Abstract
Background
Despite increasing knowledge about the optimal treatment for patients with severe COVID‐19, data from different cohorts suggested that survival of patients treated with ECMO seemed to decline over the course of the pandemic.
Methods
In this non‐interventional retrospective single‐center registry study we analyzed all consecutive patients tested positive for SARS‐CoV‐2 infection and supported with VV ECMO in our center during the first three waves of the pandemic. From March 2020 through June 2021, 59 patients have been included.
Results
Overall 90‐day survival was 32%. Besides changes in drug treatment for COVID‐19 and a lower PaO2/FiO2 ratio before ECMO initiation during the third wave, all other patient baseline characteristics were similar during the three waves. Survival rate was highest during the first wave and lowest during the third wave, yet this difference was not statistically significant.
Conclusions
VV ECMO has shown to be a feasible and safe support option for patients with severe respiratory failure due to COVID‐19. The results from this single‐center study confirm findings from other cohorts showing declining survival rates of patients treated with VV ECMO during the COVID‐19 pandemic, however, the specific reasons for this finding remain unclear.
Keywords: COVID‐19, extracorporeal membrane oxygenation, survival
The results from this comparably large single‐center cohort confirm findings from other cohorts showing declining survival rates of patients treated with VV ECMO during the COVID‐19 pandemic. Thus far, the specific reasons for this observation remain unclear.
1. BACKGROUND
Venovenous extracorporeal membrane oxygenation (VV ECMO) is an established support option for patients with severe respiratory failure. 1 Recent studies suggested a survival benefit for patients supported with ECMO in severe acute respiratory distress syndrome (ARDS) with persistent hypoxemia or hypercapnia when combined with optimized mechanical ventilation support. 2 , 3 , 4 , 5
Early during the coronavirus disease 2019 (COVID‐19) pandemic skepticism was raised about the role of ECMO for the treatment of severe COVID‐19 ARDS. 6 Concerns not only related to the low survival rates initially observed but also to the relationship between the effort and the outcomes, in light of dwindling resources and shortage of staff during the pandemic. 7 , 8 , 9 , 10 However, data from larger cohorts confirmed a benefit for patients with severe presentations of the disease, when appropriately selected. 11 , 12 , 13 , 14 Clinical parameters, such as age or time on mechanical ventilation prior to ECMO, were described as relevant factors for the outcome of ECMO support. 15 , 16 , 17
From the onset of the pandemic, evidence was eagerly collected and rapidly reported, and guidelines were developed and continually revised to provide up‐to‐date evidence to clinicians at the bedside. 18 , 19 Although there are some unique features in COVID‐19, much of the experience with severe respiratory failure in COVID‐19 patients is not different from that with other forms of ARDS, and therefore similar recommendations apply to the treatment with ECMO as in non‐COVID‐19 ARDS. 20 , 21
In our center, the first COVID‐19 patient was admitted to the intensive‐care unit (ICU) in March, 2020. Ever since, following the regional incidence rates, we treated varying numbers of patients on our wards over the course of the first three waves of the pandemic through June 2021. Here we report treatment characteristics and 90‐day survival data of all patients with COVID‐19 treated with ECMO in our center during the first three waves of the pandemic from March 2020 until the end of June 2021.
2. METHODS
This is a non‐interventional retrospective single‐center registry study. All data were collected retrospectively from patient records at the Freiburg University Medical Center Interdisciplinary Medical Intensive‐Care Unit. All patients with reverse transcriptase polymerase chain reaction (rtPCR)‐confirmed severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) infection supported with VV ECMO from the beginning of the COVID‐19 pandemic in our center in March 2020 until the end of the third wave of the pandemic by the end of June 2021 were included.
The study was approved by the institutional ethics committee of the University of Freiburg (EK 151/14) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The need for informed consent was waived due to the retrospective and observational nature of the study and anonymous data evaluation. The RECORD statement was followed for the reporting of this study. 22
Indications for VV ECMO for patients with COVID‐19 ARDS in our center followed previous recommendations and have been applied unchanged during the COVID‐19 pandemic. 2 , 23 These indications include a partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ratio less than 80 mm Hg, or a pH less than 7.25 with a hypercapnic acidosis. Prior to considering ECMO, ventilator support should be optimized and patients should have had a trial of prone positioning, if appropriate. Duration of ECMO support was not limited by pre‐defined time limitations. Treatment was discontinued if events occurred that could not be managed even with maximum support, such as refractory circulatory shock, persistent severe hypercapnia or hypoxemia despite mechanical ventilation and ECMO support, unmanageable multi‐organ failure and severe bleeding complications. Based on clinical judgment, we aimed at weaning from ECMO first and subsequently weaning from mechanical ventilation was attempted.
Four different ECMO systems were used: Sorin SCPC (LivaNova PLC, London, UK), CardioHelp (Getinge AB, Rastatt, Germany), CARL (Resuscitec GmbH, Freiburg, Germany), and CentriMag (Thoratec, Zürich, Switzerland). For connection of the ECMO with the patient blood circulation, a double‐lumen venous cannula (Avalon Elite [Getinge AB, Rastatt, Germany] or Crescent [Medtronic, Minneapolis, MN, USA]) was inserted in the right jugular vein in Seldinger's technique. If a double‐lumen cannula was not feasible, bi‐femoral or femoral‐jugular cannulation using HLS cannula (Getinge AB, Rastatt, Germany) were performed.
As reported previously, in our center patients on venovenous ECMO received anticoagulation treatment with unfractionated heparin or argatroban, aiming for an activated prothrombin time (aPTT) of 40–50 s. In patients with bleeding complications, a lower aPTT was accepted (40 s, if acceptable with regard to the bleeding complications). In case of signs of ECMO circuit thrombosis (not requiring immediate or timely system exchange) an aPTT range of 50–60 s was aimed for. In case of patient thromboembolism (e.g. pulmonary embolism or deep vein thrombosis), the aPTT target was 60–80 s. 24
For all patients included in this study, sex, age, time of hospital and ICU admission, time of initiation of mechanical ventilation, time of initiation of ECMO and decannulation from ECMO, and survival time were recorded. Furthermore, pre‐existing comorbidities, laboratory and treatment parameters required for the calculation of SOFA, RESP and PRESERVE scores and blood gas analysis parameters before initiation of ECMO were collected. 25 , 26 , 27 , 28 Follow‐up period for all patients was 90 days. All data were entered into an electronic chart (Microsoft Excel 2010, Microsoft Corp., Redmond, WA, USA) by members of the study team and cross‐checked after entry by a second study team member for accuracy. For estimation of inspired oxygen fraction in patients not mechanically ventilated, we used the approach described in previous studies to determine the concentration of oxygen supply. 25 , 29 , 30
For statistical analyses, Prism (version 9; GraphPad Software Inc., San Diego, CA, USA) and SPSS Statistics (version 27; IBM Corp., Armonk, NY, USA) were used. Survival time was visualized using Kaplan–Meier plots and statistical differences between the groups were calculated using the Log‐rank (Mantel‐Cox) test. For these analyses, patients were grouped according to the first three waves of the pandemic in our center (first wave: March 2020 – July 2020; second wave: October 2020 – February 2021; third wave: March 2021 – June 2021) or according to the duration of mechanical ventilation before VV ECMO (<3 days vs. 3–7 days vs. >7 days and ≤7 days vs. >7 days).
Baseline and outcome parameters were compared between the groups of patients treated during the three waves of the pandemic. Continuous variables were compared using one‐way analysis of variance (ANOVA). Categorical variables were evaluated using Freeman–Halton tests.
Finally, PaO2/FiO2 ratio and time of treatment with respect to the three waves were entered in a multivariate logistic regression model assessing 90‐day survival as the dependent variable. Results are expressed as odds ratios (OR) with 95% confidence intervals, and p‐values. In all evaluations, a p‐value at or below 0.05 was considered statistically significant.
3. RESULTS
In our center, from March 18, 2020 through June 26, 2021, 59 patients with rtPCR‐confirmed SARS‐CoV‐2 infection were supported with VV ECMO. Of all 59 patients, 15 (25%) were treated during the first wave, 21 (36%) during the second wave and 23 patients (39%) were treated during the third wave of the pandemic (Figure 1). Median age (IQR) of all patients was 59 (53–63) years and 16 (26%) were female. 30 patients (51%) survived until day 30, 23 patients (39%) survived until day 60, and 19 patients (32%) survived until day 90 (Tables 1 and 2).
FIGURE 1.
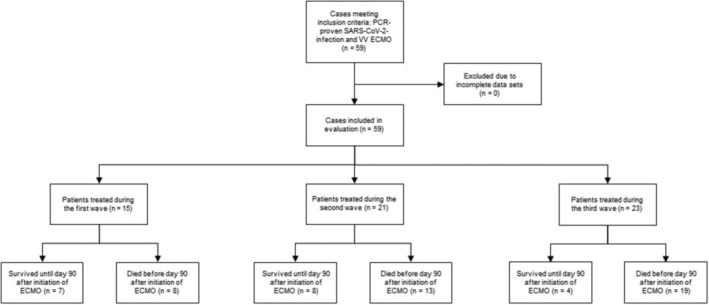
CONSORT flowchart illustrating patient recruitment, case exclusion, and number of survivors and non‐survivors treated during the three waves of the COVID‐19 pandemic reported in this analysis. ECMO, extracorporeal membrane oxygenation
TABLE 1.
Patient baseline characteristics
| All patients (n = 59) | First wave (n = 15) | Second wave (n = 21) | Third wave (n = 23) | p‐value | |
|---|---|---|---|---|---|
| Age (years) | 59 (53–63) | 59 (53–66) | 62 (52–66) | 56 (51–61) | 0.722 |
| Sex | |||||
| Male | 43 (73%) | 11 (73%) | 15 (71%) | 17 (74%) | >0.99 |
| Female | 16 (27%) | 4 (27%) | 6 (29%) | 6 (26%) | >0.99 |
| Body‐mass index (kg/m2) | 29.39 (27–35) | 27.44 (25–30) | 30.86 (28–39) | 30.86 (27–35) | 0.189 |
| Comorbidities | |||||
| Hypertension | 24 (41%) | 6 (40%) | 7 (33%) | 11 (48%) | 0.594 |
| Diabetes | 15 (25%) | 4 (27%) | 4 (19%) | 7 (30%) | 0.696 |
| Coronary heart disease | 7 (12%) | 3 (20%) | 3 (14%) | 1 (4%) | 0.310 |
| Hematological malignancy | 1 (2%) | 1 (7%) | 0 | 0 | 0.254 |
| Solid organ malignancy | 1 (2%) | 0 | 0 | 1 (4%) | >0.99 |
| Immunosuppressive therapy | 3 (5%) | 1 (7%) | 1 (5%) | 1 (4%) | >0.99 |
| Scores | |||||
| SOFA | 9 (7–10) | 10 (8–11) | 8 (7–10) | 8 (7–10) | 0.315 |
| RESP | 1 (0–2) | 1 (0–3) | 1 (0–2.5) | 1 (0–2) | 0.630 |
| PRESERVE | 4 (3–6) | 5 (3–6) | 4 (3–5.5) | 4 (3–6) | 0.588 |
| Pre‐ECMO patient conditions | |||||
| Days of in‐hospital treatment before ECMO | 8.7 (5.6–14.7) | 6.5 (5–11.5) | 10.7 (6.3–17.5) | 10.6 (5.6–14.7) | 0.344 |
| Days of ICU‐treatment before ECMO | 7.65 (4–13) | 5.6 (3–10.4) | 8.0 (5.5–16) | 8.7 (4.6–11.7) | 0.353 |
| Duration of mechanical ventilation before ECMO (days) | 7.8 (4–12.7) | 5.6 (3.7–10.5) | 8.0 (4.1–16) | 8.7 (5.6–11.7) | 0.553 |
| Prone positioning | 49 (83%) | 11 (73%) | 16 (76%) | 22 (96%) | 0.109 |
| Pre‐ECMO ventilation parameters | |||||
| FiO2 (%) | 1.0 (0.9–1.0) | 1.0 (0.85–1.0) | 1.0 (0.88–1.0) | 1.0 (0.95–1.0) | 0.452 |
| Positive end–expiratory pressure (mbar) | 15 (14–16) | 15 (13–18) | 15 (13.5–16) | 15 (14–16) | 0.240 |
| Peak pressure (mbar) | 34 (31–36) | 32 (30–35) | 34 (32–36.5) | 35 (31–36) | 0.772 |
| Dynamic driving pressure (mbar) | 18 (16–21) | 16 (14–19) | 20 (17–23) | 19 (16–22) | 0.281 |
| Tidal volume (ml) | 431 (346–517) | 450 (273–517) | 431 (366–484) | 419 (340–553) | 0.883 |
| Breathing rate (1/min) | 27 (22–32) | 22 (20–27) | 27 (22–32) | 30 (24–34) | 0.055 |
| Pre–ECMO arterial blood gas analysis | |||||
| pH | 7.31 (7.21–7.40) | 7.28 (7.12–7.36) | 7.32 (7.16–7.40) | 7.35 (7.26–7.41) | 0.295 |
| PaO2 (mm Hg) | 64.2 (50.9–75.4) | 69.1 (56–84.2) | 67.0 (57.8–78.8) | 54.1 (47.6–67.9) | 0.018 |
| PCO2 (mm Hg) | 60.0 (46.9–71.7) | 63.8 (42.9–80) | 61.9 (49–72.9) | 56.2 (46.9–69.6) | 0.498 |
| PaO2/FiO2 | 67.58 (51.9–84.2) | 83.78 (56–94.5) | 71.06 (58.96–89) | 56.90 (47.6–74.13) | 0.047 |
| Bicarbonate (mmol/l) | 24.1 (22.5–28.2) | 23.90 (21.8–26.1) | 24.1 (21–29.65) | 24.70 (23.5–29.3) | 0.329 |
| Lactate (mmol/l) | 1.56 (1.2–2.0) | 1.9 (1.2–2.2) | 1.5 (1.1–1.85) | 1.50 (1.4–1.9) | 0.934 |
| Medical treatment | |||||
| Hydroxychloroquin | 11 (19%) | 10 (67%) | 0 | 1 (4%) | <0.001 |
| Lopinavir–ritonavir | 6 (10%) | 6 (40%) | 0 | 0 | <0.001 |
| Tocilizumab | 9 (15%) | 3 (20%) | 0 | 6 (26%) | 0.031 |
| Remdesivir | 8 (14%) | 2 (13%) | 5 (24%) | 1 (4%) | 0.267 |
| Methylprednisolone | 46 (78%) | 2 (13%) | 21 (100%) | 23 (100%) | <0.001 |
Note: Data are median (IQR) or n (%). Continuous variables were compared using one‐way analysis of variance (ANOVA). Categorical variables were evaluated using Freeman–Halton tests.
Abbreviations: ECMO, exatrcorporeal membrane oxygenation; ICU, intensive–care unit; PRESERVE, predicting death for severe ARDS on venovenous ECMO; RESP, respiratory extracorporeal membrane oxygenation survival prediction; SOFA, sequential organ failure assessment.
TABLE 2.
ECMO support and outcome
| All patients (n = 59) | First wave (n = 15) | Second wave (n = 21) | Third wave (n = 23) | p‐value | |
|---|---|---|---|---|---|
| ECMO—cannulation strategy | |||||
| Dual–lumen, jugular | 43 (73%) | 8 (54%) | 17 (81%) | 18 (78%) | 0.178 |
| Femoral–femoral | 12 (20%) | 5 (33%) | 4 (19%) | 3 (13%) | 0.360 |
| Femoral–jugular | 4 (7%) | 2 (13%) | 0 | 2 (9%) | 0.289 |
| ECMO support duration (days) | 29.4 (27–35) | 17.9 (10.3–31.7) | 13.8 (7.7–31.1) | 19.7 (10.9–33.6) | 0.677 |
| Causes of death | |||||
| Intracranial hemorrhage | 9 (15%) | 2 (13%) | 4 (19%) | 3 (13%) | 0.902 |
| Other major bleeding | 3 (5%) | 1 (7%) | 0 | 1 (4%) | 0.718 |
| Respiratory failure | 10 (7%) | 2 (13%) | 2 (10%) | 6 (26%) | 0.343 |
| Septic shock | 11 (19%) | 2 (13%) | 5 (24%) | 4 (27%) | 0.766 |
| Multiorgan failure | 7 (12%) | 1 (7%) | 1 (5%) | 5 (22%) | 0.235 |
| Unknown | 1 (2%) | 0 | 1 (5%) | 0 | 0.610 |
| Died on ECMO | 36 (61%) | 7 (47%) | 12 (57%) | 17 (74%) | 0.244 |
| Survival rate after ECMO initiation | |||||
| 30 days | 30 (51%) | 10 (67%) | 9 (43%) | 11 (48%) | 0.424* |
| 60 days | 23 (39%) | 9 (60%) | 9 (43%) | 5 (22%) | 0.165* |
| 90 days | 19 (32%) | 7 (47%) | 8 (38%) | 4 (17%) | 0.205* |
Note: Data are median (IQR) or n (%). Continuous variables were compared using one‐way analysis of variance (ANOVA). Categorical variables were evaluated using Freeman–Halton tests.
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive–care unit.
p–values are derived from Log–rank (Mantel–Cox) tests.
For patient baseline characteristics during the three waves see Table 1. Most baseline parameters did not differ between the three groups. Statistically significant differences were found for the partial pressure of arterial oxygen (PaO2) and the PaO2/FiO2 ratio before initiation of ECMO with lowest values during the third wave of the pandemic. Additionally, COVID‐19 specific medical treatment was different during the three waves. At the beginning of the pandemic, according to the recommendations at that time, most patients were treated with hydroxychloroquine and lopinavir/ritonavir, and only few patients received corticosteroids. During the second wave and the third wave all patients received methylprednisolone. Only few patients received remdesivir, but during the third wave, following recent evidence and recommendations, selected patients were treated with tocilizumab (Table 1).
During the first wave, the SARS‐CoV‐2 wild‐type virus was common. At that time, in our laboratories no sequencing of SARS‐CoV‐2 samples was done, but it can be assumed that all patients were infected with the wild‐type virus. During the second wave of the pandemic, the B.1.1.7 (alpha) variant of concern spread and soon dominated in Germany. In our laboratories, we started sequencing of SARS‐CoV‐2 samples end of December, 2020, but none of the samples from the second wave were positive for B.1.1.7. During the third wave sequencing was done for 14/23 patients (61%), and all were tested positive for B.1.1.7; however, for 9/23 patients (39%) no sequencing data were available.
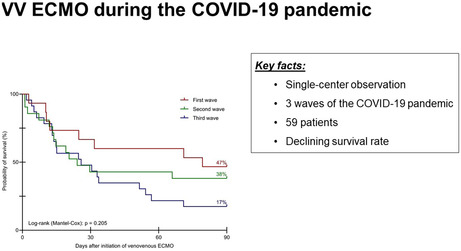
90‐day survival decreased during the course of the pandemic. During the first wave, 7 of 15 patients (47%), during the second wave, 8 out of 21 patients (38%), and during the third wave, 4 out of 23 patients (17%) survived until day 90. Yet, these differences did not reach statistical significance (Figure 2).
FIGURE 2.
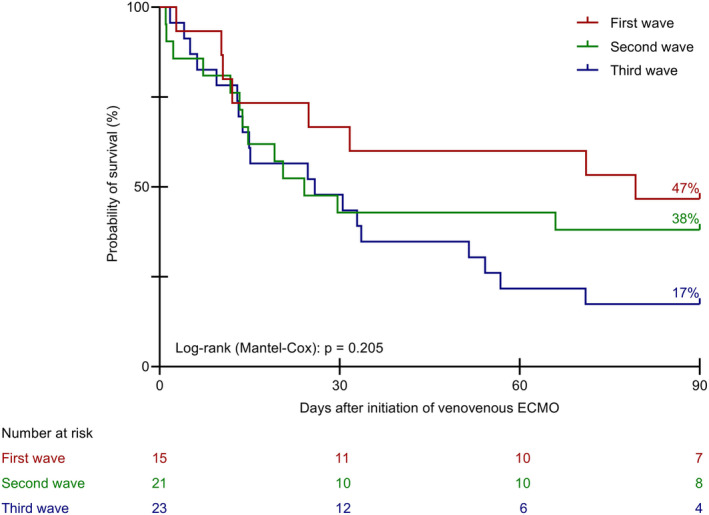
Kaplan–Meier curves for survival time until day 90 during the three waves of the COVID‐19 pandemic. ECMO, extracorporeal membrane oxygenation
The duration of mechanical ventilation before ECMO was not different between the three cohorts (Table 1). Furthermore, we did not reveal a meaningful impact of the duration of mechanical ventilation before initiation of ECMO on the survival of the patients (<3 days vs. 3–7 days vs. >7 days and ≤7 days vs. >7 days, Figures 3 and 4).
FIGURE 3.
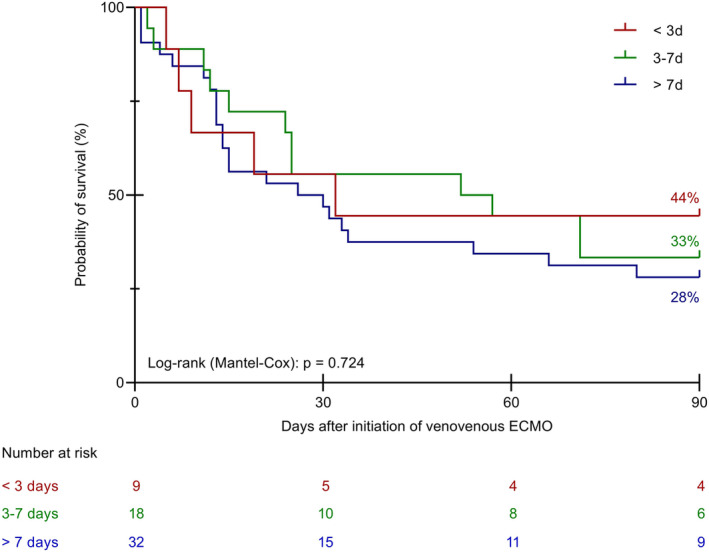
Kaplan–Meier curves for survival time until day 90 relative to the duration of mechanical ventilation prior to VV ECMO (<3 days vs. 3–7 days vs. >7 days). VV ECMO, venovenous extracorporeal membrane oxygenation
FIGURE 4.
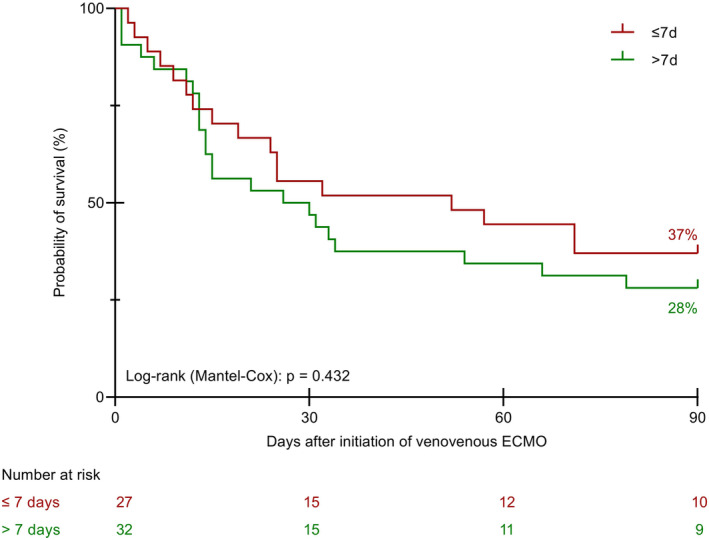
Kaplan–Meier curves for survival time until day 90 relative to the duration of mechanical ventilation prior to VV ECMO (≤7 days vs. >7 days). VV ECMO, venovenous extracorporeal membrane oxygenation
In a multiple logistic regression for the effect of PaO2/FiO2 ratio and treatment during the different waves of the pandemic no statistically significant effect on 90‐day survival was detected (Table 3).
TABLE 3.
Multiple logistic regression for 90–day survival: Effects of pre–ECMO PaO2/FiO2–ratio and treatment during different waves of the pandemic
| Odds ratio (95% CI) | p‐value | |
|---|---|---|
| PaO2/FiO2 | 1.015 (0.995, 1.036) | 0.157 |
| Wave (first vs. second) | 0.634 (0,156, 2.578) | 0.524 |
| Wave (first vs. third) | 0.328 (0.069, 1.549) | 0.159 |
Note: Multiple logistic regression for the effect of PaO2/FiO2–ratio and treatment during different waves of the pandemic on 90–day survival. For both factors no statistically significant effect was detected in this model. For both factors no statistically significant effect was detected in this model.
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen.
4. DISCUSSION
We report treatment data and 90‐day survival from 59 consecutive patients with COVID‐19 supported with VV ECMO in our center during the first three waves of the pandemic between March 2020 and June 2021. Overall survival until day 90 was 32%. Compared with larger cohorts previously reported, the survival rate in our cohort was low. This finding warrants explanation.
90‐day survival in the largest reported cohort of COVID‐19 patients with ECMO extracted from the Extracorporeal Life Support Organization (ELSO) registry was around 50%, and 90‐day survival in another large cohort treated in Paris, France, was 46%. 11 , 31 As a major difference, the patients' median age was higher in our cohort (59 years) compared with the other cohorts (50 and 52 years, respectively). Patient age is known to be an important factor for the probability of survival in COVID‐19; therefore, the higher age in our cohort could at least partly explain lower survival rates. 17 , 32 , 33 Interestingly, a recent analysis of all ECMO treatments during the first three waves in Germany also reported a 32% survival rate. 34 This analysis was based on billing data of all hospitals in Germany, therefore, the risk of selection bias or underreporting of negative results was lower than in previous cohorts. In contrast to this, in the ELSO cohort reporting bias might skew the results to some extent. Participation in the registry was voluntary and complete reporting of all ECMO runs from the participating centers could not be guaranteed. This could lead to underreporting of negative results.
In some places, local guidelines recommend that patients over a certain age limit (e.g., over 70 years) do not receive ECMO. 11 While regarding age as a relevant prognostic factor to be considered when selecting a patient for ECMO, ELSO guidelines do not specify an age limit above which ECMO should not be considered. 18 , 20 However, an expert consensus document, agreed on early during the COVID‐19 pandemic, recommended a patient age at or above 65 years as a relative contraindication for ECMO. 35 In our center, we did not have a specific age limit for patients considered for ECMO. In the here reported cohort, 14/59 patients (24%) were aged 65 years or older. Five of these patients (36%) were alive at day 90 ‐ representing 8% (5/59) of all patients included in this analysis. Probably, most of these patients would have died without ECMO.
In addition to that, we observed declining survival rates of patients treated with ECMO over the course of the three waves of the pandemic. This observation was also reported from other cohorts. 31 , 36 , 37 Yet, this finding is surprising, since it contradicts intuition and previous observations. One would expect that over the course of the pandemic experience and knowledge for the optimal treatment of the disease increased, and therefore outcomes should have improved. This expectation is supported by the observation of higher survival rates for patients with COVID‐19 ARDS treated with ECMO at centers with a caseload of at least 30 ECMO patients in the year before compared with centers with lower numbers in the previous year. 11 Likewise, in an analysis before the COVID‐19 pandemic, a higher annual hospital ECMO volume was associated with lower mortality. 38
A potential reason for the decreasing probability of survival in our cohort may have been pathophysiological changes of COVID‐19 over time due to the evolution of SARS‐CoV‐2 variants. In our center, we started sequencing of SARS‐CoV‐2 samples at the end of December 2020, when the B.1.1.7 (alpha) variant of concern emerged and rapidly spread globally. During the first two waves, we did not detect B.1.1.7 in any of the patients of the here reported cohort. During the third wave, we performed sequencing of the virus genome for 14/23 patients (61%) and all were positive for B.1.1.7. Assuming that most of the remaining patients during this wave in whom the virus was not sequenced were also infected with B.1.1.7, it is conceivable that the B.1.1.7 variant had a negative impact on patient survival.
Fluctuations in quality of care due to increased workload for the personnel may have had an additional impact on the decreasing survival rates during the course of the pandemic. During the first wave, activities in our hospital were focused on the care for an expected very high number of COVID‐19 patients and many non‐emergency admissions and interventions were deferred or canceled. As a result, the available structural and human resources were at all times well above the resources needed to care for the patients – supply bottlenecks and staff overload were avoided. During the second and third waves, in addition to the care for severely diseased COVID‐19 patients, most of the other routine and emergency patient care continued at regular levels, so that the overall workload for physicians and nurses was significantly higher. This, at least in part, might also have had an effect on survival rates.
Ongoing vigilance and continuous observation of survival data, complications, and prognostic factors is not only important on the national level but also regionally and in single centers. This data may be an important basis for decisions about prioritization and limitation of specific services, such as ECMO, when it comes to dwindling resources and shortage of staff during the pandemic. 9
There is uncertainty about the effect of the duration of mechanical ventilation before initiation of ECMO on the survival of the patients. Results from two independent large cohort analyses suggest a poorer outcome for patients that received mechanical ventilation before ECMO for more than 3 days. 11 , 15 Yet, these findings could not be confirmed in a recent analysis of a comparably large cohort from Vienna, Austria. 39 Similarly, in our cohort we did not identify a meaningful effect of the duration of mechanical ventilation before initiation of ECMO on patient survival.
In our cohort, all patients treated during the second and third wave of the pandemic received methylprednisolone. However, this treatment alone did not reflect in an improvement of survival probability compared with patients from the first wave. Hydroxychloroquine and lopinavir/ritonavir were only given to our patients during the first wave, however, one patient during the third wave had also taken hydroxychloroquine as self‐medication before he was admitted to our hospital. The rate of prone positioning was high during all three waves. From the other baseline factors, only PaO2 and the PaO2/FiO2 ratio differed significantly between the cohorts from the three waves. To further analyze the impact of the PaO2/FiO2 ratio on 90‐day survival of the patients we performed a regression analysis, which did not yield a significant result.
During all three waves, intracranial hemorrhage occurred more frequently in our cohort than in other cohorts reported previously. 12 , 31 , 36 We cannot explain with certainty if this is due to baseline differences between our cohort and the other cohorts, such as higher age, or if it is due to any differences in the treatment the patients received.
Several limitations of this study need to be mentioned and considered. First, the data come from a single‐center cohort and the number of patients is comparably small. This limitation is particularly challenging when looking at each wave individually or when comparing the patients from the three different waves—in these analyses each group was particularly small limiting the informative value of statistical comparisons. Furthermore, our hospital is serving as a major referral center for ECMO treatment in our region; therefore, most of the patients included in this analysis were transferred from other centers for initiation of ECMO treatment. Therefore, the treatment for the patients before ECMO may well have been different depending on local standards in the respective center. Finally, data of COVID‐19 ICU patients without ECMO was not available for this work. Therefore, important questions remain with respect to patient selection for ECMO and survival of patients not eligible for ECMO. 40 Consequently, all our findings need to be viewed and interpreted with caution. Nevertheless, the here reported data may be of particular interest in a regional and national context with regard to resource allocation and prognostic factors.
5. CONCLUSIONS
The results from this single‐center cohort of COVID‐19 patients treated with ECMO confirm a role for VV ECMO in the treatment of patients with severe respiratory failure due to COVID‐19. Yet, mortality is high; therefore, patients have to be selected carefully considering appropriate inclusion criteria and available resources.
CONFLICT OF INTEREST
All authors have completed the ICMJE form (available upon request from the corresponding author). AS reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside the submitted work.
AUTHORS CONTRIBUTIONS
Concept/design: Eugen Widmeier and Alexander Supady, Data analysis/interpretation: Eugen Widmeier and Alexander Supady, Drafting article: Alexander Supady, Critical revision of article: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady, Approval of article: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady, Statistics: Eugen Widmeier and Alexander Supady, Data collection: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Data collection was approved by the institutional ethics committee of the University of Freiburg (EK 151/14), due to the retrospective and observational nature of the study the need for informed consent was waived.
Widmeier E, Wengenmayer T, Maier S, Benk C, Zotzmann V, Staudacher DL, et al. Extracorporeal membrane oxygenation during the first three waves of the coronavirus disease 2019 pandemic—A retrospective single‐center registry study. Artif. Organs. 2022;00:1–10. 10.1111/aor.14270
Funding information
This work was financed by internal funds from the Department of Medicine III –Intensive Care Medicine at the University of Freiburg Medical Center.
DATA AVAILABILITY STATEMENT
All data will be available from the corresponding author on reasonable request.
REFERENCES
- 1. Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–68. [DOI] [PubMed] [Google Scholar]
- 2. Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75. [DOI] [PubMed] [Google Scholar]
- 3. Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, et al. ECMO for severe ARDS: systematic review and individual patient data meta‐analysis. Intensive Care Med. 2020;46:2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Juni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–9. [DOI] [PubMed] [Google Scholar]
- 5. Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta‐analysis. Lancet Respir Med. 2019;7:163–72. [DOI] [PubMed] [Google Scholar]
- 6. Henry BM. COVID‐19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID‐19): pooled analysis of early reports. J Crit Care. 2020;58:27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent JL, Creteur J. Ethical aspects of the COVID‐19 crisis: how to deal with an overwhelming shortage of acute beds. Eur Heart J Acute Cardiovasc Care. 2020;9:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Supady A, Badulak J, Evans L, Curtis JR, Brodie D. Should we ration extracorporeal membrane oxygenation during the COVID‐19 pandemic? Lancet Respir Med. 2021;9:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Supady A, Curtis JR, Abrams D, Lorusso R, Bein T, Boldt J, et al. Allocating scarce intensive care resources during the COVID‐19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med. 2021;9:430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID‐19 pandemic in greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the extracorporeal life support organization registry. Lancet. 2020;396:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID‐19: a systematic review and meta‐analysis. Crit Care. 2021;25:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, et al. High in‐hospital mortality in COVID patients receiving ECMO in Germany ‐ a critical analysis. Am J Respir Crit Care Med. 2021;204:991–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy D, Lebreton G, Pineton de Chambrun M, Hékimian G, Chommeloux J, Bréchot N, et al. Outcomes of patients denied extracorporeal membrane oxygenation during the COVID‐19 pandemic in greater Paris, France. Am J Respir Crit Care Med. 2021;204:994–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Supady A, Taccone FS, Lepper PM, Ziegeler S, Staudacher DL, COVEC‐Study Group . Survival after extracorporeal membrane oxygenation in severe COVID‐19 ARDS: results from an international multicenter registry. Crit Care. 2021;25:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. Extracorporeal membrane oxygenation for COVID‐19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajagopal K, Keller SP, Akkanti B, Bime C, Loyalka P, Cheema FH, et al. Advanced pulmonary and cardiac support of COVID‐19 patients: emerging recommendations from ASAIO—a “living working document”. ASAIO J. 2020;66:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo‐Sidron JA, Usman A, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO). ASAIO J. 2021;67:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maslove DM, Sibley S, Boyd JG, Goligher EC, Munshi L, Bogoch II, et al. Complications of critical COVID‐19: diagnostic and therapeutic considerations for the mechanically ventilated patient. Chest. 2021;161:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abrams D, Ferguson ND, Brochard L, Fan E, Mercat A, Combes A, et al. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med. 2019;7:108–10. [DOI] [PubMed] [Google Scholar]
- 24. Supady A. Cytokine adsorption and ECMO in patients with COVID‐19 ‐ Author's reply. Lancet Respir Med. 2021;9:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score‐development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (sepsis‐related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis‐related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The respiratory extracorporeal membrane oxygenation survival prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–82. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long‐term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sendagire C, Lipnick MS, Kizito S, Kruisselbrink R, Obua D, Ejoku J, et al. Feasibility of the modified sequential organ function assessment score in a resource‐constrained setting: a prospective observational study. BMC Anesthesiol. 2017;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. [DOI] [PubMed] [Google Scholar]
- 31. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID‐19: evolving outcomes from the international extracorporeal life support organization registry. Lancet. 2021;398:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID‐19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karagiannidis C, Slutsky AS, Bein T, Windisch W, Weber‐Carstens S, Brodie D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66:707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt M, Langouet E, Hajage D, James SA, Chommeloux J, Bréchot N, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID‐19 ARDS in Sorbonne hospitals. Paris Crit Care. 2021;25:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R. Extracorporeal membrane oxygenation for COVID‐19 during first and second waves. Lancet Respir Med. 2021;9:e80–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital‐level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hermann M, Laxar D, Krall C, Hafner C, Herzog O, Kimberger O, et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care. 2022;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Supady A, Biever PM, Staudacher DL, Wengenmayer T. Choosing the right reference cohort for assessing outcome of venovenous ECMO. Crit Care. 2022;26:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available from the corresponding author on reasonable request.


