduced activity of GABAergic and cholinergic circuits in M1, as demonstrated by alterations in SICI, LICI and SAI [8]. Therefore, a search was made for alterations of the same neurophysiological markers in the present sample of patients after mild COVID‐19. A clear impairment of LICI 100 circuits that index GABAB‐ergic interneuronal activity was found [34], but none in the GABAA‐ergic neurotransmission (SICI 2 did not differ significantly between patients and HCs). SICI is affected by chronic hypoxia [35] and its alteration in patients with cognitive impairment and fatigue after severe COVID‐19 could have been due to prolonged hypoxia [8].
Intracortical facilitation tested at ISI 15 ms (ICF 15), which reflects mainly excitatory glutamatergic transmission through the N‐methyl‐d‐aspartate receptors, did not differ between patients and HCs, in line with previous findings [8].
GABA is the principal inhibitory neurotransmitter in the human nervous system and plays a fundamental role in neuronal coding and processing. Maladaptation of cortical processes related to degeneration of intracortical inhibitory GABAergic circuits within M1 has been implicated with central fatigue in various affections of the central nervous system [36, 37, 38]. Furthermore, different cognitive abilities, mainly executive functions, are sensitive to cerebral GABA concentrations in the frontal cortex [39, 40]. Altered GABA neurotransmission has been implicated in frontotemporal dementia, in which impairment of executive functions is a prominent feature [41, 42, 43, 44].
As both mechanisms are mediated by GABAB interneurons, SP and LICI should change accordingly; therefore our results of prolonged SP and diminished LICI in patients seem contradictory. A first consideration is that SP is measured during a tonic voluntary contraction and can therefore be influenced by the level of contraction and by afferent inhibition mechanisms [45], whilst LICI is measured at rest. Furthermore, although SP and LICI are both GABAB‐mediated, SP duration is dose‐dependent to the administration of GABAB‐selective agonists [46], whilst LICI becomes saturated at low GABA levels [47], suggesting that SP and LICI can behave differently based on the GABA level in the synaptic cleft. The amount of GABA can be autoregulated by presynaptic GABAB autoreceptors. At any rate, a definite explanation cannot be inferred from the present findings.
Compared to HCs, SAI 20 mechanisms were also impaired in patients. SAI is a marker of motor cortex inhibition induced by sensory afferents through inhibitory connections from the primary somatosensory cortex to M1 [48]. This inhibitory sensorimotor integration is under excitatory modulation of cholinergic projections [49, 50]. Abnormal SAI findings concurring with central cholinergic dysfunction are a recognized biomarker of Alzheimer's disease from prodromal stages [41, 51]. The impairment of cholinergic neurotransmission could be responsible for the neuropsychological alterations. A weak negative correlation between LICI 100 and SAI 20 was found. No univocal interpretation of this finding is actually possible and it will require further consideration. The two forms of inhibition may compensate for each other, or they may suggest a distinction into two different subgroups, one with predominant LICI 100 dysfunction and another with predominant SAI 20 dysfunction.
Patients presented an average of five symptoms including cognitive difficulties and/or fatigue lasting more than 3 months and seriously disturbing the sense of well‐being. The BDI‐II score highlighted a condition of mild depression in post COVID‐19 patients. Notably, an in‐depth analysis pointed out that the items contributing most to the global score were indeed those which strongly overlap with complaints typically reported by post COVID‐19 patients [1, 2].
Scores in the ‘cognitive difficulties’ 4‐point Likert scale indicated that long COVID‐19 patients complain of high levels of perceived cognitive difficulties. Based on neuropsychological data, patients presented with subclinical global cognitive impairment but, concurrently, with clear deficits in sustained and executive attention. A deeper analysis of MoCA scores confirmed deficits in executive functions and attention. Moreover, it disclosed impairments in language: notably, 65.7% of patients failed in the sub‐item of phonemic fluency, which is considered a measure of both executive and language abilities [52]. Otherwise, orientation, memory and visuo‐spatial functions were not affected in our population.
The impairment of executive functions is furthermore highlighted by the lower performances at the frontal assessment battery. Analysing RT data, significant alterations in sustained attention emerged: patients were slower in responding to visual stimuli than HCs and showed great intra‐individual variability in RTs. These results define a condition characterized by both global slowing and increased fluctuation in response time. Moreover, defective executive attention emerged from the analysis of both the computerized Stroop Task and the Navon Task: RTs collected in patients were significantly longer than those in HCs, in all conditions. More interestingly, the interference suppression capability was significantly lower in patients than HCs. This deficit was also confirmed by the significant slowing of the patients' response time in those conditions of the Stroop and Navon Task, which reflect the ability to inhibit inappropriate or irrelevant responses, to monitor conflicts and to evaluate stimuli or resource allocation [53]. Taken together, these data confirm previous findings [17, 18] and disclose an impairment of the attentional system in post COVID‐19 patients [54, 55, 56] even after mild SARS‐CoV‐2 infection.
Selective impairment of executive function and attention, central fatigue and motor cortex physiology abnormalities following mild SARS‐CoV‐2 infection point towards a dysfunction of frontal networks. There is emerging evidence that SARS‐CoV‐2 could preferentially and directly target the frontal lobes probably via retrograde axonal transport from olfactory epithelium, as suggested by magnetic resonance imaging [57, 58], electroencephalography [59], 18F‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography imaging [10, 60] and post‐mortem studies [61]. An inflammatory or dysimmune parainfectious process involving preferentially the frontal lobes could underpin these clinical and neurophysiological findings in post COVID‐19 patients with fatigue and cognitive difficulties.
A limitation of this study consists in the lack of a control group of patients who previously suffered from COVID‐19 without complaining of fatigue and/or cognitive difficulties. Another limitation is the relatively low number of HCs. Moreover, the investigation of further ISIs in the paired‐pulse TMS tests could have provided more information on intracortical circuit activity.
CONCLUSIONS
This study highlights for the first time that patients with fatigue and cognitive difficulties following mild COVID‐19 present abnormal motor cortex neurophysiological findings. The neuropsychological examination unveils the presence of executive‐attentive deficits even in these otherwise mildly affected patients.
CONFLICT OF INTEREST
The authors report no competing interests.
AUTHOR CONTRIBUTIONS
Paola Ortelli: Conceptualization (lead); investigation (equal); methodology (equal); project administration (equal); software (equal); supervision (equal); validation (equal); writing—original draft (lead); writing—review and editing (equal). Davide Ferrazzoli: Conceptualization (equal); writing—original draft (equal); writing—review and editing (equal). Luca Sebastianelli: Resources (equal); supervision (equal); validation (equal). Roberto Maestri: Data curation (equal); formal analysis (equal); methodology (equal). Sabrina Dezi: Data curation (equal). Danny Spampinato: Conceptualization (equal); visualization (equal). Leopold Saltuari: Supervision (equal); validation (equal); visualization (equal). Alessia Alibardi: Data curation (supporting); writing—review and editing (supporting). Michael Engl: Resources (equal). Markus Kofler: Methodology (supporting); supervision (equal); writing—review and editing (supporting). Angelo Quartarone: Formal analysis (supporting); methodology (supporting); supervision (supporting). Giacomo Koch: Investigation (supporting); methodology (supporting); validation (supporting); visualization (supporting). Antonio Oliviero: Investigation (supporting); methodology (equal); visualization (equal); writing—review and editing (supporting). Viviana Versace: Conceptualization (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal).
Supporting information
ACKNOWLEDGEMENT
Annelies Gruber is acknowledged for her excellent secretarial support.
Ortelli P, Ferrazzoli D, Sebastianelli L, et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID‐19. Eur J Neurol. 2022;29:1652–1662. doi: 10.1111/ene.15278
Paola Ortelli and Davide Ferrazzoli share first authorship.
Giacomo Koch, Antonio Oliviero and Viviana Versace share senior authorship.
Funding information
This work was supported by Südtiroler Sanitätsbetrieb—Azienda Sanitaria dell'Alto Adige (SABES‐ASDAA) (grants 1767_2020 and 997_2021).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Alwan NA. The road to addressing long COVID. Science. 2021;373(6554):491‐493. [DOI] [PubMed] [Google Scholar]
- 2. Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 3. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after COVID‐19: qualitative study of 114 ‘long COVID’ patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized COVID‐19 'long haulers'. Ann Clin Transl Neurol. 2021;8(5):1073‐1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy‐associated morbidity in COVID‐19 patients. Ann Clin Transl Neurol. 2020;7(11):2221‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ortelli P, Ferrazzoli D, Sebastianelli L, et al. Neuropsychological and neurophysiological correlates of fatigue in post‐acute patients with neurological manifestations of COVID‐19: insights into a challenging symptom. J Neurol Sci. 2021;420:117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Versace V, Sebastianelli L, Ferrazzoli D, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID‐19. Clin Neurophysiol. 2021;132(5):1138‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain. 2021;144(4):1263‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guedj E, Campion JY, Dudouet P, et al. (18)F‐FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sollini M, Morbelli S, Ciccarelli M, et al. Long COVID hallmarks on [18F]FDG‐PET/CT: a case–control study. Eur J Nucl Med Mol Imaging. 2021;48:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck AT, Steer RA, Brown GK. BDI‐II, Beck Depression Inventory: manual. 1996.
- 14. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435‐437. [DOI] [PubMed] [Google Scholar]
- 15. Mordillo‐Mateos L, Soto‐Leon V, Torres‐Pareja M, et al. Fatigue in multiple sclerosis: general and perceived fatigue does not depend on corticospinal tract dysfunction. Front Neurol. 2019;10:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borg E, Borg G. A comparison of AME and CR100 for scaling perceived exertion. Acta Psychol (Amst). 2002;109(2):157‐175. [DOI] [PubMed] [Google Scholar]
- 17. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becker JH, Lin JJ, Doernberg M, et al. Assessment of cognitive function in patients after COVID‐19 infection. JAMA Netw Open. 2021;4(10):e2130645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76(2):159‐200. [DOI] [PubMed] [Google Scholar]
- 20. Nardone R, Versace V, Sebastianelli L, et al. Cortical involvement in myopathies: insights from transcranial magnetic stimulation. Clin Neurophysiol. 2017;128(10):1971‐1977. [DOI] [PubMed] [Google Scholar]
- 21. Badawy RA, Loetscher T, Macdonell RA, Brodtmann A. Cortical excitability and neurology: insights into the pathophysiology. Funct Neurol. 2012;27(3):131‐145. [PMC free article] [PubMed] [Google Scholar]
- 22. Kuppuswamy A, Clark EV, Turner IF, Rothwell JC, Ward NS. Post‐stroke fatigue: a deficit in corticomotor excitability? Brain. 2015;138(Pt 1):136‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiew FL, Rajabally YA. Sural sparing in Guillain–Barré® syndrome subtypes: a reappraisal with historical and recent definitions. Clin Neurophysiol. 2016;127(2):1683‐1688. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka M, Watanabe Y. Supraspinal regulation of physical fatigue. Neurosci Biobehav Rev. 2012;36(1):727‐734. [DOI] [PubMed] [Google Scholar]
- 25. Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6(3):342‐353. [DOI] [PubMed] [Google Scholar]
- 26. Boecker H, Jankowski J, Ditter P, Scheef L. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. NeuroImage. 2008;39(3):1356‐1369. [DOI] [PubMed] [Google Scholar]
- 27. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357‐381. [DOI] [PubMed] [Google Scholar]
- 28. Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128(4):539‐542. [DOI] [PubMed] [Google Scholar]
- 29. Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521‐534. [PMC free article] [PubMed] [Google Scholar]
- 30. Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115(5):1076‐1082. [DOI] [PubMed] [Google Scholar]
- 31. Chaves AR, Kelly LP, Moore CS, Stefanelli M, Ploughman M. Prolonged cortical silent period is related to poor fitness and fatigue, but not tumor necrosis factor, in multiple sclerosis. Clin Neurophysiol. 2019;130(4):474‐483. [DOI] [PubMed] [Google Scholar]
- 32. Knorr S, Rice CL, Garland SJ. Perspective on neuromuscular factors in poststroke fatigue. Disabil Rehabil. 2012;34(26):2291‐2299. [DOI] [PubMed] [Google Scholar]
- 33. Morris AJ, Christie AD. The effect of mental fatigue on neuromuscular function is similar in young and older women. Brain Sci. 2020;10(4):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126(10):1847‐1868. [DOI] [PubMed] [Google Scholar]
- 35. Oliviero A, Corbo G, Tonali PA, et al. Functional involvement of central nervous system in acute exacerbation of chronic obstructive pulmonary disease. A preliminary transcranial magnetic stimulation study. J Neurol. 2002;249(9):1232‐1236. [DOI] [PubMed] [Google Scholar]
- 36. Liepert J, Mingers D, Heesen C, Bäumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler. 2005;11(3):316‐321. [DOI] [PubMed] [Google Scholar]
- 37. McDonald C, Newton J, Lai HM, Baker SN, Jones DE. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. J Hepatol. 2010;53(6):1095‐1100. [DOI] [PubMed] [Google Scholar]
- 38. Vucic S, Cheah BC, Kiernan MC. Maladaptation of cortical circuits underlies fatigue and weakness in ALS. Amyotroph Lateral Scler. 2011;12(6):414‐420. [DOI] [PubMed] [Google Scholar]
- 39. Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13(7):825‐827. [DOI] [PubMed] [Google Scholar]
- 40. Porges EC, Woods AJ, Edden RA, et al. Frontal gamma‐aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benussi A, Grassi M, Palluzzi F, et al. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann Neurol. 2020;87(3):394‐404. [DOI] [PubMed] [Google Scholar]
- 42. Benussi A, Di Lorenzo F, Dell'Era V, et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89(7):665‐672. [DOI] [PubMed] [Google Scholar]
- 43. Benussi A, Grassi M, Palluzzi F, et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul. 2021;14(2):241‐249. [DOI] [PubMed] [Google Scholar]
- 44. Assogna M, Casula EP, Borghi I, et al. Effects of palmitoylethanolamide combined with luteoline on frontal lobe functions, high frequency oscillations, and GABAergic transmission in patients with frontotemporal dementia. J Alzheimers Dis. 2020;76(4):1297‐1308. [DOI] [PubMed] [Google Scholar]
- 45. Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 46. McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173(1):86‐93. [DOI] [PubMed] [Google Scholar]
- 47. Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tokimura H, Lazzaro V, Tokimura Y, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alle H, Heidegger T, Kriváneková L, Ziemann U. Interactions between short‐interval intracortical inhibition and short‐latency afferent inhibition in human motor cortex. J Physiol. 2009;587(Pt 21):5163‐5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Lazzaro V, Oliviero A, Profice P, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135(4):455‐461. [DOI] [PubMed] [Google Scholar]
- 51. Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta‐amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci. 2004;29(6):427‐441. [PMC free article] [PubMed] [Google Scholar]
- 52. Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo Z, Chen R, Liu X, et al. The impairing effects of mental fatigue on response inhibition: an ERP study. PLoS One. 2018;13(6):e0198206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mackie MA, Van Dam NT, Fan J. Cognitive control and attentional functions. Brain Cogn. 2013;82(3):301‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Östberg A, Ledig C, Katila A, et al. Volume change in frontal cholinergic structures after traumatic brain injury and cognitive outcome. Front Neurol. 2020;11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology. 2020;297(1):E232‐E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID‐19: a retrospective multicenter study. Neurology. 2020;95(13):e1868‐e1882. [DOI] [PubMed] [Google Scholar]
- 59. Antony AR, Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID‐19. Seizure. 2020;83:234‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karimi‐Galougahi M, Yousefi‐Koma A, Bakhshayeshkaram M, Raad N, Haseli S. (18)FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID‐19. Acad Radiol. 2020;27(7):1042‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2021;24(2):168‐175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


