Abstract
The concentration of SARS‐CoV‐2‐specific serum antibodies, elicited by vaccination or infection, is a primary determinant of anti‐viral immunity, which correlates with protection against infection and COVID‐19. Serum samples were obtained from 25 897 participants and assayed for anti‐SARS‐CoV‐2 spike protein RBD IgG antibodies. The cohort was composed of newly vaccinated BNT162b2 recipients, in the first month or 6 months after vaccination, COVID‐19 patients and a general sample of the Israeli population. Antibody levels of BNT162b2 vaccine recipients were negatively correlated with age, with a prominent decrease in recipients over 55 years old, which was most significant in males. This trend was observable within the first month and 6 months after vaccination, while younger participants were more likely to maintain stable levels of serum antibodies. The antibody concentration of participants previously infected with SARS‐CoV‐2 was lower than the vaccinated and had a more complex, non‐linear relation to age, sex and COVID‐19 symptoms. Taken together, our data supports age and sex as primary determining factors for both the magnitude and durability of humoral response to SARS‐CoV‐2 infection and the COVID‐19 vaccine. Our results could inform vaccination policies, prioritizing the most susceptible populations for repeated vaccination.
Keywords: antibodies, COVID‐19, SARS‐CoV‐2, severity, vaccination, vaccine
Abbreviations
- AU
arbitrary units
- Chisq
chi‐square
- CLIA
chemiluminescent immunoassay
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- mRNA
messenger RNA
- RBD
receptor‐binding domain
- RR
relative rate
- S1
SARS‐CoV‐2 spike protein subunit 1
- S2
SARS‐CoV‐2 spike protein subunit 2
1. BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative pathogen of the coronavirus disease 2019 (COVID‐19) pandemic, has rapidly spread worldwide, causing millions of deaths, massively impacting the economy and society. The BNT162b2 vaccine, which consists of two doses of modified SARS‐CoV‐2 mRNA delivered in lipid nanoparticles, was proven to be effective in prevention of COVID‐19 both in randomized clinical trials and nationwide, mass‐vaccination settings. 1
The humoral immune response to infection and vaccination relies on the production of antibodies specific to pathogen antigens, starting with acute‐response IgM antibodies, succeeded by long‐term IgG antibodies, produced by memory B cells. 2 The concentration and neutralizing capacity of SARS‐CoV‐2‐specific antibodies are primary predictors of protection against infection and severe COVID‐19. 3
While the antibody‐producing memory B‐cells persist for over 6 months, 4 serum antibody levels are rapidly declining in the months following vaccination, 5 a decline correlated with waning immunity. 6
In addition to decreasing serum antibody levels, new emerging SARS‐CoV‐2 variants are increasingly more resistant to antibody‐mediated neutralization, when compared to past strains. 7
The gradual loss of humoral immunity over time, amplified by the rapid rise of neutralization‐resistant viral variants, poses an unprecedented public health challenge. Current efforts to curb the pandemic are focused on bolstering humoral immunity by administration of repeated “booster” vaccine doses and the development of next generation COVID‐19 vaccines, with increased efficacy against currently circulating SARS‐CoV‐2 variants. 8 , 9
COVID‐19 susceptibility and the risk for severe disease are strongly influenced age and sex, with older males having higher rates of infection and significantly worse outcomes. 10 These major immunological disparities, while common to a broader range of viral infections and vaccines, 11 are being studied in an effort to personalize and improve COVID‐19 treatment and vaccination strategy. 12 , 13 , 14
In this study, we measured the concentration of SARS‐CoV‐2 RBD‐specific serum antibodies in an Israeli cohort, composed of BNT162b2 vaccine recipients, patients recovering from COVID‐19 and unvaccinated patients from the general population. We provide a statistical analysis of serum antibody concentrations arising from the immune response to vaccination and viral infection, with respect to symptomatic and asymptomatic individuals, and the interplay with sex, age and time since vaccination.
2. METHODS
2.1. Participants' samples and study design
Whole blood samples were collected into SST gel tubes using a standard technique at Shamir Medical Center outpatient clinic. Specimens of blood were kept at 2–8°C until processing within two hours. Samples were centrifuged for at least 10 min at 3000 RPM for serum separation for downstream analysis. Participants' medical and demographic data were obtained from the hospital and outpatient clinic medical records.
COVID‐19 serological tests were performed using the following commercially available, FDA approved, automated immunoassays, The LIAISON® SARS‐CoV‐2 S1/S2 IgG was in use for the first three month and till March 2021, The LIAISON® SARS‐CoV‐2 TrimericS IgG assay was in use starting March 2021 till present, all tests were performed on the Liaison XL Diasorin:
The LIAISON® SARS‐CoV‐2 S1/S2 IgG (311450, DiaSorin, Saluggia, Italy): A chemiluminescent immunoassay (CLIA) for quantitative determination of anti‐S1 and anti‐S2 specific IgG antibodies using magnetic beads coated with S1 and S2 antigens. SARS‐CoV‐2 S1/S2 IgG antibody concentrations are automatically calculated and expressed as arbitrary units (AU/ml), with a positive cutoff level of 15.0 AU/ml (according to manufacturer declaration diagnostic sensitivity above 15 days of symptoms onset is 97.4% and specificity is 98.9%).
The LIAISON® SARS‐CoV‐2 TrimericS IgG assay (Emergency Use Authorization, EUA) is able to identify patients diagnosed for COVID‐19 by virus variants (Lineage B.1.1.7 and Lineage P.1). SARS‐CoV‐2 S1/S2 IgG antibody concentrations are automatically calculated and expressed as arbitrary units (AU/ml), with a positive cutoff level of 15.0 AU/ml. The principal components of the test are paramagnetic particles (solid phase) coated with recombinant trimeric SARS‐CoV‐2 spike protein and a conjugate reagent containing an anti‐human IgG mouse monoclonal antibody linked to an isoluminol derivative (according to manufacturer declaration the test clinical sensitivity is 98.7% and specificity is 99.5%).
The data for these two assays was pooled together and was not treated separately as previous studies have shown that the two assays are in strong agreement and highly correlated. 15
2.2. Data processing and filtering
All analyses were performed using R 4.1.0, on a 64‐bit Linux system, the libraries used and their respective versions are listed in the Supporting Information (S2).
Some observations were incomplete, missing parameters such as sex and time since vaccination. In order to retain as much data as possible, only the largest subset of complete data was used on a per‐analysis basis.
Epitools 0.5‐10.1 was used for contingency testing and odds ratio calculation, while adjusted odds ratios (aOR) were derived from a multivariate, binomial regression model.
Modeling antibody levels was performed using generalized Poisson models and visualized using ggeffects 1.1.1. Grouping by age ranges was done empirically, using percentiles. Correlation analysis was performed using Pearson tests and group‐wise comparisons were performed using Wilcox or kruskal tests, as stated in the text. All confidence intervals (textual or graphical) were made with 95% confidence.
3. RESULTS
3.1. Study population
Serum samples were collected between November 8, 2020, and May 5, 2021, from a cohort of 25 097 patients, and an additional cohort of 800 participants was tested 6 months after vaccination, August 5th to September 14th, 2021. The cohort is composed of 1652 patients recovering from symptomatic (PCR‐verified) COVID‐19, 2339 BNT162b2 vaccine recipients (received at least one vaccine dose) and 21 104 samples from the general population of patients visiting the hospital for regular checkups and other non‐COVID‐19 related reasons. An additional cohort of 800 participants was sampled 6 months after receiving the second dose of the vaccine, with an additional N protein antibody assay, to verify they were not infected by the virus.
Samples taken before the Israeli national vaccination campaign (which started on 20/12/2020) are guaranteed to be unvaccinated, therefore, seropositive general population samples from this timespan are assumed to originate from an asymptomatic infection. We split the general population samples into subgroups named: “General unvaccinated” and “General vaccinated”, for unvaccinated (N = 8180) and possibly vaccinated (N = 12 926) individuals, respectively. The general population cohorts were collected primarily from hospital patients; therefore they are not representative of the wider population (Table 1).
TABLE 1.
Cohort demographic characteristics, by groups
| Group | General unvaccinated (N = 8,180) | General vaccinated (N = 12,926) | Recovered (N = 1,652) | Vaccinated (N = 2,339) | 6 months post‐vaccination (N = 800) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | |
| Age | 1.0 – 100 | 40 ± 0.20 | 4.0–93 | 38 ± 0.13 | 6 – 88 | 41 ± 0.40 | 16 – 99 | 55 ± 0.32 | 7 – 97 | 50 ± 0.48 |
| S1/S2 Antibodies (AU/ml) | 4.9 – 374 | 21 ± 0.38 | 4.9 – 800 | 79 ± 1.75 | 4.9 – 800 | 65 ± 2.61 | 4.9 – 800 | 298 ± 13.51 | 4.9 – 800 | 136.7 ± 4.8 |
| Male sex—no. (%) a | 4,902 (61%) | N/A | 323 (42%) | 1,040 (44%) | 261 (32%) | |||||
Observations with missing sex data are excluded.
IgG antibodies against S1/S2 SARS‐CoV‐2 antigens were measured from serum samples, yielding AU/ml values in the 4.9‐800 range, with 15 AU/ml being the cutoff for seropositivity (see Section 2).
3.2. Differences in humoral response to vaccination and infection
The fraction of seropositive participants varied significantly between the groups. The mixed, general population group was 28.4% seropositive, participants recovering from symptomatic COVID‐19 were 73.5% seropositive (OR = 6.98 [95% CI = 6.23–7.82]) and vaccine recipients were 94% seropositive (OR = 39.5 [95% CI = 33.3–47.2]) (Figure 1C) (Table S1).
FIGURE 1.
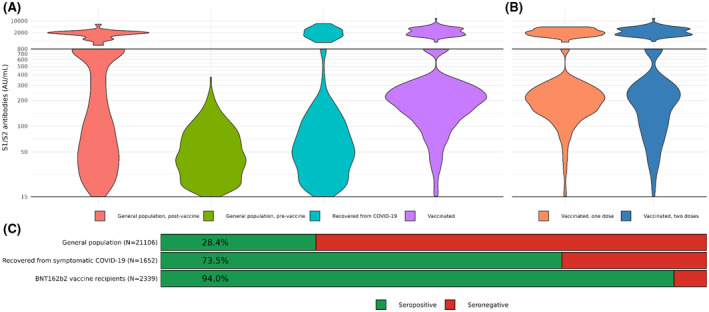
S1/S2 IgG antibody serum concentration (AU/ml) of seropositive participants from (A) The general population before and after the introduction of the vaccine, patients recovering from COVID‐19 and BNT162b2 vaccine recipients, with the latter expanded to (B) participants who received a single dose and those who received two doses of vaccine. The lines mark the range of detection for serology results (15–800), with the subset of high concentrations displayed above the line. (C) Bar plots of seropositivity between the groups. General population and vaccinated sub‐groups are similar in their seropositive fractions and are therefore combined
Among the seropositive participants, serum concentrations of IgG antibodies were also highly correlated with their group association. Individuals that were previously infected with SARS‐CoV‐2, but remained asymptomatic had the lowest antibody concentrations, 59 ± 1.8 AU/ml (Mean ± CI 95%), while those recovering from a recent symptomatic disease, had significantly higher concentrations and variance: 110 ± 20.2 AU/ml (Figure 1A).
Serum antibody concentrations of the vaccinated group, 316.3 ± 27.9 AU/ml, were significantly higher than the unvaccinated and with greater variance. Antibody levels of vaccinated participants were significantly higher for double‐vaccinated (395 ± 53.6 AU/ml), compared to participants who received a single dose (244 ± 20.9 AU/ml) (Figure 1B).
The distribution of antibody concentrations in the general population group undergoes a major shift after the introduction of the BNT162b2 vaccine. While the pre‐vaccine group is centered around a single low‐to‐moderate peak, the post‐vaccine group has a bimodal distribution, with an added peak around the higher value range, in agreement with results from recently vaccinated participants (Figure 1A).
Some participants had antibody concentration that is higher than the assay's upper limit of detection (>800 AU/ml; see Section 2), these results were commonly seen in the vaccinated groups and a minority of recovering COVID‐19 patients, but not in asymptomatic infections (Figure 1A,B).
3.3. BNT162b2‐elicited short‐term antibody response
We focus on twice‐vaccinated participants only, due to their greater relevance to long‐term immunity and availability of exact vaccination dates (N = 772).
Antibody levels of second vaccine dose recipients displayed a distinct, two‐phase pattern of a rapid increase in antibody levels in the seroconversion phase, followed by stabilization or decline. A week after the second vaccine dose, all participants were seropositive, save for one (Figure 2A).
FIGURE 2.
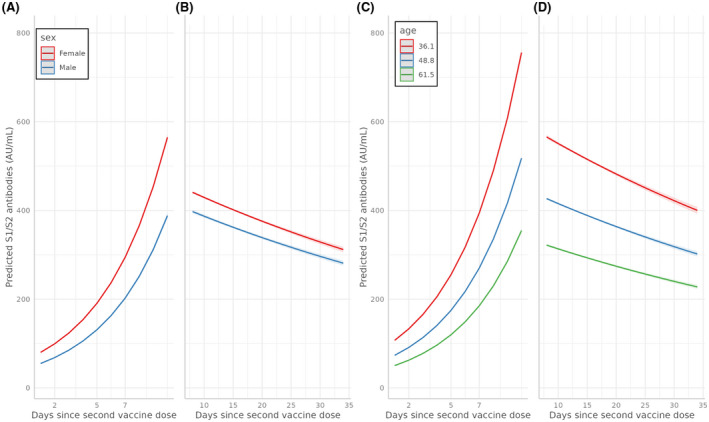
Predicted S1/S2 IgG antibody serum concentration (AU/ml) by sex (A, B) and age group (C, D), in the initial 10 days seroconversion phase after the second vaccine dose (A, C) and in the subsequent decline, over a 35‐day period (B, D)
Age, sex, and the number of days that passed from vaccine administration, were all significantly associated with antibody concentration results, both independently and in interaction with each other (p < e−16). The most significant factor influencing antibody concentration is age, which is negatively correlated with antibody concentration (p < 1e−16), followed by a negative correlation with the male sex (p < 1e−16). The effects of age and sex on the antibody response are significant from seroconversion, when the increase in antibodies is steeper in young vaccine recipients, with females having higher concentrations compared to their male counterparts (Figure 2A).
After seroconversion, 7–10 days after vaccination, antibody concentrations reach their peak in all groups and remain significantly lower in males (250 ± 51.1 AU/ml vs. 409 ± 94.1 AU/ml; Wilcox p < .005) and older participants (Pearson = −0.31; p < 3e−5).
At the latter phase, 7–35 days after vaccination, we see significant waning of antibody concentrations in all participants, but seropositivity persists and remains relatively high. Despite maintaining relative stability, antibody decline is more pronounced and significant in older participants (p < 1e−16) and males (p < 1e−16).
3.4. BNT162b2‐elicited long‐term antibody response
To examine the long‐term durability of humoral antibodies following vaccination with BNT162b2, we tested serum samples of 800 participants, 6 months after receiving the second vaccine. All participants were also tested for lack of IgG antibodies specific to SARS‐CoV‐2 Nucleocapsid protein, to assure none were infected by the virus and the humoral response was associated with the vaccine alone.
We found that 42/800 (5.2%) of participants were seronegative 6 months after vaccination, compared with 2/455 (0.4%) who were tested within the first 7–35 days.
Seropositive participants, while having significantly decreased antibody concentrations within the 6 months period, maintained a mostly stable and less variable antibody concentration across age groups (Figure 4). Age alone remains the most significant factor influencing antibody concentrations (p < 1e−16), followed by time since vaccination (p < 1e−16). The reduction of antibody concentration in males is less significant in the 6‐months group and more age‐dependent, as evident by the greater negative correlation between age and antibody concentration in males at the 6‐months group (pearson = −1.18; p = .003), compared with their female counterparts (pearson = −0.09; p = .03).
FIGURE 4.
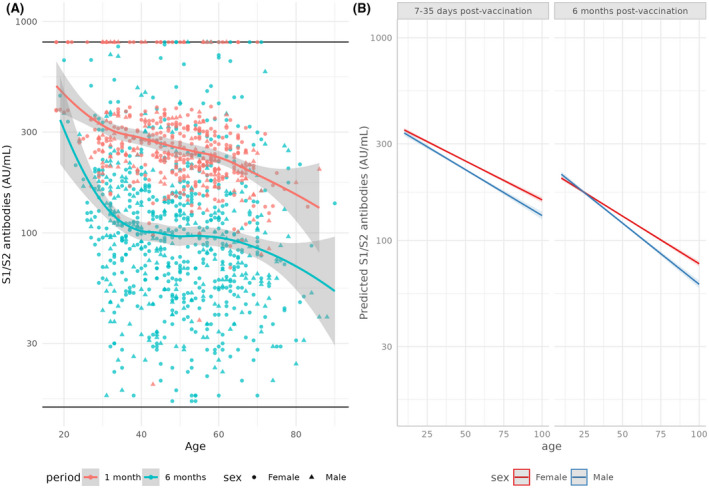
Levels of serum S1/S2 antibodies (AU/ml) in recipients of the BNT162b2 vaccine, by age, tested 7–35 days or 6 months after administration of the second dose, (A) as measured in our cohort and (B) as predicted by a generalized Poisson model. (A) Horizontal lines mark the upper (800 AU/ml) and lower (15 AU/ml) limits of detection. Trends are illustrated by loess curves, with 95% confidence intervals
3.5. Serum antibodies elicited by SARS‐CoV‐2 infection
We compared results from seropositive convalescent individuals recovering from symptomatic COVID‐19, with seropositive unvaccinated individuals suspected to have had asymptomatic COVID‐19.
There is a distinct U‐curve association between age and antibody concentration in both groups, starting with high concentrations in children, declining to a stable low level in young adults, then rising again around age 35 (Figure 3).
FIGURE 3.
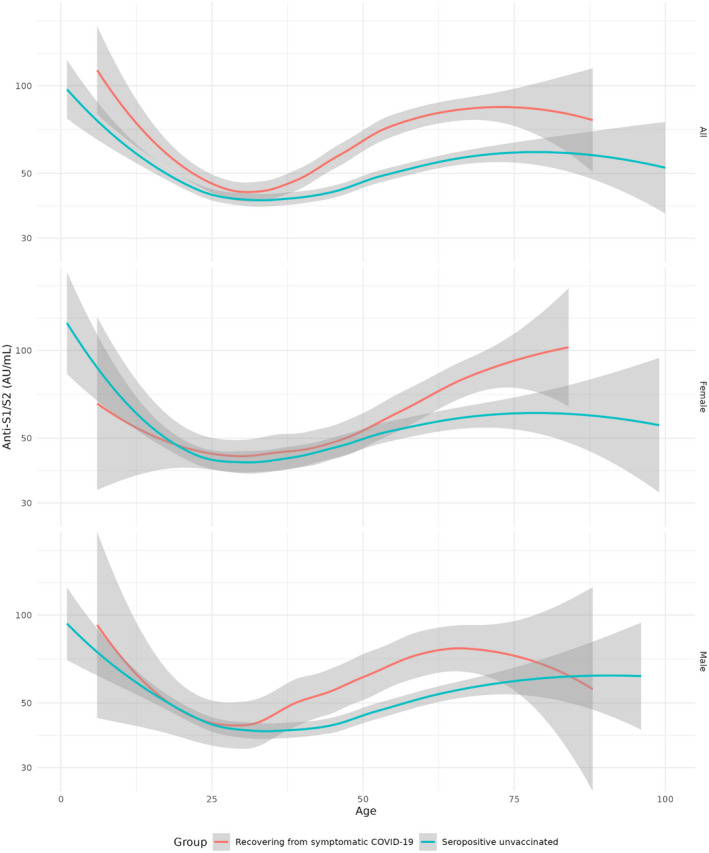
Levels of serum S1/S2 antibodies (AU/ml) in unvaccinated convalescent individuals, grouped by sex, split to individuals recovering from symptomatic and suspected asymptomatic COVID‐19
Regardless of age, individuals recovering from symptomatic COVID‐19 had significantly higher antibody levels compared to asymptomatic ones (Kruskal p = 2.3e−09). Among males the difference between the symptomatic and asymptomatic COVID‐19 groups is highly significant (Kruskal p = .0005), while in females, it is weak and only marginally significant (Kruskal p = .06). Symptom‐related differences in antibody levels of males are significant after age 30 (Kruskal p = 8e−6), while in females the difference is apparent much later, in ages 50 or older (Kruskal p = 6e−4). A closer examination reveals that the difference in antibody levels between the symptomatic and asymptomatic groups is generally insignificant in ages 30 or younger (Kruskal p = .44), but becomes highly significant after the age of 30 (Kruskal p = 7.1e−11).
3.6. Hyper‐responders
A small fraction of participants had antibody concentrations beyond the upper limit of detection (800 AU/ml), we shall name them hyper‐responders. Hyper‐responders were most prevalent in the vaccinated groups, making up 5.2% of participants in both the vaccinated group and general population samples taken after the vaccination campaign (122/2339 and 681/12926). Among those infected with the virus, only 1.2% were hyper‐responders (21/1652), all of which had symptomatic COVID‐19.
Among the vaccinated, hyper‐responder were younger (45 ± 2.8 vs. 55.1 ± 0.6 (Mean ± CI 95%); aOR = 0.96; p < 2e−6) and more likely to have taken the second vaccine (aOR = 2.8; p < 6e−6).
The group of hyper‐responders recovering from COVID‐19 was too small to draw statistically conclusive results. No significant sex‐biases were detected in any of the groups.
A small minority of hyper‐responders can also be found in the cohort tested 6 months after vaccination (1.3%; 11/800), a rather surprising discovery, considering the substantial reduction in antibody concentrations, which was significant regardless of age and sex. The small group of persistent hyper‐responders, 6 months after vaccination, were not significantly different in terms of age or sex when compared with non‐hyper‐responders.
4. DISCUSSION
In this study, we characterized the antibody response to the BNT162b2 vaccine and SARS‐CoV‐2 infection, in relation to age, sex and the presence of COVID‐19 symptoms. The study was conducted between November 2020 and May 2021, whilst the population was exposed mainly to the B.1.617.2 (Delta clade) and B.1.1.7 (Alpha clade) variants of concern. 16
Serum anti‐RBD IgG antibodies are highly accurate markers of infection 17 and strongly correlate with neutralizing activity 18 and disease severity, 3 but they cannot be used as sole predictors for anti‐SARS‐CoV‐2 neutralizing ability. 19 Convalescent individuals recovering from symptomatic COVID‐19 typically have low plasma titers of RBD‐specific antibodies, however, the antigen‐specific memory B cells that facilitate the antibody response, maintain and enhance their potency for at least a year. 4 , 18 , 20
In agreement with previous studies, levels of IgG serum antibodies elicited by the mRNA vaccine were significantly higher than those of convalescent individuals 18 and inversely correlated with age 21 (Figure 1). In the month following the second BNT162b2 dose, older individuals had lower antibody levels in the seroconversion phase, followed by a steeper decline compared to younger age‐groups (Figure 2). In agreement with past reports, this age‐dependent decline was more significant among male vaccine recipients 22 (Figure 3). Despite apparent decreasing quantities, the potency of the antibodies is mostly unaffected by aging, 23 and neutralizing activity is present at much lower concentrations. 18 Taken together with the results of large‐scale epidemiological studies, 24 our current analysis suggests that BNT162b2 maintains its efficacy in older age groups.
Among unvaccinated convalescent individuals, antibody levels were highest in young children, decreasing to low levels in young adulthood, then increasing again during adulthood (Figure 4). The prevalent association between antibody levels and increased COVID‐19 severity is contradicted by the highest antibody concentration levels belonging to the youngest and least vulnerable age‐group 25 —children (age). The elevated antibody levels might be partially explained by greater specificity of IgG antibodies for the S protein. 26
Overall, seropositive unvaccinated individuals recovering from symptomatic COVID‐19 had higher antibody levels than their asymptomatic counterparts, this difference was highly sex and age‐dependent (Figure 4). Among children and young adults, there was a slight, insignificant increase in antibody levels of symptomatic patients, which was previously associated with asymptomatic and mild COVID‐19. 27 In later adulthood, the antibody levels of symptomatic COVID‐19 patients increased significantly more than the asymptomatic group and the gap between the two grew wider with age.
Among females, the difference in antibody levels between the symptomatic and asymptomatic COVID‐19 groups was the most significant at age 50 and older, while in males this separation occurs much earlier, around 35 years of age. Higher antibody levels are significantly correlated with greater protection against COVID‐19 following vaccination, 28 but among infected adults, they are indicative of severe disease and excessive inflammation. 29
Females are at significantly lower risk of developing severe COVID‐19, this is the result of several factors, including the immunomodulatory effect of estrogen, which serves as a positive protective factor 30 along with other factors like genetic differences 31 and cultural habits. 32 According to our results, the increase in antibody levels of women recovering from symptomatic COVID‐19 starts at age 50, coinciding with menopause. 33 It requires additional studies in order to ascertain whether the rising antibody levels in women over the age of 50 might be the result of the menopausal drop in estrogen. 34
Following this conclusion, it can be postulated that the earlier separation of antibody levels between the symptomatic and asymptomatic males reflects increased susceptibility, coinciding with increased COVID‐19 susceptibility. It is currently estimated that male testosterone levels decrease significantly by age 40, 35 approximately matching with the age at which the antibody concentration difference between symptomatic and asymptomatic males becomes significant.
The previous association of higher testosterone with immunosuppression and weaker antiviral response suggests that a similar association might explain the higher COVID‐19 mortality in males, 36 , 37 however, the results of current COVID‐19 studies do not support this association. Preliminary studies found that lower serum testosterone increased the risk of COVID‐19 hospitalization and mortality in males, 38 , 39 suggesting that, in contrast to other viral diseases such as influenza, low testosterone is a risk factor for severe disease. This is further supported by our findings, indicating that antibody response to vaccination is lower in males, but the difference becomes significant only among the older age groups, after the age‐related decline in testosterone (Figure 3).
In conclusion, the humoral response to SARS‐CoV‐2 infection and vaccination is distinct and strongly influenced by age, sex and COVID‐19 symptoms, and is correlated to findings in previous studies. 40 , 41 Large‐scale antibody testing 42 could be beneficial for prioritization of susceptible individuals, increasing the effectiveness of vaccine distribution. 43 Disparities in antibody levels between the various groups are reflective of numerous under‐explored phenomena with potential clinical implications, such as the pediatric immune response to SARS‐CoV‐2 infection, disease severity, hospitalization time, and the status of underlying impaired immunity deficiencies such as MS and other autoimmune diseases. 44 , 45
5. LIMITATIONS
The study has several limitations. First, the exclusion of some clinical, demographic and lifestyle parameters, such as ethnicity, BMI, smoking and medical conditions, which were previously shown to affect the magnitude and duration of the humoral response. Second, the generalization of COVID‐19 patients to symptomatic and asymptomatic is reductive and not very indicative of disease severity or outcome. Furthermore, the exact timing of sample collection relative to initial infection and hospitalization is not known. Third, the relatively few young vaccine recipients (a result of national vaccine prioritization policy) made them under‐represented in the analysis. Finally, it is highly probable that at least some members of the cohort had undocumented and possibly asymptomatic COVID‐19 in the past, leading to altered response to repeat infection and/or vaccination.
DISCLOSURES
The authors have declared no competing interest.
ACKNOWLEDGEMENTS
The Shomron Laboratory is supported by the Israel Science Foundation (ISF; 1852/16); Horizon 2020 ‐ Research and Innovation Framework Programme, PSY‐PGx; Israeli Ministry of Defense, Office of Assistant Minister of Defense for Chemical, Biological, Radiological and Nuclear (CBRN) Defense; Foundation Fighting Blindness; The Edmond J. Safra Center for Bioinformatics at Tel Aviv University; The Koret‐UC Berkeley‐Tel Aviv University Initiative in Computational Biology and Bioinformatics; The QBI/UCSF‐Tel Aviv University joint Initiative in Computational Biology and Drug Discovery; Zimin Institute for Engineering Solutions Advancing Better Lives; Tel Aviv University Richard Eimert Research Fund on Solid Tumors; Collaborative clinical Bioinformatics research of the Edmond J. Safra Center for Bioinformatics and Faculty of Medicine at Tel Aviv University; Djerassi‐Elias Institute of Oncology; Canada‐Montreal Friends of Tel Aviv University; Donations from Harold H. Marcus, Amy Friedkin, Natalio Garber, Tal Zohar; Kirschman Dvora Eleonora Fund for Parkinson's Disease; Joint funding between Tel Aviv University and Yonsei University; Israeli Ministry of Science and Technology, Israeli–Russia; The Center for Combating Pandemics at Tel Aviv University; Aufzien Family Center for the Prevention and Treatment of Parkinson’s Disease; and a generous donation from the Adelis Foundation.
AUTHOR CONTRIBUTIONS
Guy Shapira did all statistical analysis, figure creation and wrote most of the manuscript. Ramzia Abu Hamad was the primary researcher in charge of lab‐work and data collection. Chen Weiner, Nir Rainy, Reut Sorek‐Abramovich and Patricia Benveniste‐Levkovitz orchestrated sample collection and high‐throughput testing. Rachel Rock and Eden Avnat added literature reviews and expert opinions. Osnat Levtzion‐Korach, Adina Bar Chaim and Noam Shomron designed and administrated the research and provided small additions to the manuscript.
ETHICS APPROVAL
The study was approved by the Institutional Review Board of Shamir Medical Center (Assaf Harofe) and followed the tenets of the Declaration of Helsinki (146‐21‐asf).
Supporting information
Supplementary Material
Shapira G, Abu Hamad R, Weiner C, et al. Population differences in antibody response to SARS‐CoV‐2 infection and BNT162b2 vaccination. FASEB J. 2022;36:e22223. doi: 10.1096/fj.202101492R
Guy Shapira and Ramzia Abu Hamad are equal first authors.
Adina Bar Chaim and Noam Shomron are equal last authors.
Funding information
The Shomron Laboratory is supported by the Israel Science Foundation (ISF; 1852/16); Horizon 2020—Research and Innovation Framework Programme, PSY‐PGx; Israeli Ministry of Defense, Office of Assistant Minister of Defense for Chemical, Biological, Radiological and Nuclear (CBRN) Defense; Foundation Fighting Blindness; The Edmond J. Safra Center for Bioinformatics at Tel Aviv University; The Koret‐UC Berkeley‐Tel Aviv University Initiative in Computational Biology and Bioinformatics; The QBI/UCSF‐Tel Aviv University joint Initiative in Computational Biology and Drug Discovery; Zimin Institute for Engineering Solutions Advancing Better Lives; Tel Aviv University Richard Eimert Research Fund on Solid Tumors; Collaborative clinical Bioinformatics research of the Edmond J. Safra Center for Bioinformatics and Faculty of Medicine at Tel Aviv University; Djerassi‐Elias Institute of Oncology; Canada‐Montreal Friends of Tel Aviv University; Donations from Harold H. Marcus, Amy Friedkin, Natalio Garber, Tal Zohar; Kirschman Dvora Eleonora Fund for Parkinson's Disease; Joint funding between Tel Aviv University and Yonsei University; Israeli Ministry of Science and Technology, Israeli–Russia; The Center for Combating Pandemics at Tel Aviv University; Aufzien Family Center for the Prevention and Treatment of Parkinson's Disease; and a generous donation from the Adelis Foundation
Contributor Information
Adina Bar Chaim, Email: adinab@shamir.gov.il.
Noam Shomron, Email: nshomron@tauex.tau.ac.il.
DATA AVAILABILITY STATEMENT
Individual‐level data is protected under patient confidentiality laws and cannot be shared publicly. Requests for data should be sent to the corresponding author.
REFERENCES
- 1. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akkaya M, Kwak K, Pierce SK. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20:229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia‐Beltran WF, Lam EC, Astudillo MG, et al. COVID‐19‐neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476‐488.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591:639‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberg Y, Mandel M, Bar‐On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody‐mediated neutralization: Implications for control of the COVID‐19 pandemic, Cell. 2022;185(3):447–456.e11. doi: 10.1016/j.cell.2021.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung K, Wu JT. Managing waning vaccine protection against SARS‐CoV‐2 variants. Lancet. 2022;399:2‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID‐19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID‐19 outcomes. Nat Rev Immunol. 2020;20:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shapiro JR, Li H, Morgan R, et al. Sex‐specific effects of aging on humoral immune responses to repeated influenza vaccination in older adults. Npj Vaccines. 2021;6:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aging in COVID‐19: vulnerability, immunity and intervention—PubMed. https://pubmed.ncbi.nlm.nih.gov/33137510/ [DOI] [PMC free article] [PubMed]
- 13. Fathi A, Addo MM, Dahlke C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens, Frontiers in Immunology. 2021;11. doi: 10.3389/fimmu.2020.601170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cylus J, Panteli D, van Ginneken E. Who should be vaccinated first? Comparing vaccine prioritization strategies in Israel and European countries using the Covid‐19 Health System Response Monitor, Israel Journal of Health Policy Research. 2021;10(1). doi: 10.1186/s13584-021-00453-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perkmann T, Perkmann‐Nagele N, Koller T, et al. Anti‐spike protein assays to determine SARS‐CoV‐2 antibody levels: a head‐to‐head comparison of five quantitative assays. Microbiol. Spectr. 2021;9:e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mishra S, Mindermann S, Sharma M, et al. Changing composition of SARS‐CoV‐2 lineages and rise of Delta variant in England, EClinicalMedicine. 2021;39:101064. doi: 10.1016/j.eclinm.2021.101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients, Science Immunology. 2020;5(52). doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Z, Muecksch F, Schaefer‐Babajew D, et al. Naturally enhanced neutralizing breadth against SARS‐CoV‐2 one year after infection, Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naïve and recovered individuals after mRNA vaccination, Science Immunology. 2021;6(58). doi: 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature. 2020;584:437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller L, Andrée M, Moskorz W, et al. Age‐dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination, Clinical Infectious Diseases. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS‐CoV‐2 BNT162b2 vaccine, EClinicalMedicine. 2021;36:100928. doi: 10.1016/j.eclinm.2021.100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blomberg BB, Frasca D. Quantity, not quality, of antibody response decreased in the elderly. J Clin Invest. 2011;121:2981‐2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age‐specific mortality and immunity patterns of SARS‐CoV‐2. Nature. 2021;590:140‐145. [DOI] [PubMed] [Google Scholar]
- 26. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS‐CoV‐2 in children and adults across the COVID‐19 clinical spectrum. Nat Immunol. 2021;22:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jonsdottir HR, Bielecki M, Siegrist D, et al. Titers of Neutralizing Antibodies against SARS‐CoV‐2 Are Independent of Symptoms of Non‐Severe COVID‐19 in Young Adults, Viruses. 2021;13(2):284. doi: 10.3390/v13020284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 29. Hoepel W, Chen H‐J, Geyer CE, et al. High titers and low fucosylation of early human anti–SARS‐CoV‐2 IgG promote inflammation by alveolar macrophages, Science Translational Medicine. 2021;13(596). doi: 10.1126/scitranslmed.abf8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costeira R, Lee KA, Murray B, et al. Estrogen and COVID‐19 symptoms: associations in women from the COVID Symptom Study. PLoS One. 2021;16:e0257051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asano T, Boisson B, Onodi F, et al. X‐linked recessive TLR7 deficiency in ~1% of men under 60 years old with life‐threatening COVID‐19, Science Immunology. 2021;6(62). doi: 10.1126/sciimmunol.abl4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penna C, Mercurio V, Tocchetti CG, Pagliaro P. Sex‐related differences in COVID‐19 lethality. Br J Pharmacol. 2020;177:4375‐4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parazzini F. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas. 2007;56:280‐287. [DOI] [PubMed] [Google Scholar]
- 34. Al‐kuraishy HM, Al‐Gareeb AI, Faidah H, et al. The Looming Effects of Estrogen in Covid‐19: A Rocky Rollout, Frontiers in Nutrition. 2021;8. doi: 10.3389/fnut.2021.649128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelsey TW, Li LQ, Mitchell RT, et al. A Validated Age‐Related Normative Model for Male Total Testosterone Shows Increasing Variance but No Decline after Age 40 Years, PLoS ONE. 2014;9(10):e109346. doi: 10.1371/journal.pone.0109346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furman D. Sexual dimorphism in immunity: improving our understanding of vaccine immune responses in men. Expert Rev. Vaccines. 2015;14:461‐471. [DOI] [PubMed] [Google Scholar]
- 37. Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci. 2014;111:869‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID‐19) in SARS‐CoV‐2 infected male patients: a cohort study. Aging Male. 2020;23:1493‐1503. [DOI] [PubMed] [Google Scholar]
- 39. Salonia A, Pontillo M, Capogrosso P, et al. Severely low testosterone in males with COVID‐19: A case‐control study, Andrology. (2021);9(4):1043–1052. doi: 10.1111/andr.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID‐19 in the New York City Area | Critical Care Medicine | JAMA | JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/2765184 [DOI] [PMC free article] [PubMed]
- 42. Vogl T, Leviatan S, Segal E. SARS‐CoV‐2 antibody testing for estimating COVID‐19 prevalence in the population. Cell Rep Med. 2021;2:100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujimoto AB, Keskinocak P, Yildirim I. Significance of SARS‐CoV‐2 specific antibody testing during COVID‐19 vaccine allocation. Vaccine. 2021;39:5055‐5063. doi: 10.1016/j.vaccine.2021.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med. 2021;27:1990‐2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Longitudinal dynamics of SARS‐CoV‐2‐specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. https://journals.plos.org/plospathogens/article?id= 10.1371/journal.ppat.1010211 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Individual‐level data is protected under patient confidentiality laws and cannot be shared publicly. Requests for data should be sent to the corresponding author.


