Abstract
With dermatologic side effects being fairly prevalent following vaccination against COVID‐19, and the multitude of studies aiming to report and analyze these adverse events, the need for an extensive investigation on previous studies seemed urgent, in order to provide a thorough body of information about these post‐COVID‐19 immunization mucocutaneous reactions. To achieve this goal, a comprehensive electronic search was performed through the international databases including Medline (PubMed), Scopus, Cochrane, Web of science, and Google scholar on July 12, 2021, and all articles regarding mucocutaneous manifestations and considerations after COVID‐19 vaccine administration were retrieved using the following keywords: COVID‐19 vaccine, dermatology considerations and mucocutaneous manifestations. A total of 917 records were retrieved and a final number of 180 articles were included in data extraction. Mild, moderate, severe and potentially life‐threatening adverse events have been reported following immunization with COVID vaccines, through case reports, case series, observational studies, randomized clinical trials, and further recommendations and consensus position papers regarding vaccination. In this systematic review, we categorized these results in detail into five elaborate tables, making what we believe to be an extensively informative, unprecedented set of data on this topic. Based on our findings, in the viewpoint of the pros and cons of vaccination, mucocutaneous adverse events were mostly non‐significant, self‐limiting reactions, and for the more uncommon moderate to severe reactions, guidelines and consensus position papers could be of great importance to provide those at higher risks and those with specific worries of flare‐ups or inefficient immunization, with sufficient recommendations to safely schedule their vaccine doses, or avoid vaccination if they have the discussed contra‐indications.
Keywords: acute, adverse effect, adverse event, adverse event following immunization, allergy, angioedema, AstraZeneca, AstraZeneca/Oxford, atopic dermatitis, Bharat, collagen vascular disease, Comirnaty, COVID‐19 vaccine, cutaneous, cyanosis, delayed, delayed‐type hypersensitivity, dermatology, ecchymosis, edema, erythema multiforme, exanthematous rash, herpes, hidradenitis suppurativa, inflammatory bowel disease, injection site reaction, Janssen, Johnson & Johnson, late, local site reaction, maculopapular rash, mastocytosis, Moderna, morbilliform rash, mucocutaneous, mucosal, pemphigoid, pemphigus, Pernio, Petechia, Pfizer, Pfizer‐BioNTech, pityriasis rosea, pruritus, psoriasis, purpura, remote site reaction, rheumatic disorders, SARS‐Cov‐2, side effect, Sinopharm, Sinovac, Sputnik, urticaria, vaccine, Vaccine Adverse Event Reporting System, zoster
1. INTRODUCTION
1.1. Rationale
The global impact of the Coronavirus Disease 2019 (COVID‐19) pandemic does not need to be underscored. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spread rapidly throughout the world and left tragic consequences, and vaccination appears to be a mainstay for overcoming this contagious calamity. Many candidate vaccines have been developed against SARS‐COV‐2, using different vectors and methods of production, which fall into different vaccine types. To name a few:
-
1.
mRNA vaccines
Pfizer‐BioNTech “Comirnaty” (BNT162b2, tozinameran) 1 , 2 , 3 , 4
Moderna (mRNA‐1273) 5 , 6 , 7 , 8
-
2.
Adenovirus viral vector vaccines
Oxford–AstraZeneca (Covishield, Vaxzevria,ChAdOx1SnCoV‐19, AZD1222) 9 , 10 , 11 , 12 , 13 , 14
Sputnik V (Gam‐COVID‐Vac) 15 , 16
Janssen (Ad26.COV2.S, JNJ‐78436735)(Johnson & Johnson) 19 , 20
-
3.
Protein subunit vaccines
MF59‐adjuvanted spike glycoprotein‐clamp vaccine 23
SCB‐2019 24
CoV2 preS dTM‐AS03 25
ZF2001 (ZIFIVAX or ZF‐UZ‐VAC‐2001) 26
V‐01 27
EpiVacCorona (Aurora‐CoV) 28
-
4.
Inactivated virus vaccines
Sinovac (CoronaVac) 29 , 30 , 31
Sinopharm BIBP (BBIBP‐CorV) 32 , 33
Sinopharm WIBP (WIBP‐CorV) 34
Covaxin (BBV152, Bharat Biotech) 35
KCONVAC (Minhai) 36
IBMCAMS vaccine (Institute of Medical Biology) 37
-
5.
Virus‐like particle vaccines
CoVLP 38 From these many candidates, seven COVID‐19 vaccines have been approved by WHO, 39 namely:
Pfizer‐BioNTech “Comirnaty” (BNT162b2, tozinameran),
Moderna (mRNA‐1273),
Janssen (Ad26.COV2.S, JNJ‐78436735)(Johnson & Johnson),
Oxford–AstraZeneca (AZD1222),
Covishield (Serum Institute of India, Oxford–AstraZeneca formulation),
Sinopharm BIBP (BBIBP‐CorV)(Vero Cells),
and Sinovac CoronaVac. 39
Although studies have showed overall acceptable efficacy, safety, and tolerability of all available COVID‐19 vaccines, 3 , 8 , 13 , 14 , 30 , 40 , 41 with the accelerated pace of vaccine production, distribution, and administration, several steps of vaccine development were condensed and got fast‐tracked which increased the probability of unsolicited adverse reactions, warranting further attention to the potential side effects of these vaccines, 42 , 43 and an international effort to report the observed reactions, through the Vaccine Adverse Event Reporting System (VAERS), 44 or other registries. Previous studies have revealed the main side effects to include localized pain, swelling or redness at the injection site, along with constitutional or COVID‐like symptoms, mostly comprised of generalized weakness, myalgia, headache, fever and chills, joint pain, nausea, and diarrhea. 45 Of note, mucocutaneous adverse events encompass a large number of post‐vaccination reactions: local injection site reactions as previously mentioned, delayed large local reactions, morbilliform rashes, urticaria, erythema multiforme, delayed inflammatory reactions to dermal fillers, erythromelalgia, lichen planus, varicella‐zoster, herpes simplex, pityriasis rosea, petechiae, purpura, and mimickers of COVID‐19 infection cutaneous manifestations (e.g., pernio or chilblains), which have predominantly been insignificant and self‐limited. 46 , 47
1.2. Objective
With dermatologic side effects being fairly prevalent after COVID vaccination, and the multitude of studies aiming to report and analyze these events, the need for an extensive investigation on previous studies seemed urgent, in order to provide a comprehensive body of information about these post‐COVID‐19 immunization mucocutaneous reactions.
Therefore, the main objective of this qualitative systematic review is to recapitulate and categorize the clinical characteristics of mucocutaneous reactions following COVID vaccination, provide an update on the state of underlying mucocutaneous diseases after vaccination, their diagnoses and biopsies, therapeutic strategies, patients' outcomes, and further integrated guidance for approach to patients who have previously experienced these side effects or flares of underlying diseases with other vaccines.
We have also tried to classify experts' recommendations and consensus guidelines on COVID‐19 vaccination in those with immune‐mediated dermatologic disorders, allergic disorders, along with systemic disorders with probable mucocutaneous presentations, for example, autoimmune inflammatory rheumatic diseases (AIIRD); as these disorders could be underlying factors that may affect vaccine immunogenicity, either by themselves, or indirectly, with the use of immunosuppressive and immunomodulatory treatment for their control. Being knowledgeable and updated on the non‐critical, critical or potentially life threatening mucocutaneous adverse effects of COVID‐19 vaccine and the mutual effects of vaccination and dermatologic disorders on each other is a must for dermatologic, as well as general medical practice today, and we hope the present article provides a stepping stone to that aim.
This study is the first systematic review that thoroughly assesses all aspects of the various dermatological concerns regarding COVID‐19 vaccination, condensing the results of all study types with a detailed categorization of the results.
2. METHODS
2.1. Protocol and registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines to conduct and report this review. 48
2.2. Search strategy
A comprehensive electronic search was performed through the international databases including Medline (PubMed), Scopus, Cochrane, Web of science, and Google scholar from the beginning to July 12, 2021, and all articles regarding mucocutaneous manifestations and considerations after COVID‐19 vaccine administration were initially retrieved using the following major keywords and their MeSH terms: COVID‐19 vaccine, dermatology considerations and mucocutaneous manifestations. The search strategy is illustrated in Appendix S1 of supplement file. In addition, a manual search through the references of included reviews was conducted to identify any missing related studies. Two researchers separately performed the search and screening, and the details of each step in the search and screening process is provided in our PRISMA flow diagram, 49 depicted in Figure 1.
FIGURE 1.
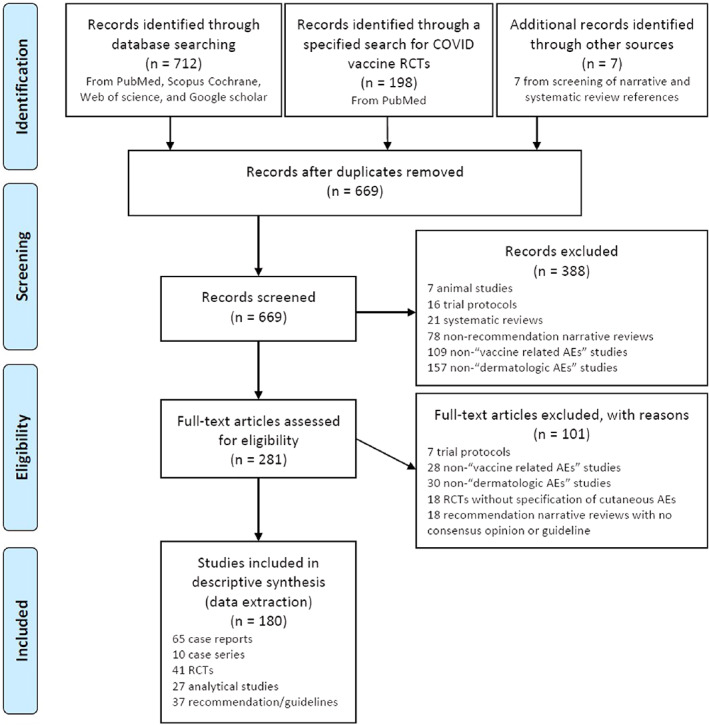
PRISMA flow diagram of the study
2.3. Eligibility criteria
Our inclusion criteria were studies or reports on any dermatology‐related adverse events following the administration of COVID‐19 vaccines and vaccine related concerns and consideration for those with dermatologic disorders. Inclusion was not limited by the type of COVID‐19 vaccine. Exclusion criteria were in vitro studies, animal studies, basic science studies, studies on non‐dermatologic adverse events (AEs) of vaccines, COVID‐19 disease manifestations, and any non‐COVID‐19 vaccine study.
2.4. Screening and data extraction
After duplication removal of the primary search results, two reviewers independently screened the title and abstract of retrieved articles based on the above eligibility criteria. They then separately studied the full‐text of the selected studies in detail, for evaluation of eligibility and data extraction. In case of disagreement, they discussed the subject and if they did not reach a consensus, another researcher expert in the field joined the discussion. The data extraction sheet contained the following information: first author name, patient characteristics in case reports, or number of patients, gender distribution and mean age in other studies, vaccine type, dose of vaccine, history of previous mucocutaneous conditions, constitutional symptoms after vaccine, characteristics and location of mucocutaneous reactions, mean time of onset, diagnosis, management of reactions, duration of reactions, and final outcomes. Studies regarding COVID‐19 vaccination considerations and recommendations among dermatologic patients were assessed separately. The study design, data reporting, and validity of included RCTs were assessed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement.
3. RESULTS
3.1. Overview of the studies
A total of 917 articles were retrieved from all databases and 248 duplicates were identified and removed. A total of 669 articles went through title and abstract screening. From those, 388 articles were excluded, including 7 animal studies, 16 trial protocols, 21 systematic reviews, 78 non‐recommendation narrative reviews, 109 non‐“vaccine related AEs” studies, and 157 non‐“dermatologic AEs” studies. The remaining281 articles were selected for full text screening. From those, 101 studies were excluded, including 7 trial protocols, 28 non‐“vaccine related AEs” studies, 30 non‐“dermatologic AEs” studies, 18 RCTs without specification of cutaneous AEs, and 18 recommendation narrative reviews with no consensus opinion or guideline. Also, references of 20 retrieved narrative and systematic reviews, comprised of 152 articles, were manually screened for any missing articles and 7 related articles from those were also added to our included papers.
Finally, a total of 180 studies were included in our data extraction and descriptive synthesis, including 65 case reports, 10 case series, 41 RCTs, 27 analytical studies, and 37 recommendations or guidelines.
3.2. Case reports
In total, 116 cases were included in the case reports table from a total of 65 articles, as depicted in Table 1. The mean age of participants was 47.37 years, with a female‐dominant gender distribution (F/M: 1.7, F = 73[62.9%] M = 43[37.1%]). The vaccines studied in order of number of participants having received them were BNT162b2 (n = 76, 65.5%), mRNA‐1273 (n = 19, 16.4%), ChAdOx1S nCoV‐19 (n = 9, 7.8%), CoronaVac (n = 7, 6%), Ad26.COV2.S (n = 3, 2.6%), and BBV152 (n = 2, 1.7%).
TABLE 1.
Mucocutaneous reaction after COVID‐19 vaccination reported in “Case reports” studies
| Supplemental references a | First author | Case age | Case gender | Patients' mucocutaneous disease history | Patients' other comorbidity | Drug history at the time of vaccination | Vaccine dose | Any sign after vaccine | Description of mucocutaneous reactions | reactions onset | Skin or mucosal biopsy | Diagnosis | Resolution after (time) | Management of reactions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Injection site reactions, “Covid arm” (n = 12) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 1 | Gyldenløve, M. | 33 | F | NM | DM, obesity | NM | 1 | Neg | Asymptomatic rash at the injection site | 12d | Perivascular, lymphocyte infiltration in the dermis | Reactions after incorrect subcutaneous administration | NM | Neg |
| 2 | Tammaro, Antonella | 64 | F | Neg | Neg | Neg | 2 | Neg | A nodule surrounded by an erythematous halo, extremely painful and pruritic | 1d | NM | Localized reaction | 4d | Topical Corticosteroid cream |
| 2 | Tammaro, Antonella | 56 | F | Neg | Neg | Neg | 2 | Neg | Small vesicular lesions surrounded by erythema | 1d | NM | Localized reaction | 7d | Topical Corticosteroid cream |
| 2 | Tammaro, Antonella | 60 | F | Neg | Neg | Neg | 2 | Neg | Severe xerosis and pruritus in the area injected, extensive erythematous pruritic and painful rash, on the shoulder and chest | 7d | NM | Localized reaction | 7d | Topical Corticosteroid cream |
| 3 | López‐Valle, A. | 27 | F | Neg | NM | NM | 1 & 2 | 1st: fever, 2nd: fatigue after 24 h of injection | 1st: pain at the injection site, erythematous–edematous firm plaque over the deltoid area, 2nd: pain and an erythematous–edematous plaque on the injection site | 1st:7d / 2nd:6 h | NM | NM | 1st: 2d | Paracetamol for 2nd dose symptoms (resolved after 2d) |
| 4 | Baeck, M. | 38 | F | BNT | NM | NM | 1 | Pain at the injection site had completely resolved within 2 days, numbness of the fingers | Only after 1st dose: erythema of the upper arm | 6d |
sparse, superficial, deep lymphohistiocytic infiltrates with CD3+ (including CD8+ and CD4+) T cells and some eosinophils and very rare CD20+ B cells |
Delayed local reaction | 5d | Spontaneous resolution |
| With mRNA‐1273 | ||||||||||||||
| 5 | Sidlow, J. S. | 67 | F | NM | mild atopy | NM | 1 | NM | Only after 1stt: itchy 7‐cm erythematous red patch at the vaccine injection site of the upper portion of patient's left arm | 7d | Spongiosis perivascular, interstitial infiltrate, mixed cell type with rare eosinophils, occasional neutrophils within the reticular dermis | Localized reaction | 7d | Topical Corticosteroid use |
| 5 | Sidlow, J. S. | 40 | F | NM | atopic family history | NM | 1 & 2 | NM | 1st: sharply demarcated warm urticarial oval patch, swelling and progressive erythema on the arm, 2nd: mild swelling at the injection site | 8d | Spongiosis perivascular, interstitial infiltrate, mixed cell type with rare eosinophils, occasional neutrophils within the reticular dermis | Localized reaction | NM | Erythromycin for presumed erysipelas |
| 5 | Sidlow, J. S. | 53 | F | NM | mild atopic background | NM | 1 & 2 | 2nd: weakness, diarrhea, patient could not raise her arm above 90° angle | 1st: some mild sensitivity at the injection site and tenderness, 2nd: tender erythematous urticarial red ring on the injected arm | NM | Spongiosis perivascular, interstitial infiltrate, mixed cell type with rare eosinophils, occasional neutrophils | Localized reaction | 3d | Neg |
| 6 | Lindgren, A. L. | 60 | F | NM | Neg | NM | 1 | Neg | Swollen, painful, extremely pruritic, erythematous plaque with minute papules in vaccination site | 6d | NM | Hypersensitivity reaction | 14 h | Clobetasol 0.05% cream twice |
| 6 | Lindgren, A. L. | 44 | F | NM | Neg | NM | 1 | fever, chills, headache, and myalgias | Erythema, pain, pruritus, induration and swelling at the vaccination site on patient's left arm | 7d | NM | Hypersensitivity reaction | 2d | Triamcinolone 0.1% cream |
| 6 | Lindgren, A. L. | 33 | F | NM | NM | NM | 1 | Neg | Redness, pain, itching, and swelling at the injection site | 7d | NM | Hypersensitivity reaction | 4d | 1% Hydrocortisone cream |
| 2. Non‐injection site reactions (n = 104) | ||||||||||||||
| 2.1. Hypersensitivity reaction type 1 (n = 14) | ||||||||||||||
| 2.1.1. Urticaria (n = 5) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 7 | Bianchi, L. | 24 | F | NM | Allergic rhinitis | NM | 1 | NM | Generalized acute urticaria | 5 min | NM | urticaria | NM | Betamethasone Sodium Phosphate IV |
| 7 | Bianchi, L. | 28 | M | NM | Allergic rhinitis | NM | 1 | NM | Generalized acute urticaria | 5 min | NM | urticaria | NM | Neg |
| 8 | Pitlick M. | 24 | F | NM | NM | NM | 1 | NM | Urticaria | 3 h | NM | NM | 4d | antihistamines |
| 8 | Pitlick M. | 45 | F | NM | Food allergy | NM | 1 | Throat tightness | Urticaria | 8 h | NM | NM | NM | Neg |
| 8 | Pitlick M. | 33 | F | NM | Asthma, venom anaphylaxis | NM | 1 | Tachycardia | Urticaria | 15 min | NM | NM | 12 h | antihistamines |
| 2.1.2. Flushing (n = 3) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 7 | Bianchi, L. | 58 | F | NM | Allergic rhinitis and asthma | NM | 1 | NM | Flushing of the face | 30 min | NM | Flushing | NM | Neg |
| 7 | Bianchi, L. | 44 | F | NM | Allergic rhinitis | NM | 1 | NM | Flushing of the face | 20 min | NM | Flushing | NM | Neg |
| 8 | Pitlick M. | 36 | F | NM | NM | NM | 1 | NM | Facial flushing | 5 min | NM | NM | 1 h | antihistamines |
| 2.1.3. Angioedema(n = 3) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 7 | Bianchi, L. | 31 | F | NM | Allergic rhinitis | NM | 1 | NM | Angioedema (tongue, gums) | 24 h | NM | Angioedema | NM | Neg |
| 7 | Bianchi, L. | 54 | F | atopic dermatitis,contact allergy | Allergic rhinitis | NM | 1 | NM | Angioedema (tongue, lips) | 10 min | NM | Angioedema | NM | Neg |
| With mRNA‐1273 | ||||||||||||||
| 8 | Pitlick M. | 20 | M | NM | Vaccine allergy | NM | 1 | NM | Angioedema | 3 h | NM | NM | 1d | Steroids, Antihistamines |
| 2.1.4. Anaphylaxis(n = 3) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 9 |
Daou, Christophe Abi Zeid |
30 | M | Allergies to Meperidine, Amocixillin‐Clavulonate Acid, pollen, and dust mites | NM | NM | 1 | Tachycardia, tachypnea, dysphagia, dyspnea, severe chills, dysphagia | Diffuse maculopapular rash + urticaria, diaphoresis, palate pruritis, rash, pruritus,diaphoresis, sudden onset of rash followed by urticaria, diaphoresis | a few minutes | NM | Biphasic anaphylaxis | 1d | Diphenhydramine, Prednisone, Dexamethasone, Hydrocortisone Sodium Succinate, Epinephrine |
| 10 | Restivo, V. | 30 | F | Poly‐allergic subject, urticaria‐angioedema, immediate cutaneous reaction | NM | Prednisone, Chlorphenamine Maleate before vaccination | 1 | the feeling of a slurred mouth and hoarseness | erythematous spots on the face and neck | 5 h | NM | Severe allergic reaction | NM | Dexamethasone, Chlorphenamine Maleate, 0.9% NaCl, Oxygen |
| With mRNA‐1273 | ||||||||||||||
| 8 | Pitlick M. | 22 | F | NM | Allergic rhinitis | NM | 1 | wheezing, throat pruritus | Angioedema | 20 min | NM | Level 1 Anaphylaxis | 6 h | Antihistamines, Steroids |
| 2.2. Generalized eruptions (n = 21) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 11 | Edriss, Manar | 54 | M | NM | NM | NM | 1 | Neg | Clustered erythematous papules and nodules on posterior upper left arm that extended to left elbow and forearm | 5d | NM | erythematous papules and nodules | NM | Clobetasol BD |
| 12 | Ackerman, M. | 55 | M | NM | Neg | NM | 1 | injection‐site soreness, slight hepatic cytolysis (ASAT and GGT 2 N) | Localized pruritic erythematous eruption, later spread on the face, trunk, upper extremities and thighs, 30% of body surface area involved | 3 h | slight lymphocytic perivascular infiltrate, compatible with non‐severe maculopapular toxidermia late biopsied | Persistent maculopapular exanthem | NM | one dose of Dermocorticoid Treatment, withholding of 2nd dose |
| 13 | Akinosoglou, K. | 32 | F | Neg | NM | NM | 1 & 2 | Neg | Itchy annular granulomatous rash over both elbows | 2d | Cutaneous small cell vasculitis, possibly of leukocytoclastic origin | Annular rash | 3d | Neg |
| 14 | Zafar, M. | 84 | M | NM | BPH | NM | 2 | Rise in D‐dimers and eosinophils | Widespread disseminated mildly itchy rash | 11d | NM | Rash with eosinophilia | NM | Oral Antihistamines, Topical Steroids |
| 15 | Patruno, C. | 42 | F | Neg | NM | Neg | 1 | NM | Wheals (acute urticaria) on the trunk and limbs | 7d | NM | Acute urticaria | 7d | Antihistamine, Prednisone 25 mg |
| 15 | Patruno, C. | 55 | M | NM | Neg | Neg | 1 | NM | Multiple itchy erythematous papules, vesicles, and blisters | 10d | spongiosis, epidermal exocytosis of lymphocytes, apoptotic keratinocytes, dermal edema | Erythema Multiforme‐like eruption | 10d | Systemic Prednisone 25 mg/day |
| 16 | Lavery, M. J. | 58 | F | Erythema Multiforme (quiescent), recurrent episodes of herpes labialis | RA, Endometriosis, HTN, thyroid goiter | Abatacept, famciclovir | 1 & 2 | NM | A painful cutaneous eruption, erythematous concentric targetoid plaques on the palms of her hands and soles of the feet bilaterally | 1st: 12 h,2nd:1d | NM | Erythema Multiforme‐like eruption | NM | Topical Clobetasol |
| 17 | Gambichler, T. | 74 | F | NM | Severe dementia syndrome | Pantoprazole | 1 | NM | Erythematous partly violaceous coalescing macules and papules with slightly indicated cocarde formation on the trunk and extremities | 1d | epidermal atrophy, vacuolar interface dermatitis, lymphocytic infiltrates, dyskeratoses of basal keratinocytes | Rowell's syndrome | NM | NM |
| 18 | Ohsawa, R. | 55 | F | NM | Neg | NM | 1 | pain at the injection |
d2: pruritic papules and erythematous lesions developed over the entire body except for the face d6: mild pruritic vesicopapular, erythematous macular and morbilliform eruption on the bilateral flanks and extremities |
2d | perivascular lymphocytic infiltrates, basal cell vacuolization and intraepidermal vesicle with mild spongiotic change containing collections of Langerhans cells and degenerated acantholysis‐like keratinocytes,microthrombi in small vessels in the mid‐ and deep dermis,perivascular and intraepidermal lymphocytic infiltrates with CD8+ > CD4+ cells | Morbilliform rash | 7d |
2d: topical betamethasone dipropionate and oral antihistamine: ineffective, 6d: 15 mg of oral predonisolone, withholding 2nd dose of vaccine |
| 19 | Farinazzo, E. | 37 | F | NM | NM | NM | NM | NM | Morbilliform eruption | NM | NM | Morbilliform eruption | NM | NM |
| 20 | Weinstock‐Guttman, B. | 31 | F | history of allergic reaction to cefixime | history of MOGSD and biopsy‐confirmed smoldering myeloma with monoclonal gammopathy | IV RTX for the last 2 years, monthly doses of IVIG (1st vaccination 12 weeks after the RTX infusion) | 1 | local warmth |
1st: multiple urticarial papules and plaques located on both lower extremities and gluteal area, primarily left side (unilateral side of the vaccination), local 2nd: lesions increased in size and became more evident Resolution: minimal hyperpigmentation residue |
1st:7d, 2nd: 2d | NM | Late onset erythema | NM | oral corticosteroids tapered within 3 weeks (15 mg/10 mg/5 mg prednisone) |
| 19 | Farinazzo, E. | 44 | F | NM | NM | NM | 2 | NM | Purplish macule on the third finger of one hand | 10d | NM | Fixed Drug Eruption | NM | NM |
| With mRNA‐1273 | ||||||||||||||
| 5 | Sidlow, J. S. | 45 | M | NM | atopy and seasonal allergies | NM | 1 | Neg | Only after 1st: pruritic morbilliform rash with spread to the arms and abdomen | 8d | spongiosis and a superficial and deep, perivascular and interstitial infiltrate, mixed cell type with numerously abundant eosinophils and occasional neutrophils within the reticular dermis | Generalized reaction | 7d | Neg |
| 5 | Sidlow, J. S. | 31 | F | History of guttate psoriasis | NM | NM | 1 & 2 | 2nd: low‐grade fever, generalized achiness, and malaise | Both doses: urticarial papular eruption on the contralateral aspect of the right arm | 3d | spongiosis and a superficial and deep, perivascular and interstitial infiltrate, mixed cell type with eosinophils and occasional neutrophils within the reticular dermis | Generalized reaction | 7d | Neg |
| 5 | Sidlow, J. S. | 88 | F | NM | multiple drug allergies | NM | 1 | 1st: pins and needles sensation on the limbs and dysesthesia on the tongue | Only after 1st: generalized and progressing pruritus | 3d | spongiosis and a superficial and deep, perivascular and interstitial infiltrate, mixed cell type with eosinophils and occasional neutrophils within the reticular dermis | Generalized reaction | NM | Benadryl (Johnson and Johnson) and Sarna (Stiefel) lotion |
| 21 | Kong, Joyce | 66 | M | NM | HTN, hyperlipidemia, DM, CKD, CAD, idiopathic hypothyroidism | Amlodipine, Aspirin, Brimonidine, Clopidogrel, Furosemide, Gabapentin, Insulin, Levothyroxine | 2 | fever, myalgias, malaise; After 5d: lower extremity muscle tenderness, stiffness with preserved strength | Painful blistering rash on torso, arms, legs, violaceous, poorly demarcated patches on trunk, arms, thighs, large flaccid bullae, erosion on buttocks, posterior shoulder, and scrotum |
1d |
epidermal necrosis, with detachment from the underlying dermis forming a subepidermal blister, a very sparse lymphocytic inflammatory infiltrate and a sparse perivascular dermatitis | Extensive Bullous Fixed Drug Eruption | 5d | Ibuprofen, High‐dose Oral Prednisone, drainage of patient's bullae, Mupirocin Ointment with Vaseline |
| With Ad26.COV2.S | ||||||||||||||
| 22 | Lospinoso, K. | 74 | M | Known allergy to sulfa drugs and amoxicillin‐clavulanic acid, no prior vaccination‐related reactions | Panhypopituitarism, adrenal insufficiency, neurogenic bladder, obstructive sleep apnea | Prednisone (20 mg, daily) | 1 | ipsilateral arm discomfort, including the axilla, within 24 h of administration | Generalized distribution (50% of body) of erythematous plaques, numerous small, non‐follicular pustules, with sparing of the face, genitals, and mucosae; Significant acral swelling | 3d | epidermal spongiosis with focal, dermal neutrophilic inflammation and occasional eosinophils, scattered subcorneal neutrophilic pustules | Spongiotic and pustular eruption | 20d | Oral Prednisone (20 mg, daily), Topical Steroids |
| 23 | Song, E. J. | 83 | F | Neg | HTN, hypothyroidism, breast cancer | Palbociclib, Letrozole, Vitamin D | 1 | Neg | pruritic erythematous annular patches with central clearing on the breast, abdomen, and axilla; scattered petechiae on previous sites of involvement | 2d | a mixed spongiotic and interface dermatitis with rare eosinophils and extravasated erythrocytes | Widespread annular eruption | 14d | Fexofenadine 360 mg daily, Cetirizine 20 mg daily, Triamcinolone 0.1% cream BD |
| With ChAdOx1 nCoV‐ 19 | ||||||||||||||
| 24 | Dash, S. | 60 | M | NM | DM, HTN | Teneligliptin, Metformin and Amlodipine | 1 | Fever, oral ulceration and skin rash | Multiple purpuric macules present all over the body with peri‐lesional erythema; lesions coalesced to form large sheets of necrosed skin over front and back of trunk, with bullae on few areas; Mucosal involvement: oral erosions, hemorrhagic crusting over the lips, eye congestion, erosions over the glans | 3d | Orthokeratosis, epidermal atrophy, infiltration of lymphocytes, neutrophils spongiosis, scattered degenerated apoptotic keratinocytes, basal cell degeneration, interface dermatitis, perivascular and peri‐adnexal inflammatory cell infiltrate, extravasation of erythrocytes in dermis | Steven‐Johnson syndrome | 7d | Oral Cyclosporine 300 mg |
| 14 | Zafar, M. | 55 | F | stable psoriasis and scleroderma | NM | NM | 1 | NM | new‐onset non‐itchy rashes, with progressive worsening over the past month on hands and back | 2d | NM | Rash without eosinophilia | NM | oral antihistamines and topical steroid creams |
| 25 | Tammaro, A. | 35 | F | NM | Prior COVID‐19 + | NM | 1 | fever, nausea, and pain on the site of injection for 2d |
extended erythematous rash on the legs with pruritus and warmth 2d later: vesicular lesions on top of that rash Resolution: vesicular lesions were substituted by white discolorations |
6d | NM | Local nonspecific inflammatory reaction to vaccination | NM | ebastine 10 mg and 2 tablets of betamethasone 1 mg (tapered for 4d) |
| 2.3. Chilblain‐like lesions (CBLL) (n = 9) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 26 | Lesort, C | 82 | F | psoriasis | NM | Methotrexate for more than 10 years | 1 | Neg | slightly painful macular violaceous and erythematous lesions of the fingers and toes, suggestive of CBLL | 24 h | A partly necrotic epidermis, overlying a dense dermal lymphocytic infiltrate forming rather well‐circumscribed aggregates around blood vessels, eccrine sweat glands and occasionally nerves | CBLL | NM | NM |
| 27 | Piccolo, V. | 41 | F | Neg | Neg | Neg | 2 | NM | CBLL on the volar aspects of, the second and the third fingertip of right hand along with an acrocyanosis of the hands | Soon after | NM | CBLL | NM | NM |
| 28 | Pileri, A. | 42 | M | NM | NM | NM | 1 | NM | Nonpainful erythematous, purplish patches located on his distal phalanges and nail beds | 12d | NM | CBLL | NM | NM |
| 29 | Davido, Benjamin | 41 | F | NM | Bipolar disorder | Valproate for more than 10 years | 1 | Sudden toe pain with walking impairment, otherwise asymptomatic | chilblain‐like skin changes on toes, itching at night; 10d after vaccination: non‐tender violaceous toes of the left foot, with no other dermatological lesion | 4d | NM | CBLL | 28d | NM |
| 19 | Farinazzo, E. | 27 | F | NM | NM | NM | 1&2 | NM |
1st: Chilblain‐like rash on the first and third finger of one foot + urticarial rash 2nd: urticarial rash |
1st: 4d,2nd:1d | NM | Chilblain‐like rash | NM | NM |
| With mRNA‐1273 | ||||||||||||||
| 30 | Watad A. | 48 | F | NM | Neg | NM | 1 | NM | Painful chilblains like lesions on fingers; itchy urticarial‐type lesions (resembling urticarial multiforme)over volar aspect of both wrists and feet; evolving over thighs (asymptomatic) and extensor surfaces of both elbows; painful macular/nodular lesions over palmar surface of both hands | 10d | NM | CBLL and Urticarial‐type lesions | 7d | Hydrocortisone 0.5%, Naproxen 500 mg |
| 31 | Kha, C. | 70 | M | NM | Pityriasis lichenoides chronica, clinically stable | clobetasol 0.05% ointment | 1 & 2 | erythema, swelling, and pain with movement of the right PIP joints of the 4th and 5th digits for 10d |
few scattered pruritic red edematous papules on an erythematous/violaceous background on palmar and lateral aspects of the fingers on right hand |
1st: 2d, 2nd: 3d | dense and predominantly perivascular lymphocytic infiltrate (majority of CD31 T cells) within the superficial‐to‐deep reticular dermis, epidermis normal with no vacuolar changes at the epidermal‐dermal junction, notable papillary dermal edema, some slightly thickened vessels walls with trophism of lymphocytes within the superficial dermis | Chilblains | 1st: 14d, 2nd: 7d |
1st: Clobetasol 0.05% ointment applied twice daily 2nd: Topical Steroid therapy |
| WithCoronaVac | ||||||||||||||
| 32 | Selami Aykut Temiz | 44 | M | Neg, Prior COVID ‐ | Neg | Neg | NM | Neg | Mildly pruritic, edematous violaceous plaques and nodules on the dorsal hands | 7d | NM | Acral CBLL | 21d | Topical corticosteroids, Antihistamines |
| 32 | Selami Aykut Temiz | 53 | M | Neg, Prior COVID ‐ | Neg | Neg | NM | Neg | Erythematous‐to‐violaceous, patches on the marginal side of the fingers of both hands | 7d | NM | Acral CBLL | 21d | Topical corticosteroids, Antihistamines |
| 2.4. Pityriasis rosea (n = 9) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 33 | Cyrenne, Benoit | 20 | F | Alopecia areata | NM | Neg | 1 & 2 | NM | 1st: large, pruritic, red and scaly plaque at the inoculation site, small, red, scaly lesions on the trunk, 2nd: increased pruritus and an increasing number of lesions, multiple oval pink‐to‐tan‐colored thin plaques with peripheral scale on the trunk and proximal extremities | 2d | Parakeratosis with minimal acanthosis and spongiosis of the epidermis, few scattered dyskeratotic keratinocytes in the lower epidermis, Melanin incontinence, perivascular lymphocytic infiltrate and rare scattered extravasated red blood cells in the papillary dermis | Pityriasis rosea‐like eruption | 14d | Topical corticosteroid therapy |
| 33 | Cyrenne, Benoit | 40 | M | NM | NM | Neg | 2 | Neg | 2nd: red and scaly plaque on lateral left axilla, widespread eruption of pruritic, symmetrically distributed, smaller plaques with peripheral scale on the trunk and proximal extremities | 21d | NM | Pityriasis rosea | 21d | Doxycycline and Bilastine |
| 34 | Busto‐Leis, J.M. | 29 | M | NM | NM | NM | 2 | NM | Herald patch, typical oval‐shaped macules, appeared along skin tension lines | 1d | Mild spongiosis with foci of parakeratosis and a lymphohistiocytic infiltrate around superficial vessels | Pityriasis rosea | NM | NM |
| 34 | Busto‐Leis, J.M. | 26 | M | NM | NM | NM | 2 | NM | Herald patch, typical oval‐shaped macules appeared along skin tension lines | 7d | Mild spongiosis with foci of parakeratosis and a lymphohistiocytic infiltrate around superficial vessels | Pityriasis rosea | NM | NM |
| 35 | Carballido Vazquez, A.M. | 35 | M | NM | NM | NM | 1 & 2 | NM | Extremely itchy exanthema, a single oval erythematous lesion on the thigh, papulo‐squamous rash on the trunk and proximal extremities | NM | NM | Pityriasis rosea like eruption | 14d | Antihistamines, Topical Betamethasone |
| 19 | Farinazzo, E. | 42 | F | NM | NM | NM | 2 | NM | Pityriasis rosea‐like rash on the thighs and abdomen | 4d | NM | Pityriasis rosea‐like rash | NM | NM |
| 19 | Farinazzo, E. | 64 | M | NM | NM | NM | 1 | NM | Pityriasis rosea‐like rash on the neck, upper limbs, and trunk | 5d | NM | Pityriasis rosea‐like rash | NM | NM |
| 36 | Abdullah, Lina | 40 | M | NM | Neg | Neg | 2 | Neg | Rash, single larger erythematous patch with scale on the back, papules on the arms, thighs, chest, abdomen, and flanks in a Blaschkoid distribution | 7d | Neg | Pityriasis rosea | 21d | 0.1% Triamcinolone cream |
| With CoronaVac | ||||||||||||||
| 37 | Akdas, E. | 45 | F | Neg | Neg | Neg | 1 & 2 | NM | Plaques, with a peripheral collarette scaling, herald patch on the right scapula and the right breast, salmon‐colored plaques over the trunk and proximal extremities, many of which had peripheral scales; distribution of plaques: along cleavage lines reminiscent of a Christmas tree pattern on the patient's body | 1st& 2nd: 4d | Focal parakeratosis in mounds with exocytosis of lymphocytes, spongiosis in the epidermis and extravasated red blood cells in the dermis | Pityriasis rosea | 1st:21d | 1st: oral Antihistamine, Topical corticosteroid |
| 2.5. Herpes zoster (n = 8) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 38 | Tessas, Ioannis | 44 | M | Mild varicella during a stressful period in childhood | Dyslipidemia and active smoking | NM | 1 | 1st: neuropathic pain extending to the neck and the left hand, tiredness | Only after 1st: pain and redness, herpetiform vesicular and erythematous rash on the left upper back and lateral side and inner side of the left arm (C5–C6 dermatomes) | 7d | NM | Ipsilateral Herpes zoster | NM | Oral Valaciclovir TDS for 14d |
| 39 | Burlando, Martina | 42 | M | NM | Neg | NM | 1 | NM | Unilateral small papulovesicular lesions, heralded by a burning sensation, on right hemithorax | 2d | NM | Herpes zoster | NM | Acyclovir 800 mg five times a day for 7d |
| 40 | Nanova, Krassimira | 33 | F | Chickenpox in childhood | NM | NM | 1 | High‐grade fever | Widespread rash, Multiple vesicular lesions with an erythematous rim, observed on the trunk, scalp, and limbs | 7d | NM | Herpes zoster | NM | NM |
| 19 | Farinazzo, E. | 34 | F | NM | NM | NM | 1 | NM | Herpes Zoster of the scalp | NM | NM | Herpes zoster | NM | NM |
| 19 | Farinazzo, E. | 48 | F | NM | NM | NM | 1 | NM | Herpes Zoster | NM | NM | Herpes zoster | NM | NM |
| With CoronaVac | ||||||||||||||
| 41 | Bostan, E. | 78 | M | NM | CHD, CVA, HTN, COPD, radical cystectomy, prostatectomy | Neg | NM | Neg | Erythematous, painful, pruritic, stinging crusted, hemorrhagic vesicles upon an erythematous base involving the left mammary region (an area corresponding to T3‐ T4 dermatomes on the chest and the back) | 5d | NM | Herpes zoster | NM | Oral valacyclovir TDS for 7d |
| With BBV152 | ||||||||||||||
| 42 | Arora, P. | 60 | M | NM | DM, HTN | NM | NM | Neg | Multiple grouped vesicles on an erythematous base, present over the knee, and anterior aspect of right thigh | 4d | Intraepidermal spongiotic vesicle containing acantholytic cells with large vesicular nuclei neutrophils and dyskeratotic cells; Occasional multinucleate cell with ground‐glass chromatin and molded nuclei seen within the blister | Herpes zoster | 14d | Oral Valacyclovir 1 g TDS for 7d, Topical Fusidic Acid BD |
| With ChAdOx1 nCoV‐ 19 | ||||||||||||||
| 43 | Algaadi, S. A. | 65 | M | Chicken pox at childhood | CHD, HTN, DM | NM | 1 | NM | Painful grouped vesicles and ulcerations with a burning sensation on the right side of chest | 6d | NM | Herpes zoster | NM | 7d Acyclovir, Topical Fusidic Acid |
| 2.6. Purpuric lesions (n = 16) | ||||||||||||||
| 2.6.1. Immune thrombocytopenic purpura (ITP) (n = 14) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 44 | Ganzel, C. | 53 | M | NM | Morbid obesity, DM, HTN | Lercanidipin, Losartan, Doxazocin, Hydrochlorothiazide, Aspirin | NM | Epistaxis and low PLT count | Wet purpura on palate and petechial and purpuric rash on the trunk and limbs | 14d | NM | ITP | NM | Dexamethasone 20 mg/d and IVIG, 1 g/kg, |
| 45 | Tarawneh, O. | 22 | M | NM | Neg | Neg | NM | Neg | Widespread petechiae and gum bleeding | 3d | NM | ITP | 3d | Dexamethasone, PLT transfusion, IVIG at 1 g/kg |
| 46 | Mazzatenta, C | 44 | F | NM | NM | NM | 2 | NM | Asymptomatic purpuric lesions on the right and left eyelid, circumscribed on the upper eyelid | 23d | NM | Purpuric lesions on the eyelids | 10d | Neg |
| 46 | Mazzatenta, C | 63 | M | NM | NM | NM | 2 | NM | Asymptomatic purpuric lesions on the right and left eyelid, circumscribed on the upper eyelid | 14d | NM | Purpuric lesions on the eyelids | 15d | Neg |
| 46 | Mazzatenta, C | 67 | F | NM | NM | NM | 1 | NM | Moderately itchy ecchymotic lesions on upper eyelids | 10d | NM | Purpuric lesions on the eyelids | 12d | Neg |
| 47 | de Bruijn, S. | 38 | F | NM | previously TTP‐ naïve | Neg | 1 & 2 | blurred vision in the left eye (central serous chorioretinopathy) | Bruises after 1st dose, increased bruising and petechiae, diffuse ecchymosis after 2nd dose | 14d | NM | Immune‐ mediated TTP (iTTP) | NM | plasma exchange, methylprednisolone, Asprin, RTX, and caplacizumab |
| With mRNA‐1273 | ||||||||||||||
| 48 | Toom, S. | 36 | F | Allergy to acetaminophen | ITP | Etonogestrel‐Ethinyl Estradiol | 1 | A mild headache 7d post vaccination | Diffuse petechiae of the extremities and trunk, easy bruising, oral ecchymosis | 14d | NM | ITP | 3d | Dexamethasone 40 mg IV daily for 4d, IVIG 1 mg/kg for 3d, monitoring |
| 49 | Julian, J. A. | 72 | F | Seasonal contact dermatitis | Gout, DM | Allopurinol, Sitagliptin | NM | NM | Diffuse petechiae across arms, legs, and abdomen and hemorrhagic bullae of the gingival mucosa | 1d | NM | ITP | NM | Dexamethasone, IVIG, aminocaproic acid, RTX, PLT transfusions |
| 50 | Helms, J. M. | 74 | M | NM | HTN, gout, hyperlipidemia and nonischemic cardiomyopathy | NM | 1 | acute epistaxis | diffuse cutaneous purpura | Few hours | NM | Severe, Refractory Immune Thrombocytopenia | NM | High dose Dexamethasone, IVIG, PLT transfusions, RTX, Eltrombopag, plasma exchange, high dose Methylprednisolone, Romiplostim, Cefazolin |
| With ChAdOx1 nCoV‐ 19 | ||||||||||||||
| 51 | Candelli, M | 28 | M | NM | Neg | Neg | 1 | fatigue and headache for 10 days and fever for 2 days | oral bleeding and the appearance of petechiae over the trunk, arms, and legs for 3d purpura over the trunk, all four limbs, and bleeding lesions within the oral cavity | 19d | NM | ITP | NM | dexamethasone (40 mg/day) |
| 52 | Ryan, E. | 35 | F | NM | migraine | Neg | 1 |
general myalgia and extreme fatigue,headache (different from her known migraine headaches |
onset of bruising and petechiae | 10d | Marrow aspirate revealed some reactivefeatures with no evidence of an underlying infiltrative disorder | VITT | NM | preemptively anticoagulated with apixaban |
| 53 | Thaler, J. | 62 | F | NM | substituted hypothyroidism of unresolved genesis since age 20 | NM | 1 | flu‐ like symptoms including aching joints, moderate headache, and moderate dizziness, self‐ medicated with 1 g paracetamol, fever: 400 mg aspirin twice | unusually large hematoma after slight biting of lip, gum bleedings, an atraumatic hematoma at the right ankle, small hematomas and petechiae of the limbs | 8d | NM | VIPIT | NM | low dose fibrinogen concentrate (1 g for 2d), danaparoid‐ sodium 750 IE IV bolus + 1500 IE SC, followed by 1500 IESC every 8 h). high dose IVIG (1 g/kg),prednisolone (0.75 mg/kg)(2d) |
| With Ad26.COV2.S | ||||||||||||||
| 54 | Costello, A. | 40 | F | NM | migraines, obesity | NM | 1 | sudden headache (intermittent, worsening with sinus pressure, prescribed amoxicillin/clavulanate, methocarbamol, and methylprednisolone), body aches, fever, chills, bilateral lower‐extremity pain without edema, intermittent vertigo | swollen red cheeks, petechiae on her right cheek and bilateral breasts and spontaneous bruising in extremities | 10‐12d | NM | vaccine‐induced thrombotic thrombocytopenia | NM | Bivalirudin, Prednisone, (1 mg/kg/day), IVIG (1 g/kg/day), discharged on Rivaroxaban and a Prednisone taper |
| With CoronaVac | ||||||||||||||
| 55 | Cebeci, F. | 82 | F | NM | seronegative RA, HTN | Hydroxychloroquine 3 years, Olmesartan 2 years Prednisolone 5 mg 6 months (discontinued 3 weeks vaccination) | 1 | weakness, burning in the legs |
1st: diffuse petechial rash on both lower extremities 2nd: uneventful |
1d | NM | petechial rash as a vaccine‐ induced hypersensitivity reaction | 7d | Prednisolone restarted 1 week after complete remission of the rash |
| 2.6.2. Vasculitis associated purpura (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 30 | Watad A. | 53 | M | NM | Neg | NM | 1 | Mild abdominal pain, arthralgia | Palpable purpura | 3d | Leukocytoclastic cutaneous vasculitis (IgA and C3 deposits in the vessel walls) | Henoch‐Schonlein purpura | NM | Dexamethasone 10 mg, Prednisone 60 mg thereafter |
| With BBV152 | ||||||||||||||
| 56 | Kharkar, V. | 31 | F | Neg | Neg | Neg | 2 | NM | painful palpable purpura on legs, predominantly on the left leg, Dermoscopy: irregularly arranged red blotches, with an orange–red background | 4d | Perivascular infiltrate comprised of eosinophils and lymphocytes with a few neutrophils, along with erythrocyte extravasation, perivascular fibrin and perivascular edema | Cutaneous small vessel vasculitis (cSVV) | 10d | Wait‐and‐watch policy |
| 2.7.Inflammatory flare‐ups (n = 27) | ||||||||||||||
| 2.7.1. Autoimmune inflammatory rheumatic diseases (n = 13) | ||||||||||||||
| 2.7.1.1. Psoriasis (n = 3) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 57 | Cohen, Stephanie R | 46 | F | Psoriasis (2 years in remission) | Psoriatic arthritis, IBS, leukocytoclastic vasculitis (biopsy‐proven) | Prednisone | 1 & 2 | Neg | 1st: mild exacerbation of palpable purpuric papules on the bilateral lower legs, 2nd: exacerbated again with significant exquisitely tender palpable purpuric papules distributed bilaterally on the lower legs, feet, upper extremities, lower back, and abdomen | 1st& 2nd: 2d | Perivascular mixed inflammatory infiltrate with numerous neutrophils, lymphocytes, occasional eosinophils, leukocytoclasia and erythrocyte extravasation, no fibrinoid necrosis of vessels | Small vessel vasculitis such as IgA vasculitis (Leukocytoclastic vasculitis flare) | NM | Topical steroids and a Prednisone taper |
| With mRNA‐1273 | ||||||||||||||
| 30 | Watad A. | 36 | F | Psoriasis since childhood (mild) | NM | NM | 1 | Dactylitis, stiffness and tightness, finger joint pain | Painful erythematous macules over palmar surfaceof several fingers on both hands, painful chilblains like lesions (CBLL) on fingers | 10d | NM | NM | NM | Ibuprofen 800 mg |
| With CoronaVac | ||||||||||||||
| 58 | Onsun, Nahide | 72 | M | Plaque psoriasis | Prerenal acute injury, HTN | Indapamide | 1 | Fever | Diffuse erythema, desquamation, and coalescing pustules over the entire body | 4d | Compatible with generalized pustular psoriasis | Generalized pustular psoriasis | NM | Acitretin (25 mg/d), IV infliximab 5 mg/kg |
| 2.7.1.2. Lichen planus or lichenoid reactions (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 59 | Hiltun, I. | 56 | F | Lichen planus lesions 7 years prior (successfully treated with topical therapy) | NM | Neg | 2 | NM | Polygonal, well‐delimited, erythematous papules in the ankles, periumbilical area, flexural wrist and forearms and mammary and axillary folds, Dermoscopy: slight desquamation and Wickham's striae, no mucosal or nail involvement | 2d | Typical findings of lichen planus with epidermal hyperplasia forming a characteristic saw‐tooth appearance with wedge‐shaped hypergranulosis, vacuolar degeneration of basal layer and dense lymphocytic infiltrate in the superficial dermis | Lichen planus | NM | High‐potency Topical corticosteroids |
| 39 | Burlando, Martina | 47 | M | Previously diagnosed with lichen planus located on both forearms | Neg | NM | 1 | NM | Sudden worsening of the preexisting papules, which spread to both arms and trunk | 1d | Refused by patient | Lichenoid reactions | NM | Topical corticotherapy BD for 10d |
| 2.7.1.3. Behçet's (n = 4) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 30 | Watad A. | 21 | M | Behcet's disease for 3 years | NM | Colchicine | 1 | NM | Oral aphthous ulcers | 5d | NM | Behcet's disease flare | NM | 20 mg Prednisone for 7d |
| 30 | Watad A. | 55 | M | Behcet's disease for 20 years | Chronic lymphocytic leukemia | Apremilast | 2 | Synovitis of small joints | Oral aphthous ulcers(on the tongue) | 7d | NM | Behcet's disease flare | NM | Colchicine 0.5 mg BD, 5 mg Prednisone |
| 30 | Watad A. | 20 | M | Behcet's disease for 2 years | NM | Colchicine | 1 | NM | Oral aphthous ulcers | 3d | NM | Behcet's disease flare | NM | Colchicine 2 mg daily |
| 30 | Watad A. | 34 | M | Behcet's disease for 10 years | Neg | Colchicine, Humira | 1 | NM | Pustular skin lesions | 5d | NM | Behcet's disease flare | NM | NSAIDs, increase of Colchicine dose |
| 2.7.1.4. Systemic lupus erythematosus (SLE) (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 30 | Watad A. | 78 | F | Laboratory SLE | NM | NM | 1 | Fever and arthritis | Erythematous rash (generalized acute cutaneous lupus), purpura, oral aphthous ulcers | 2d | Biopsy from purpura: leukocytoclastic vasculitis | Leukocytoclastic vasculitis | NM | Hydroxychloroquine |
| With ChAdOx1 nCoV‐ 19 | ||||||||||||||
| 30 | Watad A. | 50 | F | SLEwith arthritis, mucosal ulcers and hemolysis | NM | NM | 1 | Severe hemolysis and arthralgia | Oral and nasal ulceration | 14d | NM | SLE flare | NM | Prednisolone 60 mg daily, RTX |
| 2.7.1.5. Other AIIRDs (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 30 | Watad A. | 62 | F | Dermatomyositis | NM | Methotrexate, Plaquenil | 1 | NM | Skin rash similar to the dermatomyositis rash | 7d | NM | Dermatomyositis flare | 7d | Local Steroid cream |
| 30 | Watad A. | 42 | F | NM | Transient synovitis | NM | 1 | Migratory arthritis of small joints | Painless hemorrhagic rash on toes, erythema on small joints | 4d | NM | Florid clinical arthritis of the PIP joints | 7d | Prednisolone 10 mg, daily |
| 2.7.3. Ulcerative colitis (UC) (n = 1) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 30 | Watad A. | 28 | M | NM | UC for 10 years, HES for 5 years, (well controlled) | Vedolizumab, Cyclosporin | 1 | Hemorrhagic diarrhea | Vesicular skin rash, oral aphthosis | 4d | NM | Flare of HES and UC | NM | 1 gr of Sulomedrol daily for 3d, Prednisone 60 mg/day |
| 2.7.4. BCG scar local skin inflammation (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 60 | Lopatynsky‐Reyes, E. Z. | 31 | F | NM | Neg | NM | 1 | headaches, chills, myalgias, pain at injection site, lymphadenopathies | Only after 2nd: inflammation area of 1.5 cm on the BCG scar site with erythema, induration, but painless to palpation (5 cm from the COVID vaccine injection site) | 2nd: 2d | NM | BCG Scar Local Skin Inflammation | 4d | 2nd: three doses of Oral Paracetamol |
| With mRNA‐1273 | ||||||||||||||
| 60 | Lopatynsky‐Reyes, E. Z. | 28 | F | NM | NM | NM | 1 & 2 | 1st: pain, redness at the injection site, myalgias, arthralgias, malaise, 2nd: headache, nausea, myalgias, arthralgias, and malaise | 1st: redness at injection site, 2nd erythematous reaction followed by pain, induration, and mild edema at BCG scar site (3 cm below COVID vaccine injection site) | 2nd: 36 h | NM | BCG Scar Local Skin Inflammation | 2d | 2nd: four doses of Oral Paracetamol |
| 2.7.5. Radiation recall phenomenon (n = 3) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 61 | Soyfer, V. | 68 | M | NM | Metastatic soft tissues sarcoma in the posterior chest wall and 1 lesion in the right lung | Preoperative radiation therapy, resection in the posterior chest wall | 2 | NM | pain, burning sensation, redness, and mild skin exfoliation in the area of the posterior chest wall electron field (the electron port shape closely resembles the erythematic area) | 5d | NM | Radiation Recall Phenomenon | a few days | Topical steroids and painkillers |
| 61 | Soyfer, V. | 64 | M | NM | Metastatic solitary fibrous tumor | Radiation of lumbar vertebrae after spinal cord surgery | 2 | NM | Only after 2nd dose (14d after RT): skin redness and itching sensation, sparing the skin covering the lumbar spine | 6d | NM | Radiation Recall Phenomenon | 7d | Neg |
| With CoronaVac | ||||||||||||||
| 62 | Afacan, E | 60 | F | Melanoma | NM | Dabrafenib/Trametinib combination therapy | 1 | Sudden onset painful lesion on the medial side of the right leg | Well demarcated, erythematous, indurated plaque confined to an area of previous irradiation | 5d | Epidermal intercellular edema, lymphocyte exocytosis, and rare necrotic keratinocytes as well as increased dermal collagenization and fibrosis | Radiation Recall Phenomenon | NM | NM |
| 2.7.6. Inflammatory reaction to hyaluronic acid (HA) soft tissue fillers (n = 2) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 63 | Michon, A. | 39 | F | NM | Neg | Neg | 1 | flu‐ like illness symptoms (fatigue, headache, myalgias, and anorexia), which resolved within 4d | tender, erythematous swelling at the left tear trough area (areas previously treated with filler) | 2d | NM | Delayed inflammatory reaction to HA fillers | 5d | Spontaneous resolution |
| 63 | Michon, A. | 61 | F | NM | intermittent benign vertigo | Neg | 1 | Flu‐like illness symptoms | intermittent facial swelling mostly on cheeks and undereye (areas previously treated with filler), lasting for almost 1d per episode, and once for 72 h | 1d | NM | Delayed inflammatory reaction to HA fillers | 2d after hyaloronidase | 75 units of hyaluronidase at a concentration of 150 units/mL |
| 2.7.7. No Flare‐up in Autoimmune Disorders (n = 6) | ||||||||||||||
| With BNT162b2 | ||||||||||||||
| 64 | Iannone, Michela | 48 | F | HS for 6 years (vaccination was after 90D of treatment with a single HS flare), anti–TNF‐ α‐induced lupus syndrome | Concomitant endometriosis | Surgical drains, systemic Clindamycin/Rifampicin, Ixekizumab SC 160 mg | 1 & 2 | NM | No adverse events and no HS flares | NM | NM | NM | NM | Routine continuation of Ixekizumab |
| 65 | Pacifico, A | 48 | M | Psoriasis, with flares of both PsO and PsA during asymptomatic COVID‐19 infection, spontaneous resolution 10 days after COVID‐19 remission | Psoriatic arthritis | Apremilast, achieving stable remission, maintained for 8 months | 1 & 2 | NM | No psoriasis flares with either dose | NM | NM | NM | NM | NM |
| 65 | Pacifico, A | 36 | F | Plaque psoriasis, concurrent pustular palmoplantar psoriasis area | NM | Apremilast and narrowband UVB for 3 years | NM | Neg | No psoriasis flares with either dose | NM | NM | NM | NM | NM |
| 66 | Rama, T. A. | 37 | F | Adult‐onset monomorphic maculopapular cutaneous mastocytosis lesions with generalized pruritus, flare up of lesions, and osteopenia | severe mast cells mediator‐related symptoms including abdominal colicky pain, bloating and diarrhea | Antihistamines, Montelukast | 1 | Neg | No mastocytosis flare | NM | NM | NM | NM | NM |
| 66 | Rama, T. A. | 47 | F | monomorphic maculopapular cutaneous mastocytosis, anaphylaxis with multiple drugs, pruritus | MC mediator‐related symptoms including migraines, gastroesophageal reflux, and osteopenia | Antihistamines, montelukast | 1 | Myalgia | No mastocytosis flare | 1d | NM | NM | NM | NM |
| With ChAdOx1 nCoV‐19 | ||||||||||||||
| 65 | Pacifico, A | 76 | M | Psoriasis for 4 years: stable residual Psoriasis area severity index (PASI) of 3 | NM | Apremilast for 4 years | 1 | 1st: fever (38.5°C) and myalgia for 3 days | No psoriasis flares with either dose | NM | NM | NM | NM | NM |
Abbreviations: 1st, after the first dose; 2nd, after the second dose; AIIRD, autoimmune inflammatory rheumatic diseases; ASAT, aspartate aminotransferase; BD, twice a day; BPH, benign prostatic hypertrophy; CAD, coronary artery disease; CBLL, chilblain‐like lesions; CHD, coronary heart disease; CKD, chronic kidney disease; CVA, cerebrovascular accident; d, days; GGT 2N, gamma‐glutamyl transferase twice normal upper limit; HA, hyaluronic acid; HES, hypereosinophilic syndrome; HS, hidradenitis suppurativa; HTN, hypertension; IBS, irritable bowel syndrome; ITP, immune thrombocytopenic purpura; IVIG, intravenous immunoglobulin; MC, mast cell; MOGSD, myelin oligodendrocyte glycoprotein spectrum disorder; Neg, negative; NM, not mentioned; PIP, proximal interphalangeal; PLT, platelet; PO, by mouth; PsA, psoriatic arthritis; PsO, plaque psoriasis; RT, radiation therapy; RTX, rituximab; SC, subcutaneously; SLE, systemic lupus erythematosus; SLE, systemic lupus erythematosus; TDS, three times a day; UVB, ultraviolet B.
Supporting information Table S1.
A total of 73 cases (62.9%) developed mucocutaneous reactions after receiving the 1st dose of the vaccines, 19 cases (16.4%) after the 2nd dose, and 15 cases (13%) after both doses. Nine reports (7.7%) had not specified the administered dose.
Cases were further categorized into sections based on their clinical and pathological diagnosis:
3.2.1. Injection site reactions, “Covid arm” (n = 12)
Injection site skin reaction (Covid arm) consisted of 12 cases (mean age: 47.9 years, 100% female). Three of them (25%) had atopic background (either themselves or their family). They developed symptoms including painful or pruritic erythematous swelling, urticarial oval patch, patch, vesicle, nodule, or induration at the vaccine injection site after 6.27 days (50% BNT162b2 and 50% mRNA‐1273; 50% after 1st dose, 25% after 2nd dose and 25% after both doses). Resolution of symptoms was achieved in an average of 4.15 days, mainly with the use of topical corticosteroid cream (58.3%).
3.2.2. Non‐injection site reactions (n = 104)
Hypersensitivity reaction type 1 (n = 14)
A total of 14 patients were incorporated in the section of type 1 hypersensitivity reaction.
Urticaria (n = 5)
Isolated urticaria occurred in 5 patients (mean age: 30.8 years, F/M: 4) in a range of 5 min to 8 h after inoculation (100% BNT162b2; 100% 1st dose), 80% had an allergic background and they were mainly treated by antihistamines and then oral corticosteroids.
Flushing (n = 3)
Three patients experienced Flushing of the face (mean age: 48 years, all female) in a range of 5–30 min after inoculation (100% BNT162b2; 100% 1st dose). Two had an allergic background and 1 was treated with antihistamines.
Angioedema (n = 3)
Three patients (mean age: 35 years, F/M:2) presented with angioedema within 10 min to 24 h of immunization (66.7% BNT162b2 and 33.3% mRNA‐1273; 100% 1st dose). For two patients, no treatment was conducted and for the other one antihistamines and corticosteroids were prescribed and symptom relief was achieved in 24 h.
Anaphylaxis (n = 3)
Three patients encountered anaphylaxis (mean age: 27.3 years, F/M:2) all of whom had an allergic background and developed symptoms pertaining to anaphylaxis, with systemic reactions such as tachycardia, tachypnea, dysphagia, dyspnea, severe chills, dysphagia, the feeling of a slurred mouth and hoarseness, wheezing and throat pruritus, along with mucocutaneous reactions such as diffuse maculopapular rash, urticaria, diaphoresis, palate pruritis, generalized rash and pruritus, sudden onset of rash followed by urticaria and angioedema in a span of a few minutes to 5 h after vaccination (66.7% BNT162b2 and 33.3% mRNA‐1273; 100% after 1st dose). They were diagnosed as Biphasic anaphylaxis, Severe allergic reaction and Level 1 Anaphylaxis. Resolution was achieved 6 h to 1 day after the onset of symptoms using steroids, antihistamines, one patient was treated with an Epinephrine injection and Sodium Succinate, and one was given oxygen.
Generalized Eruptions (n = 21)
In the generalized eruptions section, we considered patients with miscellaneous presentations and diagnoses who could not be further categorized in other groups. A total of 21 patients were included (mean age: 55.14 years, F/M:1.63)(57.1% BNT162b2, 19% mRNA‐1273, 14.3% ChAdOx1 nCoV‐19, 9.6% Ad26.COV2.S; 66.7% after 1st dose, 14.3% after 2nd dose, 14.3% after both doses and 4.7% dose not mentioned).
Among the more distinguished presentations were:
Steven‐Johnson syndrome was a diagnosis of a patient (male, 60‐year‐old) who presented with fever, oral ulceration, eye congestion, erosions over the glans, and multiple purpuric macules all over the body with perilesional erythema which progressed to necrosis after 3 days of immunization (ChAdOx1 nCoV‐19, 1st dose); resolution of his symptoms was achieved in 7 days on oral cyclosporine.
Rowell's syndrome was diagnosed in a 74‐year‐old man with erythematous and partly violaceous coalescing macules and papules with slightly indicated cocarde formation on the trunk and extremities along with positive antinuclear autoantibodies (ANA) with 1:640 in a speckled pattern, anti‐Ro/SSA (60), anti‐Ro/SSA (52), and anti‐La/SSB antibodies within 1 day following vaccination (BNT162b2, 1st dose).
Two patients (mean age: 56.5 years, F/M: 1) encountered Erythema multiforme (EM)‐like eruption following inoculation (100% BNT162b2; 50% 1st dose, 50% both doses); The first patient was a 55‐year‐old male without previous history of EM who developed symptoms within 10 days and treated with systemic corticosteroids within 10 days, but the second patient was a 58‐year‐old female with a past medical history of EM and recurrent episodes of herpes labialis, presented her symptoms in 12 h after first and 24 h after the second dose and was cured with topical corticosteroids.
Two patients were diagnosed with Fixed Drug Eruption (FDE). One a 44‐year‐old female who developed purplish macule on the third finger of one hand 10 days after her 2nd dose of BNT162b2, and the other, a 66‐year‐old male who developed painful blistering rashes, violaceous, poorly demarcated patches, large flaccid bullae, and erosions all over his body along with fever, myalgias, malaise, muscle tenderness and stiffness in lower extremity with preserved strength, following his 2nd dose of mRNA‐1273 and was diagnosed with extensive bullous FDE. Resolution was achieved in 5 days using ibuprofen, high‐dose oral prednisone, along with drainage of patient's bullae, and administration of mupirocin ointment with Vaseline.
Chilblain‐like lesions (CBLL) (n = 9)
CBLLs were reported in 9 patients (mean age: 49.8 years, F/M: 1.25) with manifestations as violaceous/ erythematous lesions in the spectrum of macules, nodules to patches or plaques, located on the fingers and/or toes almost symmetrically which could be painful or to a lesser extent, pruritic. The patient's presentation onset was 5.22 days on average after injection (55.6% BNT162b2, 22.2% CoronaVac, and 22.2% mRNA‐1273; 44.5% after 1st dose, 11.1% after 2nd dose, 22.2% after both doses and 22.2% dose not mentioned) and their symptoms resolved in an average of 18.2 days, treated with topical corticosteroids (44.5%), antihistamines (22.2%) and NSAIDs (11.1%).
Pityriasis rosea (n = 9)
Nine patients (mean age: 37.9 years, F/M: 0.67) developed pityriasis rosea manifestations (herald patch, oval erythematous thin plaques with peripheral scale dominantly on the trunk and proximal extremities) without previous or relevant history after 6.38 days on average of immunization (88.9% BNT162b2 and 11.1% CoronaVac; 11.1% after 1st dose, 55.6% after 2nd dose and 33.3% after both doses), and resolution took place after 17.5 days on treatment with topical corticosteroids (44.4%) and antihistamines (33.3%) (either separately or in combination).
Herpes zoster (n = 8)
Eight patients (mean age: 50.5 years, F/M: 0.6) presented with painful (burning sensation) grouped vesicles on erythematous background in the various dermatomes and were diagnosed with herpes zoster. 37.5% of them reported a history of varicella infection in childhood, the patients became symptomatic following vaccination (62.5% BNT162b2, 12.5% CoronaVac, 12.5% ChAdOx1S nCoV‐19, 12.5% BBV152; 75% 1st dose and 25% doses not mentioned) after 5.17 days on average and mainly managed with Acyclovir.
Purpuric lesions (n = 16)
Immune thrombocytopenic purpura (ITP) (n = 14)
A total of 14 patients (mean age: 51.14 years, F/M: 1.8) developed ITP with the presentation of widespread petechiae, purpura or ecchymosis (78.6%) or local purpuric lesions on the eyelids (21.4%) after 9.14 days on average of vaccination (33.3% BNT162b2, 25% mRNA‐1273, 25% ChAdOx1S nCoV‐19, 8% Ad26.COV2.S and 8% CoronaVac for diffuse lesions; and 100% BNT162b2 for local eyelid purpuric lesions; 57.1% after 1st dose, 14.4% after 2nd dose, 7.1% after both doses and 21.4% dose not mentioned). ITP was treated mostly by dexamethasone and IVIG and purpuric lesions on the eyelids were managed conservatively.
Vasculitis associated purpura (n = 2)
Two patients encountered vasculitis symptoms: A 31‐year‐old female without rheumatologic background developed painful palpable purpura on her legs after 4 days of injection (BBV152, dose 2). She was diagnosed with cutaneous small vessel vasculitis (biopsy proven) and her symptoms resolved within 10 days by wait & watch strategy. Another patient was a 53‐year‐old male who experienced abdominal pain, arthralgia, and palpable purpura after 3 days of vaccination (BNT162b2, 2nddose), received the diagnosis of Henoch Schoenlein Purpura and biopsy revealed leukocytoclastic vasculitis. He was managed with corticosteroids.
Inflammatory flare‐ups (n = 27)
Autoimmune inflammatory rheumatic diseases (AIIRD) (n = 13)
A total of 13 patients with AIIRD had flare ups of their disease, including known cases of psoriasis (23%), lichen planus (15.33%), Behcet's disease (31%), SLE (15.33%) and other (dermatomyositis, arthritis)(15.33%),
Psoriasis (n = 3)
Three psoriatic patients (mean age: 51.3 years, F/M: 1.5) experienced disease flare after 4.5 days on average of inoculation (33.3% BNT162b2, 33.3% mRNA‐1273 and 33.3% CoronaVac, 75%after 1st dose and 25% after 2nd dose). One of them was a 46‐year‐old woman with in remission psoriasis (for 2 years, under prednisolone) and leukocytoclastic vasculitis who developed palpable purpuric papules after both BNT162b2 vaccine doses, and biopsy from the lesions revealed leukocytoclastic vasculitis flare, thereafter it was treated by topical and oral corticosteroids. Another patient was a 72‐year‐old man with a history of plaque psoriasis, who developed biopsy‐proven generalized pustular psoriasis after CoronaVac and was afterward treated by acitretin and infliximab.
Lichen planus or lichenoid reactions (n = 2)
Lichen planus was a medical history of 2 patients (mean age: 51.5 years, F/M:1) who encountered their disease relapse after 1.5 days on average of their immunization (100% BNT162b2, 50% after 1st dose, and 50% after 2nd dose).
Behçet's (n = 4)
In 4 patients with Behcet's disease (mean age: 32.5 years, 100% males), flare occurred with a presentation of aphthous ulcers (75%) and pustular lesions (25%) 5 days on average after injection (100% BNT162b2, 75% after 1st dose, 25% after 2nd dose) and their treatments were with colchicine, oral corticosteroids and NSAID either single or in combination.
Systemic lupus erythematosus (SLE) (n = 2)
Two patients experienced SLE flares. A 78‐year‐old woman with a past history of Systemic lupus erythematosus, presented with fever and arthritis, besides erythematous rash (generalized acute cutaneous lupus), purpura and oral aphthous ulcers after 2 days of inoculation with 1st dose of BNT162b2; biopsy from purpura showed leukocytoclastic vasculitis and consequently treatment was done with hydroxychloroquine. Another patient with SLE was a 50‐year‐old woman who received 1st dose of ChAdOx1S nCoV‐19 and presented with severe hemolysis and arthralgia in addition to oral and nasal ulceration 14 days post‐vaccination. Eventually, she was treated with oral corticosteroid and rituximab.
Other AIIRDs (n = 2)
A 62‐year‐old woman with a history of dermatomyositis, developed a characteristic rash of dermatomyositis (which she had experienced prior) after 7 days of receiving her 1st dose of BNT162b2 and was subsequently treated by topical corticosteroids leading to the resolution in 1 day.
A 42‐year‐old woman with history of transient synovitis developed a painless hemorrhagic rash on toes and erythema along with migratory arthritis on small joints 4 days after her 1stdose of BNT162b2, leading to a diagnosis of florid clinical arthritis of the PIP joints. She was treated with daily prednisolone 10 mg, and the symptoms resolved within 7 days.
Ulcerative colitis (UC) (n = 1)
A 28‐year‐old male with history of UC for 10 years, and hyper eosinophilic syndrome (HES) for 5 years (which were both well controlled under vedolizumab and cyclosporin), experienced vesicular skin rash, oral aphthosis and hemorrhagic diarrhea 4 days following his 1st dose of BNT162b2, which was treated with daily 1 gr of sulomedrol for 3 days and prednisone 60 mg/day.
BCG scar local skin inflammation (n = 2)
Two patients (mean age: 29.5 years, both female) experienced BCG scar local skin inflammation after 1.75 days of their 2nd injection (50% BNT162b2 and 50% mRNA‐1273; 50% after 1st dose, 50% after both doses) and it resolved within 3 days on average with paracetamol use.
Radiation recall phenomenon (n = 3)
Three patients with a medical history of malignancy (mean age: 64 years, F/M: 0.5) who had undergone radiotherapy, experienced Radiation Recall Phenomenon 5.3 days on average following injection (66.7% BNT162b2 and 33.3% CoronaVac, 66.7% after 1st dose, 33.3% after 2nd dose).
Inflammatory reaction to hyaluronic acid (HA) soft tissue fillers (n = 2)
Two women, one 39 and the other 61 years old, experienced delayed inflammatory reaction to HA fillers after their 1st doses of BNT162b2, presenting with flu‐like symptoms such as headache, fatigue, myalgias, and anorexia, along with tender, erythematous swelling at areas previously treated with filler. One resolved spontaneously after 5 days, but the other was administered 75 units of hyaluronidase (at a concentration of 150 units/mL), as her swelling was intermittent and lasted for 1–3 days at a time, and she had a larger volume of filler injected in her face; her symptoms subsided 2 days after administration of the hyaluronidase.
No flare‐up in autoimmune disorders (n = 6)
In the “No flare‐up” group, three patients (mean age: 53.3 years, F/M: 0.5) with a history of psoriasis, under control with apremilast (and narrowband type B ultraviolet [UVB] therapy in one of them), did not experience any flare‐ups after vaccination (66.7% BNT162b2 and 33.3% ChAdOx1S nCoV‐19, either dose). Two patients (mean age: 42 years, both female) with history of mastocytosis and also one patient with a history of hidradenitis suppurativa (HS) did not suffer from a flare‐up following inoculation (BNT162b2, 1st dose in patients with mastocytosis and both doses in the one with HS).
In the viewpoint of comparing side‐effects following the three most administered vaccine groups, the most common mucocutaneous eruptions among mRNA vaccine recipients presented in case reports (n = 90) were generalized eruptions (17.7%), hypersensitivity reactions (15.5%), injection site reactions (14%), purpuric lesions (11%), Pityriasis Rosea (8%), Chilblain‐like lesions (7.7%), and inflammatory flare‐ups (20%). Among Adenovirus viral vector vaccines (n = 11), generalized Eruptions (45%) and purpuric lesions (36%) were most common. Considering inactivated virus vaccines (n = 9), Chilblain‐like lesions (22%), Herpes Zoster (22%), and purpuric lesions (22%) were more prevalent.
3.3. Case series
A total of 10 case series were identified, comprised of 314 cases (mean age: 44.49 years; 83.76% F), as depicted in Table 2. History of previous allergy or allergic reaction was present in 42% to 90% of patients. 63.4% and 36.3% of patients received BNT162b2 and mRNA‐1273 vaccines, respectively, and one participant's (3%) vaccine was unknown. The mucocutaneous reactions appeared on first or both doses.
-
1.
Injection site reaction (n = 17, 5.4%)
TABLE 2.
Mucocutaneous reaction after COVID‐19 vaccination reported in “Case series” studies
| Supplemental references a | First author | Number of patients | Women percentage | Mean of Age (year) | Patients' mucocutaneous disease history | Dose | Any symptoms after vaccine | Mucocutaneous lesions characteristic | Mean time of onset reaction | Location of mucocutaneous reaction | Duration of reaction | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Injection site reaction (n = 17) | |||||||||||||
| With BNT162b2 | |||||||||||||
| 1 | Farinazzo, E. | 17 | 82.4 | 47 | NM | 1 or 2 | Fever (24%) | Erythema (30%), wheals (some burning, itchy, painful, with axillary lymphadenopathy [n = 2]) (24%), swelling (18%), nodules (painful and itchy erythematous subcutaneous nodule, Painful hardening)(18%), Itching (10%) | 1.7d (of those reported) | Injection site | NM | NM | NM |
| 2. Generalized reaction (n = 33) | |||||||||||||
| With BNT162b2 | |||||||||||||
| 2 | Corbeddu, M. | 11 | 64 | 50 | Allergy or allergic diathesis (72.7%) | 1 & 2 | Extracutaneous manifestations (36.3%), such as laryngospasm | Erythematous reactions, morbilliform rash, mild erythema, positive dermographism, urticarial rash, periorbital edema, angioedema, an atopic dermatitis flare up | 1.66 d | Diffuse (27.5%), Injection site (27.5%), face (9%), chest, trunk (18%), legs (9%), dorsum of foot (9%) | 2–3 d | Short oral steroids course | Mostly spontaneous remission |
| 1 | Farinazzo, E. | 22 | 95.5 | 43 | NM | 1 &/or 2 | Fever (4.5%) | Erythema (some itching) (23%), urticarial rash (23%), diffuse urticaria (14%), generalized itching (14%), swelling (eyelids, face, with mandibular lymphadenopathy)(14%), Rash (erythematous macular)(7.5%), Dermatitis (itchy)(4.5%) | 5.2 d | Generalized, localized other than injection site | NM | NM | NM |
| 3. Delayed localized hypersensitivity reactions (n = 32) | |||||||||||||
| With BNT162b2 | |||||||||||||
| 3 | Coto‐Segura, P. | 4 | 0 | 81.5 | NM | 1 | Neg |
Bullous drug‐induced reactions with severe pruritus Patients 1–3: urticated and erythematous plaques and tense bullae on erythematous base (1–3 cm in diameter), Patient 4: small vesicles‐bullae/bleb, some in a rosette‐like pattern; (Biopsy: subepidermal/subcorneal blisters, positivity in DEJ for IgG and C3) (Mucous membranes and eyes spared) |
3–17 d |
Patients 1–3: trunk, forearms and thighs Patient 4: limited to forearms |
NM | NM | NM |
| With mRNA‐1273 | |||||||||||||
| 4 | Blumenthal, K. G. | 12 | 83.3 | 43.3 | Allergy (42%), rhinitis (20%) | 1 & 2 |
1st: Fatigue (33%), myalgias (17%), headache, chills, lymphadenopathy, fever, Postural tachycardia, HTN 2nd: Fever (67%), myalgias (58%), chills (75%), fatigue (25%), headache (50%), lymphadenitis (17%), lymphadenopathy (9%), nausea (9%) |
1st: Pruritus, warmth, burning, swelling, rash, erythema, induration, hyperpigmentation (9%) 2nd:(in 50% of those with 1st dose reactions, either similar or lower grade) rash (50%), erythema (50%), itching (9%), urticaria (9%) Biopsy: Large T‐cell–mediated |
1st: 8.3 d | Near the injection site, palmar, near elbow | 6d | Amoxicillin (875 mg), clavulanic acid (125 mg), cetirizine 10 mg, loratadine 10 mg, diphenhydramine 25–50 mg, triamcinolone 0.1% topical, prednisone, famotidine 20 mg, clobetasol propionate 0.05% topical, hydrocortisone 1% topical |
58%resolution, 25% Hyperpigmentation, 17% Pain, Itching |
| 5 | Johnston, M. S. | 16 | 81 | 48.1 | NM | 1 & 2 |
1st: Fevers, chills, arthralgias, myalgias, headache, fatigue (19%),Sore arm (38%) 2nd:Nausea, chills (38%), myalgias (31%), headache (63%), sore arm (31%), fatigue (44%),decreased appetite, arthralgias (13%), Lethargy, rigors |
1st: (94% skin reaction) pruritic and variably painful erythematous reactions,typically homogenous,less commonly annular 2nd: (75% skin reaction, 73% similar to 1st dose reactions), pruritic, painful, and edematous pink plaques |
1st: 7d 2nd: 2.3 d |
at or near the injection site |
1st: 8.2 d 2nd: 3d |
Topical steroids (clobetasol ointment,hydrocortisone cream), oral antihistamines, cool compresses, cephalexin | NM |
| 4. Anaphylaxis (n = 66) (those in the preceding reports are counted once) | |||||||||||||
| With BNT162b2 | |||||||||||||
| 6 | Shimabukuro, T. | 21(first report) | 90 | 40.5 | Allergic reactions (81%), anaphylactic episode (33%) | 1 | Swollen airway, swollen lips (19%) and tongue (10%), wheezing (20%), stridor, nausea (14%), hoarseness (10%), difficulty swallowing, cough (10%) |
Diffuse erythematous rash (35%), urticaria (50%), diffuse pruritic rash (10%), pruritus (5%) (of 95% of all patients) |
46 min | Generalized | NM | Epi in 90% of patients | recovery |
| 7 | Shimabukuro, T. | 47(updated) | 94 | 39 | allergy or allergic reactions (77%), anaphylaxis (34%) | 1 & 2 | respiratory and airway obstruction symptoms, and nausea | Generalized urticaria, diffuse erythematous rash; facial, tongue, or laryngeal angioedema | 10 min | Generalized | NM | Epi (92%), endotracheal intubation (11%), corticosteroids (86%), antihistamines (72%) | 92% recovery |
| With mRNA‐1273 | |||||||||||||
| 8 | Shimabukuro, T. | 10(first report) | 100 | 46.2 | allergy or allergic reaction (90%), previous anaphylactic episode (50%) | 1 | Respiratory failure, vomiting, decreased peripheral perfusion, persistent dry cough, nausea, hypotension, wheezing |
(of 50% of all patients): Diffuse erythematous rash (80%),Generalized urticarial rash (20%); Tongue swelling (40%), throat swelling (10%), periorbital edema (20%) |
10.8 min | Generalized | NM | Epi in 100% of patients | 80% recovery |
| 7 | Shimabukuro, T. | 19(updated) | 100 | 42.3 | allergy or allergic reactions (84%), prior anaphylaxis (26%) | 1 & 2 | Respiratory and airway obstruction symptoms, nausea |
Generalized urticaria, Diffuse erythematous rash Facial, tongue, or laryngeal angioedema |
16.8 min | Generalized | NM | Epi (92%), endotracheal intubation (11%), Corticosteroids (86%), Antihistamines (72%) | 92% recovery |
| 5. Non‐anaphylaxis allergic reaction (n = 126) | |||||||||||||
| With BNT162b2 | |||||||||||||
| 6 | Shimabukuro, T. | 83 | 90 | 43 | allergies or allergic reactions (67%) | 1 | mild respiratory symptoms | Rash, pruritus, itchy and scratchy sensations in the throat | 12 min | Generalized, throat | NM | NM | NM |
| With mRNA‐1273 | |||||||||||||
| 8 | Shimabukuro, T. | 43 | 91 | 43 | allergies or allergic reactions (67%) | 1 | sensations of throat closure | Rash, pruritus, itchy sensations in the mouth and throat | 15 min | Generalized, mouth, throat | NM | NM | NM |
| 6. Herpes zoster (n = 20) | |||||||||||||
| 6 with BNT162b2 and 14 with mRNA‐1273 | |||||||||||||
| 9 | Lee, C. | 20 | 50 | 55.9 | Psoriasis, Atopic Eczema, Melanoma, Shingles, Chickenpox Actinic keratosis, BCC, SCC, previous vaccination with Zostavax and Shingrix, rubella, measles | NM | Deep painat injection site and body aches | Unilateral dermatomal herpetiform skin eruption, injection site itchiness |
6.85 d (itching, burning), 10.2 d (herpetiform eruption) |
Generalized | NM | Valacyclovir, gabapentin, LMX, terrasil, shingles cream,prednisone,mupirocin,tramadol,hydrocodone/acetaminophen,acyclovir, desonide cream | NM |
| 7. Immune thrombocytopenic purpura (ITP) (n = 20) | |||||||||||||
| 9 with BNT162b2, 11 with mRNA‐1273, 1 unknown | |||||||||||||
| 10 | Lee, E. J. | 20 | 58 (of 19 reports) | 44.4(of 19 reports) | ITP in remission (2%), mild–moderate thrombocytopenia (10%), inherited thrombocytopenia (2%), autoimmune conditions (hypothyroidism, Crohn's disease, or anti‐tg Ab +) (15%) | 1 & 2 | Thrombocytopenia | Petechiae, bruising or mucosal bleeding (gingival, vaginal, epistaxis) | 8.7 d | Generalized | NM | Corticosteroids (70%), IVIG (60%), PLT transfusions (40%), rituximab (10%), romiplostim, vincristine, aminocaproic acid | 88% improvement, 6% death, 6% no improvement |
Abbreviations: 1st, after the first dose; 2nd, after the second dose; anti‐tg Ab, anti‐thyroglobulin antibodies; BCC, basal cell carcinoma; d, days; DEJ, dermo‐epidermal junction; Epi, epinephrine injection; HF, heart failure; HTN, hypertension; ITP, immune thrombocytopenic purpura; IVIG, intravenous immunoglobulin; KF, kidney failure; NM, not mentioned; PLT, platelet; SCC, squamous cell carcinoma.
Supporting information Table S2.
Injection site reactions, including erythema, wheals (some burning, itchy, painful, with axillary lymphadenopathy), swelling, painful and itchy erythematous subcutaneous nodule, painful hardening and itching were present among 17 patients (5.4%)(100% BNT162b2), appearing after an average of 1.7 days (for whom a day of onset was reported).
-
2.
Generalized reaction (n = 33, 10.5%) (100% BNT162b2)
Other nonspecific cutaneous reactions were reported in 33 patients (10.5%). They included generalized urticaria, morbilliform rash and widespread erythematous lesions, appearing after an average of almost 4 days (for whom a day of onset was reported).
-
3.
Delayed localized hypersensitivity reactions (n = 32, 10.2%) (12.5% BNT162b2 and 87.5% mRNA‐1273)
Delayed reactions were reported in 32 patients (10.2%) occurring almost a week after injection. From these, 12 patients developed Delayed large T‐cell mediated hypersensitivity presented with rash and pruritis in extremities which persisted for 6 days and left hyperpigmentation in 17% of the patients. A total of 16 patients showed delayed localized hypersensitivity reactions at injection site, characterized with painful erythematous lesions which disappeared within 8.2 days after the 1st dose and in 3 days after the 2nd dose. Four patients developed bullous drug‐induced reactions with severe pruritus. Delayed reactions were managed with topical steroids, oral antihistamines and oral antibiotics.
-
4.
Anaphylaxis (n = 66, 21%) (71% BNT162b2 and 29% mRNA‐1273)
Three articles by Shimabukuro, et al. report anaphylaxis after mRNA vaccines in different time frames. 50 , 51 , 52 The patients in the preceding reports are only counted once, together with the ones reported in the updated article. Anaphylaxis was reported in 66 patients (21%), developed an average of about 13 min (10–16.8 min) after the injections; presenting with respiratory distress, wheezing and nausea, along with generalized urticaria, diffuse erythematous rash and angioedema (facial, tongue, or laryngeal), and they were treated with epinephrine (92%), endotracheal intubation (11%), corticosteroids (86%) and antihistamines (72%). History of prior allergy or allergic reactions was present in 80% of patients, and 30% had a history of prior anaphylaxis. Either vaccine was associated with anaphylaxis and no deaths from anaphylaxis were reported.
-
5.
Non‐anaphylaxis allergic reaction (n = 126, 40.1%) (65.9% BNT162b2 and 34.1% mRNA‐1273)
A total of 126 patients developed rash, pruritus, itchy and scratchy sensations in the throat and mild respiratory symptoms onset 12–15 min, indicative of an allergic reaction, but not to the degree of anaphylaxis. 67% of these people had prior history of allergies or allergic reactions.
-
6.
Herpes zoster (n = 20, 5.8%) (30% BNT162b2 and 70% mRNA‐1273)
A total of 20 patients (5.8%) developed Herpes Zoster after 10 days of injection, which were treated with antivirals, local or systemic corticosteroids and analgesics.
-
7.
Immune thrombocytopenic purpura (ITP) (n = 20, 5.8%) (45% BNT162b2 and 55% mRNA‐1273)
A total of 20 patients (5.8%) developed Idiopathic thrombocytopenic purpura (ITP) presented with diffuse petechia and bruising, 8.7 days after injection which were managed with corticosteroids, platelet transfusions, and rituximab so that 88% had improvements and 6% were deceased.
3.4. RCTs
We included a total of 41 RCTs as depicted in Table 3, having enrolled a cumulative number of 160,464 participants who received vaccines, with a cumulative mean age of 38.28 years (weighted average age from 40 studies), 51.53% of them being women.
TABLE 3.
Mucocutaneous reaction after COVID‐19 vaccination reported in “RCTs” studies
| Supplemental references a | First author | Phase of RCT | Number of participants (n) | Women percentage | Mean of Age (year) | Participants' comorbidity and (age group in study) | Vaccine dose | Days between doses | Dose number | Any symptoms after vaccine | Percentage of mucocutaneous reaction | Time of onset the reactions (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. mRNA vaccines | ||||||||||||
| 1.1. BNT162b2 (n = 39,144) | ||||||||||||
| 1 | Walsh, E. E. | Phase 1 | 36 | 39 | 36.7 | NM (18–55 years) | 10, 20, and 30 μg | 21 | 1 | Injection site pain, fever, fatigue, chills, small numbers of severe systemic events (fatigue, headache, chills, muscle pain, and joint pain) |
Injection site redness: (20 μg:0%, 30 μg:8%) Injection site swelling (10 μg:17%) |
Peak at d2 |
| 2 | Sahin, U. | Phase 1/2 (extended) | 12 | 66.7 | 34.8 | NM | 10 μg | 21 | 1&2 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea | Injection site: swelling (8%) |
0–7, 22–28 |
| 2 | Sahin, U. | Phase 1/2 (extended) | 12 | 33.3 | 46.7 | NM | 30 μg | 21 | 1&2 | Injection site pain, chills, headache, fatigue, muscle pain, joint pain, diarrhea | Injection site: swelling (58.3%),redness(8%) |
0–7, 22–28 |
| 3 | Polack | Phase 2/3 | 18,860 | 48.9 | 51.2 | Obesity (34.8%) | 30 μg | 21 | 1 | Injection site pain, fever, fatigue, headache, chills, vomiting, diarrhea, muscle pain, jointpain, severe systemic events (<2%) | Injection site: redness (16‐55 years:5%, ≥55 years: 5%), swelling (16‐55 years:6%, ≥55 years: 7%, lymphadenopathy (0.3%), | NM |
| 3 | Polack | Phase 2/3 | 18,556 | 48.9 | 51.2 | Obesity (34.8%) | 30 μg | 21 | 2 | Injection site pain, fever, fatigue, headache, chills, vomiting, diarrhea, muscle pain, jointpain, severe systemic events: <2% except for fatigue (3.8%) and headache (2.0%) | Injection site: redness (16‐55 years:6%, ≥55 years: 7%), swelling (16‐55 years:6%, ≥55 years: 7%) | NM |
| 4 | Frenck, R. W., Jr. | Phase 3 | 1131 | 49.9 | 13.6 | Baseline COVID +(4.1%) (12–15 years) | 30 μg | 21 | 1&2 | Injection site pain, fatigue, headache, chills, vomiting, diarrhea, muscle pain, joint pain,fever | Injection site: redness(1st:6%, 2nd:5%), swelling(1st:7%, 2nd:5%) | 0–7 |
| 4 | Frenck, R. W., Jr. | Phase 3 | 537 | 52.5 | 19.4 | Baseline COVID +(5.6%) (16–25 years) | 30 μg | 21 | 1&2 | Injection site pain, fatigue, headache, chills, vomiting, diarrhea, muscle pain, joint pain,fever | Injection site: redness(1st:6%, 2nd:6%), swelling(1st:8%, 2nd:7%) | 0–7 |
| 1.2. BNT162b2 booster in ChAdOx1‐S‐primed participants (CombiVacS) (n = 450) | ||||||||||||
| 5 | Borobia, A. M. | Phase 2 | 450 | 57 | 43.93 | Hypothyroidism, Allergies, Asthma, HTN, Hypercholesterolaemia, Psychiatric, Migraine, Insomnia, Arthralgia, Back pain, Drug hypersensitivity, baseline COVID + (0%) |
30 μg |
50–84 | 2 | Injection site pain and discomfort, malaise, pyrexia, headache, myalgia, arthralgia, chills, cough, nausea | Injection site: erythema(31%), pruritus(10.9%), urticaria(15%), hardness(35.5%), pustule(0.2%), general pruritus (2%), rash (1.3%) (among 448 subjects) | 0–7 |
| 1.3. BNT162b1 (n = 324) | ||||||||||||
| 6 | Li, J. | Phase 1 | 24 | 50 | 37.9 | Cardiac Ischemia,Sinus Bradycardia,Hyperuricemia, Nasopharyngitis, Increasedblood uric acid,HTN (18–55 years) | 10 μg | 21 | 1&2 | Injection site pain, fever, headache, fatigue, malaise, joint pain, muscle pain, chills, nausea, anorexia, diarrhea, | Injection site: redness (1st:12.5%, 2nd:16.7%), swelling(1st: 12.5%, 2nd:8.3%) | 0–7 |
| 6 | Li, J. | Phase 1 | 24 | 50 | 70.5 | Hyperuricemia, HTN, DM (65–85 years) | 10 μg | 21 | 1&2 | Injection site pain, fever, headache, fatigue, malaise, chills | Injection site: redness (1st:12.5%) | 0–7 |
| 6 | Li, J. | Phase 1 | 24 | 50 | 39.7 | Sinus Bradycardia, Hyperuricemia, Blood uric acid increased (18–55 years) | 30 μg | 21 | 1&2 | Injection site pain, fever, headache, fatigue, malaise, joint pain, muscle pain, chills, nausea, anorexia, diarrhea, vomiting | Injection site: redness (1st:25% 2nd:20.9%), swelling(1st:20.8%, 2nd:16.7%), induration(1st:8.3%, 2nd:4.2%) | 0–7 |
| 6 | Li, J. | Phase 1 | 24 | 50 | 68.5 | Hyperuricemia, HTN, DM (65–85 years) | 30 μg | 21 | 1&2 | Injection site pain, fever, headache, fatigue, malaise, joint pain, muscle pain, chills, anorexia | Injection site: redness (1st: 16.6%),swelling(2nd: 21.7%), induration(1st: 4.2%) | 0–7 |
| 1 | Walsh, E. E. | Phase 1 | 36 | 39 | 36.7 | NM (18–55 years) | 10 μg, 20 μg, 30 μg | 21 | 1 | Injection site pain, fever, fatigue, chills |
Injection site redness: (30 μg:17%) Injection site swelling: (10 μg:25%, 20 μg:17%) |
Peak at d2 |
| 1 | Walsh, E. E. | Phase 1 | 36 | 39 | 36.7 | NM (18–55 years) | 10 μg, 20 μg, 30 μg | 21 | 2 |
Injection site pain, fever, fatigue, chills *dose‐dependent (greater after the 2nd dose than after the 1st dose, both systemic and local reactions) |
Injection site redness: (30 μg:17%) Injection site swelling: (20 μg:8%, 30 μg:25%) |
Peak at d2 |
| 1 | Walsh, E. E. | Phase 1 | 36 | 67 | 70.1 | NM (65–85 years) | 10 μg, 20 μg, 30 μg | 21 | 1 | Injection site pain, fever, fatigue, chills (systemic events milder than in the younger participants) | Injection site swelling: (10 μg:8%, 20 μg:8%, 30 μg:17%) | Peak at d2 |
| 1 | Walsh, E. E. | Phase 1 | 36 | 67 | 70.1 | NM (65–85 years) | 10 μg, 20 μg, 30 μg | 21 | 2 | Injection site pain, fever (33% after the 2nd dose, 1 severe), fatigue, chills |
Injection site redness: (20 μg:8%, 30 μg:8%) Injection site swelling: (10 μg:8%, 20 μg:17%, 30 μg:25%) |
Peak at d2 |
| 7 | Sahin, U. | Phase 1/2 | 12 | 58.3 | 38.21 | NM | 1 μg | 21 | 1&2 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea | Injection site: swelling(17%) |
0–7, 22–28 |
| 7 | Sahin, U. | Phase 1/2 | 12 | 66.7 | 43.62 | NM | 10 μg | 21 | 1&2 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea | Injection site: swelling(4%) |
0–7, 22–28 |
| 7 | Sahin, U. | Phase 1/2 | 12 | 33.3 | 35.74 | NM | 30 μg | 21 | 1&2 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea | Injection site: redness(54.1%), swelling(41.6%) |
0–7, 22–28 |
| 7 | Sahin, U. | Phase 1/2 | 12 | 50 | 33.88 | NM | 50 μg | 21 | 1&2 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea, vomiting | Injection site: redness(62.5%), swelling(50%) |
0–7, 22–28 |
| 7 | Sahin, U. | Phase 1/2 | 12 | 41.7 | 35.81 | NM | 60 μg | 21 | 1 | Injection site pain, fever, chills, headache, fatigue, muscle pain, joint pain, diarrhea, vomiting | Injection site: swelling(33%) |
0–7, 22–28 |
| 8 | Mulligan, M. J. | Phase 1&2 | 12 | 50 | 35.8 | NM | 30 μg | 21 | 1&2 |
Injection site pain, fever, fatigue, headache, chills, diarrhea, muscle pain, joint pain Severe adverse events: (8.3%) |
Injection site redness:(both doses: <20%) Injection site swelling: (1st:<20%, 2nd:<30%) |
0–7, Peak at d2 |
| 8 | Mulligan, M. J. | Phase 1&2 | 12 | 58.3 | 38.3 | NM | 100 μg | 21 | 1 |
Injection site pain, fever, fatigue, headache, chills, diarrhea, muscle pain, joint pain Severe adverse events: (8.3%) |
Injection site redness(<40%), Injection site swelling(<50%) |
0–7, Peak atd2 |
| 1.4. mRNA‐1273 (n = 30,359) | ||||||||||||
| 9 | Anderson, E. J. | Phase 1 | 10 | 70 | 65.8 | NM (56–70 years) | 25 μg | 28 | 1&2 | Injection site pain, fever, headache, fatigue, arthralgia, myalgia, chills, nausea, | Injection site erythema/redness(2nd ≤ 10%), swelling/induration(1st ≤ 10% and 2nd ≤ 20%) | 0–1 |
| 9 | Anderson, E. J. | Phase 1 | 10 | 20 | 72.8 | NM (≥71 years) | 25 μg | 28 | 1&2 | Injection site pain, headache, fatigue, arthralgia, myalgia | Injection site erythema/redness (2nd ≤ 20%), swelling/induration(2nd ≤ 20%), skin and subcutaneous tissue disorders (collective n = 3 in age group) | 0–1 |
| 9 | Anderson, E. J. | Phase 1 | 10 | 50 | 63.8 | NM (56–70 years) | 100 μg | 28 | 1&2 | Injection site pain, fever, headache, fatigue, arthralgia, myalgia, chills, nausea, | Injection site erythema/redness(2nd ≤ 10%), swelling/induration(2nd ≤ 20%) | 0–1 |
| 9 | Anderson, E. J. | Phase 1 | 10 | 70 | 72.6 | NM (≥71 years) | 100 μg | 28 | 1&2 | Injection site pain, fever, headache, fatigue, arthralgia, myalgia, chills, nausea, | Injection site erythema/redness(2nd ≤ 20%), swelling/induration(2nd ≤ 30%), skin and subcutaneous tissue disorders(collective n = 3 in age group) | 0–1 |
| 10 | Jackson, L. A. | Phase 1 | 15 | 51 | 33 | NM | 25 μg | 28 | 1 | Injection site pain, fatigue, headache, myalgia, nausea | Transient urticaria on both legs in one participant | 5 |
| 10 | Jackson, L. A. | Phase 1 | 15 | 51 | 33 | NM | 100 μg | 28 | 1 | Injection site pain, arthralgia, fatigue, chills, headache, myalgia, | Erythema/redness (13.4%), induration/swelling (13.3%) | NM |
| 10 | Jackson, L. A. | Phase 1 | 15 | 51 | 33 | NM | 100 μg | 28 | 2 | Injection site pain, fever, nausea, arthralgia, fatigue, chills, headache, myalgia | Erythema/redness (13.4%), induration/swelling (6.7%) | NM |
| 10 | Jackson, L. A. | Phase 1 | 15 | 51 | 33 | NM | 250 μg | 28 | 1 | Injection site pain, arthralgia, fatigue, chills, headache, myalgia, nausea | Erythema/redness (6.7%), induration/swelling (13.4%) | NM |
| 10 | Jackson, L. A. | Phase 1 | 14 | 51 | 33 | NM | 250 μg | 28 | 2 | Injection site pain, fever, arthralgia, fatigue, chills, headache, myalgia, nausea | Erythema/redness (21.4%), induration/swelling (21.4%) | NM |
| 11 | Chu, L. | Phase 2 | 100 | 64 | 36.6 | NM (≥18 ‐ < 55 years) | 50 μg | 28 | 1&2 | Injection site pain,headache,fatigue,myalgia,arthralgia,nausea/vomiting,chills | Local: erythema(1st:2%, 2nd:5%), swelling(1st:5%, 2nd:6%), axillary swelling /tenderness(1st:7%, 2nd:7%), generalized rash(1st:4%, 2nd:7%) | 0–7 |
| 11 | Chu, L. | Phase 2 | 100 | 73 | 64.6 | NM (≥55 years) | 50 μg | 28 | 1&2 | Injection site pain,headache,fatigue,myalgia,arthralgia,nausea/vomiting,chills | Local: erythema (1st:3%, 2nd:5%), swelling(1st:3%, 2nd:6%), axillary swelling /tenderness(1st:3%, 2nd:12%), generalized rash(1st:2%, 2nd:3%) | 0–7 |
| 11 | Chu, L. | Phase 2 | 100 | 53 | 38.3 | NM (≥18 ‐ < 55 years) | 100 μg | 28 | 1&2 | Injection site pain,headache,fatigue,myalgia,arthralgia,nausea/vomiting,chills | Local: erythema (1st:3%, 2nd:8%), swelling(1st:5%, 2nd:11%), axillary swelling /tenderness(1st:15%, 2nd:10%), generalized rash(1st:4%, 2nd:4%) | 0–7 |
| 11 | Chu, L. | Phase 2 | 100 | 71 | 63.9 | NM (≥55 years) | 100 μg | 28 | 1&2 | Injection site pain, fever, headache, fatigue, myalgia, arthralgia, nausea/vomiting, chills | Local: erythema(1st:2%, 2nd:7%), swelling(1st:3%, 2nd:10%), axillary swelling /tenderness(1st:3%, 2nd:10%), generalized rash(1st:1%, 2nd:2%) | 0–7 |
| 12 | Baden, L. R. | Phase 3 | 15,168 | 47.8 | 51.4 | CLD, Cardiac disease, Obesity, DM, Liver disease, HIV | 100 μg | 28 | 1 | Injection site pain, fever, headache, fatigue, myalgia, arthralgia, nausea or vomiting, chills, serious adverse events: (<0.1%) | Erythema (2.8%), swelling (6.1%), lymphadenopathy (10.2%), hypersensitivity (1.1%), urticaria (0.2%), rash (0.2%), contact dermatitis (0.2%) | 2.6 |
| 12 | Baden, L. R. | Phase 3 | 14,677 | 47.8 | 51.4 | CLD, Cardiac disease, Obesity, DM, Liver disease, HIV | 100 μg | 28 | 2 | Injection site pain, fever, headache, fatigue, myalgia, arthralgia, nausea or vomiting, chills, serious adverse events: (<0.1%) | Erythema (8.6%), swelling (12.2%), lymphadenopathy(14.2%), hypersensitivity (1.1%), urticaria (0.2%), rash (0.2%), contact dermatitis (0.2%) | 3.2 |
| 2. Adenovirus viral vector vaccines | ||||||||||||
|
2.1. ChAdOx1 nCoV‐19 (n = 13,995) (SD: 3.5–6.5 [mostly 5] × 1010 virus particles, LD: 2.2 × 1010 viral particles) | ||||||||||||
| 13 | Barrett, J. R. | Phase 1/2 | 20 | 70 | 36 | NM | SD/SD | 56 | 1&2 | Injection site pain, chills, fatigue, fever, feverish, headache, joint pain, malaise, muscle ache, nausea | Itch(1st& 2nd), tenderness(1st&2nd), warmth(1st& 2nd) | 0–7 |
| 13 | Barrett, J. R. | Phase 1/2 | 32 | 52 | 44 | NM | SD/LD | 56 | 1&2 | Injection site pain, chills, fatigue, fever, feverish, headache, joint pain, malaise, muscle ache, nausea | Induration(1st), itch(1st& 2nd), redness(1st& 2nd), swelling(2nd), tenderness(1st& 2nd), warmth(1st& 2nd) | 0–7 |
| 14 | Folegatti, P. M. | Phase 1/2 | 487 | 49 | 34 | NM | SD | single | 1 | Injection site pain, feverishness,fever, chills,joint pain,muscle ache,fatigue,headache,malaise,nausea | Local: redness(3%), warmth(25%), itch(7%), swelling(4%), induration(3%), tenderness(83%) | 0‐7 peak at d1 |
| 14 | Folegatti, P. M. | Phase 1/2 | 56 | 49 | 34 | NM | SD + prophylactic paracetamol | single | 1 | Injection site pain, feverishness,fever, chills,joint pain,muscle ache,fatigue,headache,malaise,nausea | Local: redness(2%), warmth (20%), itch(12%), swelling(2%), tenderness(77%) | 0–7 peak at d1 |
| 14 | Folegatti, P. M. | Phase 1/2 | 10 | 49 | 34 | NM | SD | single | 1&2 | Injection site pain, feverishness,fever, chills,joint pain,muscle ache,fatigue,headache,malaise,nausea | Local: warmth(20%), itch(10%), tenderness(50%) (after both doses) | 0–7 peak at d1 |
| 15 | Madhi, S. A. | Phase 1b–2 | 1011 | 43.2 | 31 | Obesity(18.8%), current smoker (42.9%), HTN (3.1%), Chronic respiratory condition (3.5%), DM (0.4%) | SD | 21–35 | 1&2 | Cough, feverish, headache, joint pain, muscle pain, sweating, tenderness, weakness | Injection site: redness, swelling, bruising, hardness,mild itching, epidermal and dermal conditions(0.9%), skin appendage conditions(0.5%), angioedema and urticaria(0.2%), dental and gingival conditions(0.2%), oral soft tissue conditions(0.4%), allergic conditions(0.2%) | 0–6 |
| 16 | Frater, J., | Phase 2/3 | 54 | 0 | 42.5 | HIV + | NM | 28–56 | 1&2 | Injection site pain,feverish, fever, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Local: warmth(1st:11.3%, 2nd:5.9%), itch(1st:3.8%, 2nd:2%), swelling(1st:1.9%), induration(1st:1.9%), tenderness(1st:62.3%, 2nd:43.1%) | 0–7 |
| 16 | Frater, J., | Phase 2/3 | 50 | 48 | 38.5 | HIV ‐ | NM | 28–56 | 1&2 | Injection site pain,feverish, fever, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Local: redness(2nd:2.0%), warmth(1st:16%, 2nd:12.2%), itch(1st:4.0%, 2nd:12.2%), tenderness(1st:76.0%, 2nd:61.2%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 50 | 70 | 44.5 | NM (18–55 years) | SD/SD | 28 | 1&2 | Injection site pain,tenderness, feverish, fever, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: redness(2nd:2%), warmth(1st:14%, 2nd:12%), itch(1st:4%, 2nd:12%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 30 | 33 | 60.4 | NM (56–69 years) | SD/SD | 28 | 1&2 | Injection site pain,tenderness, feverish, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: warmth(1st:7%, 2nd:14%), itch(1st:7%, 2nd:3%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 46 | 35 | 73 | NM (≥70 years) | SD/SD | 28 | 1&2 | Injection site pain,tenderness, feverish, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: redness(1st:2%, 2nd:2%), warmth(1st:14%, 2nd:4%), itch(1st:4%, 2nd:2%), swelling(1st:4%, 2nd:4%), induration(1st:2%, 2nd:2%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 49 | 47 | 39 | NM (18–55 years) | LD/LD | 28 | 1&2 | Injection site pain,tenderness, feverish, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: redness(2nd:2%), warmth(1st:18%, 2nd:6%), itch(1st:4%, 2nd:2%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 30 | 53 | 59.5 | NM (56–69 years) | LD/LD | 28 | 1&2 | Injection site pain,tenderness, feverish, fever, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: warmth(1st:3%), itch(1st: 7%) | 0–7 |
| 17 | Ramasamy, M. N. | Phase 2/3 | 49 | 43 | 73 | NM (≥70 years) | LD/LD | 28 | 1&2 | Injection site pain,tenderness, feverish, fever, chills, joint pain, muscle ache, fatigue, headache, malaise, nausea | Injection site: redness(1st:2%, 2nd:4%), warmth(1st:2%, 2nd:2%), itch(1st:2%), swelling(1st:2%, 2nd:4%), induration(1st:2%, 2nd:4%) | 0–7 |
| 18 | Voysey, M. | Different phases (1,2,3) | 12,021 | 56 | NM | CVD, Respiratory disease, DM, some Baseline COVID + (18–55 years, 56–69 years, 70+ years) | SD/SD or LD/SD | 28 | 1&2 | Cardiac, sensory, GI, general and injection site, procedural complications, infections and infestations, neoplasms, nervous system, renal and urinary, musculoskeletal and connective tissue, reproductive, respiratory side effects | Cellulitis1 (<0.1%),anaphylactic reaction1 (<0.1%),psoriasis 1 (<0.1%), rosacea 1 (<0.1%), vitiligo 1 (<0.1%), Raynaud's phenomenon 1 (<0.1%), uveitis 2 (<0.1%) | any time during study |
| 2.2.Gam‐COVID‐Vac (n = 15,011) (all 1 × 1011 viral particles) | ||||||||||||
| 19 | Logunov, D. Y. | Phase 1/2 | 9 | 78 | 27 | NM | LyophilisedrAd5‐S | 21 | 1&2 | Injection site pain, hyperthermia,headache,muscle and joint pain,changes in laboratory variables | Local: edema(11%), hyperthermia(11%),mucosal abnormality | 0–28 |
| 19 | Logunov, D. Y. | Phase 1/2 | 9 | 0 | 27.8 | NM | rAd26‐S | 21 | 1&2 | Injection site pain, hyperthermia,headache,asthenia,muscle and joint pain,heartbeat (subjective palpitation),diarrhea,loss of appetite,changes in laboratory variables | Local: itch(11%), generalized: hives(11%) | 0–28 |
| 19 | Logunov, D. Y. | Phase 1/2 | 9 | 44 | 31.4 | NM | LyophilisedrAd26‐S | 21 | 1&2 | Injection site pain, hyperthermia,headache,muscle and joint pain,changes in laboratory variables | Local: edema(22%) | 0–28 |
| 19 | Logunov, D. Y. | Phase 1/2 | 20 | 30 | 26.4 | NM |
rAd26‐S plus rAd5‐S (heterologous prime‐boost) |
21 | 1&2 | Injection site pain, hyperthermia,headache,asthenia,muscle and joint pain,diarrhea,rhinorrhea,loss of appetite,pain in the oropharynx, malaise,sore throat, nasal congestion, cough, sneezing, changes in laboratory variables | Local: hyperthermia(10%), swelling(5%) | 0–28 |
| 20 | Logunov, D. Y. | Phase 3 | 14,964 | 38.9 | 45.3 | DM, HTN, IHD, Obesity |
rAd26‐S plus rAd5‐S (heterologous prime‐boost) |
21 | 1&2 | Vascular disorders, infections and invasions, reproductive, heart disorders, injury, intoxication and complications of procedures, GI, renal, liver and biliary tract, muscle, skeletal and connective tissue and nervous system side effects | Hypersensitivity (0.01%), acne‐form dermatitis (0.01%), allergic skin reaction(0.01%), allergic rash(0.05%), alopecia(0.02%), itching (0.04%), skin rash (0.1%), petechial rash(0.01%), rash(0.03%), eczema (0.01%), keratoconjunctivitis(0.01%),chalazion (0.01%), allergic reaction (0.15%), herpes (0.09%), benign neoplasm of the eyelid (0.01%), axillary lymphadenitis (0.07%) | any time during study |
| 2.3. Ad5‐nCoV (n = 490) (LD: 5 × 1010 viral particles, MD: 1 × 1011 viral particles, HD: 1.5 × 1011 viral particles) | ||||||||||||
| 21 | Zhu, F. C. | Phase 1 | 36 of 108 | 49 | 36.3 | Baseline COVID + (0%) | LD | single | 1 | Injection site pain, fever, headache, fatigue, vomiting, diarrhea, muscle pain, joint pain, throat pain, cough, nausea, impaired appetite, functional gi disorder,dizziness | Injection site: induration(6%), redness(6%), swelling(11%), itch (6%), general pruritis: (3%) | 0–7 |
| 21 | Zhu, F. C. | Phase 1 | 36 of 108 | 49 | 36.3 | Baseline COVID + (0%) | MD | single | 1 | Injection site pain, fever, headache, fatigue, diarrhea, muscle pain, joint pain, throat pain, cough, nausea, impaired appetite | Injection site: induration(3%), redness(3%), swelling(11%), itch(8%), general pruritis:(3%) | 0–7 |
| 21 | Zhu, F. C. | Phase 1 | 36 of 108 | 49 | 36.3 | Baseline COVID + (0%) | HD | single | 1 | Injection site pain, fever, headache, fatigue (6% grade 3), vomiting, diarrhea, muscle pain (3% grade 3), joint pain (3% grade 3), muscular weakness, throat pain, cough, nausea, impaired appetite, dyspnoea (3% grade 3), dizziness | Injection site: induration(3%), redness(3%), general pruritis:(3%), mucosal abnormality (3%) | 0–7 |
| 22 | Zhu, F. C. | Phase 2 | 253 | 50 | 40 | Underlying diseases (3%), Baseline COVID + (0%) | MD | single | 1 | Injection site pain, fever, headache, fatigue, vomiting, diarrhea, muscle pain, joint pain, oropharyngeal pain, cough, nausea, dyspnoea, appetite impaired, syncope | Injection site reactions: induration (5%), redness (2%), swelling (4%), itching (6%), general: pruritus (2%) | 0–14 |
| 22 | Zhu, F. C. | Phase 2 | 129 | 50 | 39.7 | Underlying diseases (6%), Baseline COVID + (0%) | LD | single | 1 | Injection site pain, fever, headache, fatigue, vomiting, diarrhea, muscle pain, joint pain, oropharyngeal pain, cough, nausea, dyspnoea, appetite impaired, syncope | Injection site reactions: induration (2%), redness (1%), swelling (4%), itching (2%), general: pruritus (3%) mucosal abnormality | 0–14 |
|
2.4. Ad26.COV2.S (n = 22,537) (LD: 5 × 1010 viral particles, HD: 1 × 1011 viral particles) | ||||||||||||
| 23 | Sadoff, J., | Phase 1–2a | 162 | 52 | 36.1 | NM 18–55 years | LD | 56 | 1&2 | Injection site pain, fatigue, headache, myalgia, nausea, pyrexia | Local: erythema (<5%), swelling(<5%) | 0–28 |
| 23 | Sadoff, J., | Phase 1–2a | 158 | 54 | 34.8 | NM 18–55 years | HD | 56 | 1&2 | Injection site pain, fatigue, headache, myalgia, nausea, pyrexia | Local: erythema(<5%), swelling(<5%) | 0–28 |
| 23 | Sadoff, J., | Phase 1–2a | 161 | 48 | 69.6 | NM ≥ 65 years | LD | 56 | 1&2 | Injection site pain, fatigue, headache, myalgia, nausea, pyrexia | Local: erythema(<10%), swelling(<10%) | 0–28 |
| 23 | Sadoff, J., | Phase 1–2a | 161 | 51 | 70 | NM ≥ 65 years | HD | 56 | 1&2 | Injection site pain, fatigue, headache, myalgia, nausea, pyrexia | Local: erythema(<10%), swelling(<10%) | 0–28 |
| 24 | Sadoff, J. | Phase 3 | 21,895 | 44.9 | 52 | Baseline COVID + (9.8%), Obesity (28.6%) | LD | 56 | 1 | Injection site pain, headache, fatigue, myalgia, nausea, fever | Local: erythema (0.23%), swelling(0.2%), urticaria (n = 8), hypersensitivity(n = 9) | 0–7 |
| 3. Protein subunit vaccines | ||||||||||||
| 3.1. NVX‐CoV2373 (n = 1060) (Ad: Adjuvant 50 μg Matrix‐M1) | ||||||||||||
| 25 | Shinde, V. | Phase 1 | 334 | 43.1 (all 2199) | 31.9 (all 2199) | HTN, DM, HIV, Baseline COVID + (0%) | 5 μg + Ad | 21 | 1 | Injection site pain and tenderness, fever, headache, fatigue, malaise, joint pain, muscle pain,nausea or vomiting | Injection site: erythema(1.5%), swelling(1.2%) | 0–6 |
| 25 | Shinde, V. | Phase 1 | 329 | 43.1 (all 2199) | 31.9 (all 2199) | HTN, DM, HIV, Baseline COVID + (0%) | 5 μg + Ad | 21 | 2 | Injection site pain and tenderness, fever, headache, fatigue, malaise, joint pain, muscle pain,nausea or vomiting | Injection site: erythema(1.8%), swelling(2.7%) | 21–27 |
| 25 | Shinde, V. | Phase 1 | 150 | 43.1 (all 2199) | 31.9 (all 2199) | HTN, DM, HIV, Baseline COVID +(100%) | 5 μg + Ad | 21 | 1 | Injection site pain and tenderness, fever, headache, fatigue, malaise, joint pain, muscle pain,nausea or vomiting | Injection site: erythema(1.3%), swelling(4%) | 0–6 |
| 25 | Shinde, V. | Phase 1 | 142 | 43.1 (all 2199) | 31.9 (all 2199) | HTN, DM, HIV, Baseline COVID +(100%) | 5 μg + Ad | 21 | 2 | Injection site pain and tenderness, fever, headache, fatigue, malaise, joint pain, muscle pain,nausea or vomiting | Injection site: swelling(0.7%) | 21–27 |
| 26 | Keech, C. | Phase 1/2 | 25 | 52 | 27.2 | NM | 25 μg, Un‐adjuvanted | 21 | 1&2 | Injection site pain, arthralgia,fatigue,malaise,headache,myalgia,nausea or vomiting | Local: erythema or redness(2nd:4%), induration or swelling(2nd:4%) | 0–7 |
| 26 | Keech, C. | Phase 1/2 | 26 | 50 | 29.5 | NM | 5 μg + Ad | 21 | 1&2 | Injection site pain, arthralgia,fatigue,malaise,headache,myalgia,nausea or vomiting | Local: erythema or redness(2nd:7.6%), induration or swelling(2nd:3.8%),skin and subcutaneous tissue disorders(3.8%) | 0–7 |
| 26 | Keech, C. | Phase 1/2 | 3 | 33.3 | 23.7 | NM | 5 μg + Ad | 21 | 1&2 | Injection site pain, fatigue,malaise,headache,myalgia | Local: erythema or redness(1st:33.3%,2nd:33.3%) | 0–7 |
| 26 | Keech, C. | Phase 1/2 | 25 | 32 | 35.6 | NM | 25 μg + Ad | 21 | 1&2 | Injection site pain, arthralgia,fatigue,malaise,fever,headache,myalgia,nausea or vomiting | Local: induration or swelling(2nd:8.3%)skin and subcutaneous tissue disorders(4%), rash(4%) | 0–7 |
| 26 | Keech, C. | Phase 1/2 | 26 | 65.4 | 33 | NM |
1st: 25 μg + Ad 2nd: placebo |
21 | 1&2 | Injection site pain, arthralgia,fatigue,malaise,headache,myalgia,nausea or vomiting | Local: erythema or redness(1st:3.8%,2nd:3.8%)skin and subcutaneous tissue disorders(7.7%) | 0–7 |
| 3.2. MF59‐adjuvanted sclamp (n = 96) | ||||||||||||
| 27 | Chappell, K. J. | Phase 1 | 24 | 54 | 34.1 | NM | 5 μg | 28 | 1&2 | Injection site pain, chills, nausea, vomiting, fever, headache, fatigue/somnolence, myalgia, arthralgia, malaise |
Local: tenderness(1st:50.0%, 2nd:62.5%), induration/swelling(1st:8.3%); Alopecia (4.2%), pruritis (4.2%) |
0–7 |
| 27 | Chappell, K. J. | Phase 1 | 24 | 54 | 31 | NM | 15 μg | 28 | 1&2 | Injection site pain, nausea, vomiting, headache, fatigue/somnolence, diarrhea, myalgia, arthralgia, malaise | Local: tenderness(1st:54.2%, 2nd:58.3%), induration/swelling(1st:8.3%) | 0–7 |
| 27 | Chappell, K. J. | Phase 1 | 1st: n = 48 2nd: n = 24 | 33.5 | 32.3 | NM | 45 μg | 28 | 1&2 | Injection site pain, chills, nausea, vomiting, headache, fatigue/somnolence, diarrhea, myalgia, arthralgia, malaise |
Local: tenderness(1st:47.9%, 2nd:58.3%), erythema/redness (1st:2.1%), induration/swelling(1st:4.2%); Macular rash(4.2%), contact dermatitis(4.2%) |
0–7 |
| 3.3. SCB‐2019 (n = 88) | ||||||||||||
| 28 | Richmond, P. | Phase 1 | 8 | 38 | 35.8 | NM (18–54 years) | 3 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, myalgia, nausea, vomiting, fatigue | Local: erythema (12.5%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 38 | 62.8 | NM (55–75 years) | 3 μg + AS03 | 21 | 1&2 | Injection site pain, headache, fatigue | Local: erythema (6.25%), swelling(6.25%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 100 | 41.9 | NM (18–54 years) | 3 μg + CpG/Alum | 21 | 1&2 | Injection site pain, headache, myalgia, nausea, fatigue | Local: erythema (6.25%), swelling(6.25%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 50 | 37 | NM (18–54 years) | 9 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, myalgia, fatigue | Local: erythema (6.25%), swelling (18.75%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 38 | 59.1 | NM (55–75 years) | 9 μg + AS03 | 21 | 1&2 | Injection site pain fever headache myalgia fatigue | Local: erythema (25%), swelling(18.75%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 50 | 36.1 | NM (18–54 years) | 9 μg + CpG/Alum | 21 | 1&2 | Injection site pain, headache, myalgia, fatigue | Local: erythema (12.5%), swelling(6.25%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 38 | 60.3 | NM (55–75 years) | 9 μg + CpG/Alum | 21 | 1&2 | Injection site pain, fever, headache, myalgia, fatigue | Local: erythema (6.7%), swelling(6.7%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 75 | 31.3 | NM (18–54 years) | 30 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, myalgia, diarrhea, nausea, vomiting, fatigue | Local: erythema (6.25%), swelling(18.75%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 75 | 59.8 | NM (55–75 years) | 30 μg + AS03 | 21 | 1&2 | Injection site pain, headache, myalgia, diarrhea nausea, vomiting | Local: erythema (6.25%), swelling(6.25%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 75 | 39.1 | NM (18–54 years) | 30 μg + CpG/Alum | 21 | 1&2 | Injection site pain, headache, fatigue | Local: erythema (18.75%), swelling(6.25%) | 0–7 |
| 28 | Richmond, P. | Phase 1 | 8 | 50 | 61.5 | NM (55–75 years) | 30 μg + CpG/Alum | 21 | 1&2 | Injection site pain, myalgia, fatigue | Local: swelling(6.25%) | 0–7 |
| 3.4. CoV2 preS dTM (n = 336) (LD: 1.3 μg, HD: 2.6 μg) | ||||||||||||
| 29 | Goepfert, P. A. | Phase 1/2 | 18 | 50 | 35.3 | NM (18–49 years) | LD + AF03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia |
1st: injection site: erythema(6.3%) 2nd: injection site: erythema(12.5%), swelling(18.8%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 54 | 46 | 33.7 | NM (18–49 years) | LD + AS03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia |
1st: injection site:swelling(2%) 2nd: injection site: erythema(19.6%), swelling(15.7%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 28 | 39 | 59.8 | NM (≥50 years) | LD + AS03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia |
1st: injection site: erythema(3.6%) 2nd: injection site: erythema(32.1%),swelling(25%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 17 | 65 | 32.5 | NM (18–49 years) | HD + AF03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia | 2nd: injection site: erythema(17.6%), swelling(17.6%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 10 | 70 | 58.7 | NM (≥50 years) | HD + AF03 | 21 | 1&2 | Injection site pain, headache, malaise, myalgia |
1st: injection site: erythema(11.1%) 2nd: injection site: erythema(11.1%),swelling(11.1%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 54 | 43 | 34.9 | NM (18–49 years) | HD + AS03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia |
1st: injection site: erythema(3.7%),swelling(7.4%) 2nd: injection site: erythema(50%),swelling(33.3%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 31 | 68 | 61.7 | NM (≥50 years) | HD + AS03 | 21 | 1&2 | Injection site pain, fever, headache, malaise, myalgia |
1st: injection site: erythema(6.5%),swelling(3.2%) 2nd: injection site: erythema(32.3%),swelling(25.8%) |
0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 18 | 56 | 34.8 | NM (18–49 years) | HD Un‐adjuvanted | 21 | 1&2 | Injection site pain, headache, malaise, myalgia | 2nd: injection site: erythema(5.9%),swelling(5.9%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 24 | 58 | 36 | NM (18–49 years) | LD + AF03 | single | 1 | Injection site pain, headache, malaise, myalgia | Injection site: erythema(3.8%), swelling(3.8%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 10 | 80 | 59.3 | NM (≥50 years) | LD + AF03 | single | 1 | Injection site pain, headache, malaise, myalgia | Injection site: erythema(10%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 24 | 38 | 35.4 | NM (18–49 years) | LD + AS03 | single | 1 | Injection site pain, headache, malaise, myalgia | Injection site: swelling(7.7%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 24 | 54 | 32 | NM (18–49 years) | HD + AF03 | single | 1 | Injection site pain, headache, malaise, myalgia | Injection site: swelling(4.3%) | 0–7 |
| 29 | Goepfert, P. A. | Phase 1/2 | 24 | 58 | 29.7 | NM (18–49 years) | HD + AS03 | single | 1 | Injection site pain, headache, malaise, myalgia | Injection site: erythema(12.5%), swelling(8.3%) | 0–7 |
| 3.5. ZF2001(n = 640) | ||||||||||||
| 30 | Yang, S. | Phase 1 | 20 | 30 | 31.7 | NM | 25 μg | 30 | 1&2&3 | Injection site pain, fever, headache, cough, muscle pain | Swelling(5%), induration(10%), redness(20%), itch(20%) | 0–7 |
| 30 | Yang, S. | Phase 1 | 20 | 45 | 33.6 | NM | 50 μg | 30 | 1&2&3 | Injection site pain, headache, fatigue, weakness, cough, nausea | Swelling(15%), induration(25%), redness(20%, 5% grade ≥3), rash(5%), itch(35%) | 0–7 |
| 30 | Yang, S. | Phase 2 | 150 | 57 | 43.04 | NM | 25 μg | 30 | 1&2 | Injection site pain, fever, headache, fatigue, cough, nausea, muscle pain | Swelling(4%), induration(3%), redness(8%), rash(2%), itch(6%) | 0–7 |
| 30 | Yang, S. | Phase 2 | 150 | 62 | 44.4 | NM | 50 μg | 30 | 1&2 | Injection site pain, fever, headache, fatigue, cough | Swelling(6%), induration(5%), redness(8%, 1% grade ≥3), rash(3%), itch(9%) | 0–7 |
| 30 | Yang, S. | Phase 2 | 150 | 53 | 42.7 | NM | 25 μg | 30 | 1&2&3 | Injection site pain, fever, headache, cough, muscle pain | Swelling(14%), induration(9%), redness(16%, 1% grade ≥3), rash(1%), itch(19%) | 0–7 |
| 30 | Yang, S. | Phase 2 | 150 | 58 | 43.2 | NM | 50 μg | 30 | 1&2&3 | Injection‐site pain, fever, nausea | Swelling(13%, 2% grade ≥3), induration(7%, 1% grade ≥3), redness(14%, 3% grade ≥3), rash(1%, grade ≥3), itch(17%) | 0–7 |
| 3.6. V‐01 (n = 24) | ||||||||||||
| 31 | Zhang, J. | Phase 1 | 24 | 75 | 40 | NM | 10 μg | 21 | 1&2 | Injection site pain, fever, anorexia, vomiting, nausea, headache, fatigue | Local: pruritus(4.17%) | 0–30 |
| 4. Inactivated virus vaccines | ||||||||||||
| 4.1. CoronaVac (n = 7497) | ||||||||||||
| 32 | Wu, Z. | Phase 1 | 24 | 54 | 65.6 | NM | 3 μg | 28 | 1&2 | Injection site pain, muscle pain, fatigue, nausea, fever, abdominal distention | Injection site pruritus(4.2%), hypersensitivity(4.2%) | 0–28 |
| 32 | Wu, Z. | Phase 1 | 24 | 46 | 67.5 | NM | 6 μg | 28 | 1&2 | Injection site pain, muscle pain, fatigue,headache, decreased appetite, cough | Injection site pruritus(4.2%),mucocutaneous eruption(8.3%), rash(4.2%) | 0–28 |
| 32 | Wu, Z. | Phase 2 | 100 | 51 | 66.8 | NM | 1.5 μg | 28 | 1&2 | Injection site pain,fever, fatigue, diarrhea, cough | Injection site: erythema(2%), pruritus(1%) | 0–28 |
| 32 | Wu, Z. | Phase 2 | 100 | 51 | 66.5 | NM | 3 μg | 28 | 1&2 | Injection site pain, fever, fatigue, diarrhea, cough | Injection site: swelling(1%) | 0–28 |
| 32 | Wu, Z. | Phase 2 | 99 | 55 | 66.2 | NM | 6 μg | 28 | 1&2 | Injection site pain | Injection site: erythema(1%), swelling(1%) | 0–28 |
| 33 | Zhang, Y. | Phase 1 | 24 | 58.3 | 45 | NM | 6 μg | 14 | 1&2 | Injection site pain, fatigue, diarrhea, fever, abdominal pain | Injection site discoloration(4.2%), acute hypersensitivity with manifestation of urticaria (1st dose, one case, 4% of 24), general hypersensitivity(4.2%) | Mostly 0–2 |
| 33 | Zhang, Y. | Phase 2 | 120 | 55 | 42 | NM | 3 μg | 14 | 1&2 | Injection site pain, fatigue, fever, diarrhea, nausea, headache, muscle pain,vomiting, chest pain,dizziness, decreased appetite | Injection site: swelling(1.7%), redness(0.8%)hypoaesthesia(0.8%),pruritus(0.8%),induration(0.8%) | Mostly 0–2 |
| 33 | Zhang, Y. | Phase 2 | 120 | 60 | 42.4 | NM | 6 μg | 14 | 1&2 | Injection site pain, fatigue, fever, diarrhea, nausea, headache, muscle pain,cough,chest pain, drowsiness,palpitations | Injection site: swelling (2.5%), redness (1.7%), hypoaesthesia (0.8%), discoloration (0.8%), pruritus (0.8%), general hypersensitivity (0.8%) | Mostly 0–2 |
| 33 | Zhang, Y. | Phase 2 | 120 | 47.5 | 41.5 | NM | 3 μg | 28 | 1&2 | Injection site pain, fatigue, fever, diarrhea,headache, muscle pain,cough | General hypersensitivity (0.8%) | Mostly 0–2 |
| 33 | Zhang, Y. | Phase 2 | 120 | 47.5 | 40.6 | NM | 6 μg | 28 | 1&2 | Injection site pain, fatigue, fever, diarrhea,headache, muscle pain,decreased appetite | Injection site: swelling (0.8%), redness (0.8%), discoloration (0.8%), pruritus (0.8%) | Mostly 0–2 |
| 34 | Tanriover, M. D. | Phase 3 | 6646 | 42.6 | 45 | HTN, CVD, Chronic respiratory disease, DM, Malignancy, Autoimmune | 3 μg | 14 | 1&2 | Injection site: pain and paraesthesia, fatigue, headache, myalgia, chill, fever, diarrhea, cough, arthralgia, nausea, vomiting, seizure(n = 1),encephalitis(n = 1) | Injection site: erythema(0.18%), swelling(0.06%), induration(0.05%), pruritus(0.03%), generalized: rash(0.11%), allergic reaction(0.08%), pruritis and swelling (<0.0%), redness and swelling in the mouth(n = 1) | 0‐unblinding |
| 4.2. WIBP‐CorV (n = 132) | ||||||||||||
| 35 | Xia, S. | Phase 1 | 24 | 54.2 | 36 | Baseline COVID + (0%) | 2.5 μg | 28 | 1&2&3 | Injection site pain | Local: swelling(4.2%) | 0–28 |
| 35 | Xia, S. | Phase 1 | 24 | 54.2 | 43.1 | Baseline COVID + (0%) | 10 μg | 28 | 1&2&3 | Injection site pain,fever,nausea and vomiting, anorexia | Local: redness(4.2%), swelling(4.2%) | 0–28 |
| 35 | Xia, S. | Phase 2 | 84 | 61.9 | 43.8 | Baseline COVID + (0%) | 5 μg | 21 | 1&2 | Injection site pain, diarrhea, fever, nausea and vomiting | Local: itching(1.2%), swelling(1.2%) | 0–28 |
| 4.3. BBIBP‐CorV (n = 27,367) | ||||||||||||
| 36 | Xia, S. | Phase 1 | 24 | 66 | 42.7 | NM (18–59 years) | 2 μg, HB02 strain | 28 | 1&2 | Injection site pain, fever,fatigue,headache | Injection site: redness(4%), mucocutaneous abnormalities(4%),itch (non‐injection site)(4%) | 0–7 |
| 36 | Xia, S. | Phase 1 | 24 | 50 | 37.7 | NM (18–59 years) | 4 μg, HB02 strain | 28 | 1&2 | Injection site pain, fever, vomiting | Injection site: itch (4%) | 0–7 |
| 36 | Xia, S. | Phase 1 | 24 | 47 | 40.1 | NM (18–59 years) | 8 μg, HB02 strain | 28 | 1&2 | Injection site pain, fever, inappetence, nausea, constipation | Injection site: swelling (8%) | 0–7 |
| 36 | Xia, S. | Phase 1 | 24 | 72 | 67.5 | NM (≥60 years) | 8 μg, HB02 strain | 28 | 1&2 | Injection site pain, fever, fatigue,headache | Injection site: induration (8%) | 0–7 |
| 36 | Xia, S. | Phase 2 | 84 | 54 | 40.8 | NM (18–59 years) | 8 μg, HB02 strain | single | 1 | Injection site pain, fever, fatigue,headache, diarrhea, muscle pain, dizziness, anaphylaxis(1%) | Injection site: swelling(2%),itch(2%),redness(1%),rash(1%) | 0–7 |
| 36 | Xia, S. | Phase 2 | 84 | 55 | 41 | NM (18–59 years) | 4 μg, HB02 strain | 14 | 1&2 | Injection site pain, fever, fatigue,headache, diarrhea, muscle pain | Injection site:itch(1%),itch (non‐injection site)(2%) | 0–7 |
| 36 | Xia, S. | Phase 2 | 84 | 53 | 41.7 | NM (18–59 years) | 4 μg, HB02 strain | 21 | 1&2 | Injection site pain, fever, fatigue,nausea,headache, drowsiness | Injection site: swelling(4%),redness(1%) | 0–7 |
| 36 | Xia, S. | Phase 2 | 84 | 57 | 43.7 | NM (18–59 years) | 4 μg, HB02 strain | 28 | 1&2 | Injection site pain, fever fatigueheadache, cough | Injection site: swelling(1%),itch(1%),redness(1%), itch (non‐injection site)(1%) | 0–7 |
| 37 | Al Kaabi, N. | Phase 3 | 13,464 | 15.9 | 36.2 | Baseline COVID PCR + (0.2%), Baseline IgG +(5.0%) | 5 μg, WIV04 strain | 21 | 1&2 | Injection site pain, headache, fatigue, myalgia, diarrhea, coughing, fever, dyspnea, arthralgia, constipation, nausea, vomiting, dysphagia, anorexia | Injection site: induration(1.0%), swelling(1.5%), rash(0.7%), redness (1.1%), itching(0.4%), pruritus (non‐inoculated site)(1.3%), skin and mucosal abnormalities(0.2%), acute allergic reactions(0.3%) | 0–7 |
| 37 | Al Kaabi, N. | Phase 3 | 13,471 | 15.4 | 36.2 | Baseline COVID PCR + (0.2%), Baseline IgG +(5.2%) | 4 μg, HB02 strain | 21 | 1&2 | Injection site pain, headache, fatigue, myalgia, diarrhea, coughing, fever, dyspnea, arthralgia, constipation, nausea, vomiting, dysphagia, anorexia | Injection site: induration(0.6%), swelling(0.8%), rash(0.7%), redness(0.9%), itching(0.5%), pruritus (non‐inoculated site)(1.5%), skin and mucosal abnormalities(0.2%), acute allergic reactions(0.3%) | 0–7 |
| 4.4. BBV152 (n = 380) | ||||||||||||
| 38 | Ella, R. | Phase 1/2 | 190 | 26 | 34 | NM | 3 μg + Algel‐IMDG | 14 | 1&2 | Injection site pain, body ache, fever, headache, malaise | Redness at injection site (1st:1%), itching (1st:1%), stiffness in upper arm(1st:1%) | 0–7 |
| 38 | Ella, R. | Phase 1/2 | 190 | 24 | 35 | NM | 6 μg + Algel‐IMDG | 14 | 1&2 | Injection site pain, weakness in injection arm, body ache, fever, headache, malaise, weakness | Redness at injection site (1st:1%), itching(1st:1%, 2nd:1%),rashes(1st:1%) | 0–7 |
| 4.5. KCONVAC (n = 424) | ||||||||||||
| 39 | Pan, H. X. | Phase 1 | 24 | 50 | 38 | NM | 5 μg | 14 | 1&2 | Injection site pain,myalgia, fatigue | Injection site:induration(4%), erythema(8%) | 0–28 |
| 39 | Pan, H. X. | Phase 2 | 100 | 47 | 45.5 | NM | 5 μg | 14 | 1&2 | Injection site pain,fever, inappetence, myalgia, headache, fatigue | Injection site:induration(2%), erythema(1%), pruritus(2%) | 0–28 |
| 39 | Pan, H. X. | Phase 2 | 100 | 55 | 44.9 | NM | 10 μg | 14 | 1&2 | Injection site pain,fever, diarrhea, inappetence, vomiting, myalgia, headache, cough, dyspnea, fatigue | Injection site:pruritus(1%), skin or mucosa abnormality(1%) | 0–28 |
| 39 | Pan, H. X. | Phase 2 | 100 | 62 | 42.4 | NM | 5 μg | 28 | 1&2 | Injection site pain,fever, diarrhea, myalgia, headache, cough, fatigue | Injection site:erythema(1%), pruritus(1%) | 0–28 |
| 39 | Pan, H. X. | Phase 2 | 100 | 54 | 44.5 | NM | 10 μg | 28 | 1&2 | Injection site pain,fever, diarrhea, nausea, cough, fatigue | Injection site:induration(%3), swelling(2%), erythema(5%), pruritus(2%) | 0–28 |
| 4.6. IBMCAMS vaccine (n = 96) (LD: 50EU, MD: 100EU) | ||||||||||||
| 40 | Pu,J. | Phase 1 | 24 | 54 | 37 | NM | LD | 14 | 1 | Injection site pain, fatigue, fever, serious adverse events (8.3%) | Injection site itching (8.3%), rash (4.2%) | 0–7 |
| 40 | Pu,J. | Phase 1 | 24 | 54 | 37 | NM | LD | 14 | 2 | Injection site pain, fatigue | Injection site redness (4.2%) | 0–7 |
| 40 | Pu,J. | Phase 1 | 24 | 67 | 38.2 | NM | MD | 14 | 2 | Injection site pain, fatigue, diarrhea | Injection site itching (4.2%) | 0–7 |
| 40 | Pu,J. | Phase 1 | 24 | 50 | 40.1 | NM | MD | 28 | 2 | Injection site pain, fatigue, fever, serious adverse events (8.3%) | Injection site swelling (4.2%) | 0–7 |
| 5. Virus‐like particle vaccines | ||||||||||||
| 5.1. CoVLP (n = 180) | ||||||||||||
| 41 | Ward, B. J. | Phase 1 | 20 | 55 | 34.9 | NM | 3.75 μgUn‐adjuvanted | 21 | 1&2 | Injection site pain, headache, fatigue, chills, discomfort or uneasiness |
Injection site: redness (5%), swelling(5%), swelling in neck(5%) 0‐21d: allergicconjunctivitis (5%), maculo‐papular rash (5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 50 | 35.3 | NM | 3.75 μg + CpG | 21 | 1&2 | Injection site pain, fever, headache, muscle aches fatigue, chills, discomfort or uneasiness |
Injection site: redness (5%), swelling(1st:5%, 2nd: 15%) swelling in neck(1st:20%, 2nd: 5%), chest wall (5%); 0‐21d: hordeolum(5%), plantar fasciitis(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 75 | 34.7 | NM | 3.75 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, muscle aches, joint aches, fatigue, chills, discomfort or uneasiness |
Injection site: redness (1st:5%, 2nd:31.6%), swelling(1st:15%, 2nd: 31.6%), swelling in neck(5.3%); 0‐21d: bacterial vaginosis(5%), vaginal infection (5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 50 | 35.6 | NM | 7.5 μg Un‐adjuvant | 21 | 1&2 | Injection site pain, headache, muscle aches, joint aches fatigue, chills |
Injection site: swelling(5%); swelling in neck (5%), axilla (5%), groin(5%); 0‐21d: rash(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 60 | 32.4 | NM | 7.5 μg + CpG | 21 | 1&2 | Injection site pain, fever, headache, muscle aches, fatigue, chills, discomfort or uneasiness |
Injection site: redness (5%), swelling(1st:20%, 2nd:15%), swelling in neck (5%) 0‐21d: vulvovaginal pruritus(5%), contact dermatitis (5%), erythema(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 60 | 37.2 | NM | 7.5 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, muscle aches, joint aches, fatigue, chills, discomfort or uneasiness |
Injection site: redness(35%), swelling(1st:30%, 2nd:40%); swelling in neck(1st:5, 2nd:10), axilla(5%), groin(5%), chest wall(5%); 0‐21d: lymphadenopathy(5%), injection site papule(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 65 | 34.1 | NM | 15 μg Un‐adjuvanted | 21 | 1&2 | Injection site pain, headache, muscle aches, fatigue |
Swelling in neck (10%); 0‐21d: oral herpes(5%), dyshidrotic eczema(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 50 | 32 | NM | 15 μg + CpG | 21 | 1&2 | Injection site pain, fever, headache, muscle aches, joint aches, fatigue, chills, discomfort or uneasiness |
Injection site: swelling(1st:10%, 2nd:5.3%); swelling in neck(1st:5%, 2nd:5.3%), axilla(5.3%), chest wall (5%); 0‐21d:hot flush(5%) |
0–7 |
| 41 | Ward, B. J. | Phase 1 | 20 | 45 | 32.7 | NM | 15 μg + AS03 | 21 | 1&2 | Injection site pain, fever, headache, muscle aches, joint aches, fatigue, chills, discomfort or uneasiness |
Injection site: redness (1st:5%, 2nd:20%), swelling(1st:20%, 2nd:25%); swelling in neck(1st:10%, 2nd:15%), axilla(1st:10%, 2nd:10%); 0–21 d:injection sitebruising(5%), erythema(5%), warmth(5%), swelling(5%) |
0–7 |
Abbreviations: 1st, after the first dose; 2nd, after the second dose; CLD, chronic lung disease; CVD, cardiovascular disease; d, days; DM, diabetes mellitus; GI, gastrointestinal; HIV, human immunodeficiency virus positive; HTN, hypertension; IHD, ischemic heart disease; NM, not mentioned.
Supporting information Table S3.
The included articles consist of phase 1 (n = 11), phase 2 (n = 3), phase 1/2 (n = 17), phase 2/3 (n = 3) and phase 3 (n = 6) trials, along with 1 study on all three phases.
The candidate vaccines in order of number of studies on them are ChAdOx1 nCoV‐19 (6 studies), BNT162b2 (5 studies), BNT162b1 (4 studies), mRNA‐1273 (4 studies), CoronaVac (3 studies), 5 candidate vaccines with 2 studies each and 10 candidate vaccines with one study each.
Among the vaccinated participant groups we included in our study, most had received BNT162b2 (n = 39,144), mRNA‐1273 (n = 30,359), BBIBP‐CorV (n = 27,367), Ad26.COV2.S (n = 22,537), Gam‐COVID‐Vac (n = 15,011), and ChAdOx1 nCoV‐19 (n = 13,995), in descending order.
For intervals between the two (or three) doses, studies on BNT162b2, BNT162b1, Gam‐COVID‐Vac, NVX‐CoV2373, SCB‐2019, V‐01 and CoVLP used an interval of 21 days; studies on mRNA‐1273 and MF59‐adjuvanted sclamp used 28 days; and studies on Ad26.COV2.S used 56‐day intervals or single doses. Other trials also tested different regimens or single doses, sometimes comparing the two. For example, studies on ChAdOx1 nCoV‐19 tested day 0–21, 0–28, 0–56 regimens or intervals anywhere between 21 to 35 days (the rest are noted in the table). Most of these trials used the same candidate vaccines for both the primer and booster doses, some using different doses of the same vaccine for each dose. Three of the studies had administered heterologous primer‐booster vaccines; rAd26 with rAd5 (making up the Gam‐COVID‐Vac), 15 , 16 and ChAdOx1‐S with BNT162b2 (named the CombiVacS). 53
Among our included groups, only one study which was on BNT162b2 had individuals under the age of 18, set in two groups, 12 to 15‐year‐olds and 16 to 25‐year‐olds. 4 The rest had only included individuals above the age of 18, some extending their age groups up to 85‐year‐olds. Also, the trials often had groups with different age ranges, measuring the side effects in each age group, which are all noted in Table 3.
We must bear in mind that the earlier expeditious vaccination with mRNA vaccines and their larger number of RCT participants is also reflected in the net number of observed side‐effects, as the number of participants in RCTs on mRNA vaccines (70,277), is almost comparable to the number of participants in the collective rest of the vaccine RCTs (90,353). This earlier jumpstart in mRNA vaccination also warrants more time for researchers to observe the side effects. We believe that these reasons have led to the larger number of studies on mRNA vaccines and therefore when evaluating the different vaccine categories, the relative risk of side‐effects should be compared between the groups, not the number of reported cases or articles.
3.5. Analytical observational studies
A total of 27 observational articles, consisting of case‐control, cohort and cross‐sectional studies were included, with a total population of 467,577 participants, as illustrated in Table 4. The studies came from different countries such as United States, the UK, Italy, Poland, Czech Republic, Jordan, Spain, South Korea, Malta, Scotland, Argentina, China and international registries. The administered candidate vaccines in these studies in order of number of collective number participants having received them were Ad26.COV2.S (n = 338,765), 54 mRNA‐1273 (n = 55,944), 47 , 55 BNT162b2 (n = 33,539), 45 , 47 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ChAdOx1 nCoV‐19 (n = 26,862), 56 , 60 , 62 , 63 , 66 , 67 , 68 CoronaVac (n = 1855), 69 , 70 Gam‐COVID‐Vac (n = 683), 71 and BBIBP‐CorV (n = 89). 56 In addition, seven studies reported combined results on more than one vaccine, collectively comprised of 9851 participants. Five of these studies were on BNT162b2 and mRNA‐1273, 72 , 73 , 74 , 75 , 76 one study with the addition of ChAdOx1 nCoV‐19 to the two previous vaccines, 77 and one study with BNT162b2 and ChAdOx1 nCoV‐19. 78 We categorized the studies base on the vaccines administered in Table 4.
TABLE 4.
Mucocutaneous reaction after COVID‐19 vaccination reported in “Observational Analytical” studies
| Supplemental references a | Patients No | Woman ratio | Mean of Age (year) | Participants' characteristics or comorbidities | Dose | Any symptoms after vaccine | Mucocutaneous lesions characteristic | Time of onset the reactions | Location of mucocutaneous reaction | Duration of reaction |
|---|---|---|---|---|---|---|---|---|---|---|
| With BNT162b2 | ||||||||||
| 1 | 141 | 70.7 | 34.99 | NM | 1 | Fever(6.4%), fatigue(31.9%), myalgia(21.3%), bone pain(7.1%), joint pain (13.5%), headache(27.7%), injection site pain(75.9%), arm numbness (12.1%), diarrhea(1.4%), shortness of breath(1.4%) | Herpes zoster (0.7%), redness and swelling (0.7%) | NM | Injection site | 1.39 days |
| 2 | 803 | 86.55 | 43 | NM | 1 & 2 | Injection site pain(88.04%), weakness(58.9%), myalgia(45.7%), headache(44.83%), chills(35.99%), fever(22.04%), joint pain(16.56%), nausea(15.94%), spasm(9.59%), sweating(9.22%), dizziness(8.34%), musculoskeletal (53.3%), GI (21.42%), neurological (12.7%), cardiovascular (5.98%), respiratory(2.61%), allergy(1.24%) lymphadenopathy(3.36%) | Swelling(5.48%), itching(5.35%), rash(2.49%), skin discoloration(1.25%), hives(0.62%), bleeding(0.37%), mouth/throat swelling(0.37%), atopic eczema(0.25%), Hair loss(0.12%), Swelling of the lips(0.12%), Flushing(7.1%) | NM | Injection site, Mouth, Lips | NM |
| 3 | 877 | 88.5 | 42.56 | Allergy(5.9%), baseline COVID + (19.3%), HTN(36.9%), thyroid disease(25.6%), asthma(21.8%), DM(9.6%), CVD(5.9%), RA(4.8%), bowel disease(4.4%), Neurologic disease(4.1%), Psychological distress(3%), Renal disease(2.2%), COPD(1.8%), Cancer(1.5%), Hepatologic disease(0.7%), Ophthalmologic disease(0.4%) | 1 & 2 | Injection site pain(89.8%), fatigue(62.2%), headache(45.6%), muscle pain(37.1%), chills(33.9%), fever(21.7%), lymphadenopathy(16.2%), nausea(13%), taste disturbance(3.5%) |
At least one skin‐related side effect(5.2%):swelling(25.6%), redness(23%), rash(62.2%), urticaria(22.2%); At least one oral side effect(13%): blisters(36%), halitosis(25.4%), ulcers(14%), bleeding gingiva(11.4%), white/red plaque(10.5%), burning gingiva(8.8%), angular cheilitis(4.4%), tongue tingling(4.4%), vesicles(3.5%), swollen lips(3.5%), xerostomia(2.6%) |
1–3 days (26.8%), 1st week (28.6%), 2nd week (16.1%), 3rd week (18.8%), 4th week (9.8%) |
Upper limb (60%), chest/trunk(33.3%), lower limb(22.2%), face(20%), back (17.8%), lips(74.1%), labial/buccal(14.8%), tongue(13%), palate(9.3%), gingiva(9.3%) |
1 day (45.1%), 3 days (35.8%), 5 days (9.4%), 1 week (5.3%), >1 week (3.0%), >4 week (1.4%) |
| 4 | 103 | 88.3 | 40.4 | NM | 1 & 2 | (47.6%) of reactions After 1st dose, (52.4%) of reactions after 2nd dose, (18.4%) of participants had reactions after both doses | Delayed injection‐site reaction (COVID‐arm) (100%), itching (68%), disseminated lesions(4.9%), slightly indurated erythematous targetoid patch(1%) | NM | Injection site, Generalized |
<8 h(22.3%), 8‐24 h(27.1%), 24‐72 h(36.9%), >72 h(13.6%) |
| 4 | 4775 | 83.4 | 43.2 | NM | 1 & 2 | NM | Vaccine‐related urticaria (0.04%) | NM | NM | <7d |
| 5 | 57 | 71 | 48.9 | Psoriatic arthritis(17.5%), Spondylo‐arthritis(5%), RA(10%), systemic sclerosis(8.7%), SLE(1.5%), Sjogren syndrome(1.7%), HTN(15.7%), obesity(8.7%), DM(3.5%) | 1 & 2 | 1st: injection site pain(29%), fatigue(8.7%), headache(28%), fever(5.2%), tachycardia(3.5%), paresthesia(3.5%), 2nd: injection site pain(41%), fatigue(58%), headache(58%), fever(58%), paresthesia(5.8%), itchy scratchy throat(5.8%), diarrhea(23.5%), lymphadenopathy(5.8%) | 2nd: cutaneous vasculitis(5.8%) | NM | NM | NM |
| 6 | 282,103 | 61·6 | 62 |
NM |
1 | Headache(7.8%), fatigue(8.4%), chills(2.5%), diarrhea(1.4%), fever(1.5%), arthralgia(3.2%), myalgia(2.3%), nausea(2.1%) | Local side‐effects(71.9%), rash(0.2%), skin burning(0.7%) | 0–8 days | NM | NM |
| 28,207 | 69·6 | 61 | 2 | Headache(13.2%), fatigue(14.4%), chills(6.4%), diarrhea(1.5%), fever(3.8%), arthralgia(7%), myalgia(5%), nausea(3.5%) | Local side‐effects(68.5%), Rash(0.4%), Skin burning(1.1%) | 0–8 d | NM | NM | ||
| 7 | 131 | 85.5 | 47 | CVD(21.4%), respiratory disease(26%),autoimmunity(16%), chronic skin conditions(2.2%), anaphylaxis(92.4%), asthma(34.4%), chronic urticaria(5.3%), contact dermatitis(9.9%) | 1 | Mild immediate reaction in (0.7%) with history of severe asthma, Nasal obstruction, Rhinolalia | Pruriginous erythematous macules (0.7%) | 10 min | Neck and upper thorax | 1 h |
| 8 | 34 |
85 85% |
42 42 |
Atopic dermatitis(7%), contact dermatitis(2.8%), psoriasis(4.2%), urticaria(2.8%), acne vulgaris(2.8%), HTN(11%), COPD(2.8%), morbid obesity(4.2%), DM(1.4%), CVD(2.8%), rheumatologic disease(5.6%), malignancy(4.2%) | 1 | Fatigue(32%), Myalgia(29%), Headache(26%), Fever(12%), Arthralgia(15%), Nausea(12%), Chills(12%), Lymphadenopathy(5.9%), Diarrheal(2.9%), Injection site pain(24%) | Delayed large local reaction(15%), Local injection site reaction(24%), swelling(18%), erythema(18%), urticaria(26%), morbilliform(18%), erythromelalgia(2.9%), Flare of existing dermatologic condition (2.4%), vesicular(8.8%), pernio/chilblains(8.8%), VZV(2.9%), pityriasis rosea(5.9%), vasculitis(2.9%), reaction in breastfed infant(5.9%), petechiae(2.9%) | Urticarial:3d, morbilliform:3d, Erythromelalgia:7d | Urticaria: arms (68%), trunk(57%), legs(46%), morbilliform: arms(62%), trunk (42%), legs(27%), erythromelalgia: arms(69%), face(31%), hands(23%), feet(15%) | Urticaria:5 days, Morbilliform:4.5 days, Erythromelalgia:5.5 days |
| 40 | 2 | Fatigue(33%), myalgia(25%), headache(15%), fever(10%), arthralgia(20%), nausea(7.5%), chills(13%), lymphadenopathy(7.5%), injection site pain(18%) | Delayed large local reaction(18%), local injection site reaction(25%), swelling(15%), erythema(20%), urticaria(20.5%), morbilliform(7.5%), erythromelalgia(5%), flare of existing dermatologic condition(7.5%), vesicular(5%), pernio/chilblains(5%), VZV(10%), angioedema(2.5%), pityriasis rosea(2.5%), filler reaction(2.5%), contact dermatitis(5%), reaction in breastfed infant(2.5%), onset of new dermatologic condition(5%) | Urticarial:2 days, morbilliform: 2 days, Erythromelalgia: 1 day | Urticaria: arms(68%), trunk(57%), legs(46%); morbilliform: arms (62%), trunk (42%), legs 27%); erythromelalgia: arms(69%), face(31%), hands(23%), feet(15%) | Urticaria: 3 days, Morbilliform: 2.5 days, Erythromelalgia:3 days | ||||
| 9 | 277 | 66.8 | NM | NM | 1 | Injection site pain(70.0%), fever(6.9%), chills(15.9%), muscle ache(33.6%), joint pain(9.4%), headache(24.2%), dizziness(14.4%), confused mentality(3.6%), anxiety(4.7%), dyspepsia(6.1%), abdominal pain(4.7%), vomiting(6.1%), diarrhea(5.8%), fatigue(37.5%), palpitation(4.3%), HTN(3.6%), hypotension(3.6%), paralysis(3.6%), paraesthesia(4.3%), nasal obstruction(5.8%), parageusia(1.4%), foreign body sensation in the throat(9.7%), hoarseness(2.2%), odynophagia(2.5%), wheezing(0.7%), chest discomfort(0.4%) | Injection site: redness(2.5%), swelling(5.1%), itch(6.1%), angioedema(4.3%), tongue edema(3.6%), throat swelling and tightness(5.8%), urticaria(0.7%), skin rash(1.8%) | 3 days | Injection site, diffuse | NM |
| 10 | 93 | 74.1 | 29 | NM | 1&2 | Injection site pain, fatigue, muscle pain, headache, diarrhea, headache, chills, joint pain, fever (only after 2nd dose) | Injection site: redness (<10%),swelling (<10%) | 0–7 days | Injection site | NM |
| 11 | 1480 | 66.69 | NM | NM | 1&2 | Pain at injection site(49.85%), fever(10.11%), chills(10.20%), fatigue(21.79%), muscle pain(24.16%), joint pain(11.00%), headache(21.91%), vomiting(12.90%), diarrhea(13.75%) | Injection site: redness(31.07%),swelling(36.44%) | Peak 1–2d | Injection site | NM |
| 12 | 151 | 48 | 73 | CVD(41%), DM(15%), underlying lung pathology(8%), malignancy (100%: solid cancer [63%], hematological cancer [37%]) | 1&2 |
Injection‐site pain, flu‐like symptoms, headaches, chills, fatigue, arthralgia, nausea/vomiting, fever, diarrhea, deranged liver function tests |
Injection site: erythema (<10%), swelling(<10%) | 0–30 days | Injection site | NM |
| 13 | 10,445 | 85 | 41 | (Study only on cutaneous reactions after mRNA COVID‐19 vaccination) | 1 | NM | Dose 1 reaction: cutaneous reaction(1.4%), itching or rash(1.2%), hives/urticaria(0.2%), swelling/angioedema(0.2%) | 0–3 days | Injection site, diffuse | NM |
| 124 | 2 | Recurrent dose 2 reaction: cutaneous reaction(16%), Itching or rash(14%), hives/urticaria(2.4%), swelling/angioedema(2.4%) | ||||||||
| 9055 | 2 | New dose 2 reaction: cutaneous reaction(1.4%), itching or rash(1.1%), hives/urticaria(0.3%), swelling/angioedema(0.2%) | ||||||||
| 14 | 4953 reports | NM | NM | NM | 1 | Thrombocytopenia (excluding ITP)(0.003%), venous thromboembolic events (including CVST)(0.03%), Arterial thromboembolic events)(0.03%), hemorrhagic events)(0.01%) | ITP (<0.001%) | 0–27 days | NM | NM |
| With mRNA‐1273 | ||||||||||
| 8 | 267 |
92 92% |
45 45 |
Atopic dermatitis(3.5%), contact dermatitis(2.9%), psoriasis(1.7%), urticaria1.5%), acne vulgaris(1.2%), HTN(16%), COPD(5.2%), morbid obesity(4.1%), DM(4.1%), CVD(2.3%), rheumatologic disease(1.7%), malignancy(1.5%) | 1 | Fatigue(22%), myalgia(21%), headache(17%), fever(6.7%), arthralgia6%), nausea(5.6%), chills(5.2%), lymphadenopathy(4.9%), diarrheal(3.4%), injection site pain(35%) | Delayed large local reaction(66%), injection site reaction(54%), swelling(44%), erythema (49%), urticaria(5.9%), morbilliform(4.1%), erythromelalgia(1.9%), flare of existing dermatologic condition(1.1%), vesicular(1.5%), pernio/chilblains(1.1%), VZV(1.9%), angioedema(1.9%), pityriasis rosea(0.4%), erythema multiforme (1.1%), filler reaction (1.1%), vasculitis (0.7%), contact dermatitis (1.1%), onset of new dermatologic condition (0.7%), petechiae(0.4%) | Urticarial:3 days, morbilliform:3 days, Erythromelalgia:7 days, delayed large local reactions:7 days | Urticaria: arms(68%), trunk(57%), legs(46%), morbilliform: arms(62%), trunk(42%), legs(27%), erythromelalgia: arms(69%), face(31%), hands(23%), feet (15%) | Urticaria: 5 days, morbilliform: 4.5 days, delayed large local reaction: 4 days, erythromelalgia: 5.5 days |
| 102 | 2 | Fatigue(62%), myalgia(62%), headache53%), fever(41%), arthralgia(27%), nausea(27%), chills(46%), lymphadenopathy(8.8%), diarrheal(3.9%), injection site pain(59%) | Delayed large local reaction (30%), Local injection site reaction(70%), swelling(68%), erythema(67%), urticaria(6.9%), morbilliform(6.9%), erythromelalgia(5.9%), Flare of existing dermatologic condition(1%), vesicular(1%), filler reaction(4.9%), contact dermatitis(1%), petechiae(2%) | Urticarial:2 days, Morbilliform:2 days, Erythromelalgia:1 day, Delayed large local reactions:2 days | Urticaria:arms(68%), trunk(57%), legs(46%); morbilliform: arms(62%), trunk(42%), legs(27%); erythromelalgia: arms(69%), face(31%), hands(23%), feet(15%) | Urticaria: 3 days, morbilliform: 2.5 days, erythromelalgia: 3 days | ||||
| 13 | 30,195 | 85 | 41 | (Study only on cutaneous reactions after mRNA COVID‐19 vaccination) | 1 | NM | Dose 1 reaction: cutaneous reaction(2.1%), itching or rash(1.6%), hives/urticaria(0.5%), swelling/angioedema(0.3%) | 0–3 days | Injection site, diffuse | NM |
| 485 | 2 | Recurrent dose 2 reaction: cutaneous reaction(17%), itching or rash(13%), hives/urticaria (3.5%), swelling/angioedema(2.7%) | ||||||||
| 24,884 | 2 | New dose 2 reaction: Cutaneous reaction(2.6%), Itching or rash(1.8%), Hives/urticaria(0.7%), Swelling/angioedema(0.4%) | ||||||||
| With ChAdOx1 nCoV‐19 | ||||||||||
| 1 |
179 409 409 |
70.7 70.7% |
34.99 34.99 |
NM |
1 |
Fever(73.7%), fatigue(84.9%), myalgia(79.9%), bone pain(44.1%), joint pain (57.0%), headache(68.7%), injection site pain(91.1%), arm numbness(22.9%), diarrhea(9.5%), shortness of breath(9.5%), dizziness (3.4%), vomiting(3.4%), nausea(6.1%) | Urticaria (0.6%) | NM | NM | 1.39 d |
| 15 | 92 | 77.2 | 35.37 | Allergy(1.1%), Baseline COVID + (8.7%), thyroid disease(5.4%), asthma (4.3%), neurologic disease(3.3%), psychologic distress(2.2%), RA(1.1%), bone disease(1.1%), HTN(1.1%) | 1 & 2 | Injection site pain(72.8%), fatigue(73.9%), muscle pain(55.4%), chills(48.9%), feeling unwell (46.7%), nausea(45.7%), headache(29.3%), fever(15.2%),lymphadenopathy(5.4%), taste alterations (5.4%) | Injection site: swelling(10.9%), redness(10.9%); skin rash(4.3%); oral: oral ulcers/blisters/vesicles(7.6%), halitosis(3.3%), bleeding gingiva(3.3%), white/red plaque(1.1%), Swollen lips(1.1%) |
1–3 days (82.6%), 1st week (13%), 4th week (4.3%) |
Injection site, lips (1.1%), labial/buccal mucosa (4.3%), tongue (2.2%) |
1–3 days |
| 6 | 345,280 | 57·7 | 63·3 | NM | 1 | Headache (22.8%), fatigue(21.1%), chills(14.7%), diarrhea(2.2%), fever(8.2%), arthralgia(11.5%), myalgia(7%), nausea(5.7%) | Local side‐effects (58.7%), Rash (0.4%), Skin burning (1.7%) | 0–8 days | NM | NM |
| 16 | 994 | 76.7 | 35.7 | NM | 1 |
Injection site: tenderness(94.5%) resting pain(88%); fatigue(92.9%), headache(77.7%), malaise(83.8%), arthralgia(61.5%), chills(67.2%), fever(27.6%), nausea/vomiting(36.4%), diarrhea(19.9%) |
Injection site: redness(34.1%), swelling(48.7%); reports of urticaria at both arms, both legs, itching sense or warmth at the injection site | 2.2 days, peak at 4 days | Injection site, diffuse | 1 day, for many |
| 9 | 5589 | 77 | NM | NM | 1 | Injection site pain(81.2%), fever(51.3%), chills(65.8%), muscle ache(79.9%), joint pain(48.6%), headache(69.5%), dizziness(46.8%), confused mentality(19.1%), anxiety(22.4%), dyspepsia(30.9%), abdominal pain (24.3%), vomiting (22.8%), diarrhea (24.1%), fatigue (76.5%), palpitation(28.3%), HTN (18.1%), hypotension(18.0%), paralysis(17.7%), paraesthesia (19.3%), nasal obstruction (27.2%), parageusia (8.1%), foreign body sensation in the throat(24.3%), hoarseness(10.4%), odynophagia(11.0%), wheezing(6.4%), chest discomfort(6.3%) | Injection site: redness (7.0%), swelling (9.6%), itch (22.2%), angioedema (19.1%), tongue edema (18.0%), throat swelling and tightness (21.1%), urticaria (5.8%), skin rash(5.7%) | 3 days | Injection site, diffuse | NM |
| 10 | 42 | 90.5 | 36 | NM | 1 | Injection site pain (93%), fatigue (81%), muscle pain (79%), headache (62%), vomiting (<20%), diarrhea (<20%), headache (~60%), chills (~60%), joint pain (~30%), fever (<40%) | Injection site: redness (<20%), swelling (<20%) | 0–7 days | Injection site | NM |
| 14 | 19,148 reports | NM | NM | NM | 1 | Thrombocytopenia (excluding ITP) (0.002%), Venous thromboembolic events (including CVST) (0.02%), Arterial thromboembolic events (0.07%), Hemorrhagic events (0.01%) | ITP (0.001%) | 0–27 days | NM | NM |
| With Ad26.COV2.S | ||||||||||
| 17 | 13,725 | 66.2 | 42 | (Percentages are among all 338,765 participants receiving the vaccine, 13,725 of which experienced adverse events) | 1 | Fatigue (59.1%), injection site pain (57.9%), headache (52.2%), myalgia (47.8%), fever (34.7%), chills (34.2%), joint pain (26.1%), nausea (18.7%), diarrhea (9.4%), abdominal pain (7.4%), vomiting (2.1%) |
Swelling (9.3%), redness (7.4%) itching (7.1%) rash (1.9%) (serious adverse reactions: 3%, including 3 reports of non‐CVST TTS)cerebral venous sinus thrombosis thrombocytopenia syndrome |
0–7 days | Injection site, diffuse | NM |
| With BBIBP‐CorV | ||||||||||
| 1 | 89 | 65.6 | 39.27 | NM | 2 | Headache (12.4%), fever (4.5%), fatigue (16.9%), myalgia1 (4.6%), injection site pain (37.1%), injection site numbness (5.6%), joint pain (3.4%), diarrhea (1.1%), shortness of breath (2.2%), bone pain (4.5%) | Herpes zoster (1.1%) | NM | NM | NM |
| With CoronaVac | ||||||||||
| 18 | 329 | 48.5 | 35.77 | NM | 2 | Injection site pain, headache (16.8%), fever (3.6%), state of sleep/fatigue (13.8%), nausea/vomiting (10.8%), myalgia (3.9%), (tachycardia, loss of taste, feeling of throat swelling, vertigo) (5.1%) | Injection site: redness/swelling/pain (9.0%), allergy (0.9%), extensive itchiness (0.6%) (serious adverse reactions: 33.2%) | 1.14 days (for serious events) | Injection site, diffuse | 1.68 days (for serious events) |
| 19 | 1526 | 79.3 | 35.4 | Allergic history (6.3%), adverse reactions to other vaccines (5.6%), BMI ≥ 28 (5.1%) | 1 | Fatigue (8.3%), Muscle pain (8.1%), headache/dizziness(6%), fever(2.9%), vomiting/diarrhea(1.6%), appetite impaired/nausea (1.4%), cough/throat pain(1.2%), stuffy runny nose(0.9%), non‐solicited adverse reactions (menstruation, chest pain, numbness of limbs): (0.6%) | Injection site adverse reactions (pain, induration, redness, swelling, or itch): (9.6%), allergic reaction/urticaria/rash(1%), lymphadenopathy(0.7%) | NM | Injection site, diffuse | NM |
| 2 | Fatigue (6.5%), muscle pain (7.8%), headache/dizziness (3.4%), fever (1%), vomiting/diarrhea (0.9%), appetite impaired/nausea (0.9%), cough/throat pain (0.5%), stuffy runny nose (0.4%), non‐solicited adverse reactions (menstruation, chest pain, numbness of limbs): (0.3%) |
Injection site adverse reactions (pain, induration, redness, swelling, or itch): (10.7%), lymphadenopathy (0.3%) (129 did not receive the 2nd dose, 16.3% of which had an adverse reaction after the 1st dose) |
NM | Injection site, diffuse | NM | |||||
| With Gam‐COVID‐Vac | ||||||||||
| 20 | 683 | 68.2 | 35 | Allergies (5.3%), DM (0.9%), hepatic disease (0.6%), renal failure (0.1%), corticosteroid treatment (0.1%), autoimmune disease (1.6%), baseline COVID‐19 + (5.0%), any vaccinations 4 months prior (1.5%), family history of reaction to vaccines (0.9%) | 1 | New or worsened muscle pain (58%, 10% severe, 1% grade 4), pain at injection site (57%, 2% severe), fever (40%), headache (33%), diarrhea (5%, n = 1 severe), vomiting (3%, n = 2 severe), breathing difficulty (2%) | Local redness or swelling (11%), swelling of face or throat (1%) 33% of all reported adverse events were local (serious adverse events: 5%) | 0–3 days | Injection site, diffuse | NM |
| Results on more than one vaccine in a study (received by noted percentage of participants) | ||||||||||
| With BNT162b2 (54%), mRNA‐1273 (46%) | ||||||||||
| 21 | 741 | 57 | 60 |
Study conducted only on solid organ transplant recipients, kidney (49%), liver (19%), heart (15%), lung (11%), pancreas (1%), multiple organs (5%) (median 7 [Refs. 3–14] years since transplant, on maintenance immunosuppression) Prior COVID +(2%) |
1&2 | Pain at the injection site (80%), fatigue (46%) (severe:2.5%), headache (49%), myalgia, chills, fever, diarrhea, vomiting | Local swelling and erythema <20% | 0–7 days | Injection site | NM |
| With BNT162b2 (93%), mRNA‐1273 (7%) | ||||||||||
| 22 | 708 | 65 | 44 |
(Study conducted only on nephrologists) Prior COVID +(17%) |
1&2 |
Local reaction (68%), followed by myalgia (44%), tiredness (39%) and headache (34%) chills (28%), Low‐grade fever (21%)(75% of all included) (69%) after the first dose (57%) after the second dose |
Lymphadenopathy (6%), rash (<1%) | 0–7 days | Diffuse | NM |
| With BNT162b2 (51%), mRNA‐1273 (49%) | ||||||||||
| 23 | 325 | 96 | 43 |
Only on patients with rheumatic and musculoskeletal diseases (RMD): inflammatory arthritis (38%), SLE (28%), overlap connective tissue disease (19%) Treated with: non‐biologic disease modifying antirheumatic drugs (44%), biologic therapy (19%) and combination therapy (37%) |
1 | Systemic symptoms (69%), fatigue: the most common systemic event, 7.4% severe), headache, myalgia, chills, fever, diarrhea, vomiting | Local symptoms (89%) including pain (<90%), swelling (<30%), and erythema (~20%) | 0–7 days | Injection site | NM |
| With BNT162b2 (95.16%), mRNA‐1273 (4.81%), unknown (0.03%) | ||||||||||
| 24 | 3908 | 80.33 | 42.75 | NM | 1&2 | (79.68%) reported after the first dose, general disorders (48.80%), such as fatigue, pain, and chills nervous system disorders (46.39%) headache (46.39%), dizziness (38.67%), and paraesthesia (25.48%) syncope (2.04%), facial nerve paralysis (0.99%), and seizure (0.66%) Gastrointestinal disorders (25.54%) nausea (56.41%), vomiting (14.73%), and diarrhea (14.13%) anaphylactic or anaphylactoid reactions (~4.15%) |
Skin and subcutaneous tissue disorders: 24.08% (rash: 40.28%) |
3 days | Diffuse | NM |
| With mRNA‐1273 (90%), BNT162b2 (7%), other or unknown (3%) | ||||||||||
| 25 | 510 | 93 | 50 | (Results only on Black, Indigenous, or People of Color [BIPOC]) | 1&2 | (96%) after 1st dose and (85%) after mRNA‐1273 | Delayed large local reactions (100%, 11% reported in BIPOC patients), diffuse itching, hives or other rash, or angioedema (20%) | 8 days | Injection site, diffuse | NM |
| With BNT162b2 (94.9%), ChAdOx1 nCoV‐19 (3.9%) and mRNA‐1273 (1.2%) | ||||||||||
| 26 | 1657 | 79 | NM | NM | 1 | Soreness (78%), limb pain (46.6%), fatigue (30%), malaise (21.3%), headache (16.65%), muscle and joint pain (15%), fever (17.3%), chills (11.5%), lymphadenopathy (4.2%), seizures (1.6%), insomnia (4.3%), nausea (3.9%), vomiting (0.8%), allergic reactions (0.6%), migraine (2.6%), diarrhea (1.3%), cough (0.9%) | Swelling (24.5%), redness (18.3%), hair loss (0.8%) | NM | Injection site | NM |
| 2 | Soreness (64.7%), fatigue (45.7%), malaise (43%), pain in the limb (38.7%), muscle and joints pain (33%), chills (31%), headache (30%), fever (42.3%), lymphadenopathy (9.5%), insomnia (7%), nausea (6.8%), migraine (3.6%), seizures (3.2%), cough (1.9%), diarrhea (1.7%), fainting (1.2%), vomiting (1.1%), allergic reaction (0.5%) | Swelling (20%), redness (16.6%), pruritus (4.8%), hair loss (0.9%) | NM | Injection site | NM | |||||
| A comparison of mRNA and viral vector vaccines: BNT162b2 (83.6%), ChAdOx1 nCoV‐19 (14.1%), other or unknown (2.3%) | ||||||||||
| 27 | 2002 | 72.1 | 45 | Prior COVID‐19 + (26.6%) |
1 1 |
More common in viral vector vaccines: Fever, flu‐like illness, shortness of breath, fatigue or tiredness | More common in viral vector vaccines: Skin rash, tingling, face and mouth swelling, generalized swelling, anaphylaxis (less common among those without a prior COVID infection) | 0–7 days | Injection site, diffuse | NM |
| More common in mRNA vaccines: Any side effect | More common in mRNA vaccines: Localized reaction (mostly mild in mRNA vaccines) | |||||||||
Abbreviations: 1st, after the first dose; 2nd, after the second dose; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; GI, gastrointestinal; HTN, hypertension; NM, not mentioned; RA, rheumatoid arthritis; SLE, systemic lupus erythematous; VZV, Varicella‐zoster virus.
Supporting information Table S4.
3.6. Recommendations and guidelines for vaccination in specific groups
A total of 37 articles regarding recommendations, guidelines and consensus opinion of experts on COVID vaccination in specific groups, such as those with underlying dermatologic or autoimmune disorders (with worries of potential flare‐ups 79 , 80 ), those with allergies, and those on immunosuppressive, immunomodulatory or biologic therapies (with worries of inefficient immunization 79 , 81 ), along with articles on certain precautions to be taken with regard to possible anaphylaxis or vaccine‐induced thrombotic thrombocytopenia (VITT) were found and their key points were extracted, as highlighted in Table 5.
TABLE 5.
Guidelines and recommendations of experts about COVID‐19 vaccination in special categories
| Supplemental references a | FA | Recommendations | Reason for recommendation | Drug dose adjustments | Any added drugs or monitoring |
|---|---|---|---|---|---|
| 1. For patients with autoimmune disorders | |||||
| 1.1. Autoimmune inflammatory rheumatic diseases | |||||
| 1 | Curtis, J.R. |
|
AIIRD patients are at a higher risk for incident viral infections hospitalization due to COVID‐ 19 compared to the general population |
|
Prophylaxis with Acetaminophen or NSAIDs to prevent post‐vaccination symptoms is not recommended |
| 2 | Park, J.K. |
|
Patients with AIIRD are immunocompromised due to underlying immune dysfunction and concomitant immunosuppressive treatment |
|
|
| 3 |
Moutsopoulos, H.M. Moutsopoulos |
|
Serum antibodies against PF‐4 in patients with SLE and APS display an association with thrombotic events |
|
|
| 4 | Tam, L.S. | For rheumatic and musculoskeletal diseases (RMD) (including SLE):
|
To allow an adequate immune response to the vaccine and also to minimize the delay in the administration of immunosuppressive therapy |
|
NM |
| 5 | Santosa, A. | For connective tissue disorders, Psoriatic arthritis, SLE, Immune mediated inflammatory myositis, Sjogren's syndrome, Systemic sclerosis:
|
B cell depleting therapy with RTX is associated with significant reduction in immunogenicity. |
|
NM |
| 6 | Bechman, K. | For immunosuppressed patients with rheumatic disorders
|
To have a more adequate immune response |
|
NM |
| 1.1.1 Psoriasis | |||||
| 7 | Gelfand, J.M. |
|
Comorbidities leading to more severe COVID‐19 are more frequent among patients with psoriasis |
|
NM |
| 8 | Mease, P. J. | Consensus opinion from rheumatologists, dermatologists, infectious disease specialists, and patient research partners:
|
Concerns of more severe outcomes of COVID‐19 among those with psoriatic diseases | Before getting the chance to vaccinate, treatment should continue without concerns of a more severe COVID course. | NM |
| 1.2. Hidradenitis Suppurativa (HS) | |||||
| 9 | Giamarellos‐Bourboulis, E.J. |
|
Metabolic diseases, which are more common in HS, may induce an increased risk of a severe course of COVID‐19 and possible fatalities. Biological drugs reduce the hyperactivity of the immune system which might result in suboptimal immunization. |
|
|
| 1.3. Patients with pemphigus on RTX | |||||
| 10 | Waldman, R. A. |
Individuals who have not initiated RTX therapy:
Individuals who are actively receiving RTX:
|
Concerns of the effect of RTX on immune response after vaccination |
|
|
| 1.4. Inflammatory bowel diseases | |||||
| 11 | Siegel, C. A. |
|
Concerns of inefficient immunization |
Patients with IBD receiving Infliximab infusions can receive non‐live vaccinations:
Patients can be vaccinated during induction or maintenance of biologic therapies irrespective of timing within their treatment cycle. |
NM |
| 2. For patients with allergic or atopic disorders | |||||
| 2.1. Atopic dermatitis | |||||
| 12 | Pfaar, O. |
|
General immune stimulation in AD, and immune suppression under drugs |
|
Apply topical anti‐inflammatory locally, using both steroids and calcineurin inhibitors |
| 13 | Ring |
|
The protein or the vector, one possible elicitor of anaphylaxis could be other ingredients such as PEG, present both in the BNT162b1 and the mRNA‐1273 vaccines | NM | NM |
| 14 | Thyssen, J.P. | AD is not a contraindication to vaccination
|
AD worsening is unlikely after vaccination, as the vaccination response is mainly skewed toward T helper cell 1. The risk of AD flares and loss of AD control increases if the systemic AD therapy is withheld or reduced in dose for longer than 3 weeks. |
Most clinicians pause these therapies as follows:
|
NM |
| 2.2. Mastocytosis | |||||
| 15 | Bonadonna, P. | Mastocytosis alone is not a contraindication to vaccines but some patients have a high risk of anaphylaxis:
|
NM | Continue anti‐mediator‐based treatment, like omalizumab, during the time of vaccination |
|
| 16 | Stingeni, L. | For cutaneous and systemic mastocytosis in general, the following criteria are suggested as contraindications for vaccination:
|
Excipients with known sensitizing potential in COVID‐19 vaccines:
|
NM |
|
| 2.3. Urticaria | |||||
| 12 | Pfaar, O. | For chronic spontaneous urticaria:
|
Effect of vaccination may be reduced by systemic immunosuppression | Vaccination is recommended between two injections of biologics with 1 week interval between vaccination and the treatment; however, vaccination can be done at any time under Omalizumab |
|
| 13 | Ring, J. |
|
Systemic allergic reactions to vaccines are rare, and are due to hypersensitivity to components of the formulation of the vaccine |
|
|
| 2.4. Allergic diseases (general) | |||||
| 17 | Klimek, L. |
|
No evidence of immunosuppressive effects in dupilumab and omalizumab |
|
Monitor patients with history of severe allergic reaction for 30 min and others for 15 min |
| 18 | Peter, J. | Consider following conditions as contraindications to vaccinationin allergic or immune‐based diseases:
|
Increased risk for severe COVID‐19 disease is considered in contraindicated conditions. The majority of drug‐ or vaccine‐induced anaphylactic reactions occur within the first 30 min following vaccination. |
Suggest a case‐by‐case risk assessment |
|
| 19 | Murphy, K.R. |
|
Adjuvants and other excipients/components in the vaccine are generally responsible for allergic reactions | The graded vaccine administration protocol as an experimental protocol: administer 0.05 ml of 1:10 dilution, and 10%, 20%, 30%, and 40% of the full dose in ascending order, in alternate arms at 15‐min intervals, followed by a minimum 30‐min observation period |
|
| 20 | Kleine‐Tebbe, J. | For atopic and/or eosinophilic airway diseases with type 2 inflammation:
|
NM |
|
NM |
| 21 | Tanno, L.K. |
Consider following conditions as contraindication for COVID‐19 vaccination:
Vaccinate with precaution in following conditions:
|
Concerns of allergies |
|
|
| 22 | Untersmayr, E. |
|
Concerns of vaccination among patients under immunomodulatory and immunosuppressive therapy or those with immunodeficiencies |
|
|
| 2.5. Precautions for anaphylaxis | |||||
| 23 | Turner, P.J. |
Recommend special precaution for following conditions:
Vaccination contraindications:
In these cases, vaccination should be proceeded as normal:
|
Adjuvants and other excipients/components in the vaccine are generally responsible for allergic reactions | NM |
|
| 24 | Kim, M. A. |
Those excluded from or candidates for a delayin vaccination:
Special considerations:
Past history of COVID‐19 infection: vaccinate at least 4 weeks after recovery In asymptomatic cases: vaccinate 4 weeks after first PCR test Patient on systemic steroids for more than 2 weeks:
|
Concerns of vaccination after a COVID infection or in special groups |
|
|
| 25 | Klimek, L. |
Contra‐indications to vaccination:
No allergy sufferer should be excluded from COVID‐19 vaccination without sufficient reason, except those with contra‐indications or high‐risk groups. High risk patients should undergo an “allergological evaluation” prior to COVID vaccination. |
Concerns of allergies in vaccination | Provide an interval of 7 days between allergologically relevant biologics and vaccination |
Monitor patients with allergies or those who belong to a defined risk groups for 30 min after vaccination. Vaccinators and vaccination staff must be prepared and have the appropriate expertisefor the recognition and treatment of anaphylaxis and severe allergic reactions and vaccination sites must have a minimum equipment of drugs and instruments. In case of (supposed) allergic reactions to vaccination, an allergological work‐up in a specialized center is indicated. |
| 26 | Klimek, L. |
|
Concerns of allergies in vaccination | NM | Vaccination personnel must always be prepared for the possibility and treatment of severe allergic/anaphylactic reactions and anaphylaxis |
| 27 | Worm, M. | A recommended set of questions for the assessment of the “allergological riskpotential” for COVID‐19 vaccination should be asked, finding details on
|
Concerns of allergies prior to vaccination | NM | NM |
| 3. For dermatology patients on immunosuppressive, immunomodulatory or biologic therapies | |||||
| 28 | Ferretti, F. |
|
Due to high risk of severe COVID‐19 or hospitalization |
|
NM |
| 29 | Gresham, L.M. |
|
Concerns of immune therapies | NM |
Consider anti‐IL‐17 biologics, anti‐IL‐12/23 biologics, Omalizumab, MTX and JAK inhibitors as safe therapies during the COVID‐19 pandemic |
| 30 | Wang, C. |
|
To maximize vaccine response and decrease the frequency of local and systemic reactions | if patient is already on a biologic or immunomodulatory agent:
|
NM |
| 31 | Hauptman, M. | For patients on biologic therapies (including patients with psoriasis):
|
Concerns of immune therapies | NM | NM |
| 32 | Chakravarthy, K. |
|
Concerns of those receiving steroids |
There is no need to defer neuraxial steroid injections when indicated in the context of COVID‐19 vaccination There is no specific guidance suggesting withholding NSAIDs or other anti‐inflammatories before vaccination |
NM |
| 4. Precautions for vaccine‐induced thrombotic thrombocytopenia | |||||
| 33 | Elalamy, I. |
|
on a Principle of Precaution |
|
|
| 34 | Oldenburg, J. |
|
Concerns of thromboses after ChAdOx1 nCoV‐19 | Labs: CBC with PLT count, blood smear, D‐dimers, further imaging in indicated (cranial MRI, ultrasound, chest and abdomen CT).
|
|
| 5. For delayed reactions to hyaluronic acid soft tissue fillers | |||||
| 35 | Gotkin, R. H. | Collected data does not support the concern for a higher risk of adverse reactions following soft tissue filler injections associated with COVID vaccination, compared to other previously described triggers or the default of adverse reactions following soft tissue filler injections | Concerns of reactions to fillers | NM | NM |
| 36 | Rice, S. M. |
A time frame should be suggested:
|
Concerns of DTRs to fillers | Dilution of fillers with saline or lidocaine or use of non‐HA fillers around the time of vaccination may also be suggested to minimize the risk of DTRs |
In case of facial swelling lasting longer than 48 h: treat with antihistamines, steroids, and/or hyaluronidase, with resolution both alone or in combination, without altering the vaccine efficacy ACE‐Is(lisinopril) have been recommended (not strongly) for the treatment of facial edema following COVID‐19 vaccination In case of symptoms after first vaccine dose, manage the side effects, reassure the patient and advise them to obtain their second dose, with pretreatment considerations (including antihistamines), and instruct them to present to the emergency department if a more severe reaction is suspected |
| 37 | Rice, S. M. | Pre‐vaccine counseling in cosmetic patients seeking fillers:
|
Concerns of DTRs to fillers |
|
|
Abbreviations: ABT, abatacept; ACE‐I, angiotensin converting enzyme inhibitors; AD, atopic dermatitis; ADA, adalimumab; AF, atrial fibrillation; AIIRD, autoimmune inflammatory rheumatic diseases; APS, antiphospholipid syndrome; AZA, azathioprine; CBC, complete blood count; CP, cyclophosphamide; CT, computed tomography; CTA, computed tomography angiography; CVD, cardiovascular disease; CYSP, cyclosporine; DIC, disseminated intravascular coagulation; DM, diabetes mellitus; DMARD, disease‐modifying anti‐rheumatic drugs; DTRs, delayed‐type hypersensitivity reactions; FA, first author; HIPA, heparin‐induced platelet activation; HIT, heparin‐induced thrombocytopenia; HUS, hemolytic uremic syndrome; IM, intramuscular; ITTP, immune thrombotic thrombocytopenic purpura; IV, intravenous; Lab, laboratory investigations; LMWH, low‐molecular‐weight heparin; MC, mast cell; MCAS, mast cell activation syndrome; MRI, magnetic resonance imaging; MTX, methotrexate; NM, not mentioned; NSIAD, non‐steroidal anti‐inflammatory drugs; PEG, polyethylene glycol; PF4, platelet factor 4; PLT, platelet; RMD, rheumatic and musculoskeletal diseases; RTX, rituximab; SC, subcutaneous; SLE, systemic lupus erythematosus; SRA, serotonin‐release assay; VTE, venous thromboembolism.
Supporting information Table S5.
These points have been categorized in the table as depicted below:
4. DISCUSSION
With the emerging knowledge of the adverse events following COVID‐19 immunization, an arising demand has been put on clinicians to be updated on the various mild to severe or potentially life‐ threatening manifestations of COVID vaccines, and whether or not certain patient groups can be vaccinated, or what precautions are to be taken with regard to their medical status. Much attention has been focused on hot topics regarding COVID‐19 prognostic and therapeutic options during the pandemic, with special focus on dermatologic concerns, 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 and now with the wide distribution of the COVID vaccines, a comprehensive assessment of the mucocutaneous eruptions associated with COVID‐19 immunization is an issue of great importance, so that with concrete knowledge, vaccination is not hindered by hesitancy through false beliefs about the extension or prevalence of adverse events or worries of flare‐ups or inefficient immunization, and that critical or potentially fatal adverse reactions are safely avoided.
We extracted any side effects of COVID‐19 vaccines reported in these studies with a special emphasis on mucocutaneous manifestations, comorbidities, lesion characteristics, time of onset, location, and duration of reactions, along with vaccine types, and further details regarding dosage, conjugated materials and age groups for the RCTs. It is important to mention that studies with no mucocutaneous manifestations were not included in our systematic review. Also, among the studies we did include, we did not extract the data on groups or subgroups that had no mucocutaneous reactions to the vaccines, so the results and numbers presented here are only on groups with mucocutaneous side effects.
Regarding the case reports and case series, we would like to also emphasize the importance of reporting registries and helping the medical community gather data on more unsolicited adverse events related to vaccination, that were perhaps less commonly observed in the initial trials, and through this cumulative international effort to report these events, many less solicited adverse reactions are now well‐known and clinicians are well aware of their potential emergence, and as expressed earlier, many guidelines, consensus recommendations and position papers have been written since.
Regarding the RCTs, the most common side effect among all trials was injection site pain, present in 95% of studies. Among the common systemic reactions were fatigue, fever, headache, chills, malaise, arthralgia, myalgia, nausea, vomiting and diarrhea. Among the common local (injection site) reactions reported in the RCTs were redness, swelling, induration, pruritis and warmth. Generalized pruritis, rash, hypersensitivity and lymphadenopathy (axillary) were also among the solicited general side effects. Less solicited or unsolicited mucocutaneous adverse events reported in the RCTs were mucocutaneous eruptions like pustules (at injection site or elsewhere), macular or maculo‐papular allergic rashes, petechial rash, urticaria, injection site discoloration or bruising, alopecia, contact dermatitis, acne‐form dermatitis, allergic conjunctivitis, hordeolum, chalazion, keratoconjunctivitis, uveitis, benign neoplasm of the eyelid, plantar fasciitis, swelling in the neck, chest wall or groin, oral herpes, dyshidrotic eczema, bacterial vaginosis, vaginal infection, vulvovaginal pruritus, anaphylactic reactions, cellulitis, psoriasis, rosacea, vitiligo, Raynaud's phenomenon, among other epidermal and dermal conditions, skin appendage conditions, dental and gingival conditions and oral soft tissue conditions. These side effects were sometimes more common in lower dose groups or younger age groups, which is an interesting observation. There was the same pattern with the primer and booster doses where sometimes side effects were more common after the booster doses and sometimes after the primers, so the trials' results were not all in the same direction on this matter. Among patients' comorbidities were obesity, hypertension, diabetes, history of or current COVID‐19, metabolic and endocrine conditions, allergies and hypersensitivities, asthma, cardiac and pulmonary conditions, psychiatric disorders, joint and back pain, positive HIV, malignancy and autoimmune illnesses. Moving on to safety assessments, most studies had observed the side effects within 7 days after vaccination, others extending the watch period to 14, 21, 28, or more days.
Regarding the analytical observational studies, a wide range of side effects after vaccination were reported in the studies. Among these studies, the most common reaction after COVID‐19 vaccination was local adverse events such as injection site pain and numbness. Apart from local events, systematic reactions such as fatigue followed by fever, myalgia, headache, bone pain, joint pain represented as the most common symptoms. Other mild adverse effects such as nausea, sweating, dizziness, diarrhea, vomiting, taste disturbance, itchy scratchy throat, insomnia, spasm, migraine, nasal obstruction, and rhinolalia were also observed in the reported results. To further categorize the reported adverse events, they mostly fell into seven groups; musculoskeletal, gastrointestinal, cardiovascular, neurological, respiratory, allergic reactions, and mucocutaneous symptoms. The mucocutaneous reactions ranged a broad spectrum from mild local reactions including swelling, redness, pruritus, rash, urticaria, and burning sensation of the skin, to more rare reactions such as erythromelalgia, morbilliform rash, contact dermatitis, oral ulcers, blisters and vesicles, bleeding and burning gingiva, angular cheilitis, swollen lips, xerostomia, flare of existing dermatologic conditions such as varicella‐zoster or herpes simplex flares, and pityriasis rosea‐like reactions. The rare mucocutaneous effects were efficiently controlled with steroids, antihistamines, and pain‐relievers. The side effects were most frequently reported within the first 3 days after vaccination, but there were also some studies reporting delayed adverse reactions, up to 4 weeks after vaccination. Duration of reactions varied from most frequently almost 1 day to as long as 4 weeks or more. The most common location of mucocutaneous lesions was upper limbs (injection site), trunk and face. Almost half of these studies reported participants' comorbidities. The most commonly reported comorbidities were hypertension, prior COVID‐19, diabetes mellitus, cardiovascular disorders, autoimmune, rheumatologic or allergic disorders, malignancies, obesity, and anaphylaxis. 47 , 57 , 59 , 65 , 67 , 70 , 71 , 72 , 73 , 74 , 78 , 118 , 136 , 137 , 138 , 139 In addition, less common co‐morbidities including asthma, thyroid disorder, psychological distress, hepatologic disease, and ophthalmologic disease were also mentioned in these studies.
Regarding the recommendations and guidelines for vaccination in specific groups, a generally positive view toward vaccination was expressed, inviting most groups to vaccinate, while having the necessary precautions in mind, and clarifying the contra‐indications so those with higher risks could safely avoid any severe adverse reactions or modify their vaccination and/or treatment schedules to achieve peak immunization, all the while having their underlying diseases under control.
In some cases, a true causality between proposed adverse events and vaccination was not concluded. In these situations, we relied on the authors' original statement, while considering patient's past medical history, notably previous allergic/hypersensitivity reactions to other vaccines or drugs, and the temporal course between vaccine administration and onset of the eurptions. Based on provided evidence, in patients with no alternative underlying source for the adverse eurptions, and in case the onset of reactions were compatible with our experience and current literature, usually occurring from 2–3 days to 3–4 weeks, a vaccine induced adverse reaction was ascertained.
The authors of this study have worked on various clinical aspects of COVID‐19, COVID‐19 vaccination and dermatology 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 140 , 141 and it seems that this hot topic could answer some questions and concerns about the most encountered specific disorder in the field of dermatology.
We hope the present article provides its audience with the state‐of‐the‐art knowledge that is essential in today's standard of care regarding the mucocutaneous adverse reactions following COVID‐19 vaccination.
5. CONCLUSION
Mild, moderate, severe, and potentially life‐threatening adverse events have been reported following immunization with COVID‐19 vaccines. It appears that although in the assessment of the pros and cons of vaccination, mucocutaneous adverse events could be one of the causes of vaccine hesitancy, they are nonetheless mostly non‐significant, self‐limiting reactions, and for the more uncommon moderate to severe reactions, guidelines and consensus position papers can be of great importance to provide those at higher risks and those with specific worries of flare‐ups or inefficient immunization, with sufficient recommendations to safely schedule their vaccine doses, or avoid vaccination if they have the discussed contra‐indications.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed to the preparation and finalization of this article.
Supporting information
Appendix S1: Search strategy.
Table S1: Supporting information – Table 1 References.
Table S2: Supporting information – Table 2 References.
Table S3: Supporting information – Table 3 References.
Table S4: Supporting information – Table 4 References.
Table S5: Supporting information – Table 5 References.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the staff of the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) for their technical and editorial assistance.
Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID‐19 vaccination and expert recommendations about vaccination of important immune‐mediated dermatologic disorders. Dermatologic Therapy. 2022;35(6):e15461. doi: 10.1111/dth.15461
Farnoosh Seirafianpour, Homa Pourriyahi, and Milad Gholizadeh Mesgarha contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly‐specific T cells in humans. Nature. 2021;595(7868):572‐577. [DOI] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frenck RW Jr, Klein Np, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. N Engl J Med. 2021;385(3):239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson EJ, Rouphael NG, Widge A, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383(20):1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA‐1273 SARS‐CoV‐2 vaccine. Vaccine. 2021;39(20):2791‐2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett JR, Belij‐Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS‐CoV‐2 vaccine ChAdOx1 nCoV‐19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 10. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV‐19 Covid‐19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 in HIV infection: a single‐arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8(8):e474‐e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voysey M, Costa Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Logunov DY, Dolzhikova IV, Shcheblyakov DM, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu FC, XH G, YH L, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396(10249):479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1‐2a trial of Ad26.COV2.S Covid‐19 vaccine. N Engl J Med. 2021;384(19):1824‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX‐CoV2373 Covid‐19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899‐1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keech C, Albert G, Cho C, et al. Phase 1‐2 trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chappell KJ, Mordant FL, Li Z, et al. Safety and immunogenicity of an MF59‐adjuvanted spike glycoprotein‐clamp vaccine for SARS‐CoV‐2: a randomised, double‐blind, placebo‐controlled, phase 1 trial. Lancet Infect Dis. 2021;21(10):1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S‐Trimer (SCB‐2019), a protein subunit vaccine candidate for COVID‐19 in healthy adults: a phase 1, randomised, double‐blind, placebo‐controlled trial. Lancet. 2021;397(10275):682‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS‐CoV‐2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo‐controlled, phase 1‐2, dose‐ranging study. Lancet Infect Dis. 2021;21(9):1257‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang S, Yan Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem‐repeat dimeric RBD‐based protein subunit vaccine (ZF2001) against COVID‐19 in adults: two randomised, double‐blind, placebo‐controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Hu Z, He J, et al. Safety and immunogenicity of a recombinant interferon‐armed RBD dimer vaccine (V‐01) for COVID‐19 in healthy adults: a randomized, double‐blind, placebo‐controlled. Phase I Trial Emerg Microbes Infect. 2021;10(1):1589‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryzhikov A, Ryzhikov EA, Bogryantseva MP, et al. A single blind, placebo‐controlled randomized study of the safety, reactogenicity and immunogenicity of the “EpiVacCorona” vaccine for the prevention of COVID‐19, in volunteers aged 18–60years (phase I–II). Russian J Infect Immun. 2021;11(2):283‐296. [Google Scholar]
- 29. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanriover MD, Levent Doğanay H, Akova A, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: A randomized clinical trial. JAMA. 2021;326(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: interim results from a double‐blind, randomised, multicentre, phase 2 trial, and 3‐month follow‐up of a double‐blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan HX, Liu JK, Huang BY, et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: randomized, double‐blind, and placebo‐controlled phase 1 and phase 2 clinical trials. Chin Med J (Engl). 2021;134(11):1289‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pu J, Yu Q, Yin Z, et al. The safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in Chinese adults aged 18‐59years: A phase I randomized, double‐blinded, controlled trial. Vaccine. 2021;39(20):2746‐2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward BJ, Gobeil P, Séguin S, et al. Phase 1 randomized trial of a plant‐derived virus‐like particle vaccine for COVID‐19. Nat Med. 2021;27(6):1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanghvi AR. COVID‐19: an overview for dermatologists. Int J Dermatol. 2020;59(12):1437‐1449. [DOI] [PubMed] [Google Scholar]
- 40. Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of covid‐19 vaccines: A systematic review and meta‐analysis of randomized clinical trials. Vaccine. 2021;9(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: a double‐blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccine. 2021;9(5):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ball P. The lightning‐fast quest for COVID vaccines ‐ and what it means for other diseases. Nature. 2021;589(7840):16‐18. [DOI] [PubMed] [Google Scholar]
- 44.FDA. Vaccine Adverse Event Reporting System (VAERS). Available from: https://vaers.hhs.gov/index.html.
- 45. Kadali RAK, Janagama R, Peruru S, Malayala SV, et al. Side effects of BNT162b2 mRNA COVID‐19 vaccine: A randomized, cross‐sectional study with detailed self‐reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun Q, Fathy R, McMahon DE, Freeman EE, et al. COVID‐19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39(4):653‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.. [DOI] [PubMed] [Google Scholar]
- 49. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine. JAMA. 2021;325(8):780‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. CDC COVID‐19 Response Team; Food and Drug Administration . Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID‐19 vaccine ‐ United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID‐19 vaccines in the US—December 14, 2020‐January 18, 2021. JAMA. 2021;325(11):1101‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Borobia AM, Carcas AJ, Pérez‐Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1‐S‐primed participants (CombiVacS): a multicentre, open‐label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID‐19 vaccine ‐ United States, march‐April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robinson LB, Fu X, Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID‐19 vaccines. JAMA Dermatol. 2021;157(8):1000‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abu‐Hammad O, Alduraidi H, Abu‐Hammad S, et al. Side effects reported by jordanian healthcare workers who received covid‐19 vaccines. Vaccine. 2021;9(6):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M, et al. Prevalence of COVID‐19 vaccine side effects among healthcare Workers in The Czech Republic. J Clin Med. 2021;10(7):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fernandez‐Nieto D, Hammerle D, Fernandez‐Escribano JH, et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. 'COVID‐arm': a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425‐e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cuomo, G. , Atteno M & Naclerio C, et al., POS1248 safety profile of Pfizer‐BioNTech Covid‐19 vaccine in patients with rheumatic diseases: preliminary assessment, BMJ Publishing Group Ltd, 2021
- 60. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rojas‐Pérez‐Ezquerra P, Crespo Quirós J, Tornero Molina P, Baeza Ochoa de Ocáriz ML. Safety of new mRNA vaccines against COVID‐19 in severely allergic patients. J Investig Allergol Clin Immunol. 2021;31(2):180‐181. [DOI] [PubMed] [Google Scholar]
- 62. Bae S, Lee YW, Lim SY, et al. Adverse reactions following the first dose of ChAdOx1 nCoV‐19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hwang YH, Song KH, Choi Y, et al . Can reactogenicity predict immunogenicity after COVID‐19 vaccination? Korean J Intern Med. 2021;36(6):1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cuschieri S, Borg M, Agius S, Souness J, Brincat A, Grech V. Adverse reactions to Pfizer‐BioNTech vaccination of healthcare workers at Malta's state hospital. Int J Clin Pract. 2021;75(10):e14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monin L, Laing AG, Muñoz‐Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Simpson CR, Shi T, Vasileiou E, et al. First‐dose ChAdOx1 and BNT162b2 COVID‐19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Riad A, Pokorná A, Mekhemar M, et al. Safety of chadox1 ncov‐19 vaccine: independent evidence from two eu states. Vaccine. 2021;9(6):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jeon M, Kim J, Oh CE, Lee JY. Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci. 2021;36(17):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaya F, Pirincci E. Determining the frequency of serious adverse reactions of inactive SARS‐COV‐2 vaccine. Work. 2021;69(3):735‐739. [DOI] [PubMed] [Google Scholar]
- 70. Zhang MX, Zhang TT, Shi GF, et al . Safety of an inactivated SARS‐CoV‐2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20(7):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pagotto V, Ferloni A, Soriano MM, et al. Active monitoring of early safety of sputnik V vaccine in Buenos Aires, Argentina. Medicina (B Aires). 2021;81(3):408‐414. [PubMed] [Google Scholar]
- 72. Elalamy I, Gerotziafas G, Alamowitch S, et al. SARS‐CoV‐2 vaccine and thrombosis: an expert consensus on vaccine‐induced immune thrombotic thrombocytopenia. Thromb Haemost. 2021;121(8):982‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Quiroga B, Sánchez‐Álvarez, Goicoechea, de Sequera, Spanish Society of Nephrology Council . COVID‐19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021;36(6):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Connolly Caoilfhionn M, Ruddy Jake A, Boyarsky Brian J, et al . Safety of the first dose of mRNA SARS‐CoV‐2 vaccines in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021;80(8):1100–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen G, Li X, Sun M, et al. COVID‐19 mRNA vaccines are generally safe in the short term: A vaccine vigilance real‐world study says. Front Immunol. 2021;12:669010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Samarakoon U, Alvarez‐Arango S, Blumenthal KG. Delayed large local reactions to mRNA Covid‐19 vaccines in blacks, indigenous persons, and people of color. N Engl J Med. 2021;385(7):662‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jęśkowiak I, Wiatrak B, Grosman‐Dziewiszek P, Szeląg A. The incidence and severity of post‐vaccination reactions after vaccination against COVID‐19. Vaccines (Basel). 2021;9(5):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mathioudakis AG, Ghrew M, Ustianowski A, et al. Self‐reported real‐world safety and reactogenicity of COVID‐19 vaccines: a vaccine recipient survey. Life. 2021;11(3):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Croce E, Hatz C, Jonker EF, et al. Safety of live vaccinations on immunosuppressive therapy in patients with immune‐mediated inflammatory diseases, solid organ transplantation or after bone‐marrow transplantation ‐ A systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35(9):1216‐1226. [DOI] [PubMed] [Google Scholar]
- 80. Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune‐mediated diseases. JAMA. 2012;308(1):43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bugatti S, De Stefano L, Balduzzi S, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80(12). [DOI] [PubMed] [Google Scholar]
- 82. Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology Guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheum. 2021;73(7):1093‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Park JK, Lee EB, Shin, et al. COVID‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean college of rheumatology. J Korean Med Sci. 2021;36(12):e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moutsopoulos HM. A recommended paradigm for vaccination of rheumatic disease patients with the SARS‐CoV‐2 vaccine. J Autoimmun. 2021;121:102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tam LS, Tanaka Y, Handa R, et al. Updated APLAR consensus statements on care for patients with rheumatic diseases during the COVID‐19 pandemic. Int J Rheum Dis. 2021;24(6):733‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Santosa A, Xu C, Arkachaisri T, et al. Recommendations for COVID‐19 vaccination in people with rheumatic disease: developed by the Singapore chapter of rheumatologists. Int J Rheum Dis. 2021;24(6):746‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bechman K, Dey M, Yates M, Bukhari M, Winthrop K, Galloway JB. The COVID‐19 vaccine landscape: what a rheumatologist needs to know. J Rheumatol. 2021;48(8):1201–1204. [DOI] [PubMed] [Google Scholar]
- 88. Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID‐19 task force guidance for management of psoriatic disease during the pandemic: version 2—advances in psoriatic disease management, COVID‐19 vaccines, and COVID‐19 treatments. J Am Acad Dermatol. 2021;84(5):1254‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mease PJ, Calabrese LH, Duffin KC, et al. Psoriasis and psoriatic arthritis in the context of the COVID‐19 pandemic: A plenary session from the GRAPPA 2020 annual meeting. J Rheumatol Suppl. 2021;97:24‐29. [DOI] [PubMed] [Google Scholar]
- 90. Giamarellos‐Bourboulis EJ, Bettoli V, Jemec GBE, et al. Anti‐COVID‐19 measurements for hidradenitis suppurativa patients. Exp Dermatol. 2021;30(1):18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Waldman RA, Creed M, Sharp K, et al. Toward a COVID‐19 vaccine strategy for patients with pemphigus on rituximab. J Am Acad Dermatol. 2021;84(4):e197‐e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Siegel CA, Melmed GY, McGovern DP, et al. SARS‐CoV‐2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70(4):635‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pfaar O, Klimek L, Hamelmann E, et al. COVID‐19 vaccination of patients with allergies and type‐2 inflammation with concurrent antibody therapy (biologicals) – A position paper of the German Society of Allergology and Clinical Immunology (DGAKI) and the German Society for Applied Allergology (AeDA). Allergologie. 2021;44(4):261‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ring J, Worm M, Wollenberg A, et al. Risk of severe allergic reactions to COVID‐19 vaccines among patients with allergic skin diseases – practical recommendations. A position statement of ETFAD with external experts. J Eur Acad Dermatol Venereol. 2021;35(6):e362‐e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Thyssen JP, Vestergaard C, Barbarot S, et al. European task force on atopic dermatitis: position on vaccination of adult patients with atopic dermatitis against COVID‐19 (SARS‐CoV‐2) being treated with systemic medication and biologics. J Eur Acad Dermatol Venereol. 2021;35(5):e308‐e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bonadonna P, Brockow K, Niedoszytko M, et al. COVID‐19 vaccination in mastocytosis: recommendations of the European Competence Network on mastocytosis (ECNM) and American Initiative in Mast Cell Diseases (AIM). J Allergy Clin Immunol Pract. 2021;9(6):2139‐2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stingeni L, Bianchi L, Zalaudek I, et al. Adverse cutaneous and mucous reactions from anti SARS‐CoV‐2 vaccines: recommendations from the Italian Society of Dermatology (SIDeMaST). J Eur Acad Dermatol Venereol. 2021;156(2):115‐117. [DOI] [PubMed] [Google Scholar]
- 98. Klimek L, Chaker AM, Cuevas M. Allergic reactions to COVID‐19 vaccinations ‐ what ENT doctors should know. Laryngorhinootologie. 2021;100(4):252‐258.33524996 [Google Scholar]
- 99. Peter J. COVID‐19 vaccination: recommendations for management of patients with allergy or immune‐based diseases. S Afr Med J. 2021;111(4):291‐294. [DOI] [PubMed] [Google Scholar]
- 100. Murphy KR, Patel NC, Ein D, et al. Insights from American College of Allergy, asthma, and immunology COVID‐19 vaccine task force: allergic reactions to mRNA SARS‐CoV‐2 vaccines. Ann Allergy Asthma Immunol. 2021;126(4):319‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kleine‐Tebbe J, Klimek L, Hamelmann E, et al. Severe allergic reactions to the COVID‐19 vaccine–statement and practical consequences. Allergol Select. 2021;5:26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tanno LK, Berard F, Beaudoin E, Didier A, Demoly P. Sars‐cov‐2 vaccination and anaphylaxis: recommendations of the french allergy community and the Montpellier world health organization collaborating center. Vaccine. 2021;9(6):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Untersmayr E, Förster‐Waldl E, Bonelli M, et al. Immunologically relevant aspects of the new COVID‐19 vaccines‐an ÖGAI (Austrian Society for Allergology and Immunology) and AeDA (German Society for Applied Allergology) position paper. Allergo J Int. 2021;30(5):155‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Worm M, Bauer A, Wedi B, et al. Practical recommendations for the allergological risk assessment of the COVID‐19 vaccination ‐ a harmonized statement of allergy centers in Germany. Allergol Select. 2021;5:72‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID‐19 vaccine‐associated anaphylaxis: a statement of the world allergy organization anaphylaxis committee. World Allergy Organ J. 2021;14(2):100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim MA, Lee YW, Kim SR, et al. COVID‐19 vaccine‐associated anaphylaxis and allergic reactions: consensus statements of the KAAACI Urticaria/angioedema/anaphylaxis working group. Allergy Asthma Immunol Res. 2021;13(4):526‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Klimek L, Bergmann KC, Brehler R, et al. Practical handling of allergic reactions to COVID‐19 vaccines: A position paper from German and Austrian allergy societies AeDA, DGAKI, GPA and ÖGAI. Allergo J Int. 2021;30(3):79‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Klimek L, Novak N, Hamelmann E, et al. Severe allergic reactions after COVID‐19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: position statement of the German allergy societies: medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2021;30(2):51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ferretti F, Cannatelli R, Benucci M, et al. How to manage COVID‐19 vaccination in immune‐mediated inflammatory diseases: an expert opinion by IMIDs study group. Front Immunol. 2021;12:656362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gresham LM, Marzario B, Dutz J, Kirchhof MG. An evidence‐based guide to SARS‐CoV‐2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol. 2021;84(6):1652‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang C, Rademaker M, Tate B, Baker C, Foley P. SARS‐CoV‐2 (COVID‐19) vaccination in dermatology patients on immunomodulatory and biologic agents: recommendations from the Australasian medical dermatology group. Australas J Dermatol. 2021;62(2):151‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hauptman M, Vasic J, Krase J. COVID‐19 vaccine and biologics: an impending dilemma. J Drugs Dermatol. 2021;20(1):115‐114. [DOI] [PubMed] [Google Scholar]
- 113. Chakravarthy K, Strand N, Frosch A, et al. Recommendations and guidance for steroid injection therapy and COVID‐19 vaccine administration from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2021;14:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41(3):184‐189. [DOI] [PubMed] [Google Scholar]
- 115. Gotkin RH, Gout U, Sattler S, et al. Global recommendations on COVID‐19 vaccines and soft tissue filler reactions: A survey‐based investigation in cooperation with the International Society for Dermatologic and Aesthetic Surgery (ISDS). J Drugs Dermatol. 2021;20(4):374‐378. [DOI] [PubMed] [Google Scholar]
- 116. Rice SM, Ferree SD, Kourosh AS. COVID‐19 vaccine reactions in dermatology: "filling" in the gaps. Cutis. 2021;107(6):288‐290. [DOI] [PubMed] [Google Scholar]
- 117. Rice SM, Ferree SD, Mesinkovska N, Shadi Kourosh A. The art of prevention: COVID‐19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7(2):209‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: A systematic review. Dermatol Ther. 2020;33(6):e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Atefi N, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N‐acetylcysteine and coronavirus disease 2019: may it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. J Res Med Sci. 2020;25(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bazargan M, Behrangi E, Goodarzi A. Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review. Iran J Dermatol. 2020;23(Suppl. 1):13–18. [Google Scholar]
- 121. Mohamadi M, Goodarzi A, Aryannejad A, et al. Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: A systematic review. Med J Islam Repub Iran. 2020;34:123‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Najar Nobari N, Seirafianpour F, Mashayekhi F, Goodarzi A. A systematic review on treatment‐related mucocutaneous reactions in COVID‐19 patients. Dermatol Ther. 2020;34(1):e14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nobari NN, Goodarzi A. Patients with specific skin disorders who are affected by COVID‐19: what do experiences say about management strategies? A systematic review. Dermatol Ther. 2020;33(6):e13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Systemic retinoids in the COVID‐19 era–are they helpful, safe, or harmful? A comprehensive systematized review. Iranian. J Dermatol. 2020;23(1):9‐12. [Google Scholar]
- 125. Seirafianpour F, Mozafarpoor S, Fattahi N, Sadeghzadeh‐Bazargan A, Hanifiha M, Goodarzi A. Treatment of COVID‐19 with pentoxifylline: could it be a potential adjuvant therapy? Dermatol Ther. 2020;33(4):e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Goodarzi A. A comprehensive review on COVID‐19 infection and comorbidities of various organs. Acta Med Iran. 2021;59(1):4‐14. [Google Scholar]
- 127. Kooranifar S, Sadeghipour A, Riahi T, Goodarzi A, Tabrizi S, Davoody N. Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: A large study from Iran with a high rate of anthracosis. Med J Islam Repub Iran. 2021;35(1):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Najar Nobari N, Seirafianpour F, Dodangeh M. A systematic review of the histopathologic survey on skin biopsies in patients with Corona virus disease 2019 (COVID‐19) who developed virus or drug‐related mucocutaneous manifestations. Exp Dermatol. 2021;30(9):1233–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nobari NN, Montazer F, Seirafianpour F, Nikkhah F, Aryanian Z, Goodarzi A. Histopathologic changes and cellular events of organs systems in COVID‐19. J Cell Mol Anesth. 2021;6(1):81‐88. [Google Scholar]
- 130. Sadeghzadeh‐Bazargan A, Rezai M, Najar Nobari N, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID‐19 patients. J Cutan Pathol. 2021;48(10):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kalantari S, Sadeghzadeh‐Bazargan A, Ebrahimi S, et al. The effect of influenza vaccine on severity of COVID‐19 infection: an original study from Iran. Med J Islam Repub Iran. 2021;35:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Riahi T, Sadeghzadeh‐Bazargan A, Shokri S, et al. The effect of opium on severity of COVID‐19 infection:an original study from Iran. Med J Islam Repub Iran. 2021;35:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lotfi Z, Haghighi A, Akbarzadehpasha A, Mozafarpoor S, Goodarzi A. Pansclerotic morphea following COVID‐19: A case report and review of literature on rheumatologic and non‐rheumatologic dermatologic immune‐mediated disorders induced by SARS‐CoV‐2. Front Med. 2021;8:728411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mashayekhi F, Seirafianpour F, Pour Mohammad A, Goodarzi A. Severe and life‐threatening COVID‐19‐related mucocutaneous eruptions: A systematic review. Int J Clin Pract. 2021;75(12):e14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tavakolpour S, Aryanian Z, Seirafianpour F, et al. A systematic review on efficacy, safety, and treatment‐durability of low‐dose rituximab for the treatment of pemphigus: special focus on COVID‐19 pandemic concerns. Immunopharmacol Immunotoxicol. 2021;43(5):507‐518. [DOI] [PubMed] [Google Scholar]
- 136. Ocáriz M, Zubeldia Ortuño J. Safety of new MRNA vaccines against COVID‐19 in severe allergic patients. J Investig Allergol Clin Immunol. 2021;31(2):180–181. [DOI] [PubMed] [Google Scholar]
- 137. Behrangi E, Ghassemi M, Sadeghzadeh‐Bazargan A, Roohaninasab M, Najar Nobari N, Goodarzi A. A systematic review of clinical studies on mucocutaneous manifestations of COVID‐19: virus‐related and drug‐related. Acta Med Iran. 2021;59(12):687‐698. [Google Scholar]
- 138. Hatami P, Aryanian Z, Nicknam Asl H, Goodarzi A. Mucocutaneous adverse effects following COVID‐19 vaccination: a comprehensive review of the literature with a presentation of some cases from Iran. Iran J Dermatol. 2021;24(4):331–338. [Google Scholar]
- 139. Kalantari Y, Sadeghzadeh‐Bazargan A, Aryanian Z, Hatami P, Goodarzi A. First reported case of delayed‐type hypersensitivity reaction to non‐hyaluronic acid polycaprolactone dermal filler following COVID‐19 vaccination: A case report and a review of the literature. Clin Case Rep. 2022;10:e05343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bidari A, Hassanzadeh M, Naderkhani M, et al. Predictors of critical COVID‐19 in an Iranian population: age and disabilities play a special role. Med J Islam Repub Iran. 2021;35(1):737‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bahadorizadeh L, Emamikhah M, Pour Mohammad A, et al. Simultaneous occurrence of cerebral venous sinus thrombosis and immune thrombocytopenic Purpura in a patient with a history of COVID‐19 infection. Neurol Ther. 2021;11(1):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Search strategy.
Table S1: Supporting information – Table 1 References.
Table S2: Supporting information – Table 2 References.
Table S3: Supporting information – Table 3 References.
Table S4: Supporting information – Table 4 References.
Table S5: Supporting information – Table 5 References.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


