Abstract
Seroconversion after COVID-19 vaccination is impaired in kidney transplant recipients. Emerging variants of concern such as the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants pose an increasing threat to these patients. In this observational cohort study, we measured anti-S1 IgG, surrogate neutralizing, and anti-receptor-binding domain antibodies three weeks after a third mRNA vaccine dose in 49 kidney transplant recipients and compared results to 25 age-matched healthy controls. In addition, vaccine-induced neutralization of SARS-CoV-2 wild-type, the B.1.617.2 (delta), and the B.1.1.529 (omicron) variants was assessed using a live-virus assay. After a third vaccine dose, anti-S1 IgG, surrogate neutralizing, and anti-receptor-binding domain antibodies were significantly lower in kidney transplant recipients compared to healthy controls. Only 29/49 (59%) sera of kidney transplant recipients contained neutralizing antibodies against the SARS-CoV-2 wild-type or the B.1.617.2 (delta) variant and neutralization titers were significantly reduced compared to healthy controls (p< 0.001). Vaccine-induced cross-neutralization of the B.1.1.529 (omicron) variants was detectable in 15/35 (43%) kidney transplant recipients with seropositivity for anti-S1 IgG, surrogate neutralizing, and/or anti-RBD antibodies. Neutralization of the B.1.1.529 (omicron) variants was significantly reduced compared to neutralization of SARS-CoV-2 wild-type or the B.1.617.2 (delta) variant for both, kidney transplant recipients and healthy controls (p< .001 for all).
KEYWORDS: clinical decision-making, clinical research, immune modulation, immunosuppression, kidney transplantation, nephrology, practice, solid organ transplantation, vaccine
Abbreviations: ID50, inhibitory dilution 50; IQR, interquartile range; MFI, mean fluorescence intensity; RBD, receptor-binding domain; snABs, surrogate neutralizing antibodies; TCID, tissue culture infectious dose; VEP, variant effect predictor; VoCs, variants of concern
1. INTRODUCTION
A coordinated innate and adaptive immune response is crucial for successfully combating SARS-CoV-2 infection.1The innate immune response slows down viral replication and spread by impeding viral replication within infected cells and creating an antiviral microenvironment in infected tissue. An adaptive immune response is then triggered by the early responses of the innate immune system resulting in highly antigen-specific effector T and B cells. However, naïve T and naïve B cells need time to proliferate and differentiate into effector cell subsets. Severe COVID-19 cases have been shown to be associated with failure of a timely coordinated immune response during natural infection, whereas rapid induction of SARS-CoV-2 specific CD4+ T cells has been shown to play a key role in milder disease and enhanced viral clearance.2 , 3Successful vaccination against COVID-19 counteracts a delayed immune response by activating the immune system before exposure to SARS-CoV-2 and is essential to prevent severe COVID courses, especially in immunocompromised individuals.
However, immune response to two-dose COVID-19 vaccination is impaired in solid organ transplant recipients compared to the general population and recent data have shown inferior real-world effectiveness of different vaccination schemes against COVID-19 disease.4, 5, 6, 7Qin et al. recently demonstrated an 82-fold higher risk of a COVID-19 breakthrough infection and a 485-fold higher risk of associated hospitalization and death for solid organ transplant recipients compared to fully vaccinated adults in the United States through April 2021.8Due to waning humoral immunity and a rapid increase in breakthrough infections, a third vaccine dose was recommended for the general population, including immunocompromised individuals with impaired vaccination response.9, 10, 11First results describe an increased immune response in transplant recipients to a third vaccine dose with an induction of serologic response in 25%–49% of previous non-responders and a significant increase in antibody titers for patients who were seropositive already before the third dose.12, 13, 14, 15
Emerging variants of concern (VoCs), such as the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants with partial immune escape are rapidly displacing other circulating strains and increasingly lead to breakthrough infections. In particular, the omicron variant escapes antibody neutralization and early real-world data indicate reduced effectiveness and reduced protection from hospitalization after two-dose vaccination or prior infection in the general South African population where omicron was first described.16 , 17Schmidt et al. and Nemet et al. recently independently demonstrated a substantial gain in neutralizing antibody activity against the omicron variant in healthy persons who were vaccinated after COVID-19 infection or received a third mRNA vaccine dose compared to individuals with standard two-dose vaccination.18 , 19
We recently first demonstrated impaired neutralization of the B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) variant in seroconverted kidney transplant recipients compared to healthy controls after two-dose vaccination.20However, little is known about neutralization of the currently predominant B.1.617.2 (delta) and B.1.1.529 (omicron) variants in kidney transplant recipients after a third mRNA vaccine dose. Data regarding neutralization against both VoCs are urgently needed to guide further vaccination strategies for non- and low-responders and ultimately help protect highly vulnerable kidney transplant recipients from severe COVID-19.
2. METHODS
2.1. Study design
In this ongoing observational cohort study to assess immunogenicity after COVID-19 vaccination in kidney transplant recipients and healthy controls, we enrolled 49 kidney transplant recipients and 25 healthy controls who received a third mRNA vaccine dose between August and October 2021 at the Department of Nephrology and at the Department of Pediatrics I at Heidelberg University Hospital. Serum was collected after a median (IQR) of 21 (20–32) and 21 (18–22) days after a third vaccine dose in kidney transplant recipients and healthy controls, respectively. Vaccine interval between a second and third dose was a median (IQR) of 138 (117–162) and 228 (218–236) days in kidney transplant recipients and healthy controls, respectively. All 25/25 (100%) healthy controls received three vaccinations with BNT162b2, whereas 40/49 (82%) kidney transplant recipients received three doses of an mRNA vaccine, 7/49 (14%) a priming dose with the replication-deficient adenoviral vector vaccine ChAdOx1 followed by two doses of an mRNA vaccine, and 2/49 (4%) two doses of ChAdOx1 followed by a third dose of an mRNA vaccine. Study participants with antibodies against the nucleocapsid protein (indicative of previous SARS-CoV-2 infection) or a medical history of SARS-CoV-2 infection were excluded from the analysis.
We determined IgG antibodies against the SARS-CoV-2 spike S1 subunit, surrogate neutralizing antibodies (snABs), and IgG antibodies against different target epitopes of the SARS-CoV-2 in all 49 kidney transplant recipients and 25 healthy controls after a third mRNA vaccine dose. In addition, IgG antibodies against the spike S1 of 4 common cold coronaviruses, namely HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43, were assessed.
Neutralizing antibodies against the SARS-CoV-2 wild-type, the B.1.617.2 (delta), and the B.1.1.529 (omicron) variants were quantified by using a live-virus neutralization assay. Neutralization against the B.1.1.529 (omicron) variants was tested in 35 kidney transplant recipients with seropositivity for anti-S1 IgG, snABs, and/or anti-receptor-binding domain (anti-RBD) antibodies. Results for neutralization against the B.1.1.529 (omicron) variants in kidney transplant recipients were compared to 10 age- and sex-matched healthy controls.
In 33/49 (67%) kidney transplant recipients with sera available before third vaccine dose, the same assays including live-virus neutralization against wild-type and the B.1.617.2 (delta) variant were performed using to analyze interindividual courses of humoral responses.
The study was approved by the Ethics Committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki. All study participants provided written informed consent. The study is registered at the German Clinical Trial Register (DRKS00024668).
2.2. Assessment of humoral responses after COVID-19 vaccination using commercially available tests
We used the SARS-CoV-2 Total Assay (Siemens) and the Elecsys anti-SARS-CoV-2 assay (Roche) to detect anti-S1 IgG and anti-nucleocapsid antibodies, respectively. A semi-quantitative index of ≥1 defines positivity for both assays.
Surrogate neutralizing antibodies were detected using a surrogate virus neutralization test (Medac) that mimics the virus interaction with the host cell by direct protein-protein interaction using purified RBD protein from the viral spike and the ACE-2 host cell receptor.21An inhibition ≥30% of RBD:ACE-2 binding defines positivity for this assay.
A bead-based multiplex assay for the Luminex platform (LabScreen Covid Plus, One Lambda Inc.) was used to detect IgG antibodies against four different SARS-CoV-2 target epitopes and IgG antibodies against the spike S1 of four common cold coronaviruses.22The mean fluorescence intensity (MFI) was analyzed using a Luminex 200 device (Luminex Corporation).
All assays were performed according to the manufacturer’s instructions and as described previously.20 , 23, 24, 25, 26, 27, 28, 29
2.3. Live-virus neutralization against wild-type, the B.1.617.2 (delta), and the B.1.1.529 (omicron) variants
All experiments have been described in detail previously.20 , 26, 27, 28,30In brief, we determined neutralization titers in titration experiments using VeroE6 cells. Virus stocks were produced by either amplification of the BavPat1/2020 strain (European Virus Archive) or isolation and amplification of the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants from nasopharyngeal and oropharyngeal swabs of PCR-confirmed SARS-CoV-2 positive patients.20 , 26, 27, 28,31BavPat1/2020 and B.1.617.2 (delta) variant were amplified in VeroE6 cells and virus titers of stocks were determined by plaque assay and Tissue Culture Infectious Dose (TCID) 50 assay in VeroE6 cells. To avoid rapid cell culture adaptation, stocks of B.1.1.5291 (omicron) were produced in Calu-3 cells and titers were determined in VeroE6 cells using TCID 50 assay. Virus stocks were validated by genome sequencing. For neutralization assays, two-fold serial dilutions of vaccine sera were incubated with 6 × 104TCID 50 of wild-type, the B.1.617.2 (delta), and the B.1.1.529 (omicron) variants. Virus replication was determined by immunostaining for the viral nucleocapsid protein using an in-cell ELISA. Data were normalized to a mock-infected (0%) and a no-serum control (100%). The inhibitory dilution 50 (ID50) is defined as the serum dilution that results in 50% reduction of normalized signal.
2.4. SARS-CoV-2 genome sequencing
SARS-CoV-2 genomes were sequenced with the ARTIC protocol using the NEBNext ARTIC FS kit to prepare sequencing libraries. To increase sample throughput the I.DOT liquid dispenser (Dispendix) was employed, and 4 × 96 libraries were pooled for sequencing in paired-end mode (2 × 75 bp) on a NextSeq instrument. Sequencing adapters were trimmed using trim_galore and host-read contamination was assessed and filtered using kraken2, as described previously.31 , 32Reads were aligned to the SARS-CoV-2 reference genome using bwa and alignments were sorted and indexed using samtools and quality-controlled using alfred.33, 34, 35Priming regions were masked with iVar, followed by variant calling with FreeBayes, normalization of variants with bcftools, and annotation of variants with the Ensembl Variant Effect Predictor (VEP).36, 37, 38, 39iVar was employed to compute a viral consensus sequence that was then classified by Pangolin and Nextclade to determine the viral lineage and clade, respectively.40 , 41
2.5. Statistics
The statistical analysis was performed using GraphPad Prism version 9.0.0 (GraphPad Software). Data are given as median and interquartile range (IQR) or number (N) and percent (%). Continuous variables were compared using the Mann-Whitney Utest for unpaired or the Wilcoxon matched-pairs rank test for paired variables. When comparing more than three paired continuous variables, we applied the Friedman’s test with Dunn’s post-test. Categorial data were compared using the Fisher’s exact test. Spearman’s rho was calculated to describe the correlation of different commercially available assays to the current gold standard to assess humoral immunity using a live virus assay. Statistical significance was assumed at a p< .05.
2.6. Role of the funding source
The funding source did not affect in the trial design, conduct, or reporting of this study.
3. RESULTS
3.1. Study population
Humoral immune response was assessed in 49 kidney transplant recipients and 25 age-matched healthy controls. Median (IQR) age was 55 (46–65) for kidney transplant recipients and 53 (39–59) years for healthy controls. With 20/49 (41%) kidney transplant recipients and 17/25 (68%) healthy female controls, the healthy controls cohort consisted of more females (p= .048). Baseline characteristics of all 49 kidney transplant recipients stratified for seropositivity in at least one of three commercially available assays after a third vaccine dose are shown in Table 1.
TABLE 1.
Clinical characteristics of kidney transplant recipients according to humoral response 3 weeks after a third vaccine dose
| Characteristic | All patients (N= 49) | Seropositive after third dose (N= 35) | Seronegative after third dose (N= 14) | pvalue |
|---|---|---|---|---|
| Female, N(%) | 20 (41) | 16 (46) | 4 (29) | .27 |
| Age, median (IQR) | 55 (46–65) | 57 (49–65) | 54 (45–67) | .67 |
| Vaccine type | ||||
| 3 × mRNAa | 40 (82) | 28 (80) | 12 (86) | >.99 |
| ChAdOx1 + 2 × mRNAb | 7 (14) | 6 (17) | 1 (7) | .66 |
| 2 × ChAdOx1 + mRNAc | 2 (4) | 1 (3) | 1 (7) | .49 |
| Time between vaccine and transplant, years (IQR) | 8.1 (2.4–13.6) | 8.5 (4.4–13.7) | 3.7 (0.8–12.7) | .12 |
| First transplant, N(%) | 46 (94) | 33 (94) | 13 (93) | >.99 |
| Type of immunosuppressive regimen, N(%) | ||||
| Calcineurin inhibitors | 44 (90) | 32 (91) | 12 (86) | .62 |
| Tacrolimus | 32 (65) | 20 (57) | 12 (86) | .10 |
| Cyclosporin A | 12 (24) | 12 (34) | 0 (0) | .01 |
| Mycophenolic acid | 39 (80) | 27 (77) | 12 (86) | .70 |
| mTOR inhibitors | 5 (10) | 5 (14) | 0 (0) | .30 |
| Belatacept | 2 (4) | 1 (3) | 1 (7) | .49 |
| Steroids | 47 (96) | 35 (100) | 12 (86) | .08 |
| Comorbidities, N(%) | ||||
| Hypertension | 35 (71) | 26 (74) | 9 (64) | .50 |
| Diabetes | 6 (12) | 3 (9) | 3 (21) | .33 |
| Chronic artery disease | 12 (24) | 9 (26) | 3 (21) | >.99 |
| Chronic lung disease | 5 (10) | 5 (14) | 0 (0) | .30 |
| Chronic liver disease | 2 (4) | 2 (6) | 0 (0) | >.99 |
| Malignancy | 8 (16) | 5 (14) | 3 (21) | .67 |
Abbreviations: mTOR, mammalian target of rapamycin; N, Number.
36 received three doses of BNT162b2; 4 received 2 doses of BNT162b2 followed by a third dose of mRNA-1273.
6 received a priming dose with ChAdOx1 followed by two doses of BNT162b2; 1 received a priming dose with ChAdOx1 followed by two doses of mRNA-1273.
2 received two doses of ChAdOx1 followed by a third dose of BNT162b2.
3.2. Humoral immune responses in kidney transplant recipients compared to healthy controls
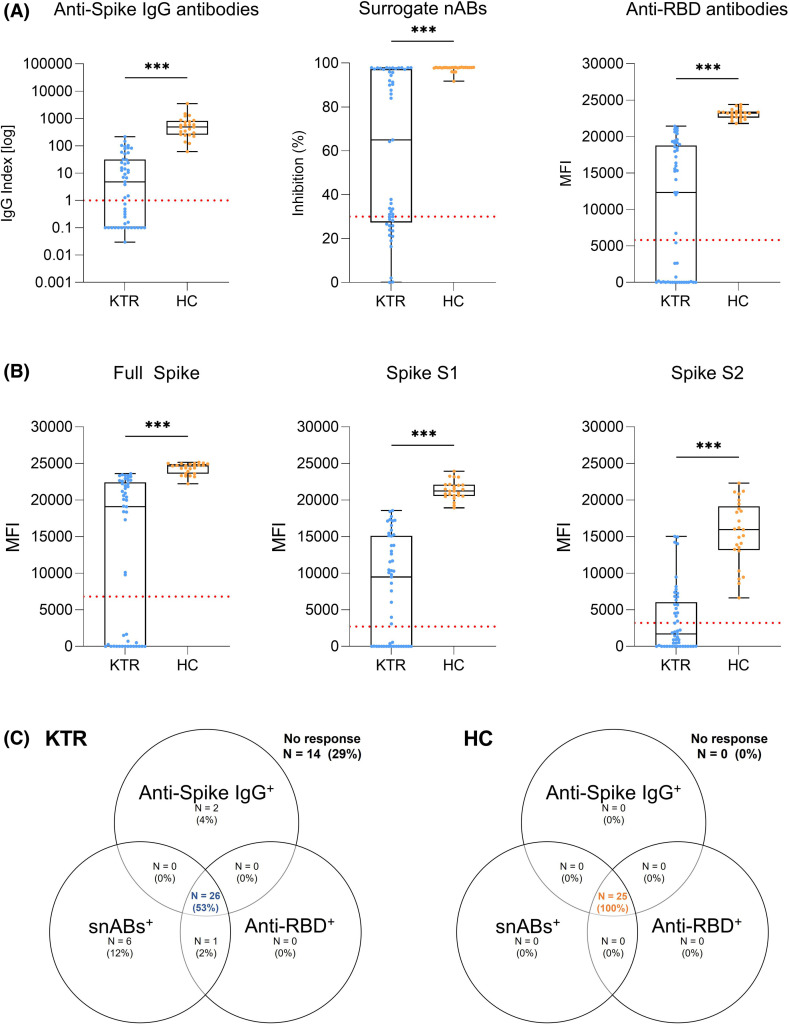
Kidney transplant recipients showed significantly impaired humoral response to a third mRNA vaccine dose in all commercially available assays with a median (IQR) anti-spike S1 IgG index of 4.79 (0.1–31.4), a median (IQR) % inhibition for surrogate neutralizing antibodies of 65 (27.4–97.2), and a median (IQR) MFI for anti-RBD antibodies of 12,322 (0–21,413) compared to 482.1 (253.6–811.9), 97.9 (97.8–98), and 23,214 (22,603–23,429), respectively, in healthy controls (p< .001 for all; Figure 1A). IgG antibodies against the full spike, the spike S1, and the spike S2 subunits as determined by a bead-based multiplex assay were also significantly lower in kidney transplant recipients with a median (IQR) MFI of 19,084 (0–22,419) for the full spike, 9482 (0–15,117) for the spike S1, and 1716 (0–6,042) for the spike S2, respectively, when compared to healthy controls (24,657 [23,619–24,913]; 21,237 [20,572–22,084]; 15,953 [13,159–19,137]; p< .001 for all; Figure 1B). Healthy controls also exhibited significantly higher IgG antibodies against the four tested common cold coronaviruses, but the difference of antibody response between healthy controls and kidney transplant recipients was less pronounced than for SARS-CoV-2 (p= .003 for HCoV-229E, p= .004 for HCoV-HKU1, p= .003 for HCoV-NL63, and p< .001 for HCoV-OC43; Figure S1).
FIGURE 1.
Humoral immune response after a third mRNA vaccine dose in kidney transplant recipients and healthy controls as determined by commercially available assays. (A) Anti-spike IgG (left panel), surrogate neutralizing (middle panel), and anti-receptor-binding domain (right panel) antibodies in 49 kidney transplant recipients and 25 age-matched healthy controls after a third mRNA vaccine dose. The dashed red line indicates the respective cut-off for each assay. (B) IgG antibodies against the SARS-CoV-2 full spike, spike S1, and spike S2 subunits in 49 kidney transplant recipients and 25 age-matched healthy controls. The y-axis represents the mean fluorescence intensity (MFI) where the dashed red line indicates the cut-off for each target. (C) Seropositivity for anti-spike IgG, surrogate neutralizing, and anti-receptor-binding domain antibodies in 49 kidney transplant recipients and 25 age-matched healthy controls after a third mRNA vaccine dose shown in a Venn diagram. HC, healthy controls; KTR, kidney transplant recipients; MFI, mean fluorescence intensity; N, number; RBD, receptor-binding domain; snABs, surrogate neutralizing antibodies. ***p< .001 [Color figure can be viewed at wileyonlinelibrary.com]
All 25/25 (100%) healthy controls were seropositive for anti-spike S1 IgG, surrogate neutralizing antibodies, and anti-RBD antibodies after a third vaccine dose whereas only 26/49 (53%) kidney transplant recipients showed concurrent seropositivity in all three commercially available tests (Figure 1C).
3.3. Neutralizing antibody response against SARS-CoV-2 wild-type and the B.1.617.2 (delta) variant
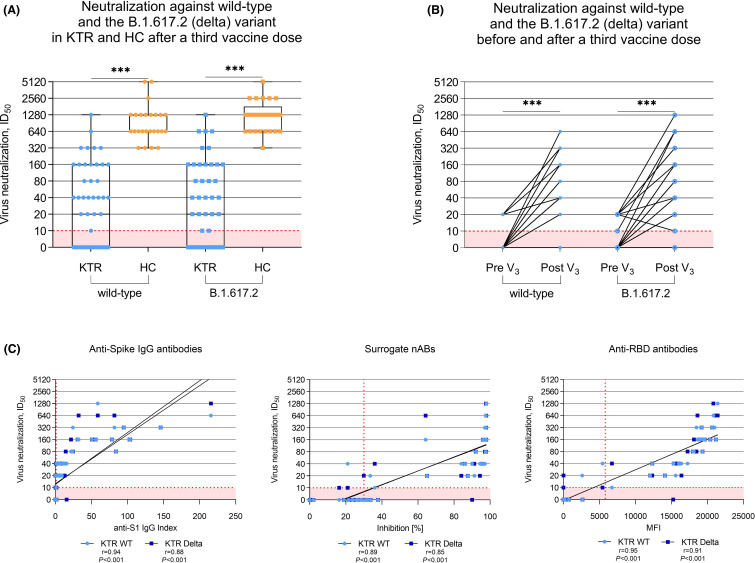
After a third mRNA vaccine dose, kidney transplant recipients showed significantly impaired neutralization against SARS-CoV-2 wild-type and the B.1.617.2 (delta) variant when compared to healthy controls (p< .001 for both; Figure 2A). The median (IQR) ID50was 1:20 (0–1:160) versus 1:640 (1:640–1:1280) for wild-type neutralization, and 1:20 (0–1:160) versus 1:1280 (1:640–1:1280) for neutralization of the B.1.617.2 (delta) variant in kidney transplant recipients compared to healthy controls, respectively. All 25/25 (100%) healthy controls were above the cut-off for detection of 1:10 for neutralization against wild-type and the B.1.617.2 (delta) variant, whereas 20/49 (41%) kidney transplant recipients remained below the threshold for wild-type and B.1.617.2 (delta) neutralization.
FIGURE 2.
Neutralization of SARS-CoV-2 wild-type and the B.1.617.2 (delta) variant by antibodies in sera of kidney transplant recipients and healthy controls taken after a third mRNA vaccine dose. (A) Vaccine-induced neutralization of the SARS-CoV-2 wild-type and cross-neutralization of the B.1.617.2 (delta) variant by antibodies in sera of 49 kidney transplant recipients and 25 age-matched healthy controls taken after a third mRNA vaccine dose as determined using a live-virus assay. The dashed red line indicates the cut-off for detection which is the 1:10 dilution in this assay. (B) Interindividual course of neutralization against SARS-CoV-2 wild-type and cross-neutralization against the B.1.617.2 (delta) variant in 33 kidney transplant recipients with sera available before and after a third mRNA vaccine dose. (C) Correlation analyses of three commercially available assays for anti-spike IgG, surrogate neutralizing, and anti-receptor-binding domain antibodies with neutralization titers of SARS-CoV-2 wild-type and cross-neutralization titers of the B.1.617.2 (delta) variant by sera of kidney transplant recipients taken after a third mRNA vaccine dose. The dashed red line indicates the respective cut-off for each assay. HC, healthy controls; ID50; inhibitory dilution 50; KTR, kidney transplant recipients; MFI, mean fluorescence intensity; RBD, receptor-binding domain; r; Spearman’s rho; snABs, surrogate neutralizing antibodies; V, vaccination; WT, wild-type. ***p< .001 [Color figure can be viewed at wileyonlinelibrary.com]
When assessing interindividual changes in neutralization against the SARS-CoV-2 wild-type strain and the B.1.617.2 (delta) variant in the 33 kidney transplant recipients with sera available before and after a third vaccine dose, neutralizing activity against wild-type and the B.1.617.2 (delta) variant increased significantly with a third mRNA vaccine dose (p< .001 for both; Figure 2B). Corresponding interindividual changes in anti-S1 IgG, surrogate neutralizing, and anti-RBD antibodies are displayed in Figure S2.
Commercially available assays suitable for clinical routine use showed a strong correlation to the ID50as determined by live-virus neutralization assay. Correlation for anti-spike S1 IgG index, surrogate neutralizing antibodies, and anti-RBD antibodies to wild-type neutralization was slightly better compared to neutralization of the B.1.617.2 (delta) variant with a Spearman’s rho of 0.94 and 0.88 for anti-spike S1 IgG, 0.89 and 0.85 for surrogate neutralizing antibodies, and 0.95 and 0.91 for anti-RBD antibodies, respectively (Figure 2C).
3.4. Neutralizing antibody response against the B.1.1.529 (omicron) variants
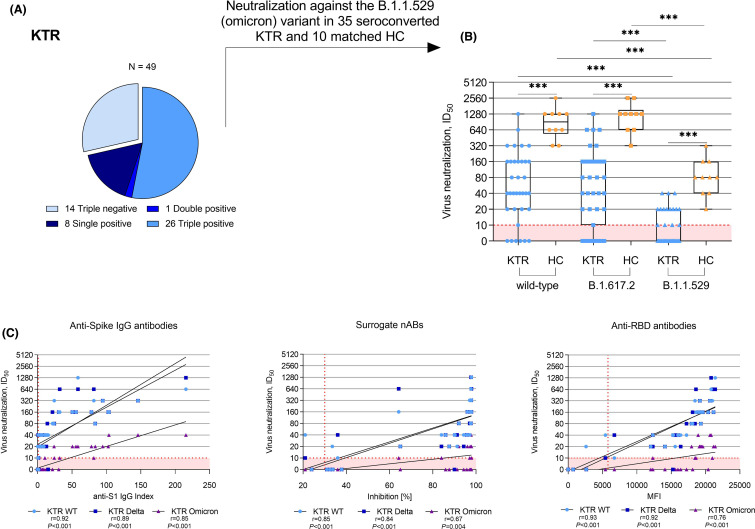
Neutralization against the B.1.1.529 (omicron) variants was assessed in 35 kidney transplant recipients that showed seroconversion for anti-S1 IgG, surrogate neutralizing, and/or anti-RBD antibodies and was compared to 10 age- and sex-matched healthy controls ( Figure 3A). Neutralization against the B.1.1.529 (omicron) variants in seroconverted kidney transplant recipients was with a median (IQR) ID50of 0 (0–1:20) significantly lower compared to healthy controls with a median (IQR) ID50of 1:80 (1:40–1:160) (p< .001; Figure 3B). For both, kidney transplant recipients and healthy controls, neutralization of the B.1.1.529 (omicron) variants was significantly reduced compared to neutralization of the wild-type or the B.1.617.2 (delta) variant (p< .001 for both; Figure 3B). Anti-S1 IgG showed the strongest correlation to neutralization of the B.1.1.529 (omicron) variants with a Spearman’s rho of 0.85 (Figure 3C).
FIGURE 3.
Neutralization of the B.1.1.529 (omicron) variants in kidney transplant recipients showing seroconversion in at least one commercially available assay and healthy controls after a third mRNA vaccine dose. (A) Neutralization of the B.1.1.529 (omicron) variants was assessed in 35 kidney transplant recipients with seropositivity for anti-S1 IgG, surrogate neutralizing, and/or anti-RBD antibodies. (B) Vaccine-induced neutralization of the SARS-CoV-2 wild-type and cross-neutralization of the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants by sera of 35 seroconverted kidney transplant recipients and 10 age- and sex-matched healthy controls after a third mRNA vaccine dose as determined with a live-virus assay. (C) Correlation analyses of anti-spike IgG, surrogate neutralizing, and anti-RBD antibodies with neutralization titers of SARS-CoV-2 wild-type and cross-neutralization of the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants in sera of seroconverted kidney transplant recipients after a third mRNA vaccine dose. The dashed red line indicates the respective cut-off for each assay. HC, healthy controls; ID50, inhibitory dilution 50; KTR, kidney transplant recipients; MFI, mean fluorescence intensity; N, number; RBD, receptor-binding domain; r; Spearman’s rho; snABs, surrogate neutralizing antibodies; WT, wild-type. ***p< .001 [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Breakthrough infections
All study participants were inquired about the occurrence of breakthrough infection at a median (IQR) of 5.9 (5.4–6.5) months after receiving a third mRNA vaccine dose. PCR-confirmed infection occurred in 12/49 (25%) kidney transplant recipients a median (IQR) of 5.2 (4.1–5.8) months after receiving their third vaccine dose. One infection occurred in December 2021, when the B.1.617.2 (delta) variant was the predominant SARS-CoV-2 variant in Germany, whereas the remaining 11/12 infections occurred in 2022 in parallel with the surge of the B.1.1.529 (omicron) variants. In 6/12 (50%) kidney transplant recipients with breakthrough infections, seroconversion was detectable in all three commercially available assays before SARS-CoV-2 infection. Notably, all patients were oligosymptomatic with no patient requiring hospitalization due to COVID-19.
4. DISCUSSION
This is the first study to describe live-virus neutralization of the SARS-CoV-2 wild-type, the B.1.617.2 (delta) variant, and the B.1.1.529 (omicron) variants by sera of kidney transplant recipients before and after a third vaccine dose in comparison to healthy controls.
In commercially available assays, we detected seropositivity for 26/49 (53%) kidney transplant recipients after a third vaccine dose. This is in line with other studies that found a 36%–68% seroresponse rate for kidney transplant recipients after a third COVID-19 vaccine dose and a 25%–49% seroconversion rate of previous non-responders.12, 13, 14, 15However, the authors of these studies only used commercially available tests that may not fully reflect the actual protection against variants of concern as they test for antibodies against the SARS-CoV-2 wild-type strain.
Only recently, Kumar et al. published results on neutralization of SARS-CoV-2 variants by sera of transplant recipients after two and three vaccine doses of mRNA-1273 (Moderna) vaccine using a SARS-CoV-2 spike-pseudotyped lentivirus-based neutralization assay.42The authors showed that neutralizing antibody positivity after two doses of mRNA-1273 vaccine is low for B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) but subsequently increases with the administration of a third vaccine dose.42In concordance with our results, the authors were not able to detect sufficient neutralization response in 25/60 (42%) and 27/60 (45%) patients against the SARS-CoV-2 wild-type or B.1.617.2 (delta) variant, respectively.42Although lentivirus-based assays show a good correlation to results obtained by live-virus neutralization, the testing performed in our study is generally considered the current gold standard to assess actual neutralization titers.43
In our results including neutralizing antibody activity detected by a live-virus assay, kidney transplant recipients with detectable seroconversion before the administration of a third vaccine dose showed significantly stronger neutralization of the SARS-CoV-2 wild-type and the B.1.617.2 (delta) variant after a third vaccine dose. However, sera of 20/49 (41%) kidney transplant recipients did not contain neutralizing antibodies above the threshold for detection of neutralization of SARS-CoV-2 wild-type or the B.1.617.2 (delta) variant even after the administration of a third mRNA vaccine dose. Notably, sera of only 15/35 (43%) kidney transplant recipients with seropositivity in any of the three used commercially available assays showed neutralizing antibody activity against the emerging B.1.1.529 (omicron) variants. Impaired neutralization of the B.1.1.529 (omicron) variants has been described previously in healthy cohorts and is explained by the various mutations in the spike region of the B.1.1.529 (omicron) variants that facilitate immune escape.16, 17, 18, 19,44Therefore, even seroconverted kidney transplant recipients may not be adequately protected against infection with the B.1.1.529 (omicron) variants. These results are in line with only recently published data by Kumar et al. analyzing 60 solid organ transplant recipients (kidney, kidney-pancreas, lung, heart, and liver) one and three months after completion of 3 doses of the mRNA-1273 vaccine.45Kumar et al. showed a significantly lower proportion of patients with detectable neutralizing antibody responses to omicron compared to neutralization against wild-type or delta using a SARS-CoV-2 spike pseudotyped lentivirus assay.45Our data with an increasing number of breakthrough infections about 6 months after reception of a third vaccine dose, even in seroconverted kidney transplant recipients, illustrate the insufficient neutralization against the B.1.1.529 (omicron) variants. As kidney transplant recipients have a higher risk for breakthrough infections, hospitalization, and death due to COVID-19 than the general population, the high proportion of non-responders even after a third vaccine dose and the significantly lower neutralization against the B.1.1.529 (omicron) variants is highly distressing and poses an urgency to optimize vaccine responsiveness in kidney transplant recipients.
One approach to optimize vaccination response in kidney transplant recipients is to combine different vaccines to a heterologous vaccination regimen. However, heterologous vaccination regimens have not yet shown any superior outcome regarding seroconversion rates in kidney transplant recipients as they are all still based on the original SARS-CoV-2 strain.46 , 47In our study cohort, 9/49 (18%) received either one or two doses of ChAdOx1 before a third mRNA vaccine dose. Among kidney transplant recipients who received heterologous vaccination, 4/9 (44%) were seropositive in all three assays, compared to 22/40 (55%) who received three doses of an mRNA vaccine. Although limited by the small number of patients vaccinated with a heterologous vaccination regimen, we did not detect a trend toward better serologic response after heterologous vaccination.
Another approach to improve the vaccine-induced immune response in kidney transplant recipients is by modulation of immunosuppression. Recent data suggest that number and type of immunosuppressive agents, especially treatment with mycophenolate mofetil and belatacept, act as major determinants of seroconversion failure in kidney transplant recipients after standard two-dose vaccination.6 , 48Both the magnitude of humoral responses and spike-specific T cells have been shown to depend on immunosuppressive treatment during vaccine administration for patients with systemic rheumatic diseases, rheumatoid arthritis, or multiple sclerosis receiving disease-modifying therapies.49, 50, 51We did not see any significant differences between responders and non-responders after a third mRNA vaccine dose for a particular type of immunosuppressive regimen, which may be due to the size of our study population. However, we found that patients who were transplanted more recently tended to remain seronegative even after a third vaccine dose, which may indicate a better vaccination response with reduced immunosuppressive maintenance therapy, as is common in long-term kidney transplant recipients. D’Offizi et al. recently reported similar findings with a higher immunologic response after two-dose vaccination in liver transplant recipients with ≥6 years since transplantation, which they also attribute to progressive dose reduction.52The two patients of our cohort with Belatacept maintenance therapy showed low antibody levels or no seroconversion which has been described previously, even after a third mRNA vaccine dose.53
The administration of a fourth vaccine dose is another attempt to optimize vaccination response in kidney transplant recipients, ideally using a vaccine formulation that is based on more recently circulating variants such as B.1.1.529 (omicron). First results indicate an improved humoral response after a fourth vaccine dose among those with a weak response after three doses but little to no improvement among those with no response after three doses.54 , 55Although this may suggest immunogenic potential for poor responders after a third vaccine dose, additional actions seem necessary to reach vaccine-induced immunity and protection from severe disease courses.55Passive immunization of those patients that do not mount immune response at all with therapeutic antibodies that have shown to inhibit SARS-CoV-2 and variants of concern including the B.1.1.529 (omicron) variants is another attempt to protect these patients from severe COVID-19 disease.56 , 57Our data show that anti-spike S1 IgG and other commercially available tests may aid in clinical decision-making for additional booster vaccination(s) as these tests show a strong correlation to live-virus neutralization which is unfortunately not yet feasible in clinical routine. A strong correlation between commercially available tests and live-virus neutralization has been described previously by us and others in different cohorts.20 , 26 , 27 , 58, 59, 60
A limitation of our study is the lack of data on B and T cell responses after vaccination. Recent studies provided evidence on highly reproducible whole-blood assays to detect SARS-CoV-2 spike specific T cell response, using a similar platform to assays measuring T cell specific responses against Mycobacterium tuberculosis.49 , 50 , 61A strong correlation between anti-RBD antibodies and SARS-CoV-2 specific IFN-yT cell response was shown for healthy cohorts, immunosuppressed patients with rheumatoid arthritis, and multiple sclerosis patients on various disease-modifying therapies.49 , 50 , 62Similarly, in kidney and liver transplant recipients, a strong correlation between quantitative and functional CD4+ T-cell responses and anti-S1 IgG antibodies was demonstrated after two vaccine doses.6 , 52These results suggest that anti-spike titers may be used as a surrogate parameter to assess immunologic response after COVID-19 vaccination as T cell studies are more resource intensive and less standardized between different laboratories.
In healthy individuals, a substantial increase in neutralizing antibody activity against omicron was observed after a third vaccine dose, possibly due to the presence of memory B cells recognizing the omicron RBD.44 , 63 , 64Tarke et al. recently demonstrated preserved T cell responses in healthy individuals against variants of concern, including the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants, up to 6 months after second vaccination.63These data provide cause for optimism at a time of rising incidence and waning humoral immunity at least for the general population.58 , 63Regarding kidney transplant recipients, Schrezenmeier et al. found significantly increased spike-reactive CD4+T helper cells with higher portions of IL-2 and IL-4 secreting and polyfunctional (IFNy +TNFα+IL-2+) T cells in seroconverted kidney transplant recipients after a third vaccine dose, however, non-responders showed only marginal improvements in antigen-specific B and T cells.65
In conclusion, a third mRNA vaccine dose increases vaccine-induced immunity in most kidney transplant recipients. However, neutralizing antibody activity against immune-escape variants such as the B.1.1.529 (omicron) variants is barely detectable even in seroconverted individuals after a third vaccine dose and poses the urgent need to optimize vaccination strategies for highly vulnerable kidney transplant recipients.
ACKNOWLEDGMENTS
We thank Iris Arnold and Sabine Bönisch at the Department of Nephrology, and Verena Backendorf and Tina Hildenbrand at the Department of Immunology (all at Heidelberg University Hospital, Heidelberg, Germany) for their technical support.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding informationFunding for this study has been received by the Dietmar Hopp Stiftung (1DH2111111). Louise Benning is funded by the Rahel Goitein-Straus Program of the Heidelberg Faculty of Medicine. Ralf Bartenschlager is supported by the program for surveillance and control of SARS-CoV-2 mutations of the State of Baden-Württemberg, the German Federal Research Network Applied Surveillance and Testing (BFAST) within the Network University Medicine and the Project “Virological and immunological determinants of COVID-19 pathogenesis—lessons to get prepared for future pandemics (KA1-Co-02 ‘COVIPA’),” a grant from the Helmholtz Association’s Initiative and Networking Fund. Claudius Speer is funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Fig S1-S2
REFERENCES
- 1.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.038. 996-1012.e19. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2 specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and Islet transplant recipients. Transplantation. 2022;106(3):436–446. doi: 10.1097/tp.0000000000004059. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Regional Heal – Europe. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(12):3980–3989. doi: 10.1111/ajt.16766. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265–e266. doi: 10.1097/tp.0000000000003907. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/s0140-6736(21)01642-1. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. New Engl J Med. 2021;385(24):e84. doi: 10.1056/nejmoa2114583. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun. 2021;12(1):6379. doi: 10.1038/s41467-021-26672-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063–1065. doi: 10.1001/jama.2021.12339. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi: 10.7326/l21-0282. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Bello AD. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. New Engl J Med. 2021;385(7):661–662. doi: 10.1056/nejmc2108861. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22(1):322–323. doi: 10.1111/ajt.16775. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. New Engl J Med. 2022;386(5):494–496. doi: 10.1056/nejmc2119270. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Science. 2022:eabn4947. doi: 10.1101/2021.11.11.21266068. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. New Engl J Med. 2022;386(6):599–601. doi: 10.1056/nejmc2119641. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. New Engl J Med. 2022;386(5):492–494. doi: 10.1056/nejmc2119358. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benning L, Morath C, Bartenschlager M, et al. Neutralization of SARS-CoV-2 variants of concern in kidney transplant recipients after standard COVID-19 vaccination. Clin J Am Soc Nephrol. 2022;17(1):98–106. doi: 10.2215/cjn.11820921. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. doi: [DOI] [PubMed] [Google Scholar]
- 22.Bray RA, Lee J-H, Brescia P, et al. Development and validation of a multiplex, bead-based assay to detect antibodies directed against SARS-CoV-2 proteins. Transplantation. 2020;105(1):79–89. doi: 10.1097/tp.0000000000003524. doi: [DOI] [PubMed] [Google Scholar]
- 23.Benning L, Töllner M, Hidmark A, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines. 2021;9(8):857. doi: 10.3390/vaccines9080857. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speer C, Göth D, Benning L, et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol. 2021;16(7):1073–1082. doi: 10.2215/cjn.03700321. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speer C, Schaier M, Nusshag C, et al. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines. 2021;9(10):1130. doi: 10.3390/vaccines9101130. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benning L, Morath C, Bartenschlager M, et al. Natural SARS-CoV-2 infection results in higher neutralization response against variants of concern compared to two-dose BNT162b2 vaccination in kidney transplant recipients. Kidney Int. 2022;101(3):639–642. doi: 10.1016/j.kint.2021.12.009. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speer C, Benning L, Töllner M, et al. Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int. 2021;100(3):700–702. doi: 10.1016/j.kint.2021.07.002. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speer C, Töllner M, Benning L, et al. Third COVID-19 vaccine dose with BNT162b2 in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2022;81(4):593–595. doi: 10.1136/annrheumdis-2021-221747. doi: [DOI] [PubMed] [Google Scholar]
- 29.Speer C, Morath C, Töllner M, et al. Humoral responses to single-dose BNT162b2 mRNA vaccination in dialysis patients previously infected with SARS-CoV-2. Front Med. 2021;8:721286. doi: 10.3389/fmed.2021.721286. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tönshoff B, Müller B, Elling R, et al. Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. JAMA Pediatr. 2021;175(6):586–593. doi: 10.1001/jamapediatrics.2021.0001. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallm JP, Bundschuh C, Kim H, et al. Local emergence and decline of a SARS-CoV-2 variant with mutations L452R and N501Y in the spike protein. Medrxiv. Published online 2021. doi: 10.1101/2021.04.27.21254849 [DOI]
- 32.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46. doi: 10.1186/gb-2014-15-3-r46. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rausch T, Fritz MHY, Korbel JO, Benes V. Alfred: interactive multi-sample BAM alignment statistics, feature counting and feature annotation for long- and short-read sequencing. Bioinformatics. 2019;35(14):2489–2491. doi: 10.1093/bioinformatics/bty1007. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grubaugh ND, Gangavarapu K, Quick J, et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019;20(1):8. doi: 10.1186/s13059-018-1618-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. Arxiv. 2012. Published online.
- 38.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–2993. doi: 10.1093/bioinformatics/btr509. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaren W, Gil L, Hunt SE, et al. The ensembl variant effect predictor. Genome Biol. 2016;17(1):122. doi: 10.1186/s13059-016-0974-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 variants in transplant recipients after two and three doses of mRNA-1273 vaccine. Ann Intern Med. 2022;175(2):226–233. doi: 10.7326/m21-3480. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. doi: [DOI] [PubMed] [Google Scholar]
- 44.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. Published online 2021. doi: [DOI] [PubMed] [Google Scholar]
- 45.Kumar D, Hu Q, Samson R, et al. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am J Transplant. 2022. doi: 10.1111/ajt.17020 [DOI] [PMC free article] [PubMed]
- 46.Masset C, Ville S, Garandeau C, et al. Observations on improving COVID-19 vaccination responses in kidney transplant recipients: heterologous vaccination and immunosuppression modulation. Kidney Int. 2022;101(3):642–645. doi: 10.1016/j.kint.2021.11.024. Published Online 2021. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients. JAMA Intern Med. 2022;182(2):165. doi: 10.1001/jamainternmed.2021.7372. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22(2):634–639. doi: 10.1111/ajt.16851. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-Cell–specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–e554. doi: 10.1212/wnl.0000000000013108. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picchianti-Diamanti A, Aiello A, Laganà B, et al. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol. 2021;12:740249. doi: 10.3389/fimmu.2021.740249. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Töllner M, Speer C, Benning L, et al. Impaired neutralizing antibody activity against B.1.617.2 (Delta) after anti-SARS-CoV-2 vaccination in patients receiving anti-CD20 therapy. J Clin Med. 2022;11(6):1739. doi: 10.3390/jcm11061739. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Offizi G, Agrati C, Visco-Comandini U, et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42(1):180–186. doi: 10.1111/liv.15089. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavarot N, Morel A, Leruez-Ville M, et al. Weak antibody response to 3 doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021. doi: 10.1111/ajt.16814 [DOI] [PMC free article] [PubMed]
- 54.Kamar N, Abravanel F, Marion O, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4(11):e2136030. doi: 10.1001/jamanetworkopen.2021.36030. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alejo JL, Mitchell J, Chiang T-Y, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105(12):e280–e281. doi: 10.1097/tp.0000000000003934. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3) doi: 10.1016/j.cell.2021.12.032. 447-456.e11. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/nejmoa2107934. doi: [DOI] [PubMed] [Google Scholar]
- 58.Benning L, Morath C, Bartenschlager M, et al. Neutralizing antibody activity against the B.1.617.2 (delta) variant 8 months after two-dose vaccination with BNT162b2 in health care workers. Clin Microbiol Infect. 2022. doi: 10.1016/j.cmi.2022.01.011 [DOI] [PMC free article] [PubMed]
- 59.Benning L, Klein K, Morath C, et al. Neutralizing antibody activity against the B.1.617.2 (delta) variant before and after a third BNT162b2 vaccine dose in hemodialysis patients. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.840136. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahrsdörfer B, Kroschel J, Ludwig C, et al. Independent side-by-side validation and comparison of four serological platforms for SARS-CoV-2 antibody testing. J Infect Dis. 2020;223(5):jiaa656. doi: 10.1093/infdis/jiaa656. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrone L, Petruccioli E, Vanini V, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infec. 2021;27(2) doi: 10.1016/j.cmi.2020.09.051. 286.e7-286.e13. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agrati C, Castilletti C, Goletti D, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. 2021;9(6):1315. doi: 10.3390/microorganisms9061315. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5) doi: 10.1016/j.cell.2022.01.015. 847-859.e11. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. doi: [DOI] [PubMed] [Google Scholar]
- 65.Schrezenmeier E, Rincon-Arevalo H, Stefanski A-L, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol. 2021;32(12):3027–3033. doi: 10.1681/asn.2021070966. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1-S2
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.