Abstract
Estimating the total coronavirus disease 2019 (COVID‐19) mortality burden of solid organ transplant recipients (SOTRs), both directly through COVID‐19 infection and indirectly through other impacts on the healthcare system and society, is critical for understanding the disease's impact on the SOTR population. Using SRTR data, we modeled expected mortality risk per month pre‐COVID (January 2015–February 2020) for kidney/liver/heart/lung SOTRs, and compared monthly COVID‐era deaths (March 2020–March 2021) to expected rates, overall and among subgroups. Deaths above expected rates were designated "excess deaths." Between March 2020 and March 2021, there were 3739/827/265/252 excess deaths among kidney/liver/heart/lung SOTRs, respectively, representing a 41.2%/27.4%/18.5%/15.0% increase above expected deaths. 93.0% of excess deaths occurred in patients age≥50. The observed:expected ratio was highest among Hispanic SOTRs (1.82) and lowest among White SOTRs (1.20); 56.0% of excess deaths occurred among Black or Hispanic SOTRs. 64.7% of excess deaths occurred among patients who had survived ≥5 years post‐transplant. Excess deaths peaked in January 2021; geographic distribution of excess deaths broadly mirrored COVID‐19 incidence. COVID‐19 likely caused over 5000 excess deaths among SOTRs in the US in a 13‐month period, representing 1 in 75 SOTRs and a substantial proportion of all deaths among SOTRs during this time. SOTRs will remain at elevated mortality risk until the COVID‐19 pandemic can be controlled.
Keywords: clinical research / practice, infection and infectious agents – viral: SARS‐CoV‐2/COVID‐19, organ transplantation in general, patient survival
Abbreviations
- SOTR
solid organ transplant recipient
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
- O/E
observed to expected
1. INTRODUCTION
As of November 2021, COVID‐19 has caused over 46 million reported infections and over 750 000 deaths in the United States. However, it is likely that even this number substantially underestimates the total number of deaths caused by COVID‐19. 1 , 2 In addition to causing death directly from infection, COVID‐19 may cause death indirectly through many mechanisms; death can be the ultimate result of pandemic‐related stresses such as loss of wages or insurance, disruption to health care due to suspension of nonemergency procedures, avoidance of hospitals or emergency departments, and even "deaths of despair"—suicides, drug overdoses, and alcohol‐related liver disease. 3 , 4 , 5
High COVID‐19 case‐fatality rates have been reported among solid organ transplant recipients (SOTRs), 6 , 7 , 8 , 9 , 10 but the total mortality burden among transplant recipients is unknown. Estimating total mortality due to the COVID‐19 epidemic among SOTRs is critical for understanding the impact of the pandemic on the transplant population, and has implications for the broader US population living with chronic disease.
To estimate deaths attributable to the COVID‐19 pandemic, we conducted a retrospective study using national registry data from the United States.
2. METHODS
2.1. Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait‐listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This dataset has previously been described elsewhere. 11 This study was classified as "exempt—not human subjects research" by the Johns Hopkins School of Medicine Institutional Review Board.
2.2. Study population
The study population consisted of kidney, liver, heart, and lung transplant recipients who were residents of the United States (the 50 US states, the District of Columbia, or Puerto Rico); who received a transplant between July 1, 2004 and March 31, 2020; and who were alive on January 1, 2015. SOTRs who received a transplant before July 1, 2004 were excluded because the state of residence was not available for such recipients.
2.3. Expected deaths
To model the expected death rate among SOTRs we used a discrete‐time proportional hazards framework. Specifically, separately for kidney, liver, heart, and lung recipients, we created a dataset of pre‐COVID person‐months January 2015–February 2020. Using this dataset, we modeled the probability of death in each person‐month using multilevel logistic regression with a random intercept for the state of residence at time of transplant and adjusting for age (categorized as <12 years, 12–17 years, 18–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, or ≥70 years), sex, self‐reported race (classified as White, Hispanic, Black, Asian, or other), pretransplant history of diabetes, primary payer (classified as private, Medicare, Medicaid, other public, or other), living vs deceased donor, elapsed time since transplant (classified as 0–1 months, 2–3 months, 4–6 months, 7–12 months, 13–36 months, 37–60 months, 61–119 months, or ≥120 months), years on dialysis prior to transplant (for kidney recipients), calendar month (as indicator variables), and calendar year (continuous). Elapsed time since transplant, calendar month, and calendar year were time‐varying; other variables were measured at the time of transplant. Then, for each COVID‐era person‐month March 2020–March 2021, we calculated the expected probability of death during that person‐month using the previously described pre‐COVID logistic regression model. Summing these probabilities for any group of patients gives an expected number of deaths for that cohort: for example, if three individuals have 5%, 10%, and 12% probability of death within a given month, the total number of expected deaths among the three is 5%+10%+12% = 0.27 expected deaths. We added up these probabilities, overall and in subgroups, to obtain the expected number of deaths per month for each month March 2020–March 2021. Data past April 1, 2021 were excluded due to lags in mortality reporting.
2.4. Statistical analysis
Overall and for various subgroups, we compared observed deaths to expected deaths between March 2020 and March 2021 using a χ2 test. We created maps of state‐level ratios of observed to expected deaths (O/E ratio) separately for the time periods of March–May 2020, June–September 2020, and October 2020–March 2021, corresponding to the first three waves of COVID infections in the United States. We calculated excess deaths as observed minus expected deaths. The estimated number of excess deaths was compared to the number of deaths reported as due to COVID‐19 when cause‐of‐death data was available. Confidence intervals are reported as per the method of Louis and Zeger. 12 All analyses were performed using Stata 17.0/MP for Linux (College Station, TX). Maps were created using the Stata shp2dta and spmap packages. The map legend was superimposed onto the map using Microsoft Paint for Windows 10 (Redmond, WA).
3. RESULTS
3.1. Study population
Data from 401 263 recipients (17 605 703 person‐months) between January 2015 and February 2020 was used to construct the models of expected deaths; specifically, 252 651 kidney recipients, 91 026 liver recipients, 35 097 heart recipients, and 22 488 lung recipients. There were 381 966 transplant recipients (242 633 kidney/87 645 liver/33 504 heart/18 184 lung) who were either alive on February 29, 2020 or who received a transplant between March 1, 2020 and March 31, 2021 and thus were at risk of death between March 2020 and March 2021.
3.2. Observed and expected deaths by organ group
Among kidney recipients, there were 12726 observed vs 8987.0 expected deaths between March 2020 and March 2021, representing 3739 excess deaths and an O/E ratio of 1.42 (Table 1). Among liver recipients, there were 3852 observed vs 3024.6 expected deaths, representing 827.4 excess deaths and an O/E ratio of 1.27. Among heart recipients, there were 1699 observed vs 1433.9 expected deaths, representing 265.1 excess deaths and an O/E ratio of 1.18. Among lung recipients, there were 1932 observed versus 1679.6 expected deaths, representing 252.4 excess deaths and an O/E ratio of 1.18.
TABLE 1.
Observed and expected deaths among SOTRs, March 2020–March 2021
| Subgroup | Observed | Expected | Excess | O/E ratio |
|---|---|---|---|---|
| Kidney | 12 726 | 8987.0 | 3739.0 | 1.42 |
| Liver | 3852 | 3024.6 | 827.4 | 1.27 |
| Heart | 1699 | 1433.9 | 265.1 | 1.18 |
| Lung | 1932 | 1679.6 | 252.4 | 1.15 |
| Age | ||||
| 0–11 | 118 | 121.9 | −3.9 | 0.97 |
| 12–17 | 80 | 72.3 | 7.7 | 1.11 |
| 18–29 | 372 | 361.2 | 10.8 | 1.03 |
| 30–39 | 584 | 527.4 | 56.6 | 1.11 |
| 40–49 | 1311 | 1027.6 | 283.4 | 1.28 |
| 50–59 | 3444 | 2524.2 | 919.8 | 1.36 |
| 60–69 | 7360 | 5281.2 | 2078.8 | 1.39 |
| 70+ | 6940 | 5209.2 | 1730.8 | 1.33 |
| Sex | ||||
| Female | 6745 | 5313.0 | 1432.0 | 1.27 |
| Male | 13464 | 9812.1 | 3651.9 | 1.37 |
| Race/Ethnicity | ||||
| Asian | 819 | 597.8 | 221.2 | 1.37 |
| Black | 4671 | 3248.9 | 1422.1 | 1.44 |
| Hispanic | 3158 | 1735.1 | 1422.9 | 1.82 |
| White | 11 177 | 9284.4 | 1892.6 | 1.20 |
| Other | 384 | 259.0 | 125.0 | 1.48 |
| Insurance type | ||||
| Medicaid | 1480 | 1190.8 | 289.2 | 1.24 |
| Medicare | 11 492 | 8216.6 | 3275.4 | 1.40 |
| Other | 179 | 150.0 | 29.0 | 1.19 |
| Other pub | 283 | 203.0 | 80.0 | 1.39 |
| Private | 6775 | 5364.7 | 1410.3 | 1.26 |
| Time since transplant | ||||
| 0–3 months | 972 | 840.5 | 131.5 | 1.16 |
| 4–6 months | 549 | 386.0 | 163.0 | 1.42 |
| 7 months−2 years | 3364 | 2478.2 | 885.8 | 1.36 |
| 3–4 years | 2413 | 1798.1 | 614.9 | 1.34 |
| 5–9 years | 6330 | 4707.6 | 1622.4 | 1.34 |
| 10+ years | 6581 | 4914.7 | 1666.3 | 1.34 |
Bold denotes O/E ratio statistically significantly different from 1.
3.3. Observed and expected deaths over time
Observed deaths exceeded expected deaths each month during the study period (Figure 1A). Excess deaths reached an initial peak in April 2020, then declined in May and June 2020. They reached a smaller secondary peak in July 2020, declined until September 2020, and then increased dramatically, reaching a maximum in January 2021. This temporal pattern corresponded to temporal patterns in COVID‐associated mortality in the United States. The ratio of observed to expected deaths followed similar temporal patterns in each organ group, although there was less temporal variation for liver recipients than for other organ groups (Figure 1B).
FIGURE 1.
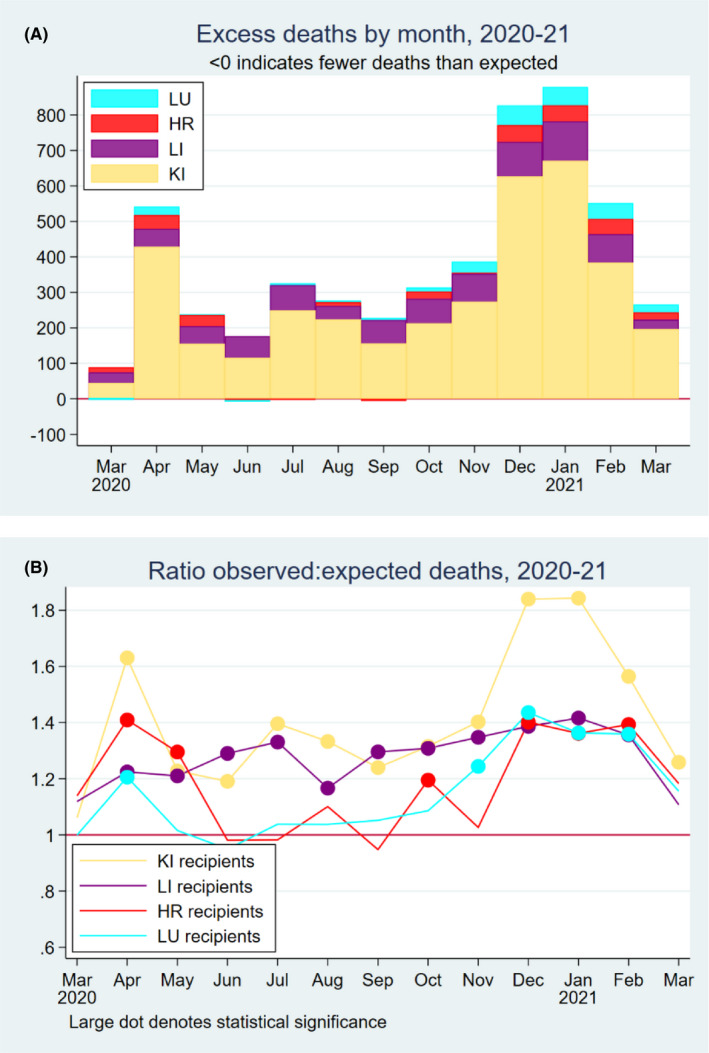
(A) Estimated excess deaths by month and organ type in 2020; (B) ratio of observed to expected deaths among solid organ transplant recipients in 2020
3.4. Observed and expected deaths by patient subgroup
The ratio of observed to expected deaths showed a strong age gradient (Table 1). The number of observed deaths was not statistically significantly different from expected deaths among patients aged 0–29, but the O:E ratio exceeded 1.30 for patients aged 50 or older (interaction p < .001). The O:E ratio was higher for male patients (1.37) than for female patients (1.27) (interaction p < .001). The O:E ratio varied substantially by race/ethnicity, being highest for Hispanic patients (1.82) and lowest for White patients (1.20) (interaction p < .001). The O:E ratio was higher for patients on Medicare (1.40) and other non‐Medicaid public insurance (1.39) than for patients on Medicaid (1.24) or private insurance (1.26) (interaction p < .001). The O:E ratio was lowest in the first three months posttransplant (1.16), highest 4–6 months posttransplant (1.42), and close to 1.34 thereafter (interaction p < .001).
3.5. Observed and expected deaths by the state of residence
During the first wave of COVID infections March‐May 2020, the O/E ratio was highest in New York (2.33), New Jersey (1.91), and Delaware (1.78) (Figure 2). During the second wave, June–September 2020, the O/E ratio was highest in Mississippi (1.87), Hawaii (1.77), and Arizona (1.62). During the third wave, October 2020–March 2021, the O:E ratio was highest in North Dakota (2.30), New Mexico (1.95), Mississippi (1.85), Arizona (1.85), Nevada (1.85), Alabama (1.84), and West Virginia (1.80). The O/E ratio exceeded 1.5 in only four states during the first wave and six states during the second wave, but in 22 states during the third wave.
FIGURE 2.
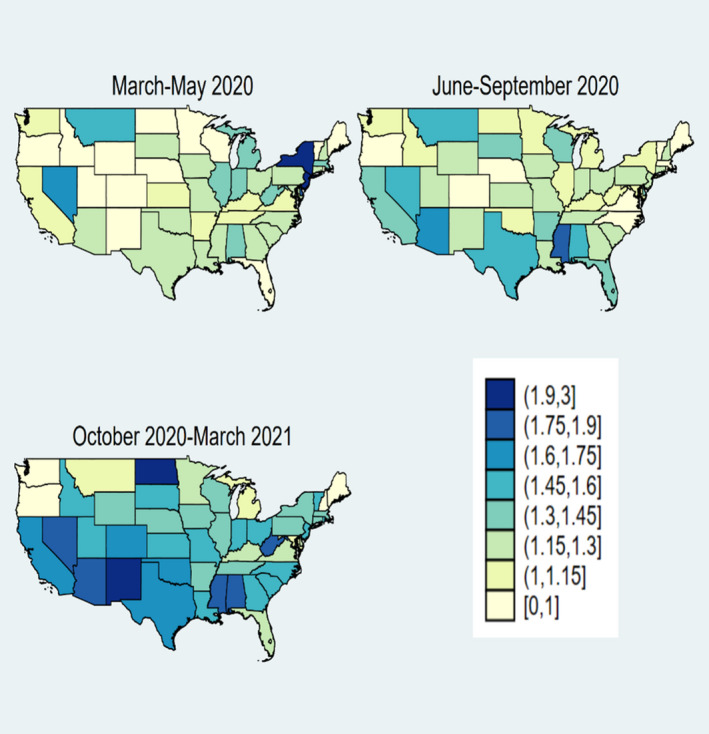
State‐level ratio of observed to expected deaths
3.6. Deaths reported as caused by COVID
Among all deaths among SOTRs between March 2020 and March 2021, cause‐of‐death information was available for 50.4% of deaths in kidney recipients, 72.1% of deaths in liver recipients, 81% of deaths in heart recipients, and 91% of deaths in lung recipients. COVID‐19 was reported as the cause of death for 1836 kidney recipients (vs 3739 estimated excess deaths), 63 liver recipients (vs 827 estimated excess deaths), 18 heart recipients (vs 265 estimated excess deaths), and 3 lung recipients (vs 252 estimated excess deaths).
4. DISCUSSION
In this national study of mortality among SOTRs during the COVID‐19 era, we estimated that there were 5083 excess deaths in the United States in the first 13 months of the pandemic, representing 1 in 75 SOTRs and a substantial proportion of all deaths among SOTRs during the study period. Excess deaths occurred in every month of the COVID era, peaking in January 2021. Although a statistically significant number of excess deaths were observed across categories of organ type, age (except for children and adults under the age of 30), sex, race, and insurance type, the apparent increase in mortality was higher for kidney recipients than for recipients of other organs; higher for older adults; and higher for men than for women. There was substantial variation by race/ethnicity, with the greatest increase seen among Hispanic SOTRs (82% above expected) and the smallest increase among White SOTRs (20% above expected). Spatio‐temporal patterns of excess deaths match patterns of COVID‐19 incidence in the United States, suggesting that the mortality increase is likely in fact due to the COVID‐19 pandemic and not some other cause.
Our study is consistent with reports of substantial excess mortality in the general population. 2 , 3 Excess deaths have also been observed among ESRD patients in the United States. 13 A study of the first wave of COVID‐19 in France estimated 275 excess deaths among 42 812 kidney transplant recipients 14 ; this represented a 78% increase above expected deaths. Our study covers a longer time period (including times of relatively low COVID‐19 incidence); moreover, treatment of COVID‐19 has improved over the course of the epidemic, while social disruption has decreased as society has adapted to the challenges of the pandemic. Nevertheless, excess deaths continued in SOTRs across the duration of our study period. The way deaths are evaluated in publicly‐reported performance evaluations for transplant programs should be considered in light of our findings.
Vaccination is a key factor in reducing death from COVID‐19, but benefits may be attenuated in some SOT recipients. 15 Antibody responses and vaccine effectiveness versus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection are dramatically lower after 2 mRNA vaccines and serious disease is more common. 16 Additional doses and booster vaccines ameliorate antibody and cellular responses in many SOT recipients, 17 , 18 , 19 yet some remain seronegative and likely at high risk of morbidity, particularly in light of the highly‐resistant omicron variant. 20 It is probable that COVID‐19 will be responsible for ongoing excess deaths in this population unless vaccine responses are improved and therapeutics including passive immunoprophylaxis and antiviral therapies are optimized.
Our study must be understood in the context of its limitations. First, we cannot demonstrate that these excess deaths occurred because of COVID‐19 and not for some other reason. However, spatio‐temporal patterns of mortality closely match patterns of COVID‐19 incidence, suggesting that COVID‐19 is the most likely cause, either directly (through fatal infection) or indirectly (through disruptions to medical care, social disruption, economic deprivation, and other causes). There may be unobserved confounding, that is, changes in the composition of the SOTR population. However, our models accounted for likely confounders captured in the national registry, and also included a temporal‐trend term to account for changes in the death rate over time. Moreover, most of the SOTRs alive during the first year of COVID were transplanted before COVID started; it is hard to see how the composition of the SOTR population in the United States could change rapidly enough in a single year to cause the patterns we observe. Our findings may not generalize completely to future waves of COVID‐19 (as treatment improves and social disruption declines) or to other countries (where both characteristics of the epidemic and the sociocultural response have been different). Nevertheless, our findings suggest that SOTRs are at substantial risk during a COVID‐19 wave. Transplant centers and other treatment providers should incorporate COVID prevention into standard care for SOTRs. Encouraging SOTRs to get tested quickly in case of suspected COVID‐19, and to contact health care providers immediately in the event of illness, may further mitigate mortality risk.
Our findings demonstrate the profound impact of COVID‐19 on the SOTR population in the first thirteen months in the United States. The advent of vaccines has led to substantially decreased incidence of COVID‐19, and novel treatments may reduce the future mortality risk for COVID patients. Nevertheless, COVID‐19 management and mitigation will continue to be an essential component of the medical treatment of SOTRs for some time to come.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENTS
This work was supported by grant number U01AI138897 (Segev), K24AI144954 (Segev) and K01DK101677 (Massie) from the National Institute of Allergy and Infectious Diseases (NIAID). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US government.
Massie AB, Werbel WA, Avery RK, Po‐Yu Chiang T, Snyder JJ, Segev DL. Quantifying excess deaths among solid organ transplant recipients in the COVID‐19 era. Am J Transplant. 2022;00:1–6. doi: 10.1111/ajt.17036
DATA AVAILABILITY STATEMENT
These data are available upon request from the Scientific Registry of Transplant Recipients.
REFERENCES
- 1. Rossen LM. Excess Deaths Associated with COVID‐19, by Age and Race and Ethnicity — United States, January 26–October 3, 2020. MMWR. Morbidity and Mortality Weekly Report [Internet] 2020; [cited 2021 Jan 22] 69. Available from: https://www‐cdc‐gov.proxy1.library.jhu.edu/mmwr/volumes/69/wr/mm6942e2.htm [DOI] [PMC free article] [PubMed]
- 2. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID‐19 and other causes, March‐April 2020. JAMA. 2020;324:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiang MV, Irizarry RA, Buckee CO, Balsari S. Every body counts: measuring mortality from the COVID‐19 pandemic. Ann Intern Med. 2020;173:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koob GF, Powell P, White A. Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID‐19. Am J Psychiatry. 2020;177:1031. [DOI] [PubMed] [Google Scholar]
- 5. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID‐19 pandemic. Lancet Psychiat. 2020;7:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID‐19‐related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA‐EDTA Registry indicate a high mortality due to COVID‐19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol. 2021;74:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: A multi‐center cohort study. Clin Infect Dis. Published online August 7, 2020. doi: 10.1093/cid/ciaa1097 [DOI] [Google Scholar]
- 10. Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID‐19 during the course of the pandemic. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics (Oxford, England). 2009;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziemba R, Campbell KN, Yang T‐H, et al. Excess death estimates in patients with end‐stage renal disease — United States, February–August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thaunat O, Legeai C, Anglicheau D, et al. IMPact of the COVID‐19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020;98:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐Dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS‐CoV‐2 infections in adult transplant recipients. Transplantation. 2021;105:e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karaba AH, Zhu X, Liang T, et al. A third dose of SARS‐CoV‐2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamar N, Abravanel F, Marion O, et al. Anti‐SARS‐CoV‐2 spike protein and neutralizing antibodies at one and 3 months after 3 doses of SARS‐CoV‐2 vaccine in a large cohort of solid‐organ‐transplant patients. Am J Transplant [Internet] [cited 2022 Jan 9] n/a. 10.1111/ajt.16950. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G. Antibody response to a fourth messenger RNA COVID‐19 vaccine dose in kidney transplant recipients: A case series. Ann Intern Med [internet]. 2022. [cited 2022 Jan 11] 10.7326/L21-0598. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tseng HF, Ackerson BK & Luo Y et al. Effectiveness of mRNA‐1273 against SARS‐CoV‐2 omicron and delta variants. medRxiv. Published online February 18, 2022. doi: 10.1101/2022.01.07.22268919v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data are available upon request from the Scientific Registry of Transplant Recipients.


