Abstract
Introduction
The COVID‐19 pandemic has had a significant impact on global surgery. In particular, deleterious effects of SARS‐CoV‐2 infection on the heart and cardiovascular system have been described. To inform surgical patients, we performed a systematic review and meta‐analysis aiming to characterize outcomes of COVID‐19 positive patients undergoing cardiac surgery.
Methods
The study protocol was registered with PROSPERO (CRD42021228533) and conformed with PRISMA 2020 and MOOSE guidelines. PubMed, Ovid MEDLINE and Web of Science were searched between 1 January 2019 to 24 February 2022 for studies reporting outcomes on COVID‐19 positive patients undergoing cardiac surgery. Study screening, data extraction and risk of bias assessment were conducted in duplicate. Meta‐analysis was conducted using a random‐effects model where at least two studies had sufficient data for that variable.
Results
Searches identified 4223 articles of which 18 studies were included with a total 44 patients undergoing cardiac surgery. Within these studies, 12 (66.7%) reported populations undergoing coronary artery bypass graft (CABG) surgery, three (16.7%) aortic valve replacements (AVR) and three (16.7%) aortic dissection repairs. Overall mean postoperative length of ICU stay was 3.39 (95% confidence interval (CI): 0.38, 6.39) and mean postoperative length of hospital stay was 17.88 (95% CI: 14.57, 21.19).
Conclusion
This systematic review and meta‐analysis investigated studies of limited quality which characterized cardiac surgery in COVID‐19 positive patients and demonstrates that these patients have poor outcomes. Further issues to be explored are effects of COVID‐19 on decision‐making in cardiac surgery, and effects of COVID‐19 on the cardiovascular system at a cellular level.
Keywords: aortic dissection, bypass grafting, cardiac surgery, coronary artery, COVID‐19, emergency surgery
Cardiac Surgery on Patients with COVID‐19 – A Systematic Review and Meta‐Analysis
Introduction
The unprecedented coronavirus (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has had a dramatic effect on the global population. 1 The advent of vaccines in late 2020 resulted in many government programs encouraging and at times mandating vaccinations for sections of the population. Increasing vaccination among elderly, immunocompromised and vulnerable people resulted in lower infections, hospitalizations and deaths from the virus. Governments which had implemented physical distancing policies to reduce transmission, proceeded to remove these restrictions in part or full. 2 Recently, new variants of concern have provided a renewed threat due to their higher transmissibility and ability to evade vaccine defence. 3 , 4 , 5 , 6
Cardiac surgery comes with attendant risks. The decision to operate is based on the perceived risks and benefits discussed between a patient and surgeon. Patient factors are taken into account, along with operative considerations, clinical urgency and expected postoperative recovery. 7 , 8 Active infection with COVID‐19 represents a serious factor with the potential to cause morbidity and mortality. Various data regarding deleterious effects of CARS‐CoV‐2 in the heart and cardiovascular system have been reported. 9 , 10 To inform the decision‐making process in cardiac surgery worldwide, we performed a systematic review aiming to characterize the outcomes of COVID‐19 positive patients undergoing cardiac surgery.
Methods
We performed a systematic review according to a protocol registered prior to commencement with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021228533). Our results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 and the Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) guidelines 11 , 12 (Data S1–S2).
Search strategy and selection criteria
The population for included studies was patients who had COVID‐19 infection, defined as a positive laboratory diagnosis. The intervention was cardiac surgery. Where reported, the comparator was outcomes from cardiac surgical procedures in patients who did not have COVID‐19 infection. Reports of patients who were previously diagnosed with COVID‐19 but were deemed by the treating team to have cleared the infection were excluded. The primary outcome was mortality (both in‐hospital or 30‐day), while secondary outcomes included postoperative length of ICU and hospital stay, and postoperative complications including major adverse cardiovascular and cerebrovascular events.
PubMed, Ovid MEDLINE and Web of Science Core Collection databases were searched between 1 January 2019 and 24 February 2022. There were no other filters or restrictions applied. Search terms included (COVID OR ‘COVID‐19’ OR coronavirus OR ‘2019‐nCoV’ OR ‘SARS‐CoV‐2’) AND (‘cardiac surg*’ OR ‘cardiothoracic surg*’ OR ‘thoracic surg*’ OR ‘heart surg*’) AND (outcome* OR mortal*). These searches were supplemented by review of the grey literature, searching the bibliographies of included studies and targeted searches of Google Scholar and Scopus.
Data extraction
After removal of duplicate items, studies were reviewed for inclusion by two independent investigators (JNH and AL). This was performed with a free‐to‐use web application (Rayyan, Qatar Computing Research Institute, Ar‐Rayyan, Qatar 13 ). Studies were screened firstly by title and abstracts and subsequently full‐text reviews. Discrepancies were resolved by a third independent investigator (AKG). Extraction of Data was performed using a pre‐designed extraction form by two independent investigators (AKG and AL) and discrepancies resolved by consensus. Extracted data included study design, setting, population demographics, surgical intervention, postoperative length of ICU and hospital stay and outcomes. The extracted data were synthesized into narrative and tabular formats.
Data analysis
Data analyses were performed using Stata Statistical Software: Release 15.1 College Station, TX: StataCorp LP. The I2 statistic was used to evaluate heterogeneity (with I2 >50% indicating significant heterogeneity) as was Cochran's Q P value (with P‐value <0.05 indicating significant heterogeneity). A random‐effects model was used throughout. A P‐value of <0.05 denoted statistical significance. A variable was included in the meta‐analysis if at least two journal articles involved had sufficient values for that variable. However, as many studies were case studies with one subject only, there were not two studies to compare when dividing the analysis into type of surgery subgroups: CABG, aortic dissection repair and aortic valve replacement. Due to only four valid studies for each comparison, Funnel plots and Eggers Tests would not be informative, and although attempted, meta‐regression was found to require more than four studies. The Downs and Black risk of bias checklist 14 was used by two independent investigators (AKG and AL) to assess risk of bias and methodological quality of the included studies.
Results
Our search strategy identified 4223 records which were screened for duplicates, with a total of 2103 articles progressing to title and abstract screening. Of these, 309 were relevant and proceeded to full‐text review (Data S3). Ten full texts were unable to be obtained, and so 299 articles were assessed for eligibility based on the inclusion criteria. From these, 100 had the wrong population group or intervention, 105 reported the wrong outcomes, 56 were non‐observational studies and 29 in which the data were not extractable. Accordingly, a total of 18 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 articles fit the inclusion criteria and were included in our systematic review and meta‐analysis. This is outlined in Fig. 1.
Fig. 1.
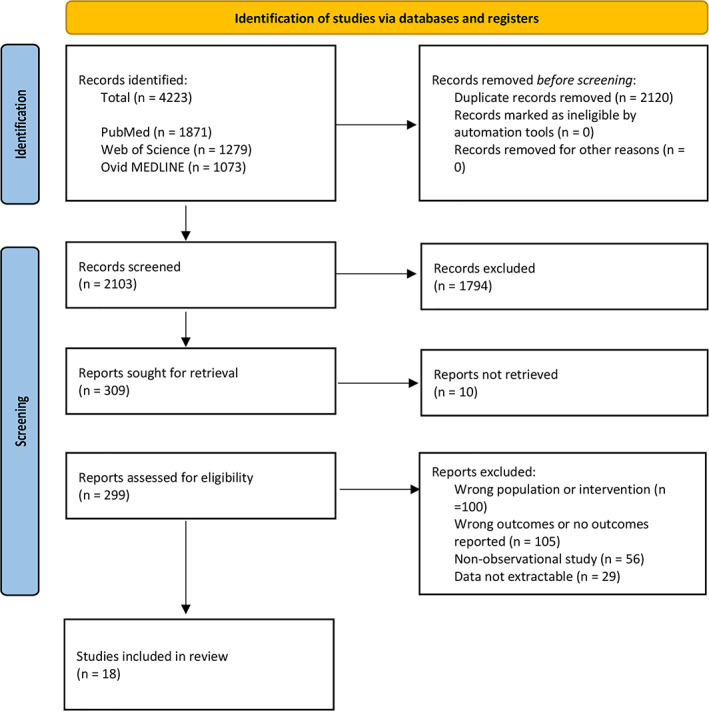
PRISMA diagram detailing the inclusion and exclusion of articles.
Study characteristics
The characteristics of the 18 included studies are outlined in Table 1. Regarding the country of origin of the included studies, three were from the United States (USA, 27.8%), three from the United Kingdom (16.7%) and two from Italy (11.1%). The total sample size across the 18 studies was 44 patients undergoing cardiac surgery. Within the included studies, 12 (66.7%) reported populations undergoing coronary artery bypass graft (CABG) surgery, three (16.7%) aortic valve replacements (AVR) and three (16.7%) aortic dissection repairs. Regarding sex in the total population, the majority of patients were male (61.4%). When it was reported, mean age was 56.7 years. Four studies reported either society of thoracic surgeons (STS) score 33 or European system for cardiac operative risk evaluation 34 (EuroSCORE), and one study reported both scores.
Table 1.
Characteristics of included studies
| Study | Country | Setting | Design | Sample size | Case | Follow‐up | Source of funding | Risk of bias (percentage)† |
|---|---|---|---|---|---|---|---|---|
| Fukuhara (2020) 15 | United States | Hospital | Case study | 1 | Aortic dissection repair | Short term (to clinic) | None | 59.38 |
| Fukuhara (2020) 16 | United States | Hospital | Case series | 24 | Aortic dissection repair | Hospital Admission | None | 67.19 |
| Hussain (2020) 17 | United Kingdom | Hospital | Case study | 1 | AVR | Hospital Admission | None | 62.50 |
| Rescigno (2020) 18 | United Kingdom | Hospital | Case study | 1 | CABG | Hospital Admission | None | 62.50 |
| Salna (2020) 19 | United States | Hospital | Case study | 1 | CABG | Hospital Admission | None | 65.63 |
| Silveira (2020) 20 | Brazil | Hospital | Case study | 1 | CABG | Hospital Admission | None | 65.63 |
| Varela Barca (2020) 21 | Spain | Hospital | Case study | 1 | AVR | Hospital Admission | None | 59.38 |
| Yandrapalli (2020) 22 | United States | Hospital | Case study | 1 | CABG | Hospital Admission | None | 65.63 |
| Farsky (2021) 23 | Brazil | Hospital | Case series | 3 | CABG | Hospital Admission | None | 62.50 |
| Romiti (2020) 24 | Italy | Hospital | Case study | 1 | CABG | Hospital Admission | None | 62.50 |
| Farina (2020) 25 | Italy | Hospital | Case study | 1 | CABG | Hospital Admission | None | 60.94 |
| Montandrau (2020) 26 | France | Hospital | Case study | 1 | CABG | Hospital Admission | None | 64.06 |
| Schwerzmann (2021) 27 | Switzerland | Hospital | Cohort | 1 | AVR + Ascending Aorta Replacement | Hospital Admission | Internal research funding | 65.63 |
| Soetisna (2021) 28 | Indonesia | Hospital | Case study | 1 | CABG | Hospital Admission | Not stated | 64.06 |
| Darvishi (2021) 29 | Iran | Hospital | Case study | 1 | CABG + Pericardiotomy | Hospital Admission | Not stated | 56.25 |
| Lopez‐Marco (2021) 30 | UK | Hospital | Cohort | 3 | Aortic Dissection Repair | Hospital Admission | None | 64.06 |
| Keaton‐Nasser (2021) 31 | USA | Hospital | Case study | 1 | CABG | Hospital Admission | Not stated | 53.13 |
| Omar(2021) 32 | Qatar | Hospital | Case Series | 3 | CABG | Hospital Admission | Corporate | 62.50 |
Average score on downs and black checklist. 28
Patient characteristics
Regarding patient characteristics in the overall cohort, 13 (29.5%) had type 2 diabetes mellitus, 29 (65.9%) had hypertension, 15 (34.1%) had a smoking history, three (6.8%) had chronic obstructive pulmonary disease COPD, two (4.5%) had preoperative stroke. The mean left ventricular ejection fraction (LVEF) was 46.8%, 10 (22.7%) had coronary artery disease and nine (20.5%) had previous cardiac surgery; of these, four (9.1%) had previous CABG surgery, and three (6.8%) had a past AVR.
Patient outcomes
Across the included studies, 28 (63.6%) patients experienced postoperative complications. Of these, 12 (27.3%) experienced ARDS, six (13.6%) experienced cerebrovascular complications specifically and four (9.1%) patients required extracorporeal membrane oxygenation (ECMO). Both in‐hospital and 30‐day mortality was experienced in 12 (27.3%) patients. When reported, mean postoperative length of ICU stay was 7.4 days, and mean postoperative hospital length of stay was 14.5 days.
Mean and standard deviation of Postoperative length of ICU and hospital stay was pooled across four studies using a random effects meta‐analysis model. Heterogeneity in the study estimates was assessed using the I‐squared statistic (0%) and Cochran's Q P‐value (0.797) which showed no heterogeneity. Because a random effects model was used degree of heterogeneity is not relevant however. The overall mean postoperative length of ICU stay was 3.39 (95% confidence interval (CI): 0.38, 6.39), while overall mean postoperative length of hospital stay was 17.88 (95% CI: 14.57, 21.19). This is shown in Figs 2 and 3. Meta‐analysis was not possible for the other planned outcomes.
Fig. 2.
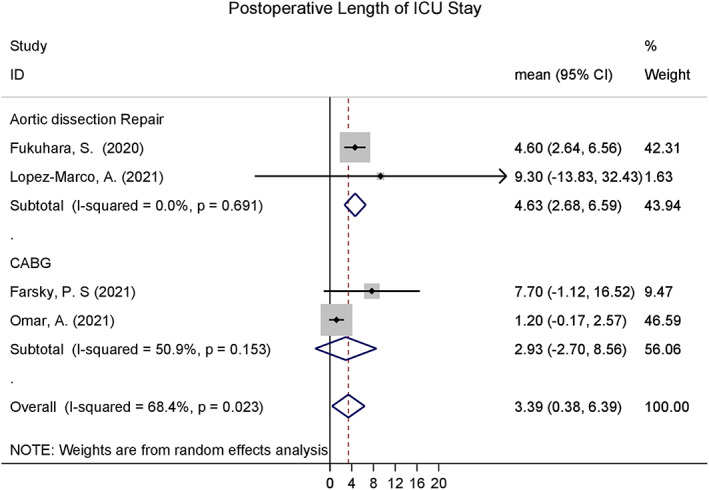
Postoperative length of ICU stay pooled across included studies.
Fig. 3.
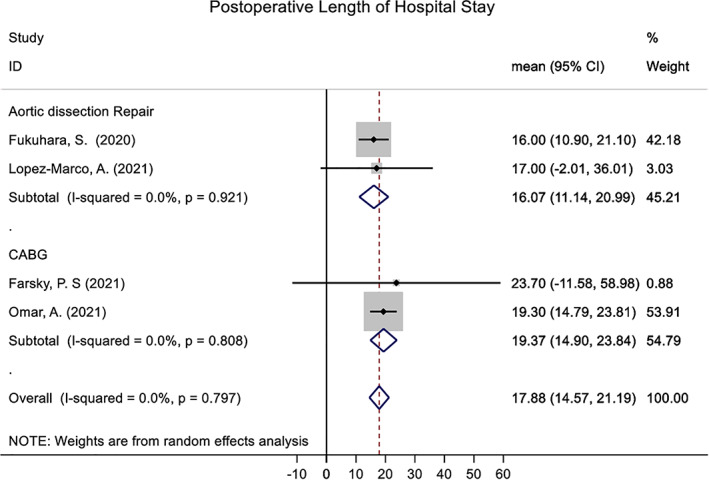
Postoperative length of hospital stay pooled across included studies.
Risk of bias
The included studies were of moderate methodological quality upon critical appraisal using the Downs and Black checklist. 14 Overall percentages on risk of bias assessments for the included studies can be found in Table 1 and the individual breakdown of these scores can be found in Data S4. Mean scores, representing the average of the two reviewer scores, were calculated for each category. Calculated means were as follows: total mean score 19.8 out of 32 (SD 1.22); reported sub‐scale mean score 6.1 out of 11 (SD 0.90); external validity sub‐scale mean score 3 out of 3 (SD 0); bias sub‐scale mean score 4.9 out of 7 (SD 0.16); confounding sub‐scale mean score 3.4 out of 6 (SD 0.36); power sub‐scale mean score 3.1 out of 5 (SD 0.51).
Discussion
This systematic review and meta‐analysis is the first to characterize the global literature regarding outcomes after cardiac surgery in COVID‐19 positive patients. Our study suggests that COVID‐19 positive patients undergoing cardiac surgery have long postoperative length of stay in hospital and ICU and overall poor outcomes of high morbidity and mortality. Our review identified poor short‐term outcomes in patients undergoing cardiac surgery with concomitant COVID‐19 infection. Myocardial damage from infection may represent one large factor, combined with the physiological and inflammatory stresses associated with cardiopulmonary bypass, need for mechanical ventilation and ischaemia reperfusion. 35 It is likely that the cohort studied represents cases of urgent and emergency surgery that could not otherwise be postponed, which has also been shown as a risk factor for poorer post‐operative outcomes. 36 , 37 Although not shown in our study, issues within hospitals and health systems may also contribute to this finding, as available resources may have been diverted to treat other COVID‐19 patients.
COVID‐19 infection causes significant damage to the cardiovascular system. A large meta‐analysis of 1527 patients found that 8% of patients with COVID‐19 had associated cardiac injury. 38 There is a growing body of evidence suggesting that endothelial dysfunction through the Angiotensin‐converting enzyme 2 (ACE‐2) receptor can cause severe myocardial injury. 39 , 40 , 41 , 42 COVID‐19 uses severe acute respiratory syndrome coronavirus (SARS‐CoV) receptor ACE‐2 to enter host cells. 41 It has been shown that susceptibility to SARS coronavirus S protein‐driven infection correlates with expression of ACE‐2, 43 and accordingly, higher ACE‐2 expression may also lead to higher risk of SARS‐CoV‐2 infection. The systemic inflammatory response syndrome (SIRS) associated with COVID‐19, in severe disease, results in a cytokine release syndrome which can cause injury to vascular endothelium and cardiac myocytes. 44 , 45 , 46 , 47 A number of pro‐inflammatory cytokines are released, including interleukin‐2 (IL‐2), IL‐6, IL‐8, IL‐10 and tumour necrosis factor alpha (TNF‐α). During severe inflammation, this can result in asystemic inflammatory response syndrome (ARDS) and multi‐organ dysfunction. 48 , 49 The elevated cardiometabolic demand from systemic inflammation, combined with ongoing hypoxia caused by pneumonia or ARDS, is associated with myocardial damage. 46 This process can also result in plaque rupture or coronary thrombosis causing acute coronary syndrome. 50 , 51 An American study of 21 patients admitted with COVID‐19 to an intensive care unit (ICU) demonstrated one‐third of patients had new‐onset cardiomyopathy with decreased left ventricular function and clinical signs of cardiogenic shock with elevated cardiac enzymes. 52 In some case series, upto half of patients who died from COVID‐19 had some form of heart failure. 53 , 54
Arrhythmias and sudden cardiac arrest have been documented in patients with COVID‐19. These pathologies are thought to stem from cardiac injury arising from hypoxia, poor coronary perfusion, myocardial injury, SIRS and the proarrhythmic effect of certain medications used to treat COVID‐19. The pro‐inflammatory state brought on by COVID‐19 infection also has a significant role. Animal studies from past pandemics due to H1N1, SARS and Middle Eastern Respiratory Syndrome (MERS) showed a significant association between a past history of cardiovascular disease and myocardial injury due to cardiomyopathy causing atrioventricular dilatation and impaired ejection fraction. 55 Severe COVID‐19 disease also manifests acute myocardial injury as a by‐product, demonstrated by the finding that patients who were admitted to ICU as a result of COVID‐19 infection had significantly higher creatine kinase myocardial band (CK‐MB) and troponin I levels. 39 Elevated cardiac troponin levels is associated with ventricular tachycardia and fibrillation. 56 Accordingly, the rate of developing cardiac arrhythmia was found to be more than six times greater for patients admitted to ICU. Given that many cardiovascular diseases are acute manifestations of underlying chronic disease processes, it is hypothesised that the hyper‐inflammatory microenvironment produced by COVID‐19 causes an imbalance between infection induced increase in metabolic demand and diminished cardiorespiratory reserve. This causes acute instability leading to coronary plaque events, arrhythmias and sudden cardiac death. 57
The majority of evidence relating to cardiac surgery during COVID‐19 is opinion‐based, usually deriving from polls of cardiac surgeons across prominent centres. A poll of cardiac surgeons in the UK produced a consensus view that all patients should be screened for COVID‐19 pre‐surgery, full PPE at all times to prevent spread between patients and importantly the decision to hold a multidisciplinary team (MDT) meeting for every case of cardiac surgery in a COVID‐19 positive patient. 58 These MDTs believed aortic and mitral surgery could be pursued in select cases however the role of CABG was more controversial amongst the surgeons. The CovidSurg Collaborative explored the impact of COVID‐19 on surgical services. Data from CovidSurg suggests that delaying surgery for 4 weeks after a positive COVID‐19 test is beneficial to outcomes in Cancer related surgeries, and this is echoed in the wider literature. 59 , 60 The CovidSurg Collaborative also found that swab testing all patients before major surgery was beneficial in preventing complications; this benefit was amplified in areas with higher COVID‐19 transmission and more radical surgeries. 59 The most marked effect on surgical decision‐making has been the introduction of COVID‐19 screening for surgical patients. Initially this screening was CT based however more rapid swab kits are now the preferred method, these swab kits are more effective than CT screening alone. 59
An important subgroup of patients who have been significantly affected by COVID‐19 are transplant recipients. These patients are routinely on immunosuppressive regimens to protect their grafts, regarding which there are limited data available, on immunosuppressive agents and their interactions with the COVID pathogen. Clearly, these are vulnerable patients. A multicentre review paper by Bottio et al. 61 found a twofold increase in the prevalence of COVID‐19 amongst heart transplant recipients compared with the general population, and attributed this to the higher susceptibility to infections due to chronic immunosuppressive therapy. They noted that the 25% case fatality rate for heart transplant recipients, regardless of age, was equivalent to that of Italians aged over 70. There is some literature which has shown that ceasing immunosuppressive therapy can be associated with positive outcomes in COVID‐19 patients, 62 however there is some contention, given that this can precipitate immunological memory and subsequent allograft rejection.
There are limitations to our study. Ten texts were identified through title and abstract screening which could not be retrieved, and given the small evidence base, the absence of these papers may impact our results. While our study aimed to include large cohort studies, more than half of included studies were single patient case reports which limits the generalizability of our results to larger, heterogeneous patient populations. Given that a large proportion of our studies originated from the USA and Western Europe, regional differences in the COVID‐19 virus and healthcare systems more broadly may not be accounted for. Further studies investigating the outcomes of COVID‐19 positive patients who undergo cardiac surgery may yield different results compared with our review, in particular more recent studies conducted after the date of our search. Our study did not capture COVID‐19 positive patients who required cardiac surgery but failed to survive in the operating room. 63
During the COVID‐19 pandemic, cardiac surgery has faced many challenges. Many cardiac surgeons have had to change their daily practice, with some offering their services in critical care units to meet the demand caused by the pandemic. 64 Not only have these placed surgeons into an unfamiliar environment, but the pandemic‐associated risks for healthcare workers contributes to an increased level of anxiety. Due to the redistribution of resources in many countries towards critical care services for patients suffering from COVID‐19, cardiac surgical procedures have been frequently delayed. 65 As a result, there has been a significant decrease cardiac surgeries performed during the pandemic. 66 Similarly, there has been a restructuring in the delivery of cardiac surgery to more centralized units. 67 The combined effect of this has been to create a significant backlog of patients who require cardiac surgery but faced cancellations due to insufficient resources, hospital beds or personal protective equipment for staff. 68 Patients who have a clinical urgency should be prioritized for treatment, a challenge given the ongoing pandemic setting.
Conclusion
This systematic review and meta‐analysis investigated studies of limited quality which characterized outcomes after cardiac surgery in COVID‐19 positive patients. Our study demonstrates that these patients have poor outcomes. Further issues to be explored are the effect of COVID‐19 on decision‐making in cardiac surgery, and the effects of COVID‐19 on the cardiovascular system at a cellular level.
Author contributions
Aashray K. Gupta: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; visualization; writing – original draft; writing – review and editing. Alasdair Leslie: Data curation; formal analysis; investigation; validation; writing – original draft; writing – review and editing. Joseph N. Hewitt: Data curation; formal analysis; investigation; methodology; visualization; writing – review and editing. Joshua G. Kovoor: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Christopher D. Ovenden: Conceptualization; formal analysis; investigation; methodology; validation; visualization; writing – review and editing. Suzanne Edwards: Formal analysis; investigation; methodology; resources; software; visualization; writing – original draft; writing – review and editing. Justin C. Y. Chan: Formal analysis; investigation; methodology; supervision; validation; visualization; writing – review and editing. Michael G. Worthington: Formal analysis; investigation; methodology; supervision; writing – review and editing.
Conflict of interest
None declared.
Supporting information
Appendix S1: Supporting Information
Acknowledgment
Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
A. K. Gupta MBBS GDipSurgAnat MS; A. Leslie MBBS; J. N. Hewitt MBBS MMed; J. G. Kovoor BHlthMedSc (Hons) MBBS; C. D. Ovenden MBBS GDipSurgAnat MS; S. Edwards BN GDipMStat GDipMa; J. C. Y. Chan MBBS MPhil FRACS; M. G. Worthington MBChB FRACS.
References
- 1. Organisation WH . Naming the Coronavirus Disease (COVID‐19) and the Virus that Causes it. Geneva: World Health Organization, 2020. [Google Scholar]
- 2. Dhama K, Sharun K, Tiwari R et al. COVID‐19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020; 16: 1232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nyberg T, Twohig KA, Harris RJ et al. Risk of hospital admission for patients with SARS‐CoV‐2 variant B. 1.1. 7: cohort analysis. BMJ 2021; 373: n1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Twohig KA, Nyberg T, Zaidi A et al. Hospital admission and emergency care attendance risk for SARS‐CoV‐2 delta (B. 1.617. 2) compared with alpha (B. 1.1. 7) variants of concern: a cohort study. Lancet Infect Dis. 2022; 22: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bager P, Wohlfahrt J, Fonager J et al. Risk of hospitalisation associated with infection with SARS‐CoV‐2 lineage B. 1.1. 7 in Denmark: an observational cohort study. Lancet Infect. Dis. 2021; 21: 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karim SSA, Karim QA. Omicron SARS‐CoV‐2 variant: a new chapter in the COVID‐19 pandemic. Lancet 2021; 398: 2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JCY, Gupta AK, Stewart S et al. "nobody told me": communication issues affecting Australian cardiothoracic surgery patients. Ann. Thorac. Surg. 2019; 108: 1801–6. [DOI] [PubMed] [Google Scholar]
- 8. Chan JC, Gupta AK, Babidge WJ, Worthington MG, Maddern GJ. Technical factors affecting cardiac surgical mortality in Australia. Asian Cardiovasc. Thorac. Ann. 2019; 27: 443–51. [DOI] [PubMed] [Google Scholar]
- 9. Puntmann VO, Carerj ML, Wieters I et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; 5: 1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajpal S, Tong MS, Borchers J et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID‐19 infection. JAMA Cardiol. 2021; 6: 116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 13. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J. Epidemiol. Community Health 1998; 52: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukuhara S, Rosati CM, El‐Dalati S. Acute type a aortic dissection during the COVID‐19 outbreak. Ann. Thorac. Surg. 2020; 110: e405–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuhara S, Tang H, Kim KM et al. Type a aortic dissection during COVID‐19 pandemic: report from tertiary aortic centers in the United States and China. Semin. Thorac. Cardiovasc. Surg. 2021; 33: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hussain A, Khan H, Lopez‐Marco A, Roberts N, Oo A. Cardiac surgery in patients with confirmed COVID‐19 infection: early experience. J. Card. Surg. 2020; 35: 1351–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rescigno G, Firstenberg M, Rudez I, Uddin M, Nagarajan K, Nikolaidis N. A case of postoperative Covid‐19 infection after cardiac surgery: lessons learned. Heart Surg. Forum 2020; 23: E231–e3. [DOI] [PubMed] [Google Scholar]
- 19. Salna M, Polanco A, Bapat V, George I, Argenziano M, Takeda K. A case of coronavirus disease 2019 (COVID‐19) presenting after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2020; 160: e193–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silveira L, Guerreiro GP, Lisboa LAF et al. Coronary artery bypass graft during the COVID‐19 pandemic. Braz. J. Cardiovasc. Surg. 2020; 35: 1003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varela Barca L, Torralba Cloquell I, Herrero Cereceda J, Sáez de Ibarra JI. An unexplained death after routine cardiac surgery: how long have we dealt with coronavirus disease 2019? Interact. Cardiovasc. Thorac. Surg. 2020; 31: 904–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yandrapalli S, Cooper HA, Malekan R. Successful coronary artery bypass operation in a SARS‐COV‐2 infected patient with acute coronary syndrome. J. Card. Surg. 2020; 35: 2361–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farsky PS, Feriani D, Valente BBP et al. Coronary artery bypass surgery in patients with COVID‐19: what have we learned? Circ. Cardiovasc. Qual. Outcomes 2021; 14: e007455. [DOI] [PubMed] [Google Scholar]
- 24. Romiti S, Totaro M, Laderchi A, Peruzzi M, Vinciguerra M, Greco E. Case report: emergency CABG following failure of PTCA in a COVID‐19 patient. Front Cardiovasc Med. 2020; 7: 620610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farina A, Uccello G, Spreafico M, Bassanelli G, Savonitto S. SARS‐CoV‐2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur. J. Intern. Med. 2020; 76: 100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montandrau O, Arana H, Ehooman F et al. Surgical revascularization with cardiopulmonary bypass on a patient with severe COVID‐19. Semin. Cardiothorac. Vasc. Anesth. 2021; 25: 46–50. [DOI] [PubMed] [Google Scholar]
- 27. Schwerzmann M, Ruperti‐Repilado FJ, Baumgartner H et al. Clinical outcome of COVID‐19 in patients with adult congenital heart disease. Heart 2021; 107: 1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soetisna TW, Buana AC, Tirta ES et al. A 48‐year‐old man at low risk for SARS‐CoV‐2 infection who underwent planned elective triple‐Vessel coronary artery bypass graft surgery at a National Heart Center in Indonesia followed by a fatal case of COVID‐19. Am J Case Rep. 2021; 22: e928900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darvishi M, Shahali H. Acute cardiac tamponade: a case of life‐threatening coronavirus disease 2019 complication during air medical transportation. Air Med. J. 2021; 40: 179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopez‐Marco A, Harky A, Malvindi PG et al. Type a aortic syndromes in COVID‐19 positive patients: case series from a UKmulticentre study. J. Card. Surg. 2021; 36: 2692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nasser K, Rana J, Ahsan C. COVID‐19 and acute coronary syndrome with multi‐VESSEL disease needing CABG. J. Am. Coll. Cardiol. 2021; 77: 2028. [Google Scholar]
- 32. Omar AS, Shoman B, Sudarsanan S, Shouman Y. Chest radiography requirements for patients with asymptomatic COVID‐19 undergoing coronary artery bypass surgery: three case reports. World J Virol. 2021; 10: 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shahian DM, O'Brien SM, Filardo G et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann. Thorac. Surg. 2009; 88: S2–S22. [DOI] [PubMed] [Google Scholar]
- 34. Nashef SA, Roques F, Sharples LD et al. Euroscore ii. Eur. J. Cardiothorac. Surg. 2012; 41: 734–45. [DOI] [PubMed] [Google Scholar]
- 35. Marini JJ, Gattinoni L. Management of COVID‐19 respiratory distress. JAMA 2020; 323: 2329–30. [DOI] [PubMed] [Google Scholar]
- 36. Chan JCY, Gupta AK, Stewart SK et al. Mortality in Australian cardiothoracic surgery: findings from a National Audit. Ann. Thorac. Surg. 2020; 109: 1880–8. [DOI] [PubMed] [Google Scholar]
- 37. Mullen MG, Michaels AD, Mehaffey JH et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining "quality" and reporting outcomes for urgent surgery. JAMA Surg. 2017; 152: 768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B, Yang J, Zhao F et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin. Res. Cardiol. 2020; 109: 531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann M, Kleine‐Weber H, Schroeder S et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tikellis C, Thomas MC. Angiotensin‐converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012; 2012: 256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hofmann H, Geier M, Marzi A et al. Susceptibility to SARS coronavirus S protein‐driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004; 319: 1216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Z, Shi L, Wang Y et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020; 8: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat. Rev. Cardiol. 2020; 17: 259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur. Heart J. 2020; 41: 1798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clerkin KJ, Fried JA, Raikhelkar J et al. COVID‐19 and cardiovascular disease. Circulation 2020; 141: 1648–55. [DOI] [PubMed] [Google Scholar]
- 48. Shi Y, Wang Y, Shao C et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020; 27: 1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin C, Zhou L, Hu Z et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin. Infect. Dis. 2020; 71: 762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schoenhagen P, Tuzcu EM, Ellis SG. Plaque vulnerability, plaque rupture, and acute coronary syndromes: (multi)‐focal manifestation of a systemic disease process. Circulation 2002; 106: 760–2. [DOI] [PubMed] [Google Scholar]
- 51. Dominguez‐Erquicia P, Dobarro D, Raposeiras‐Roubín S, Bastos‐Fernandez G, Iñiguez‐Romo A. Multivessel coronary thrombosis in a patient with COVID‐19 pneumonia. Eur. Heart J. 2020; 41: 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arentz M, Yim E, Klaff L et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA 2020; 323: 1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen T, Wu D, Chen H et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alexander LK, Small JD, Edwards S, Baric RS. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J. Infect. Dis. 1992; 166: 978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo T, Fan Y, Chen M et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; 5: 811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID‐19. J. Cardiovasc. Electrophysiol. 2020; 31: 1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benedetto U, Goodwin A, Kendall S, Uppal R, Akowuah E. A nationwide survey of UKcardiac surgeons' view on clinical decision making during the coronavirus disease 2019 (COVID‐19) pandemic. J. Thorac. Cardiovasc. Surg. 2020; 160: 968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kovoor JG, Tivey DR, Williamson P et al. Screening and testing for COVID‐19 before surgery. ANZ J. Surg. 2020; 90: 1845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bottio T, Bagozzi L, Fiocco A et al. COVID‐19 in heart transplant recipients: a multicenter analysis of the northern Italian outbreak. JACC Heart Fail. 2021; 9: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu L, Xu X, Ma K et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am. J. Transplant. 2020; 20: 1859–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tabaghi S, Akbarzadeh MA. Acute type a aortic dissection in a patient with COVID‐19. Futur. Cardiol. 2021; 17: 625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fudulu DP, Angelini GD. Cardiac surgery in the time of the coronavirus. J. Card. Surg. 2020; 35: 1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Babidge WJ, Tivey DR, Kovoor JG et al. Surgery triage during the COVID‐19 pandemic. ANZ J. Surg. 2020; 90: 1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mohamed Abdel Shafi A, Hewage S, Harky A. The impact of COVID‐19 on the provision of cardiac surgical services. J. Card. Surg. 2020; 35: 1295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bonalumi G, di Mauro M, Garatti A, Barili F, Gerosa G, Parolari A. The COVID‐19 outbreak and its impact on hospitals in Italy: the model of cardiac surgery. Eur. J. Cardiothorac. Surg. 2020; 57: 1025–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frankel WC, Nguyen TC, Weiss AJ. Charting a safe and expeditious course Back to elective cardiac surgery during the COVID‐19 pandemic. Innovations (Phila). 2020; 15: 296–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


