Abstract
Background
It is essential to know about immune response levels after booster doses of the two different types of vaccines, mRNA, and the inactivated, currently used against COVID‐19. For this purpose, we aimed to determine the effects of BNT162b2 (BNT) and CoronaVac (CV) boosters on the humoral and cellular immunity of individuals who had two doses of CV vaccination.
Methods
The study was conducted in three centers (Koc University Hospital, Istanbul University Cerrahpasa Hospital, and Istanbul University, Istanbul Medical School Hospital) in Istanbul, Turkey. Individuals who had been previously immunized with two doses of CV and no history of COVID‐19 were included. The baseline blood samples were collected 3–5 months after the second dose of CV. Follow‐up blood samples were taken 1 and 3 months after administration of third doses of CV, or one dose of BNT boosters. Neutralizing antibody titers were measured by plaque reduction assay. The CD4+ T cell, CD8+ T cell, effector CD4+CD38+CD69+ T cell, and effector CD8+CD38+CD69+ T cell ratios were determined by flow cytometry. The intracellular IFN‐γ and IL‐2 responses were measured by ELISpot assay.
Results
We found a 3.38‐fold increase in neutralizing antibody geometric mean titers (NA GMT, 78.69) 1 month after BNT booster and maintained at the third month (NA GMT, 80). Nevertheless, in the CV booster group, significantly lower NA GMT than BNT after 1 month and 3 months were observed (21.44 and 28.44, respectively) (p < .001). In the ELISpot assay, IL‐2 levels after BNT were higher than baseline and CV booster (p < .001) while IFN‐γ levels were significantly higher than baseline (p < .001). The CD8+CD38+CD69+ and CD4+CD38+CD69+ T cells were stimulated predominantly in the third month of the BNT boosters.
Conclusion
The neutralizing antibody levels after 3 months of the BNT booster were higher than the antibody levels after CV in fully vaccinated individuals. On the contrary, ratio of the effector T cells increased along with greater IFN‐γ activation after BNT booster. By considering the waning immunity, we suggest a new booster dose with BNT for the countries that already had two doses of primary CV regimens.
Keywords: SARS‐CoV, T cells, vaccines
This study determines the effects of BNT162b2 (BNT) and CoronaVac (CV) boosters on the humoral and cellular immunity of individuals who had two doses of CV vaccination. The neutralizing antibody levels after 3 months of the BNT booster are higher than the antibody levels after CV in fully vaccinated individuals. The ratio of the effector T cells increases along with greater IFN‐γ activation after BNT booster.

Abbreviations
- GMT
geometric mean titer
- PBMCs
peripheral blood mononuclear cells
- PRNT50
plaque reduction neutralization test
- SFU
spot forming units
1. INTRODUCTION
The worldwide use of effective and safe COVID‐19 vaccines is still a high priority to control the pandemic and to reduce the burden of COVID‐19. The vaccine type and vaccination schedule affect many of the cellular and molecular elements of innate and adaptive immune systems.
Estimation of the immune responses after SARS‐CoV‐2 vaccinations is one of the important parameters in order to predict the efficacy of booster vaccines. Therefore, testing the effectiveness of COVID‐19 vaccines in different vaccination schedules is necessary as there are a variety of vaccine availabilities, worldwide. Following primary vaccination, antibody and T‐cell responses have decreased over time. 1 A booster dose being administered 6 months after the second dose of various vaccines significantly increased neutralizing antibody concentrations. 2 The heterologous vaccine regimens were reported to stimulate neutralizing antibodies more than the homologous vaccine protocols. 3
In Turkey, the inactivated vaccine CoronaVac (Sinovac Life Sciences) was the first vaccine to receive approval by the Ministry of Health. Healthcare workers and individuals over the age of 65 were suggested two doses of CV administered 2 months apart in the initial phase of the vaccination program. 4 After 6 months, the Ministry of Health of Turkey recommended a booster of BNT as an alternative option to the CV booster. In early studies of the CV, effectiveness after two‐dose schedules was reported as 60%–90%. 4 , 5 Nevertheless, these studies were performed approximately 6 weeks after the second dose. Six months after the second dose of CV, neutralizing antibody titers declined below the seropositivity cut‐off value while a remarkable increase in the neutralizing antibody concentrations was observed with the administration of a third dose. 6 However, our knowledge on the humoral and cellular immune responses obtained by BNT and CV boosters following two doses of primary CoronVac vaccination is still limited. In this study, we aimed to explore the neutralizing antibody and T‐cell responses after the booster doses of CV and BNT following two doses of CV.
2. MATERIALS AND METHODS
2.1. Study design and participants
The study was conducted in three centers (Koç University Hospital, Istanbul University Cerrahpasa Hospital, and Istanbul University, Istanbul Medical School Hospital) in Istanbul, Turkey. Individuals who had been previously immunized with two doses of CV, and no history of COVID‐19 were included in the study. The baseline blood samples were collected 3–5 months after 2 doses of CV. Follow‐up blood samples were taken after the first and third months of the third dose of CV or one dose of the BNT booster (Figure 1). Participants were continuously monitored for SARS‐CoV‐2 infection. Informed consent was obtained from all participants. This study was approved by the Institutional Review Board of Koç University under the number of 2021. 151.IRB1.055.
FIGURE 1.
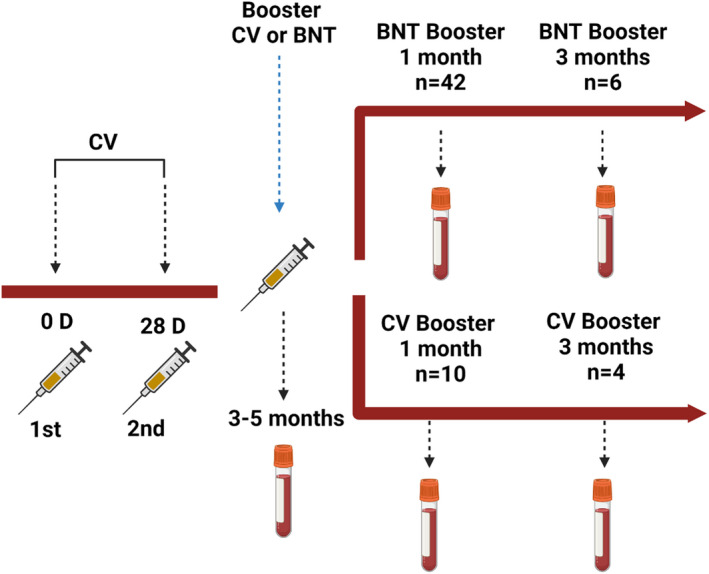
Design of the study
A total of 52 individuals received two doses of CV, and whole blood samples were collected in heparin tubes 3–5 months after vaccination (n = 19 for 3 months, n = 26 for 4 months, n = 7 for 5 months.). One month after receiving the third dose booster, whole blood samples were collected from the same 52 individuals. (n = 42 for BNT and n = 10 for CV). Due to a change in vaccine recommendations based on the variant of concerns, some participants have received the 4th dose of BNT or CV vaccine, therefore, were excluded from the study. Consequently, six participants from the BNT booster group and four participants from the CoronaVac booster group were able to donate blood samples 3 months after the booster dose.
The peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation method, 7 and the cells were stored at the −80°C 8 until use for flow cytometry and ELISpot assays. The sera were stored at the −80°C for neutralizing antibody tests.
2.2. Immunological assays
Humoral and cellular immune responses were examined to compare the serum SARS‐CoV‐2 neutralizing antibody titers and T‐cell reactivity in PBMC.
2.2.1. Plaque reduction neutralization test (PRNT50): neutralizing antibody titers
A live SARS‐CoV‐2 Wuhan strain (B303) which was previously isolated from the SARS‐CoV‐2 RdRp PCR positive nasopharyngeal specimen of a patient admitted to Koc University Hospital was used for the plaque assay. The nasopharyngeal specimen was cultured on Vero E6 cells (ATCC CRL‐1586) with DMEM High‐Glucose (Gibco, 41966–029) supplemented with 10% Fetal bovine serum (FBS), (Gibco, 10500064), 1% Penicillin‐Streptomycin, and Amphotericin B (Sigma, A2942) for 5 days. After observation of cytopathic effect, the growth of SARS‐CoV‐2 was confirmed by qRT‐PCR using primer and Taqman probe targeting SARS‐CoV‐2 nucleocapsid. Viral titer (TCID50) of the SARS‐CoV‐2 isolate was determined by Spearman–Karber method. 9 To sequence the virus, viral RNA was extracted with viral RNA isolation kit (QIAamp viral RNA), DNA library preparation was done using the Illumina TruSeq stranded total RNA kit, and the viral RNA was sequenced using Illumina MiniSeq (GenBank: MT675956). The TCID50/ml of the SARS‐CoV‐2 used for plaque assay was 2 × 106. Vero E6 cells (ATCC CRL‐1586) were cultured with DMEM High‐Glucose (Gibco, 41966–029) supplemented with 10% Fetal bovine serum (FBS), (Gibco, 10500064), 1% Penicillin‐Streptomycin, and Amphotericin B (Sigma, A2942). Serum dilutions of 300 µl from each donor were incubated with 300 µl SARS‐CoV‐2 at the multiplicity of infection (MOI) 0.01 for 1 h at 37°C, 5% CO2, and then, 600 µl mixture was inoculated onto the VeroE6 cells at 100% confluency. After 1 h of incubation at 37°C, 5% CO2, the serum‐virus mixture was discarded. The cell monolayers were coated with 2% methylcellulose (Sigma, M0512, 9004–67–5) and 5% Fetal bovine serum (FBS)/DMEM mixture (1:1). Four days after infection, methylcellulose and DMEM mixture was discarded. Plates were washed and cells were fixed with 4% PFA (Electron Microscopy Sciences, 15710‐S) followed by Gram's crystal violet solution staining (Merck milipore, 109218). Plaques were counted with the naked eye and the Celigo Image cytometer (Nexcelom, Celigo Image Cytometer 200‐BFFL‐5C). The virus control was studied in duplicate for each assay. A negative control (unexposed unvaccinated individuals' serum samples) and an internal control (serum contains SARS‐CoV‐2 neutralizing antibody at the titer of 1:80) were included in each study. 10
2.2.2. Flow cytometry for T‐cell response
For T‐cell assays, 2 × 106 PBMCs were seeded in RPMI 1640 media supplemented with 5% AB serum for the wells of the 96 well plates. T cells were activated by using a SARS‐CoV‐2 Spike protein‐specific peptivator (PepTivator®SARS‐CoV‐2 Prot S‐research grade (6 nmol/peptide), (Miltenyi Biotec,130‐126‐700) and were incubated for 20 h at 37°C, 5% CO2. T cells were stained with a viability dye and T‐cell surface marker antibodies (CD3‐FITC (Biolegend, 344804), CD4‐PerCP‐Cy5.5 (Biolegend, 367108), CD8‐Brilliant Violet 510 (877–246–5343) (Biolegend, 344732), CD38‐PE‐Cy7 (877–246–5343), (Biolegend, 303516), CD69‐APC (KLON FN50), (Biolegend, 310910), CD14‐APC‐Cy7 (Biolegend, 367108), CD19‐APC‐Cy7 (Biolegend, 877–246–5343), Zombie NIR Viability (Biolegend, 423105). PMA/Ionomycin, CMV (423302 400 µl), (Biolegend, 423302), and DMSO (Sigma, D2650‐100 ml) were used as controls. Samples were run using Attune Flow cytometer (Attune NxT Flow Cytometer).
2.2.3. ELISpot assay for T‐cell response
The IFN‐γ and IL‐2 responses of T cells were measured with Fluorospot Assay (Abcam Fluorospot, ab48452) according to the manufacturer's instructions. Plates were incubated for 30 s at room temperature with 35% ethanol and washed with DPBS 1X (Biowest, L0615‐500). The capture antibodies were added and incubated overnight at +4°C. After washing with DPBS 1X, RPMI‐1640 Medium (Sigma, R8758) containing 10% FBS cell culture media was added into the wells and incubated for 2 h at room temperature (RT). Plates were washed with DPBS 1X and 105 PBMCs were added into each well with appropriate concentration of SARS‐CoV‐2 S peptide activator (PepTivator®SARS‐CoV‐2 Prot S‐research grade (6 nmol/peptide, Miltenyi Biotec). Cells were incubated at 37°C in a CO2 incubator for 20 h and were washed 3 times using 1X DPBS containing 20% Tween‐20 (P9416, Sigma). The FITC‐labeled and biotinylated antibodies were added (for IFN‐γ/IL‐2 respectively) and incubated for 1 h and 30 min at RT. Following the wash step, FITC‐green fluorescence conjugate/streptavidin‐phycoerythrin solution (for IFN‐γ/IL‐2, respectively), was added to each well and incubated for 1 h at RT in the dark. The wash was repeated three times, and the residual buffer was removed using distilled water. After blot drying the plates, spots were read with GFP and RFP filters, under a dissection microscope (Leica M205 FA). The PMA/ionomycin was used as a positive control while DMSO was used as a negative control, eventually, CMV virus was used for cross‐reaction. The tests were performed in duplicate.
2.3. Statistical analysis
After obtaining sufficient longitudinal data, the statistical analysis of paired samples was performed by using a Wilcoxon signed rank test for the comparison of two dependent groups. For comparison of independent groups, for example, biontech vs. coronavac at first and 3 months, unpaired non‐parametric test, Wilcoxon rank‐sum (Mann–Whitney) test was performed. GraphPad Prism 8.0.2 Software was used for the analysis and visualization of the obtained data. In statistical analysis STATA 16v (USA) was used, and statistical significance was set as p < .05.
3. RESULTS
The demographic characteristics of the participants were presented in Table 1.
TABLE 1.
|
General cohort (n = 52) |
BNT162b2 group (n = 42) |
CoronaVac group (n = 10) |
|
|---|---|---|---|
| Mean age (Range) | 34 (22–72) | 31 (22–47) | 49 (27–72) |
| Female gender | 33 (63.46%) | 30 (71.43%) | 3 (30%) |
| BMI | |||
| <25 | 33 (63.46%) | 33 (78.57%) | 0 (0%) |
| 25–30 | 11 (21.15%) | 7 (16.66%) | 4 (40%) |
| 30≥ | 8 (15.38%) | 2 (4.76%) | 6 (60%) |
| Smoking | 17 (32.69%) | 13 (30.92%) | 4 (40%) |
| Comorbidity | 14 (26.92%) | 8 (21.42%) | 7 (70%) |
| Immunosuppression | 0 | 0 | 0 |
3.1. Neutralizing antibody titers
Both vaccine boosters produced detectable neutralizing antibody titers (Figure 2A). Three to five months after two doses of CV, 40 out of 52 (76.92%) participants had neutralizing antibody levels above 1/20 with a GMT of 23.27 (range between <1/10 and 1/80). One month after booster administration, BNT booster induced significantly higher neutralizing antibody with GMT of 78.69 (ranging between 20 and 1280) compared with GMT of CV with 21.44 (ranging between 10 and 40; p < .001). In this period, all participants in the BNT and 7 out of 10 (70%) of CV receivers had neutralizing antibody levels of >1/20. Meanwhile, 98% of the BNT and 40% of the CV participants had antibody titers above the 1/30 threshold. In both groups, GMTs remained at similar levels in the third month (Figure 2A). The neutralizing antibody levels of each participant in the study period were presented in Figure 2B.
FIGURE 2.
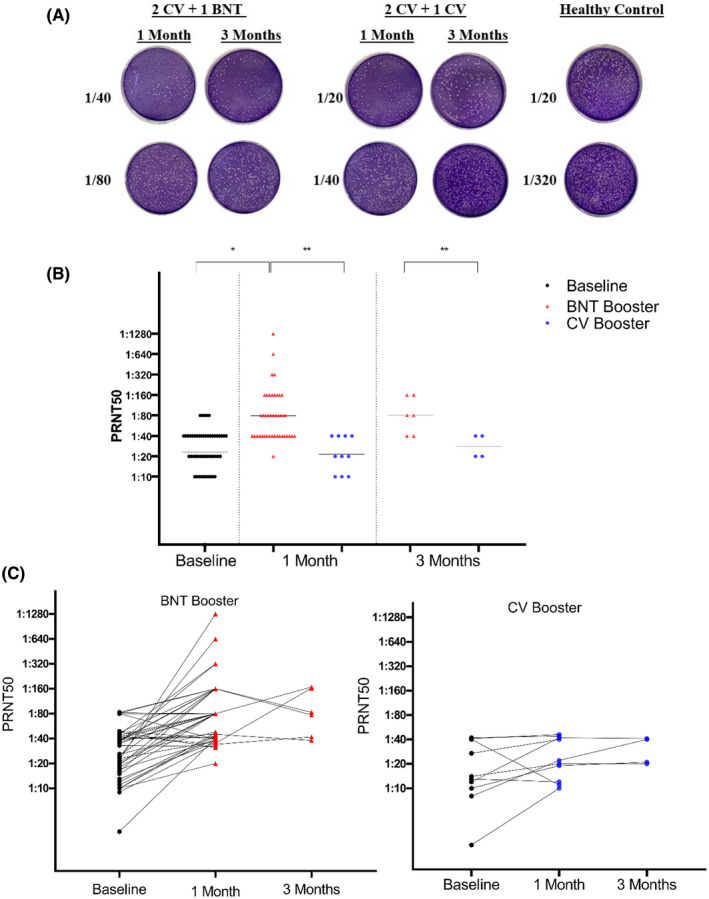
Neutralizing antibody levels after BNT162b2 and CoronaVac booster doses. (A) neutralizing antibody (PRNT50) titers at baseline, 1 month, and 3 months after boosters. Black lines are GMT = geometric mean titer (*p < .001; **p < .05). (B) Changes in neutralizing antibody levels for each individual during a 3‐month period. Dots represent neutralizing antibody titers for individuals in the population
3.2.
Reactivity and magnitude of CD4+ and CD8+ T‐cell responses to the SARS‐CoV‐2 spike S peptide pool were studied. In the assessment of CD4+ cell and CD8+ cell ratios after stimulation with a mixture of spike peptides, there were no significant change in the magnitude of CD4+ and CD8+ T cells after BNTC162b2 and CV boosters compared with baseline levels (Figure 3).
FIGURE 3.
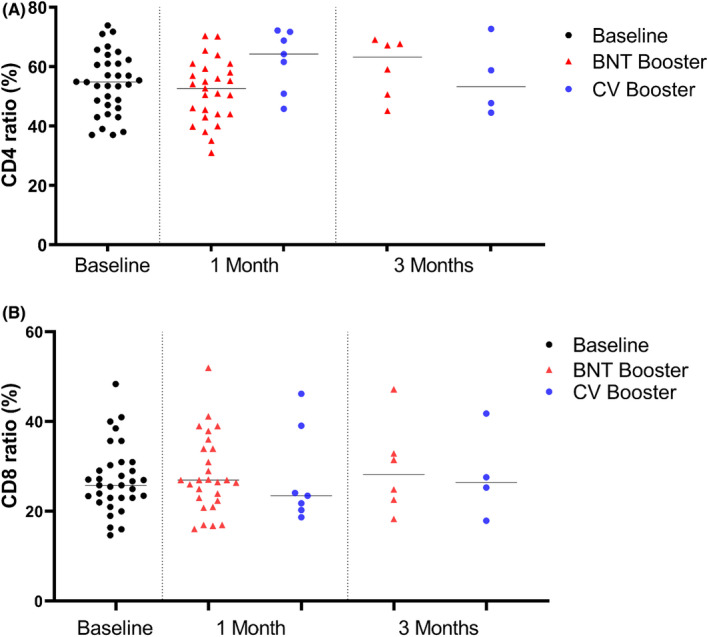
Proportion of specific T‐cell subpopulations after activation with SARS‐CoV‐2 S peptide pool. (A) CD4+ cell ratio at baseline, 1 month, and 3 months after boosters. (B) CD8+ at baseline, 1 month, and 3 months after boosters. Dots represent T‐cell ratios for individuals in the population. Black lines are median values of each population
In the ELISpot assay, T cells showed significantly higher IFN‐γ reactivity after BNT booster dose, with a median of 140 SFU per million PBMC (Range 5 SFU‐ 320 SFU) compared with baseline T‐cell response (60 SFU/106 PBMC; p < .001). CV booster dose did not cause a significant change in IFN‐γ reactivity of the T cells (80 SFU/106 PBMC) (p = .747). A month after BNT booster, the IL‐2 reactivity of the T cells (60 SFU/106 PBMC) were significantly higher than CV booster (median SFU 30/106 PBMC, p < .05) and baseline levels (20 SFU/106 PBMC; p < .001) (Figure 4).
FIGURE 4.
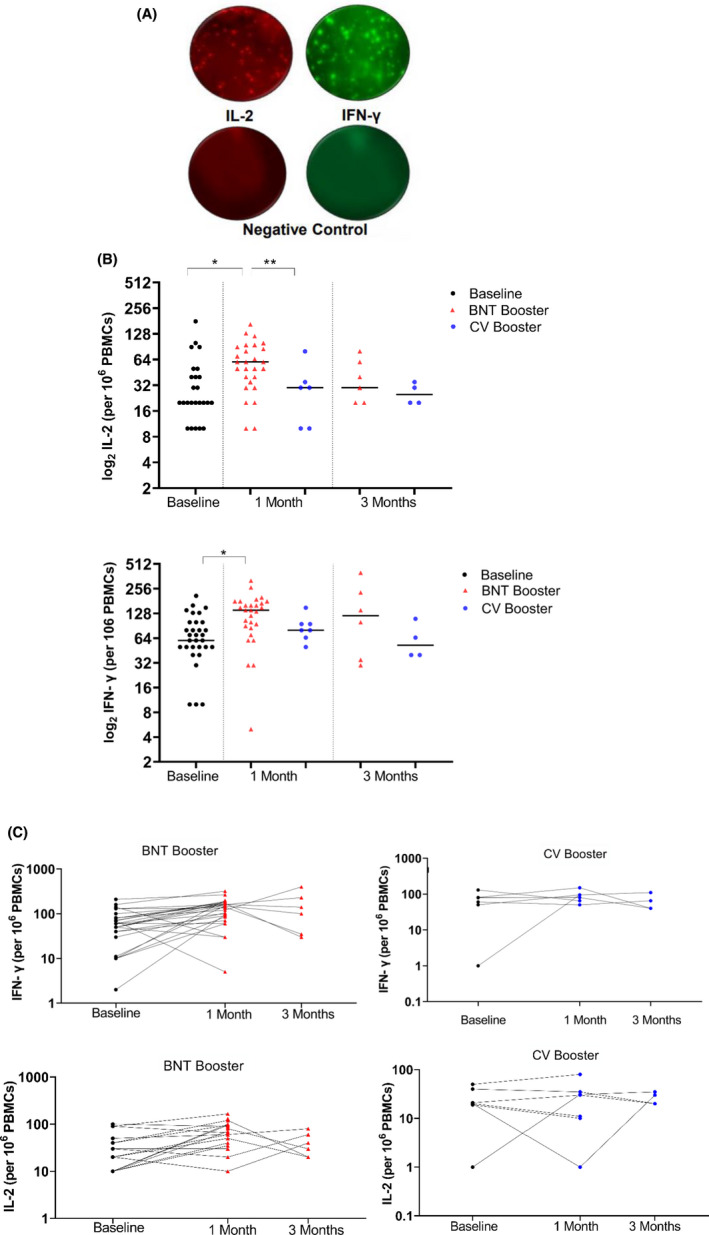
(A) IFN‐γ response at baseline, 1 month, and 3 months after boosters. (B) IL‐2 response at baseline, 1 month, and 3 months after boosters. (C) Changes in IFN‐ γ and IL‐2 levels for each individual during a 3‐month period. Dots represent T‐cell ratios for individuals in the population, (*p < .001; **p < .05)
Assessment of the effector T‐cell response after COVID‐19 vaccine booster doses was done by phenotyping S‐specific CD4+ and CD8+ T cells. Representative gating was shown in Figure S1. One month after the BNT booster, both CD8+CD38+CD69+ T cells and CD4+CD38+CD69+ T cells were stimulated. There was no significant difference compared with baseline levels whereas a significant increase was detected at the third month. After 3 months, the proportion of CD8+CD38+CD69+ T cells increased from baseline of 1.160% to 3.130% (p = .031) and CD4+CD38+CD69+ T cells from 4.37% of baseline to 10% (p = .013). Three months after the CV booster, CD4+CD38+CD69+ T cells increased from 4.37% of baseline to 10.20% (p = .12) and CD8+CD38+CD69+ T cells increased from 1.160% to 3.130% (p = .62) (Figure 5).
FIGURE 5.
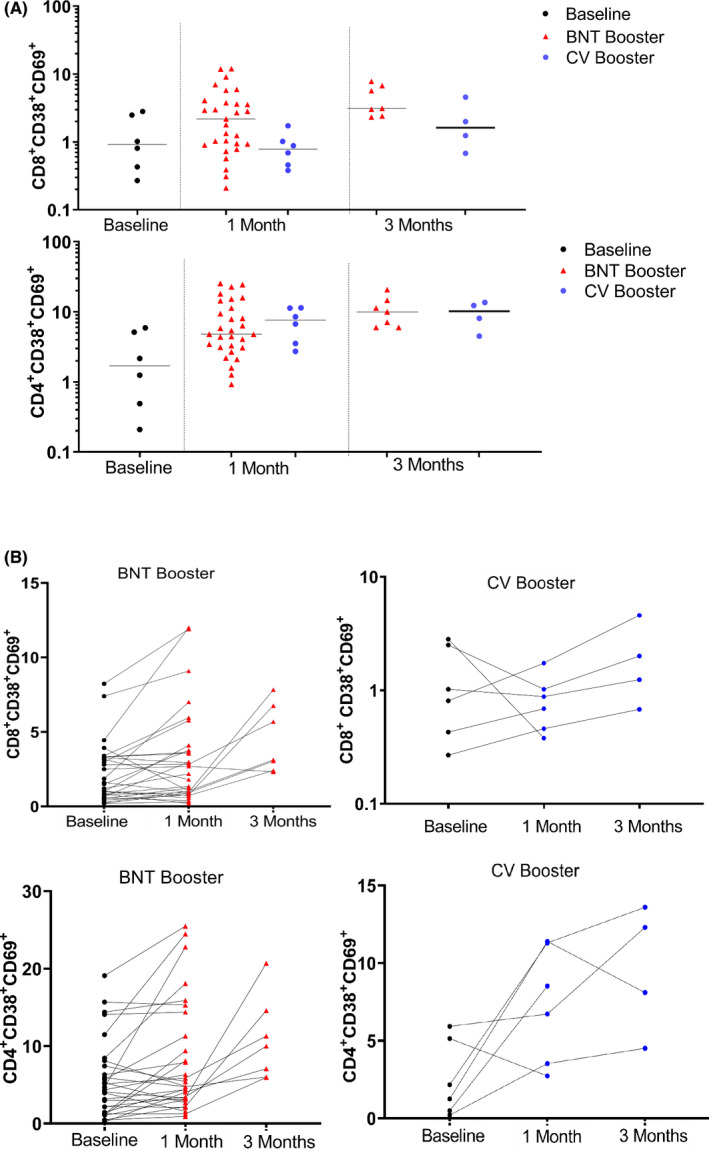
Ratio of CD8+CD38+CD69+ and CD4+CD38+CD69+ cells after BNT162b2 and CoronaVac boosters. (A) CD8+CD38+CD69+ cell ratio at baseline, 1 month, and 3 months after boosters. (B) CD4+CD38+CD69+ cell ratio at baseline, 1 month, and 3 months after boosters. (C) Changes in the ratio of CD8+CD38+CD69+ and CD4+CD38+CD69+ cells for each individual during a 3‐month period. Dots represent T‐cell ratios for individuals in the population. (*p < .05)
4. DISCUSSION
The present study addresses the lack of knowledge on the immunogenicity of BNT and CoronaVac boosters after 2 doses of CV administration. We focused on neutralizing antibody and T‐cell responses after 1 and 3 months elicited by BNT and CV boosters in fully vaccinated individuals with CV.
We found a 3.38‐fold increase in neutralizing antibody titers (GMT 78.69) one month after the BNT booster, and antibody titers were found to be maintained after 3 months.
(GMT 80). However, in the CV group, low GMTs after 1 month and 3 months (21.44 and 28.44) indicated the weak immunogenicity of the CV booster. For the protection of 50% of the individuals, a neutralizing antibody titer of 1/19 for BNT and 1/30 for CV was suggested as cut‐off levels of protection. 11 All BNT booster receivers had antibody levels above the protection threshold of 1/19 after 3 months, but only 40% of donors had antibody titers above the 1/30 threshold in the CV group. High neutralizing antibody levels following 14 days after CV booster dose 12 and decreased neutralizing antibody levels after 28 days were reported. 13 Moreover, a recent study from Turkey reported a marked increase in binding antibody response after BNT booster compared with a CoronaVac booster (104.8‐ vs. 8.7‐fold, respectively). We found low neutralizing antibody levels at a prolonged time (1–3 months) after booster doses of CV. The concern about the weak immunogenicity would be increased by considering the reduced efficacy of vaccines against the Omicron variant. 14 , 15
Current vaccine trials have focused on the stimulation of the neutralizing antibody against SARS‐CoV‐2, but CD4+ and CD8+ cells also might provide protection from severe disease and support resolution of COVID‐19. 16 In our cohort, the magnitude of post BNT booster responses was higher than the CV booster. We detected increased IFN‐γ and IL‐2 reactivity after the BNT booster, accompanied by an increase in CD8+CD38+CD69+T cells. CV booster did not cause a significant change in IFN‐γ reactivity of the T cells. In accordance with our results, the increased IFN‐γ secreting T‐cell responses were observed after the BNT booster while no increase was detected for the inactivated vaccine booster in fully Immunized healthy adults with inactivated vaccine. The ratio of CD4+CD38+CD69+ cells was also high in the third month of the BNT booster. Effector T cells have short lifespan, the high ratio of CD4+ and CD8+ effector T cells in third month was possibly due to memory cell differentiation in the population. Robust CD8 and CD4 T‐cell responses to BNT were reported in clinical studies. 17 , 18 The induction of T‐cell responses after CV vaccination was also reported by a study from Hong Kong. 19 T‐cell clones against other SARS‐CoV‐2 antigens such as N protein can also be stimulated with inactivated vaccines. 20 The type of peptide pool used for activation might have affected the results of T‐cell response measurements. 21 In this study, rather than using peptides of the virus, we used S‐pool, which might lead to the underestimation of the true magnitude of T‐cell responses.
The strength of our study is being a longitudinal study and having a prominent advantage of being performed in Turkey, of where the primary vaccine schedule consisted of two CV doses. Main limitations of this study can be stated as having a low sample size to detect the statistical significance especially in the CoronoVac booster group. However, our results are in parallel with reported studies that evaluated anti‐S antibody titers by ELISA in Turkey. 22 Some of the participants were excluded from the study since they received their fourth doses after 2 months. Moreover, we could not cover the newly emerged Omicron variant, and this will be done in future studies.
Our study has implications for the countries that have used a two‐dose regimen of CV. The neutralizing antibody levels after 3 months of the BNT booster were higher than the antibody levels after the CV booster. On the contrary, the ratio of the effector CD8+ and CD4+ T cells was increased along with greater IFN‐γ activation after the BNT booster. Taking into consideration the waning immunity, we suggest a second booster dose with BNT for the individuals who already had BNT or CV boosters as their third doses following two doses of CV.
CONFLICT OF INTEREST
The authors declare no conflict of interest in relation to this work.
AUTHOR CONTRIBUTION
ZEK, RE, MK, YT, SSY, KM, and OE involved in Data and sample collection. ZEK, RE, GGE, TB, ZGT, YT, and FC involved in laboratory work. ZEK, RE, GGE, TB, OD, OE, and FC involved in data analysis. ZEK, RE, GGE, ZGT, OE, and FC involved in manuscript preparation.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
Koc University Isbank Center for Infectious Diseases (KUISCID) funded the study.
Kuloğlu ZE, El R, Guney‐Esken G, et al. Effect of BTN162b2 and CoronaVac boosters on humoral and cellular immunity of individuals previously fully vaccinated with CoronaVac against SARS‐CoV‐2: A longitudinal study. Allergy. 2022;00:1–9. doi: 10.1111/all.15316
Zeynep Ece Kuloğlu and Rojbin El performed equal work in the study.
Contributor Information
Gulen Guney‐Esken, Email: gesken@ku.edu.tr.
Füsun Can, Email: fucan@ku.edu.tr.
REFERENCES
- 1. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID‐19. Lancet Microbe. 2021;2(9):e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barros‐Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS‐CoV‐2 variants after heterologous and homologous ChAdOx1 nCoV‐19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525‐1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N Engl J Med. 2021;385(10):875‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2009;85(1):7.1.1‐7.1.8. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Yang X, Zhong J, et al. Exposure to SARS‐CoV‐2 generates T‐cell memory in the absence of a detectable viral infection. Nat Commun. 2021;12(1):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman‐Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Toxicol. 1977;11(7):713‐771. [Google Scholar]
- 10. Mendoza EJ, Manguiat K, Wood H, Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS‐CoV‐2. Curr Protoc Microbiol. 2020;57(1):ecpmc105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 12. Cao Y, Hao X, Wang X, et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res. 2022;32(1):107‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang K, Cao Y, Zhou Y, et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS‐CoV‐2. medRxiv. 2021. doi: 10.1101/2021.09.02.21261735 [DOI] [PubMed] [Google Scholar]
- 14. Lu L, Mok BW, Chen LL, et al. Neutralization of SARS‐CoV‐2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021:ciab1041. doi: 10.1093/cid/ciab1041. Epub ahead of print. PMID: 34915551; PMCID: PMC8754807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez‐Then E, Lucas C, Monteiro VS, et al. Immunogenicity of heterologous BNT162b2 booster in fully vaccinated individuals with CoronaVac against SARS‐CoV‐2 variants Delta and Omicron: the Dominican Republic Experience. medRxiv. 2021. doi: 10.1101/2021.12.27.21268459 [DOI] [Google Scholar]
- 16. Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS‐CoV‐2‐specific T cells associates with rapid viral clearance and mild disease in COVID‐19 patients. Cell Rep. 2021;34(6):108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly‐specific T cells in humans. Nature. 2021;595(7868):572‐577. [DOI] [PubMed] [Google Scholar]
- 18. Kanokudom S, Assawakosri S, Suntronwong N, et al. Safety and immunogenicity of the third booster dose with inactivated, viral vector, and mRNA COVID‐19 vaccines in fully immunized healthy adults with inactivated vaccine. Vaccines (Basel). 2022;10(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mok CKP, Cohen CA, Cheng SMS, et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID‐19 vaccines in Hong Kong. Respirology. 2021;27(4):301‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen THO, Rowntree LC, Petersen J, et al. CD8(+) T cells specific for an immunodominant SARS‐CoV‐2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity. Immunity. 2021;54(5):1066‐1082 e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS‐CoV‐2 infection on humoral and T‐cell responses to single‐dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yalcin TY, Topcu DI, Dogan O, et al. Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID‐19 infection: prospective observational study. J Med Virol. 2022;94(1):279‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


