Abstract
Aims
To estimate the associations between high‐risk alcohol consumption and (1) severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroconversion, (2) self‐reported new SARS‐CoV‐2 infection and (3) symptomatic COVID‐19.
Design
Prospective cohort study.
Setting
Indiana University Bloomington (IUB), IN, USA.
Participants
A total of 1027 IUB undergraduate students (64% female), aged 18 years or older, residing in Monroe County, Indiana, seronegative for SARS‐CoV‐2 at study baseline.
Measurements
Primary exposure was high‐risk alcohol consumption measured with an Alcohol Use Disorders Identification Test (AUDIT) questionnaire score of 8 or more. Primary outcome was SARS‐CoV‐2 seroconversion since baseline, assessed with two SARS‐CoV‐2 antibody tests, at baseline (September 2020) and end‐line (November 2020). Secondary outcomes were (a) self‐reported new SARS‐CoV‐2 infection at the study end‐line and (b) self‐reported symptomatic COVID‐19 at baseline.
Findings
Prevalence of high‐risk alcohol consumption was 32 %. In models adjusted for demographics, students with high‐risk alcohol consumption status had 2.44 [95% confidence interval (CI) = 1.35, 4.25] times the risk of SARS‐CoV‐2 seroconversion and 1.84 (95% CI = 1.04, 3.28) times the risk of self‐reporting a positive SARS‐CoV‐2 infection, compared with students with no such risk. We did not identify any association between high‐risk alcohol consumption and symptomatic COVID‐19 (prevalence ratio = 1.17, 95% CI = 0.93, 1.47). Findings from sensitivity analyses corroborated these results and suggested potential for a dose–response relationship.
Conclusions
Among American college students, high‐risk alcohol consumption appears to be associated with higher risk for severe acute respiratory syndrome coronavirus 2 seroconversion/infection.
Keywords: American colleges, AUDIT, AUDIT‐C, COVID‐19, heavy drinking, high‐risk alcohol consumption, quantity–frequency index, respiratory disease, SARS‐CoV‐2, young adults
INTRODUCTION
Background and rationale
Coronavirus disease 2019 (COVID‐19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has a major public health burden on college campuses. As of 26 May 2021, more than 700 000 SARS‐CoV‐2 infections have been reported from colleges and universities in the United States, with the vast majority of the cases among students [1]. COVID‐19 has a wide range of symptoms, such as fever, cough, fatigue and dyspnea [2]. In some cases, COVID‐19 causes long‐lasting symptoms (long COVID [3]), such as loss of smell, impaired concentration and memory problems among young adults [4]. Acquiring COVID‐19 and outbreaks of this disease on college campuses adversely impact students’ mental health and school performance and results in an increase in missed school days through isolation or quarantine requirements [5, 6]. Finally, SARS‐CoV‐2 infection spread among college students can overflow into other segments of the community with higher risk for severe COVID‐19 outcomes. Early increases in COVID‐19 cases among college‐aged (18–24 years) adults have been followed by increases in cases among older adults who are at higher risk of severe disease [7, 8, 9]. Identifying modifiable risk factors for SARS‐CoV‐2 transmission among college students is imperative to prevent and control COVID‐19 outbreaks on college campuses as well as among more vulnerable subpopulations in the community.
Alcohol consumption is an underexplored yet plausible risk factor for SARS‐CoV‐2 infection and transmission. It is a prevalent modifiable risky behavior, particularly among college students [10]. In 2018, 51% of college‐aged adults reported drinking alcohol in the past 30 days; 24% reported binge drinking and 6% reported heavy drinking [11]. Alcohol consumption might increase individuals’ susceptibility to SARS‐CoV‐2 infection through two inter‐related pathways: cognitive/behavioral and pathophysiological.
Alcohol consumption causes cognitive distortion and brings about behavioral changes that could increase the risk of SARS‐CoV‐2 transmission and infection [12, 13, 14]. It weakens vigilance, information processing, spatial working memory and performance of complex tasks and increases impulsivity [15, 16, 17]. These cognitive changes plausibly disrupt compliance with COVID‐19 protective behaviors, including mask‐wearing and physical distancing [12, 13, 18]. Moreover, the alcohol use and cognitive distortion relationship might be cyclical. Young adults tend to drink more alcohol in groups [19], and more alcohol consumption exacerbates cognitive distortion which consequently can result in more non‐compliance with COVID‐19 protective measures [20, 21, 22].
Moreover, alcohol consumption impairs innate and adaptive immune subsystems’ responses to respiratory infections [23, 24, 25, 26] through disrupting various immunological functionalities, such as reducing T and B cell counts, impairing neutrophil production and damaging alveolar barrier function [23, 24, 25]. Similarly, we expect pathophysiological changes in the lungs due to alcohol consumption could contribute to SARS‐CoV‐2 infections [24]. Lastly, alcohol consumption has also been found to increase susceptibility to respiratory complications, such as pneumonia [27] and acute respiratory distress syndrome [28]. Hence, alcohol consumption might also worsen COVID‐19 prognosis.
The association between alcohol consumption and COVID‐19 is not well understood. In commentaries and a non‐quantitative review, researchers have suggested that the associations between alcohol use and COVID‐19 incidence as well as COVID‐19 severity need to be evaluated [24, 29, 30, 31]. However, no quantitative study has been conducted to evaluate the associations between alcohol consumption and COVID‐19 incidence and severity among college students, a population with prevalent excessive alcohol drinking and frequent COVID‐19 outbreaks.
Objectives
The primary objective was to longitudinally evaluate the association between high‐risk alcohol consumption and SARS‐CoV‐2 seroconversion among college students. We hypothesized that students with high‐risk alcohol consumption were more likely to experience SARS‐CoV‐2 seroconversion. Because seroconversion may have been imperfectly detected with our antibody tests, our secondary objective was to evaluate the association between high‐risk alcohol consumption and self‐reported positive SARS‐CoV‐2 polymerase chain reaction (PCR) with reverse transcription (RT–PCR) testing history. We further assessed the association between high‐risk alcohol consumption and symptomatic COVID‐19 as another secondary outcome. The hypotheses were not pre‐registered and consequently the results should be considered exploratory.
METHODS
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [32] guidelines in this study report. The parent study was a randomized controlled trial evaluating the effect of receiving SARS‐CoV‐2 antibody test results on participants’ compliance with protective behavior against COVID‐19 [33]. Details regarding the parent study and overall study design are published elsewhere [14, 34].
Study design
We used a prospective cohort study design. The study period was from September to November 2020. Payments (up to $30) were made to participants to compensate them for their time. The Indiana University Human Subjects and Institutional Review boards approved the study protocol (Protocol no. 2008293852). Participants provided informed consent through an on‐line eConsent framework.
Study setting, participants and procedures
We conducted this study on the Indiana University Bloomington (IUB) campus. In Fall 2020, IUB had a total undergraduate population of 32986. Many COVID‐19 restrictions were in place during the data collection phase, including mask‐wearing, physical distancing, hybrid and remote classes, class spacing, contact tracing, mitigation testing and quarantine and self‐isolation mandates. Inclusion criteria were: (1) age ≥ 18 years, (2) IUB undergraduate student in Fall 2020 and (3) residing in Monroe County, IN. We acquired a random sample of IUB undergraduate students from the IUB registrar. Upon our request, the Office of the Vice Provost for Undergraduate Education generated a random list of 7499 IUB undergraduate students (of 32 986). The list was provided to the study team in the form of a comma‐separated values (CSV) file, and included columns of students’ names, e‐mails and current addresses. Even though we sent the study invitation e‐mails to all students in this list, students were not included in the study (and were counted as ineligible) if their current address in the CSV file was not Monroe County, IN (third eligibility criterion).
We sent study invitation e‐mails with information about the study and links to an eligibility screening on‐line survey to the 7499 sampled students. Eligible students were directed to an on‐line eConsent form with more information about the study. Students who consented to participate could schedule a baseline antibody testing appointment and complete the on‐line baseline survey [34]. This survey included questions about participant demographics, SARS‐CoV‐2 testing history and alcohol use.
During 14–30 September, we conducted in‐person SARS‐CoV‐2 baseline antibody tests on the IUB campus. We asked participants to re‐schedule their appointments if they were experiencing COVID‐19 symptoms, had tested positive for SARS‐CoV‐2 in the last 2 weeks before their appointment or had been directed to isolate or quarantine. Antibody testing results were entered into the REDCap (Research Electronic Data Capture [35, 36]) data capturing system [14].
Four follow‐up on‐line surveys were administered every 2 weeks after the baseline antibody test visit, starting 28 September 2020. In each follow‐up survey, participants self‐reported the quantity and frequency of their alcohol drinking during the last week. Using the same laboratory antibody testing procedures discussed above, we tested participants for SARS‐CoV‐2 antibodies at end‐line. Lastly, on the fourth follow‐up survey (end‐line survey), participants self‐reported their RT–PCR SARS‐CoV‐2 testing history since baseline.
Variables
Primary exposure
The main exposure was high‐risk alcohol consumption, measured with Alcohol Use Disorders Identification Test (AUDIT) (self‐report version) [37]. Previous studies have established AUDIT as a valid measurement tool for use among young adults and college students [38, 39]. AUDIT has 10 questions. The first three regard frequency and quantity of alcohol consumption, questions four to six regard drinking behavior during the last year and the last four questions regard drinking problems during the last year. Each question can contribute a score from 0 to 4, and correspondingly a total AUDIT score can range from 0 to 40 [37]. In our main analysis, we used an AUDIT score of 8 or more (AUDIT ≥ 8 versus AUDIT < 8) as the cut‐off score for high‐risk drinking, as established in prior studies [37, 39]. AUDIT was measured once, in the on‐line baseline survey.
Secondary exposures for sensitivity analyses
To explore the sensitivity of our findings we used three sets of secondary exposures. We used AUDIT score as a continuous variable to avoid residual confounding and loss of power [40]. Further, AUDIT‐C is an effective and brief three‐question measurement tool for detecting high‐risk alcohol consumption [41], validated for use among college students [42]. We used a cut‐off score of 7 for males and 5 for females when using AUDIT‐C to identify at‐risk drinkers [42]. Lastly, in each of the four follow‐up surveys, we collected weekly alcohol use data using a quantity–frequency measure. The questions used for collecting these data were similar to that from the behavioral risk factor surveillance system [43], although slightly re‐worded. Using these data, we made the following secondary exposures: (a) any drinking during the study follow‐up and (b) heavy drinking (> 14 drinks per week for men and > 7 drinks per week for women in any of the four follow‐up surveys). Compared to AUDIT, quantity–frequency measurement tool captured alcohol use that occurred closer to the seroconversion outcome.
Primary outcome
The main outcome was SARS‐CoV‐2 seroconversion. For primary outcome analyses, we had an additional exclusion criterion. Participants needed to be seronegative for SARS‐CoV‐2 antibodies at baseline (n = 1027). Seroconversion was defined as having a negative SARS‐CoV‐2 antibody test result at baseline and a positive one at end‐line. We used SARS‐CoV‐2 immunoglobulin (Ig)M/IgG rapid assay kit (colloidal gold method) from BGI Genomics (Shenzhen, Guangdong, China; a biotechnology company [44]) to test participants for SARS‐CoV‐2 IgM and IgG antibodies. The antibody test result was interpreted as positive if one or both IgG and IgM antibody types were detected in the blood sample. In an external validation analysis, Beckman Coulter Access (Brea, CA, USA) SARS‐CoV‐2 IgG with high‐throughput chemiluminescent immunoassay (CLIA) [45] was used as the reference standard test to evaluate the accuracy of the rapid assay kits that we used in the current study. Compared to the reference standard test, the rapid assay kits showed a 64% of sensitivity and a 100% of specificity in detecting SARS‐CoV‐2 seroconversion.
Secondary outcomes
We chose two secondary outcomes:
Self‐reported new SARS‐CoV‐2 infections since baseline. Participants self‐reported their testing history for active SARS‐CoV‐2 infection in baseline and end‐line surveys. At baseline, they reported if they have ever been tested positive for SARS‐CoV‐2 infection. In the end‐line survey, we asked participants if they have been tested for an active SARS‐CoV‐2 infection since baseline. Among those who responded ‘yes’ to this question, we asked about the results of their test. Only participants who self‐reported a negative SARS‐CoV‐2 infection history at baseline and self‐reported testing for SARS‐CoV‐2 active infection since baseline, in the end‐line survey, were eligible for this outcome analyses (n = 518). New SARS‐CoV‐2 infection was defined as self‐reporting a negative SARS‐CoV‐2 antibody test result in baseline and a positive one in end‐line surveys.
Self‐reports of symptomatic COVID‐19. At baseline, among participants who self‐reported that they have ever been tested positive for SARS‐CoV‐2 infection (n = 128), we asked them to describe the symptoms of their SARS‐CoV‐2 infection. There were four response options for this question: ‘asymptomatic’, ‘mild’, ‘moderate’ and ‘severe’. For this dichotomized secondary outcome, participants who reported ‘mild’, ‘moderate’ or ‘severe’ symptoms were recoded as symptomatic (asymptomatic versus symptomatic).
Covariates
In the baseline survey, participants self‐reported the following demographics: age (years), sex at birth (female versus male), race (Asian, black, multi‐racial, other, white), year in school (1st–4th and 5th), residence (on‐ versus off‐campus) and Greek membership 1 (yes versus no). For descriptive analysis, we dichotomized the age variable at the legal age of drinking in the United States (21 years). We also dichotomized the race variable (white versus non‐white). Participants had the option to choose ‘do not know’ when responding to the baseline survey questions. ‘Do not know’ responses were set to missing in the analysis. Lastly, in the parent randomized controlled study (RCT), participants were randomized to receive their antibody testing results either ≤ 24 hours (group 1) or 4 weeks (group 2) after their antibody testing [33]. In our inferential analyses, we accounted for this variable.
Study size
We did not perform power analysis for the current cohort study as this study was leveraged from the RCT study.
Statistical methods
To evaluate the representativeness of our study sample, we compared the sample characteristics with that of the IUB undergraduate population using official IUB enrollment reports [46]. We used multiple imputation (MI, fully conditional method) and created 20 imputed data sets to address missingness, specifically to account for the missing values of the seroconversion outcome (Supporting information, Box S2) [47, 48]. To account for the imperfect sensitivity of the antibody tests in detecting seroconversion, we used logistic regression with a maximum likelihood analysis approach [49, 50] when evaluating the associations between high‐risk alcohol consumption (or secondary exposures) and seroconversion outcome. This approach generates the odds ratio (OR). However, the risk ratio (RR) was the measure of interest in the current prospective cohort study. Hence, we used the equation developed by Zhang & Yu [51] to convert these ORs to RRs.
We used Poisson regression with a robust error variance [52] to estimate RRs for the association between the high‐risk alcohol consumption and secondary outcome of self‐reported new SARS‐CoV‐2 infection. We used the same statistical model [52] to estimate the prevalence ratio (PR) for the association between high‐risk alcohol consumption and symptomatic COVID‐19. Poisson regression with a robust error variance is the method of choice when estimating PRs [53]. All models were adjusted for sex at birth, dichotomized race, age (continuous) and intervention group. We calculated the 95% confidence interval (CI) for all estimated RRs and PRs.
In our sensitivity analysis, we used total AUDIT score (continuous variable), AUDIT‐C and quantity and frequency of alcohol consumption (any drinking and heavy drinking) and re‐estimated the RR for the associations between these exposures and the outcomes. The data analysis was conducted using SAS software, version 9.4 (Cary, NC, USA) (analysis SAS code available in Supporting information, Box S2). We used Python version 3.7.6 for data visualization (Python Software Foundation, Beaverton, OR, USA).
RESULTS
Participants
Of the 7499 sampled IUB undergraduate students, 3430 did not meet one or more of the inclusion criteria and 2672 were non‐responders (Figure 1). A total of 1397 students consented to participate in the study. However, 130 participants did not complete any of the study procedures and 191 did not complete their baseline SARS‐CoV‐2 antibody testing. Moreover, 49 of the 1076 participants who completed their baseline antibody test appointment tested positive at baseline and were excluded from the analysis regarding the primary outcome. The response rate for the current study was estimated to be 27% (Supporting information, Box S1). This response rate is above average compared to other, similar, studies [54, 55, 56]. Of the 1027 participants who tested negative at baseline, 808 returned for their end‐line antibody test (retention rate = 79%). A total of 736 participants completed all follow‐up surveys.
FIGURE 1.
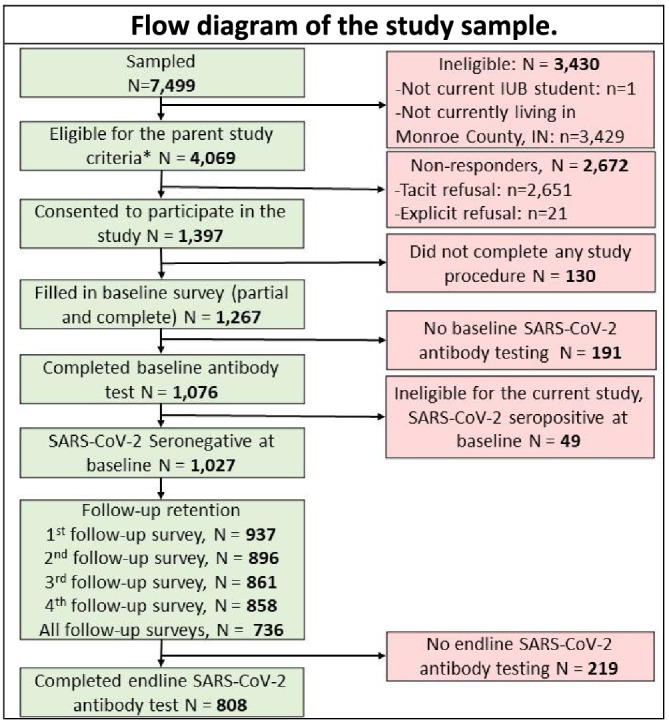
Flow diagram of the study sample. There were three eligibility criteria for the parent study and one additional criterion for the current study
Descriptive data
Age median was 20 (interquartile range = 2). Students were mostly female (64%), white (79%), senior undergraduate student (30%) and non‐Greek affiliated (77%) (Table 1). Approximately 69% of participants reported living off‐campus. The study sample seemed to be representative of the IUB undergraduate population for most demographics (Supporting information, Table S1). However, female students were over‐represented in our sample relative to their representation in the overall IUB student body.
TABLE 1.
Baseline characteristics of the study participants of 1027 Indiana University undergraduate students, September 2020
| Tota N = 1027 n (%) | Primary exposure: total AUDIT score a | P‐value b | ||
|---|---|---|---|---|
| AUDIT < 8 n = 688 (68.2%) | AUDIT ≥ 8 n = 321 (31.8%) | |||
| Socio‐demographic characteristics | ||||
| Age (years) | < 0.0001 | |||
| ≥ 21 | 332 (34.4) | 191 (29.5) | 139 (46.0) | |
| < 21 | 632 (65.6) | 457 (70.5) | 163 (54.0) | |
| Missing | 63 | 40 | 19 | |
| Sex at birth | < 0.0001 | |||
| Female | 655 (64.0) | 476 (69.2) | 171 (53.3) | |
| Male | 368 (36.0) | 212 (30.8) | 150 (46.7) | |
| Missing | 4 | 0 | 0 | |
| Race | < 0.0001 c | |||
| Asian | 77 (7.5) | 67 (9.7) | 10 (3.1) | |
| Black | 13 (1.3) | 11 (1.6) | 2 (0.6) | |
| Multi‐racial | 80 (7.8) | 60 (8.7) | 20 (6.2) | |
| Other | 43 (4.2) | 35 (5.1) | 7 (2.2) | |
| White | 809 (79.2) | 515 (74.9) | 282 (87.9) | |
| Missing | 5 | 0 | 0 | |
| Race dichotomized | < 0.0001 | |||
| White | 809 (79.2) | 515 (74.9) | 282 (87.9) | |
| Non‐white | 213 (20.8) | 173 (25.1) | 39 (12.1) | |
| Missing | 5 | 0 | 0 | |
| Year in school | < 0.0001 | |||
| 1st | 224 (21.9) | 167 (24.3) | 50 (15.6) | |
| 2nd | 235 (23.0) | 168 (24.5) | 65 (20.2) | |
| 3rd | 255 (25.0) | 173 (25.2) | 79 (24.6) | |
| 4th and 5th | 307 (30.1) | 178 (25.9) | 127 (39.6) | |
| Missing | 6 | 2 | 0 | |
| Residence | 0.0012 | |||
| Off‐campus | 701 (68.7) | 452 (65.8) | 243 (75.9) | |
| On‐campus | 320 (31.3) | 235 (34.2) | 77 (24.1) | |
| Missing | 6 | 1 | 1 | |
| Greek membership | < 0.0001 | |||
| No | 788 (77.2) | 565 (82.4) | 213 (66.4) | |
| Yes | 233 (22.8) | 121 (17.6) | 108 (33.6) | |
| Missing | 6 | 2 | 0 | |
| Intervention group | 0.2089 | |||
| Group 1 | 516 (50.2) | 355 (51.6) | 152 (47.4) | |
| Group 2 | 511 (49.8) | 333 (48.4) | 169 (52.6) | |
| Primary outcome | ||||
| SARS‐CoV‐2 seroconversion, among those who were seronegative for SARS‐CoV‐2 at baseline (n = 1027) | 0.0039 | |||
| Yes | 42 (5.2) | 20 (3.6) | 21 (8.5) | |
| No | 766 (94.8) | 531 (96.4) | 226 (91.5) | |
| Missing | 219 | 137 | 74 | |
| Secondary outcomes | ||||
| Self‐reported new SARS‐CoV‐2 infections since baseline: among those who self‐reported a negative SARS‐CoV‐2 infection history at baseline and self‐reported testing for SARS‐CoV‐2 active infection since baseline, in the end‐line survey (n = 518) | 0.0244 | |||
| Yes | 44 (8.6) | 23 (6.8) | 21 (12.8) | |
| No | 465 (91.4) | 317 (93.2) | 143 (87.2) | |
| Missing | 9 | 6 | 3 | |
| Symptomatic COVID‐19: among those who self‐reported positive SARS‐CoV‐2 testing history at baseline (n = 128) | 0.1138 | |||
| Yes | 95 (75.4) | 42 (68.9) | 48 (81.4) | |
| No | 31 (24.6) | 19 (31.1) | 11 (18.6) | |
| Missing | 2 | 2 | 0 | |
Overall, Alcohol Use Disorders Identification Test (AUDIT) score was missing for 18 participants.
χ2 and Fisher's exact tests were used to compare the groups.
Fisher's exact test due to small cell sizes. Bold type indicates significant values.
There were significant socio‐demographic differences between participants with high‐risk alcohol consumption (AUDIT score ≥ 8) and low‐risk alcohol consumption (AUDIT score < 8). In χ2 tests, age, sex at birth, race, year in school, residence and Greek membership were associated with high‐risk alcohol consumption. Participants with high‐risk alcohol consumption status tended to be ≥ 21 years old, male, white, senior students, living off‐campus and affiliated with the Greek organizations.
AUDIT score data were available for 1009 (of the 1027) participants (n missing = 18) (Table 1). AUDIT score median was 5 with an interquartile range of 7 (Supporting information, Figure S1). Approximately 32% of participants were at high‐risk alcohol consumption.
Seroconversion (primary outcome)
Of the 808 participants who tested negative at baseline and completed the end‐line antibody test, 42 (5%) seroconverted; 21 (9%) of 247 participants with high‐risk alcohol consumption status seroconverted while only 20 (4%) of 551 participants with low‐risk alcohol consumption status seroconverted (Table 1, Figure 2). The proportion of missing values for primary outcome was similar among the low‐ and high‐risk groups.
FIGURE 2.
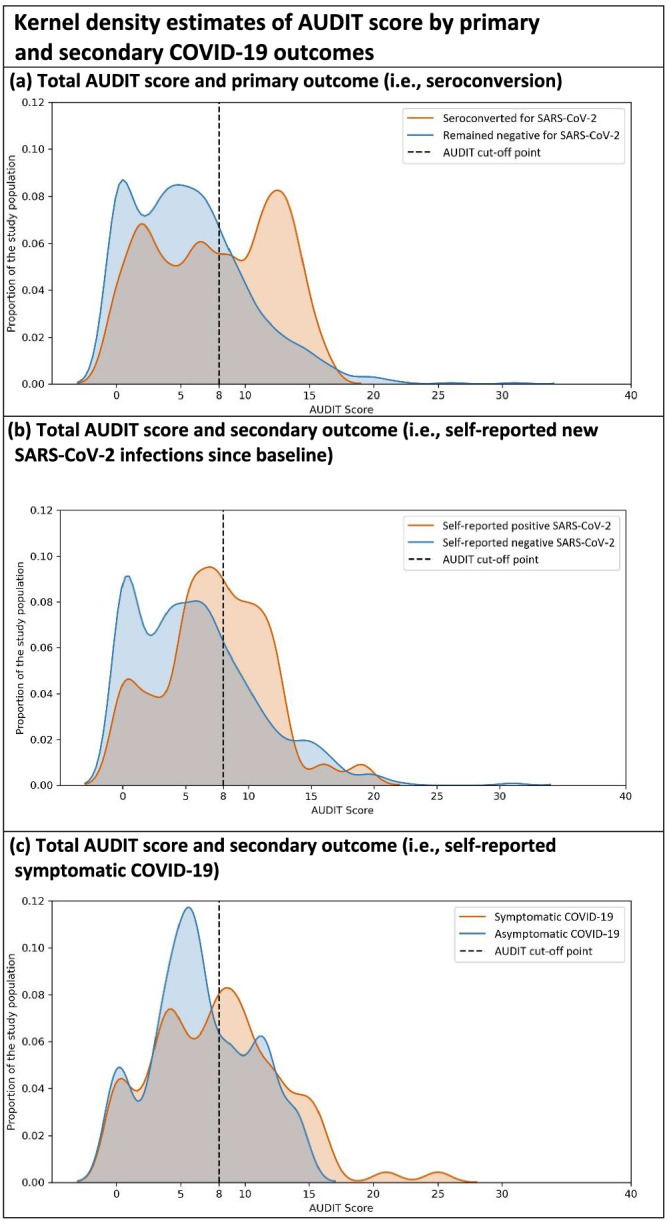
Kernel density estimates of AUDIT score by primary and secondary COVID‐19 outcomes. NB: Kernel density estimate is a non‐parametric method to visualize the distribution of a continuous variable (we used bandwidth of 1 in all figures)
Self‐reported new SARS‐CoV‐2 infection/symptomatic COVID‐19 (secondary outcomes)
Overall, 9% of participants who self‐reported a negative SARS‐CoV‐2 infection history at baseline self‐reported a positive SARS‐CoV‐2 infection in the end‐line survey. Moreover, among participants who self‐reported that they have ever been tested positive for SARS‐CoV‐2 infection in the baseline survey, 75% reported experiencing symptomatic COVID‐19.
Main results
In adjusted models and after MI (Table 2), we found that students with high‐risk alcohol consumption had 2.44 times the risk of SARS‐CoV‐2 seroconversion (corrected RR = 2.44, 95% CI = 1.35, 4.25) and 1.84 times the risk of self‐reporting a positive SARS‐CoV‐2 infection at end‐line (RR = 1.84, 95% CI = 1.04, 3.28) compared to students with low‐risk alcohol consumption. We did not identify any association between high‐risk alcohol consumption and symptomatic COVID‐19 (PR = 1.17, 95% CI = 0.93, 1.47). Similar associations were found in complete case analysis (Supporting information, Tables S2 and S3).
TABLE 2.
Adjusted associations between alcohol consumption and COVID‐19 outcome, findings after multiple imputation
| Primary exposure | Primary outcome a , b | Secondary outcomes a | |
|---|---|---|---|
| SARS‐CoV‐2 seroconversion at end‐line | Self‐reported new SARS‐CoV‐2 infections at end‐line | Symptomatic COVID‐19 self‐report at baseline | |
| Adjusted RR | Adjusted RR | Adjusted PR | |
| High‐risk alcohol consumption assessed with AUDIT | n = 1027 | n = 518 | n = 128 |
| Yes (AUDIT ≥ 8) | 2.44 (1.35, 4.25) | 1.84 (1.04, 3.28) | 1.17 (0.93, 1.47) |
| No (AUDIT < 8) | Ref. | Ref. | Ref. |
| Secondary exposures | |||
| High‐risk alcohol consumption assessed with AUDIT‐C | n = 1027 | n = 518 | n = 128 |
| Yes (AUDIT‐C ≥ 7 for males and AUDIT‐C ≥ 5 for females) | 2.54 (1.38, 4.53) | 2.28 (1.26, 4.14) | 0.98 (0.79, 1.23) |
| No (AUDIT‐C < 7 for males and AUDIT‐C < 5 for females) | Ref. | Ref. | Ref. |
| Frequency and quantity of alcohol consumption | |||
| Any drinking | n = 1027 | n = 518 | |
| Yes | 1.47 (0.60, 3.44) | 1.95 (0.78, 4.87) | NA |
| No | Ref. | Ref. | NA |
| Heavy drinking | n = 1027 | n = 518 | |
| Yes | 2.32 (1.26, 4.15) | 2.53 (1.36, 4.69) | NA |
| No | Ref. | Ref. | NA |
All models were adjusted for sex at birth, race, age and intervention group (from the parent RCT study).
For the seroconversion outcome, we first estimated the corrected odds ratios (OR) for misclassified outcomes [49, 50] and then converted these ORs to risk ratios (RRs) using the Zhang & Yu equation [51].
NA = not applicable because exposure occurred after outcome; AUDIT = Alcohol Use Disorders Identification Test; RCT = randomized controlled trial; PR = prevalence ratio.
Bold type indicates significant value (P < 0.05).
Sensitivity analyses
We found similar results when we used AUDIT‐C instead of AUDIT as the exposure variable (Table 2). Moreover, we found that participants who reported heavy drinking in one or more of the follow‐up surveys had 2.32 (95% CI = 1.26, 4.15) times the risk of SARS‐CoV‐2 seroconversion and 2.53 (95% CI = 1.36, 4.69) times the risk of self‐reporting a new positive SARS‐CoV‐2 infection compared to participants who did not report heavy drinking. Lastly, the associations between the continuous variable of AUDIT score and SARS‐CoV‐2 seroconversion were positively and statistically significant [adjusted RR (aRR) = 1.10, 95% CI = 1.03, 1.17]. Similar results were found for the association between AUDIT score and self‐reported new SARS‐CoV‐2 infection at the end‐line (aRR = 1.05, 95% CI = 1.00, 1.10).
DISCUSSION
Key results
We found that undergraduate students with high‐risk alcohol consumption were at higher risk for SARS‐CoV‐2 seroconversion, compared to students with no such risk. We found similar results when we used an alternative outcome (self‐reported new SARS‐CoV‐2 infection). In sensitivity analyses, we found similar results when we used other alcohol consumption exposures (continuous AUDIT score, AUDIT‐C and heavy drinking). This study highlights the important role that alcohol may play in the spread of COVID‐19 on college campuses.
Interpretation
Few studies have evaluated similar associations between alcohol use and COVID‐19. In our literature screening of more than 660 titles on PubMed, we identified eight relevant study reports [57, 58, 59, 60, 61, 62, 63, 64]. Two studies did not find any association between alcohol consumption and COVID‐19 [57, 60]. Four studies identified excessive alcohol use as a risk factor for COVID‐19 diagnosis [63], poor prognosis [62] and severity [58, 64]. One study found excessive alcohol use as a risk factor for COVID‐19 death in patients with obesity but not in those without obesity [52]. One study found low‐dose alcohol intake (< 100 g alcohol per week) to be a protective factor for COVID‐19 hospitalization [50]. Lastly, in a previous cross‐sectional analysis report using baseline data of the RCT study, we found that drinking alcohol more than once a week increased the likelihood of SARS‐CoV‐2 seropositivity [14].
These studies were heterogeneous in their methodology, target population and exposure and outcome measurements. Only one study was conducted in the United States (among hospital patients) [63]. No prior study was in college students or young adults, a general population among whom excessive alcohol drinking and SARS‐CoV‐2 infections are both prevalent. Previous studies measured alcohol consumption in different ways, such as quantity–frequency questionnaires [57, 58, 59] and semi‐structured interviews [62]. To our knowledge, no study used the validated AUDIT screening tool. AUDIT screens a longer period compared to other measurement tools and can identify harmful drinking patterns and chronic alcohol consumption, which are linked to adverse physical consequences [65]. Studies used different ways to measure COVID‐19 outcome, such as the RT–PCR test [61], electronic health records [63] or COVID‐19 hospitalization [57, 59, 60]. No study used SARS‐CoV‐2 seroconversion. Serological tests can detect previously infected individuals even if they were not tested for active infection using RT‐PCR test.
We further observed a statistically significant association between continuous AUDIT score and SARS‐CoV‐2 seroconversion. These findings suggest that there might be a dose–response relationship between alcohol consumption and SARS‐CoV‐2 seroconversion. Another study found that low‐dose alcohol use is associated with lower risk for COVD‐19 hospitalization [59]. Previous studies have found similar protective associations for low to moderate alcohol use and other respiratory infections, such as the common cold [66]. Further studies are needed to fully understand the relationship.
Strengths and limitations
Study design
Some aspects of our study design influence the interpretation of our findings. As our study design was observational, no causal inference should be made based on the findings, particularly because of the potential for unmeasured confounding. Moreover, the associations between high‐risk alcohol consumption and symptomatic COVID‐19 were evaluated cross‐sectionally and with a small sample size. Thus, our ability to evaluate the temporal ordering for these associations is limited. Similarly, the temporal relationships between frequency and quantity of alcohol consumption measured during the data collection weeks and the outcomes are not fully clear because we do not have data on the exact date of seroconversion. Seroconversion could have occurred at any time between the baseline and end‐line antibody testing, though more likely it occurred close to the end‐line testing date. Nonetheless, we used a more robust study design (prospective cohort) with larger sample sizes when evaluating the associations between high‐risk alcohol consumption and seroconversion and self‐reported SARS‐CoV‐2 new infection outcomes. Here, the temporal relationship is clear; that is, the exposure comes before the outcome, because AUDIT, which measures high‐risk alcohol consumption in previous years, was completed at baseline, and outcome could have only occurred after the baseline.
Measures
We chose different measurement tools for assessing the exposure and outcome, each of which have some strengths and limitations. We used biological antibody testing to measure seroconversion outcome. Antibody testing kits can capture undetected previous SARS‐CoV‐2 infections, although the antibody testing kits in this study had a low sensitivity. However, RR estimates tend to be less biased when, as in our study, the outcome is not prevalent, and it is measured with perfect (100%) specificity but low sensitivity [50]. We counted for the low sensitivity of antibody tests in our statistical analysis. Further, we used a secondary outcome (self‐reported new SARS‐CoV‐2 infection) that does not depend upon antibody positivity. Previously, we found a strong association between self‐reported SARS‐CoV‐2 infection and antibody testing variables [14]. Similarly, we used different measures to collect self‐reported data on alcohol use, AUDIT, AUDIT‐C and quantity–frequency index. Because alcohol use data were self‐reported, the data might suffer from recall and social desirability biases. However, all these measurement tools are validated. Collecting real‐time alcohol use data using ecological momentary assessment tools might help to reduce these biases [67].
Generalizability
We used random sampling to identify our potential study participants. Because the demographics of IUB undergraduates are comparable to those of other large campuses, we might be able to generalize our findings to American college students. However, there were some differences between the random sample and IUB undergraduate population, specifically in the ‘sex at birth’ variable. The response rates of 27% might seem low; however, our response rate is considered greater than average when compared to other studies on college campuses [55, 56].
CONCLUSION
Our findings suggest that high‐risk alcohol consumption is associated with higher risk for SARS‐CoV‐2 infection/seroconversion. These findings could have implications for colleges’ re‐opening planning. Even though effective interventions to reduce high‐risk alcohol consumption might take more time to be tested and implemented at individual level, university policymakers can use our findings to predict locations in college towns where transmission might be more likely to occur (e.g. college town bars or Greek houses) and implement COVID‐19 protective measures (e.g. face mask‐wearing) in such places. More studies are needed to understand the extent of a causal relationship between alcohol consumption and SARS‐CoV‐2 infection.
CLINICAL TRIAL REGISTRATION DETAILS
Not applicable.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Sina Kianersi: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; visualization. Christina Ludema: Conceptualization; data curation; funding acquisition; methodology; project administration; resources. Jonathan Macy: Conceptualization; data curation; funding acquisition; methodology; project administration; resources. Chen Chen: Data curation; investigation; validation. Molly Rosenberg: Conceptualization; data curation; formal analysis; funding acquisition; methodology; project administration; resources; supervision.
Supporting information
Table S1. Baseline characteristics of the study participants of 1027 Indiana University undergraduate students compared to IUB undergraduate population, September 2020
Table S2. Crude associations between alcohol consumption and COVID‐19 outcome, complete case analysis
Table S3. Adjusted associations between alcohol consumption and COVID‐19 outcome, complete case analys
Figure S1. AUDIT score distribution in the sample
Box S1. Response rate calculation
Box S2. Multiple Imputation Procedure
Table S4. Missing Data Patterns
ACKNOWLEDGEMENTS
This study was supported by private contributions to the Indiana University Foundation. The United Arab Emirates provided the testing kits. S.K. was a pre‐doctoral trainee of a National Institutes of Health (NIH)‐funded program (NIAAA grant no. R25DA051249, 2021) while working on this study. NIAAA had no role in the design, analysis, interpretation or publication of this study. The content is solely the responsibility of the authors. We are grateful to all participants for participating in the study. S.K. would like to thank Dr Maria Parker for her valuable comments on an earlier version of this paper.
Kianersi S, Ludema C, Macy JT, Chen C, Rosenberg M. Relationship between high‐risk alcohol consumption and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroconversion: a prospective sero‐epidemiological cohort study among American college students. Addiction. 2022;117:1908–1919. 10.1111/add.15835
Funding information National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: R25DA051249; Indiana University Foundation
ENDNOTE
Greek housing is a type of community housing for North American college students who are members of fraternity and sorority social organizations [68].
REFERENCES
- 1. The New York Times . Tracking Coronavirus Cases at U.S. Colleges and Universities 2021 [updated March 2, 2021; cited 2021 3.6.2021]. Available at: https://www.nytimes.com/interactive/2021/us/college-covid-tracker.html. Accessed 24 Dec 2021.
- 2. Centers for Disease Control and Prevention (CDC) . Symptoms of COVID‐19 2021 [updated 02.22.2021; cited 06.28.2021. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html. Accessed 24 Dec 2021.
- 3. Centers for Disease Control and Prevention . Post‐COVID Conditions 2021 [updated July 12, 2021; cited 2021 7.21.2021]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html. Accessed 24 Dec 2021.
- 4. Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home‐isolated patients. Nat Med. 2021;27:1607–13. 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nichol KL, D'Heilly S, Ehlinger EP. Influenza vaccination among college and university students: Impact on influenza‐like illness, health care use, and impaired school performance. Arch Pediatr Adolesc Med. 2008;162:1113–8. 10.1001/archpedi.162.12.1113 [DOI] [PubMed] [Google Scholar]
- 6. Son C, Hegde S, Smith A, Wang X, Sasangohar F. Effects of COVID‐19 on college students' mental health in the United States: interview survey study. J Med Internet Res. 2020;22:e21279. 10.2196/21279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boehmer TK, DeVies J, Caruso E, van Santen KL, Tang S, Black CL, et al. Changing age distribution of the COVID‐19 pandemic — United States, May–August 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69:(39):1404–9. 10.15585/mmwr.mm6939e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oster AM, Caruso E, DeVies J, Hartnett KP, Boehmer TK. Transmission dynamics by age group in COVID‐19 hotspot counties — United States, April–September 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69:(41):1494–6. 10.15585/mmwr.mm6941e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salvatore PP, Sula E, Coyle JP, Caruso E, Smith AR, Levine RS, et al. Recent increase in COVID‐19 cases reported among adults aged 18–22 years—United States, may 31–September 5, 2020. Morb Mortal Wkly Rep. 2020;69:1419–24. 10.15585/mmwr.mm6939e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Malley PM, Johnston LD. Epidemiology of alcohol and other drug use among American college students. J Stud Alcohol Suppl. 2002;23–39. 10.15288/jsas.2002.s14.23 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC) . Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2018.
- 12. Suffoletto B, Ram N, Chung T. In‐person contacts and their relationship with alcohol consumption among young adults with hazardous drinking during a pandemic. J Adolesc Health. 2020;67:671–6. 10.1016/j.jadohealth.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor S, Paluszek MM, Rachor GS, McKay D, Asmundson GJ. Substance use and abuse, COVID‐19‐related distress, and disregard for social distancing: a network analysis. Addict Behav. 2021;114:106754. 10.1016/j.addbeh.2020.106754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kianersi S, Ludema C, Macy JT, Garcia Colato E, Chen C, Luetke M, et al. A cross‐sectional analysis of demographic and behavioral risk factors of severe acute respiratory syndrome coronavirus 2 Seropositivity among a sample of U.S. college students. J Adolesc Health. 2021;69:219–26. 10.1016/j.jadohealth.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. 10.1111/j.1530-0277.1998.tb03695.x [DOI] [PubMed] [Google Scholar]
- 16. Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. 10.1016/j.ypmed.2004.04.044 [DOI] [PubMed] [Google Scholar]
- 17. Stephens DN, Duka T. Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex. Phil Trans R Soc Lond B Biol Sci. 2008;363:3169–79. 10.1098/rstb.2008.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurrieri L, Fairbairn CE, Sayette MA, Bosch N. Alcohol narrows physical distance between strangers. Proc Natl Acad Sci. 2021;118(20):e2101937118. 10.1073/pnas.2101937118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thrul J, Kuntsche E. The impact of friends on young adults' drinking over the course of the evening—an event‐level analysis. Addiction. 2015;110:619–26. 10.1111/add.12862 [DOI] [PubMed] [Google Scholar]
- 20. Rosenberg M, Luetke M, Hensel D, Kianersi S, Fu TC, Herbenick D. Depression and loneliness during April 2020 COVID‐19 restrictions in the United States, and their associations with frequency of social and sexual connections. Soc Psychiatry Psychiatr Epidemiol. 2021;56:1221–32. 10.1007/s00127-020-02002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venkatesh A, Edirappuli S. Social distancing in COVID‐19: what are the mental health implications? BMJ. 2020;369:m1379. 10.1136/bmj.m1379 [DOI] [PubMed] [Google Scholar]
- 22. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10:1206–12. 10.3201/eid1007.030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simet SM, Sisson JH. Alcohol's effects on lung health and immunity. Alcohol Res. 2015;37:199–208. [PMC free article] [PubMed] [Google Scholar]
- 24. Bailey KL, Samuelson DR, Wyatt TA. Alcohol use disorder: A pre‐existing condition for COVID‐19?. Alcohol. 2021;90:11–7. 10.1016/j.alcohol.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–32. [DOI] [PubMed] [Google Scholar]
- 26. Szabo G, Saha B. Alcohol's effect on host defense. Alcohol Res. 2015;37:159–70. [PMC free article] [PubMed] [Google Scholar]
- 27. Simou E, Britton J, Leonardi‐Bee J. Alcohol and the risk of pneumonia: a systematic review and meta‐analysis. BMJ Open. 2018;8(8):e022344. 10.1136/bmjopen-2018-022344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–77. 10.1097/01.ccm.0000055389.64497.11 [DOI] [PubMed] [Google Scholar]
- 29. Sharma A, Kroumpouzos G, Lotti T, Goldust M. COVID‐19 and alcohol use. Drug Alcohol Rev. 2021;40:683–4. [DOI] [PubMed] [Google Scholar]
- 30. Saengow U, Assanangkornchai S, Casswell S. Alcohol: a probable risk factor of COVID‐19 severity. Addiction. 2021;116:204–5. 10.1111/add.15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Testino G. Are patients with alcohol use disorders at increased risk for COVID‐19 infection? Alcohol Alcohol. 2020;55:344–6. 10.1093/alcalc/agaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 33. Indiana University . Longitudinal COVID‐19 Antibody Testing in Indiana University Undergraduate Students. 2020. Available at: https://ClinicalTrials.gov/show/NCT04620798. Accessed 24 Dec 2021.
- 34. Kianersi S, Luetke M, Ludema C, Valenzuela A, Rosenberg M. Use of research electronic data capture (REDCap) in a COVID‐19 randomized controlled trial: a practical example. BMC Med Res Methodol. 2021;21(1):175–3. 10.1186/s12874-021-01362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 38. Cook RL, Chung T, Kelly TM, Clark DB. Alcohol screening in young persons attending a sexually transmitted disease clinic. Comparison of AUDIT, CRAFFT, and CAGE instruments. J Gen Intern Med. 2005;20:1–6. 10.1111/j.1525-1497.2005.40052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kokotailo PK, Egan J, Gangnon R, Brown D, Mundt M, Fleming M. Validity of the alcohol use disorders identification test in college students. Alcohol Clin Exp Res. 2004;28:914–20. 10.1097/01.alc.0000128239.87611.f5 [DOI] [PubMed] [Google Scholar]
- 40. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–41. 10.1002/sim.2331 [DOI] [PubMed] [Google Scholar]
- 41. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT‐C): an effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 42. DeMartini KS, Carey KB. Optimizing the use of the AUDIT for alcohol screening in college students. Psychol Assess. 2012;24:954–63. 10.1037/a0028519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention (CDC) . Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 44. BGI Group . BGI Group website [cited 2022 1.4.2022]. Available at: https://en.genomics.cn/. Accessed 24 Dec 2021.
- 45. US Food and Drug Administration . Beckman Coulter, Inc. Access SARS‐CoV‐2 IgG ‐ Instructions for Use 2020 [cited 2022 1.4.2022]. Available at: https://www.fda.gov/media/139627/download. Accessed 24 Dec 2021.
- 46. University Institutional Research and Reporting . Enrollment Information—Official, First Day, and Daily 2020 [cited 2021 12.15.2021]. Available at: https://uirr.iu.edu/facts-figures/enrollment/index.html. Accessed 24 Dec 2021.
- 47. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–101. 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 49. Lyles RH, Tang L, Superak HM, King CC, Celentano DD, Lo Y, et al. Validation data‐based adjustments for outcome misclassification in logistic regression: an illustration. Epidemiology. 2011;22:589–97. 10.1097/ede.0b013e3182117c85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146:195–203. 10.1093/oxfordjournals.aje.a009251 [DOI] [PubMed] [Google Scholar]
- 51. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 52. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 53. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross‐sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:1–13. 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eastman‐Mueller H, Fu T‐c, Dodge BM, Herbenick D. The relationship between college students' campus sexual health resource utilization and self‐reported STI testing: Findings from an undergraduate probability survey. J Am Coll Health. 2020;1–9. 10.1080/07448481.2020.1775607 [DOI] [PubMed] [Google Scholar]
- 55. National Survey of Student Engagement . NSSE response rates: frequently asked questions 2021 [cited 2021 7.5.2021]. Available at: https://nsse.indiana.edu/nsse/psychometric-portfolio/responserate-faq.html. Accessed 24 Dec 2021.
- 56. Cantor D, Fisher B, Chibnall SH, Townsend R, Lee H, Thomas G Report on the AAU Campus Climate Survey on Sexual Assault and Sexual Misconduct. Rockville, MD: Association of American Universities; 2015.
- 57. Hamer M, Kivimaki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–7. 10.1016/j.bbi.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lassen MCH, Skaarup KG, Sengelov M, Iversen K, Ulrik CS, Jensen JUS, et al. Alcohol consumption and the risk of acute respiratory distress syndrome in COVID‐19. Ann Am Thorac Soc. 2021;18:1074–6. 10.1513/annalsats.202008-988rl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gao C, Zhao Z, Li F, Liu JL, Xu H, Zeng Y, et al. The impact of individual lifestyle and status on the acquisition of COVID‐19: a case‐control study. PLOS ONE. 2020;15:e0241540. 10.1371/journal.pone.0241540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li S, Hua X. Modifiable lifestyle factors and severe COVID‐19 risk: a Mendelian randomisation study. BMC Medical Genom. 2021;14:(1):38–45. 10.1186/s12920-021-00887-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fan X, Liu Z, Poulsen KL, Wu X, Miyata T, Dasarathy S, et al. Alcohol consumption is associated with poor prognosis in obese patients with COVID‐19: A mendelian randomization study using UK Biobank. Nutrients. 2021;13(5):1592–613. 10.3390/nu13051592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mehra A, Suri V, Sahoo S, Malhotra P, Yaddanapudi LN, Puri GD, et al. Relationship of substance dependence and time to RT‐PCR negative status in patients with COVID‐19 infection. Asian J Psychiatry. 2021;57:102562. 10.1016/j.ajp.2021.102562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID‐19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26:30–9. 10.1038/s41380-020-00880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saurabh S, Verma MK, Gautam V, Kumar N, Jain V, Goel AD, et al. Tobacco, alcohol use and other risk factors for developing symptomatic COVID‐19 vs asymptomatic SARS‐CoV‐2 infection: a case–control study from western Rajasthan, India. Trans Royal Soc Trop Med Hygiene. 2021;115:820–31. 10.1093/trstmh/traa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. World Health Organization (WHO) . In: Babor TF, Higgins‐Biddle JC, Saunders JB, Monteiro MG, editorsAUDIT: the Alcohol Use Disorders Identification Test: guidelines for use in primary health care. 2nd ed. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 66. Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83:1277–83. 10.2105/ajph.83.9.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rosenberg M, Ludema C, Kianersi S, Luetke M, Jozkowski K & Guerra‐Reyes L et al. Wearable alcohol monitors for alcohol use data collection among college students: feasibility and acceptability in a pilot study. medRxiv 2021. 10.1101/2021.02.17.21251959 [DOI] [PMC free article] [PubMed]
- 68. Whipple EG, Sullivan EG. Greek letter organizations: Communities of learners? New Directions for Student Services. 1998;1998(81):7–17. 10.1002/ss.8101 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of the study participants of 1027 Indiana University undergraduate students compared to IUB undergraduate population, September 2020
Table S2. Crude associations between alcohol consumption and COVID‐19 outcome, complete case analysis
Table S3. Adjusted associations between alcohol consumption and COVID‐19 outcome, complete case analys
Figure S1. AUDIT score distribution in the sample
Box S1. Response rate calculation
Box S2. Multiple Imputation Procedure
Table S4. Missing Data Patterns


