Summary
Since the start of the COVID‐19 pandemic, few studies have reported anaesthetic outcomes in parturients with SARS‐CoV‐2 infection. We reviewed the labour analgesic and anaesthetic interventions utilised in symptomatic and asymptomatic parturients who had a confirmed positive test for SARS‐CoV‐2 across 10 hospitals in the north‐west of England between 1 April 2020 and 31 May 2021. Primary outcomes analysed included the analgesic/anaesthetic technique utilised for labour and caesarean birth. Secondary outcomes included a comparison of maternal characteristics, caesarean birth rate, maternal critical care admission rate along with adverse composite neonatal outcomes. A positive SARS‐CoV‐2 test was recorded in 836 parturients with 263 (31.4%) reported to have symptoms of COVID‐19. Neuraxial labour analgesia was utilised in 104 (20.4%) of the 509 parturients who went on to have a vaginal birth. No differences in epidural analgesia rates were observed between symptomatic and asymptomatic parturients (OR 1.03, 95%CI 0.64–1.67; p = 0.90). The neuraxial anaesthesia rate in 310 parturients who underwent caesarean delivery was 94.2% (95%CI 90.6–96.0%). The rates of general anaesthesia were similar in symptomatic and asymptomatic parturients (6% vs. 5.7%; p = 0.52). Symptomatic parturients were more likely to be multiparous (OR 1.64, 95%CI 1.19–2.22; p = 0.002); of Asian ethnicity (OR 1.54, 1.04–2.28; p = 0.03); to deliver prematurely (OR 2.16, 95%CI 1.47–3.19; p = 0.001); have a higher caesarean birth rate (44.5% vs. 33.7%; OR 1.57, 95%CI 1.16–2.12; p = 0.008); and a higher critical care utilisation rate both pre‐ (8% vs. 0%, p = 0.001) and post‐delivery (11% vs. 3.5%; OR 3.43, 95%CI 1.83–6.52; p = 0.001). Eight neonates tested positive for SARS‐CoV‐2 while no differences in adverse composite neonatal outcomes were observed between those born to symptomatic and asymptomatic mothers (25.8% vs. 23.8%; OR 1.11, 95%CI 0.78–1.57; p = 0.55). In women with COVID‐19, non‐neuraxial analgesic regimens were commonly utilised for labour while neuraxial anaesthesia was employed for the majority of caesarean births. Symptomatic women with COVID‐19 are at increased risk of significant maternal morbidity including preterm birth, caesarean birth and peripartum critical care admission.
Keywords: COVID‐19, general anaesthesia, labour analgesia, pregnancy, regional anaesthesia
Introduction
COVID‐19, caused by the SARS‐CoV‐2 virus, has spread rapidly around the globe since the first reported case in late 2019. Multiple reviews published on COVID‐19 in pregnancy have now reported higher rates of emergency caesarean birth, pre‐eclampsia, admission to the intensive care unit (ICU), preterm birth and adverse fetal outcomes, particularly in symptomatic parturients [1, 2, 3, 4, 5, 6, 7, 8].
At the start of the COVID‐19 pandemic in 2020, recommendations from various national as well as international societies advocated the use of neuraxial labour analgesia and anaesthesia (where feasible) in parturients with SARS‐CoV‐2 infection [9, 10, 11]. These recommendations were based not only on decreasing the risks associated with general anaesthesia (GA) in parturients with acute respiratory illness, but also to minimise the exposure of healthcare staff to aerosol generating procedures potentially associated with infection. Our previous study in the north‐west of England found that the rate of GA for caesarean birth declined markedly in the first wave of the pandemic from 7.7% to 3.7%, though these data included only a limited number of parturients with SARS‐CoV‐2 infection [12]. Paradoxically however, a recent registry‐based study in the USA highlighted a lower rate of neuraxial labour analgesia in parturients with symptomatic COVID‐19, and a higher rate of GA for emergency caesarean birth [13]. To date, there have been no large‐scale multicentre studies of labour analgesia and anaesthetic interventions in parturients who tested positive for SARS‐CoV‐2 before childbirth in the UK, though some single centres have reported anaesthetic outcomes in a small number of parturients affected by COVID‐19 [14, 15].
Using data from 10 maternity units across the north‐west of England, we conducted a retrospective cohort study to investigate the anaesthetic and analgesic techniques used, and maternal and neonatal outcomes, among parturients who tested positive for SARS‐CoV‐2 infection between 1 April 2020 and 31 May 2021.
Methods
We collated anaesthesia, maternal and neonatal records of all parturients with antenatal SARS‐CoV‐2 infection diagnosed in hospital by respiratory tract reverse transcriptase polymerase chain reaction (PCR) between 1 April 2020 and 31 May 2021, at 10 hospitals across the north‐west of England. Participating hospitals included tertiary units with >7000 deliveries annually (St. Mary's Hospital and Liverpool Women's Hospital) and general units with <7000 deliveries annually (Wythenshawe Hospital, North Manchester General Hospital, Burnley General Teaching Hospital, Royal Preston Hospital, Stepping Hill Hospital, Royal Bolton Hospital, Royal Albert Edward Infirmary Wigan and Royal Oldham Hospital). All hospitals were participating in the UK Obstetric Surveillance Study (UKOSS) on COVID‐19 during pregnancy and the National Maternal and Perinatal Audit and contributing data to the Intensive Care National Audit and Research Centre [16]. Guidelines for provision of anaesthesia services from the Royal College of Anaesthetists and the National Institute for Health and Care Excellence recommend collecting data on provision of neuraxial analgesia for labour as well as anaesthetic technique utilised for caesarean birth as part of quality improvement [17, 18]. As a result, ethical approval was not deemed to be necessary though local governance approvals and appropriate permissions were obtained before commencing data collection at all participating hospitals.
Maternal information acquired included: baseline characteristics (age, ethnicity and body mass index (BMI) at booking); obstetric history including comorbidities; date of SARS‐CoV‐2‐positive test; presence and details of any symptoms (respiratory and or non‐respiratory reported by the patient) at the time of the test; need for oxygen supplementation at the time of hospital admission; pre‐delivery haemoglobin, white cell count, lymphocyte count and platelet count; mode of delivery; and if a caesarean birth was performed, the indication and category of caesarean birth as per the Royal College of Obstetricians and Gynaecologists classification [19]. Anaesthesia information included (as appropriate): labour analgesia technique for vaginal delivery and anaesthetic technique for caesarean delivery. Post‐partum data collected included: admission to ICU; the need for mechanical ventilation; and total length of ICU and hospital stay. Neonatal data collected included: gestational age at birth; birth weight; APGAR scores at 1 and 5 minutes of age; umbilical artery and umbilical vein pH; tracheal intubation; admission to neonatal intensive care unit (NICU); and SARS‐CoV‐2 infection status. The total number of live births and the caesarean delivery rate at the participating hospitals in the study period were also recorded.
Parturients were assigned to cohorts of symptomatic SARS‐CoV‐2 infection and asymptomatic infection. Primary outcomes included pharmacological labour analgesia techniques used (none; nitrous oxide; opioid including pethidine, diamorphine or remifentanil; labour epidural) and anaesthetic technique for caesarean delivery (spinal; epidural; GA; combined technique). Secondary outcomes included: maternal characteristics; maternal comorbidities; haematological investigations pre‐delivery; caesarean birth rates; ICU admission rates; length of ICU and hospital stay; and neonatal outcomes, using a composite of adverse neonatal outcomes of Apgar score < 7 at 5 min, umbilical arterial pH < 7.20, SARS‐CoV‐2‐positive rates, NICU admission rate, tracheal intubation, stillbirth and mortality. An overall comparison of caesarean birth rates in SARS‐CoV‐2 positive/negative parturients across the maternity units was also performed.
Rates and effect sizes were estimated from the data as stratified by hospital to obtain pooled estimates with 95%CI. Multilevel linear, quantal and logistic mixed‐effects regression models were used for analyses, stratified by hospital as random coefficients. Fisher's expanded exact p value was used to compare distributions in categories. Analyses were performed using Stata 16.1 (StataCorp Inc., College Station, TX, USA) and p < 0.05 (two‐sided) was used to define statistical significance.
Results
Over the 1‐y study period, 57,800 births were recorded at the 10 participating maternity units including 18,871 (32.65%) caesarean deliveries. A total of 836 parturients tested positive for SARS‐CoV‐2 antenatally. Of these, 263 (31.4%) were symptomatic while 573 (68.6%) were asymptomatic at the time of testing. Respiratory symptoms were reported in 134 parturients (51%). Cough was the most common respiratory symptom, reported in 125 parturients (47.5%), followed by shortness of breath in 91 (32.3%) and chest pain in 14 (5.3%). Fever was the most common non‐respiratory symptom seen in 91 parturients (34.6%) followed by anosmia in 44 (16.7%), gastro‐intestinal symptoms in 37 (14.1%) and joint pains in 27 (10.3%). Maternal baseline characteristics, comorbidities and laboratory investigations were generally comparable (Table 1), though some differences were noted: symptomatic parturients were older (p = 0.042); less likely to be primiparous (p = 0.002); and delivered at a significantly earlier gestational age (p = 0.001). The overall preterm birth rate (< 37 weeks) was 16.9%, with a higher rate in symptomatic parturients (25.4% vs. 13.2%; OR 2.16, 95%CI 1.47–3.19; p = 0.001). Parturients of Asian ethnicity were more likely to be symptomatic than White parturients (p = 0.005). Symptomatic parturients were also noted to have significantly lower haemoglobin levels, lower platelet count and lower lymphocyte count. Only 10 parturients (1.2%) had platelet counts < 100,000 μl‐1.
Table 1.
Baseline characteristics, obstetric and medical data for COVID‐19‐positive parturients. Values are mean (SD), number (proportion) and median (IQR [Range]).
|
Symptomatic n = 263 |
Asymptomatic n = 573 |
Effect size (95% CI) | p value | |
|---|---|---|---|---|
| Age; y | 30.8 (5.7) | 29.9 (5.8) | 0.9 (0.0–1.7) | 0.042 |
| BMI; kg.m‐2 | 28.1 (6.6) | 27.3 (6.2) | 0.7 (−0.2–1.7) | 0.132 |
| Gestation; weeks | 37.4 (3.1) | 38.2 (2.9) | −0.7 (−1.2 to −0.3) | 0.001 |
| Prematurity; < 37 weeks | 64 (25.4%) | 74 (13.2%) | 2.16 (1.47–3.19) | 0.001 |
| Parity | 1.0 (0.0–2.0 [0.0–9.0]) |
1.0 (0.0–2.0 [0.0–8.0]) |
0.4 (0.1–0.6) | 0.004 |
| Primiparity | 85 (33.1%) | 251 (44.6%) | 0.61 (0.45–0.84) | 0.002 |
| Ethnicity | 0.005 | |||
| White | 124 (50.2%) | 342 (62.0%) | Reference | |
| Asian | 84 (34.0%) | 127 (23.0%) | 1.54 (1.04–2.28) | 0.033 |
| Black | 26 (10.5%) | 44 (8.0%) | 1.62 (0.93–2.82) | 0.089 |
| Other | 13 (5.3%) | 39 (7.1%) | 0.98 (0.49–1.95) | 0.947 |
| Comorbidities | ||||
| Smoking | 22 (8.6%) | 53 (9.4%) | 1.0 (0.59–1.72) | 0.979 |
| Obesity; > 30 kg.m2 | 87 (32.8%) | 140 (25.1%) | 1.40 (1.01–1.94) | 0.046 |
| Anaemia * | 74 (28.7%) | 157 (28.9%) | 0.99 (0.71–1.38) | 0.937 |
| Diabetes | 27 (10.5%) | 71 (12.6%) | 0.78 (0.49–1.27) | 0.320 |
| Hypertensive disorders of pregnancy | 25 (9.7%) | 48 (8.5%) | 1.15 (0.70–1.92) | 0.580 |
| Sepsis in labour | 11 (4.3%) | 12 (2.1%) | 2.01 (0.86–4.71) | 0.109 |
| Asthma | 15 (5.8%) | 34 (6.0%) | 0.97 (0.48–1.86) | 0.999 |
| Cardiac | 3 (1.2%) | 12 (2.1%) | 0.54 (0.15–1.94) | 0.348 |
| Other | 53 (20.6%) | 89 (15.8%) | 1.39 (0.93–2.05) | 0.092 |
| Investigations | ||||
| Haemoglobin; g.l−1 | 114.9 (14.2) | 117.0 (13.7) | −2.15 (−4.26 to −0.03) | 0.047 |
| Platelet count; x109.l‐1 | 229.0 (72.2) | 240.5 (72.6) | −11.7 (−22.6 to −0.81) | 0.035 |
| Leucocyte count; x109.l‐1 | 10.2 (4.0) | 10.8 (3.7) | −0.54 (−1.11–0.03) | 0.064 |
| Lymphocyte count; x109.l‐1 | 1.61 (0.71) | 1.82 (0.78) | −0.21 (−0.32 to −0.09) | 0.001 |
Other conditions include: mental health issues; hypothyroidism; gastro‐intestinal; dermatological; autoimmune; and haematological conditions like sickle cell trait.
Defined as haemoglobin pre‐delivery <105 g.l‐1. BMI ‐ body mass index
Vaginal birth (spontaneous and instrumental) was reported in 526 parturients (62.9%) of whom 146 (27.8%) were symptomatic. Of the primary outcomes, documented in 509 patients, the most commonly used pharmacological agents for labour analgesia included a 50:50% mixture of nitrous oxide and oxygen in 339 parturients (66.6%) and opioid (including remifentanil, diamorphine or pethidine) in 181 (35.5%). Fewer parturients in the symptomatic group utilised remifentanil patient‐controlled analgesia (2.8% vs. 7.7%, OR 3.13, 95%CI 1.04–9.09; p = 0.042). Labour epidural was utilised for pain relief in 104 (20.4%) parturients, with no differences noted between symptomatic and asymptomatic parturients (20.7% vs. 20.3%, OR 1.03, 95%CI 0.64–1.67; p = 0.90) (Table 2).
Table 2.
Primary outcome: labour analgesia and anaesthesia for caesarean birth in COVID‐19 positive parturients. Values are number (proportion).
|
COVID‐19 symptomatic n = 263 |
COVID‐19 asymptomatic n = 573 |
Odds ratio (95%CI) | p value | |
|---|---|---|---|---|
| Analgesia a | n = 145 | n = 364 | 0.661 | |
| None | 26 (17.9%) | 69 (19.0%) | 0.95 (0.55–1.67) | 0.870 |
| 50:50% mixture of N2O/O2 | 98 (67.6%) | 241 (66.2%) | 0.99 (0.61–1.62) | 0.969 |
| Opioid | 43 (29.7%) | 106 (29.1%) | 1.01 (0.65–1.55) | 0.972 |
| Remifentanil PCA | 4 (2.8%) | 28 (7.7%) | 0.32 (0.11–0.96) | 0.042 |
| Epidural | 30 (20.7%) | 74 (20.3%) | 1.03 (0.64–1.67) | 0.899 |
| Anaesthesia | n = 117 | n = 193 | 0.449 | |
| General | 7 (6.0%) | 11 (5.7%) | Reference | |
| Spinal | 103 (88.0%) | 161 (83.4%) | 1.02 (0.38–2.75) | 0.974 |
| Epidural | 6 (5.1%) | 20 (10.3%) | 0.46 (0.12–1.77) | 0.260 |
| Combined spinal–epidural | 1 (0.9%) | 1 (0.5%) | 1.42 (0.07–27.61) | 0.818 |
Details available for only 509 patients that had a vaginal birth. Odds ratios and 95%CI were estimated for each mode using multilevel mixed‐effects logistic regression, stratified by hospital, as appropriate. PCA ‐ patient‐controlled analgesia.
A caesarean birth was reported in 310 parturients (37.1%) of whom 117 (37.7%) were symptomatic. Regional (neuraxial) anaesthesia was utilised successfully in 292 parturients (94.2%) with a spinal anaesthetic utilised for 85.2% of caesarean births. Regional anaesthesia to GA conversion was reported in five parturients (1.7%), comprising failed neuraxial anaesthesia in four parturients and one due to intra‐operative haemorrhage; all parturients who had regional anaesthesia to GA conversion were asymptomatic. The rates of GA for caesarean birth were similar in symptomatic and asymptomatic parturients who tested positive for SARS‐CoV‐2 (6.0% vs. 5.7%; p = 0.52) (Table 2).
Our analysis of secondary outcome measures reveals that 104 parturients (12.4%) who tested positive for SARS‐CoV‐2 had an elective caesarean birth (Table 3). Overall, symptomatic parturients (117/263, 44.5%) had a higher rate of caesarean birth (44.5% vs. 33.7%; OR 1.57, 95%CI 1.16–2.12; p = 0.003) (Table 4).
Table 3.
Secondary outcomes: mode of delivery in COVID‐19‐positive parturients. Values are number (proportion).
|
COVID‐19 symptomatic n = 263 |
COVID‐19 asymptomatic n = 573 |
Odds ratio (95%CI) |
p value | |
|---|---|---|---|---|
| Category of caesarean birth | n = 117 | n = 193 | 0.008 | |
| 1 | 24 (20.5%) | 44 (22.8%) | Reference | |
| 2 | 34 (29.1%) | 55 (28.5%) | 1.14 (0.58–2.22) | 0.703 |
| 3 | 29 (24.8%) | 20 (10.4%) | 2.63 (1.22–5.67) | 0.013 |
| 4 | 30 (25.6%) | 74 (38.3%) | 0.75 (0.39–1.46) | 0.398 |
| Vaginal birth | n = 146 | n = 380 | 0.110 | |
| Normal | 124 (84.9%) | 300 (78.9%) | Reference | |
| Assisted | 22 (15.1%) | 80 (21.1%) | 0.65 (0.38–1.10) |
Odds ratios, 95%CI and p values for each category were estimated using multilevel mixed‐effects logistic regression, stratified by hospital, as appropriate.
Table 4.
Secondary outcomes: caesarean birth rates in COVID‐19‐positive/negative parturients. Values are number (proportion).
| COVID‐19 symptomatic | COVID‐19 asymptomatic | COVID‐19‐negative | |
|---|---|---|---|
| COVID‐19 symptomatic |
117 (44.5%) |
||
| COVID‐19 asymptomatic |
1.57 (1.17–2.12) p = 0.003 |
193 (33.7%) | |
| COVID‐19‐negative |
1.73 (1.35–2.20) p = 0.001 |
1.10 (0.92–1.30) p = 0.307 |
18,561 (32.6%) |
Results are shown in a matrix format with the observed data on the diagonal. Odds ratios, 95%CI and p values were estimated using multilevel mixed‐effects logistic regression, stratified by hospital, as appropriate.
Comparing all caesarean births stratified across hospitals, parturients with SARS‐CoV‐2 infection had a higher caesarean birth rate than parturients without SARS‐CoV‐2 (37% v 32.4%; OR 1.22; 95%CI 1.04–1.43; p = 0.016) (Fig. 1). Caesarean birth was most often performed for fetal indications (31.6%), with COVID‐19 and increasing oxygen requirements contributing to 5.8% of all caesarean births (online Supporting Information Table S1).
Figure 1.
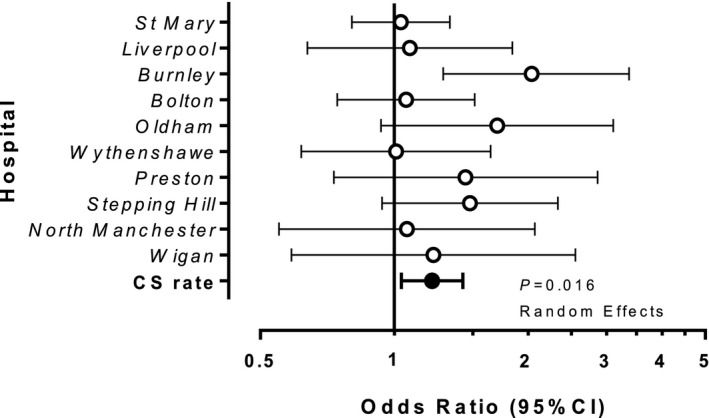
Effects of COVID‐19 on caesarean birth rates. COVID‐19‐positive parturients (n = 836) are compared with non‐exposed controls (n = 56,964). Pooled odds ratio and CI for caesarean birth are significantly increased by COVID‐19 at 1.22 (95%CI 1.04, 1.43; p = 0.016). Data were analysed using a random effects model, stratified by hospital, as appropriate.
Oxygen supplementation pre‐delivery was reported in 52 (19.8%) of all symptomatic parturients who tested positive for SARS‐CoV‐2. No difference in post‐partum haemorrhage rates was noted between symptomatic and asymptomatic parturients. Symptomatic parturients were more likely to need an ICU bed during the antepartum period (8% vs. 0%; OR ∞, 95%CI 12.2 ‐ ∞; p = 0.001) as well as during the post‐partum period (11% vs. 3.5%; OR 3.43 95%CI 1.83–6.52; p = 0.001), and more likely to require mechanical ventilation of their lungs. Length of ICU and hospital stay were longer in symptomatic parturients (Table 5). Tracheostomy was performed in two symptomatic parturients (0.85%) while extracorporeal membrane oxygenation was required in one parturient (0.42%). No maternal mortality was reported. Eight neonates (0.95%) tested positive for SARS‐CoV‐2; no differences in composite neonatal outcomes were observed between neonates whose mothers were symptomatic or asymptomatic (online Supporting Information Table S2).
Table 5.
Secondary outcomes: maternal outcomes in COVID‐19‐positive parturients. Values are number (proportion). or median (IQR[Range]).
|
COVID‐19 symptomatic |
COVID‐19 asymptomatic |
Effect size (95%CI) |
p value |
|
|---|---|---|---|---|
| Post‐partum haemorrhage | 0.241 | |||
| > 500–1000 ml | 45 (29.6%) | 87 (30.4%) | ||
| > 1000 ml | 9 (5.9%) | 30 (10.5%) | ||
| Oxygen supplementation | 52 (19.8%) | 0 | ∞ (36.4 to ∞) | 0.001 |
| ICU pre‐delivery | 21 (8.0%) | 0 | ∞ (12.2 to ∞) | 0.001 |
| Level 2 | 20 (7.6%) | 0 | ||
| Level 3 | 1 (0.4%) | 0 | ||
| ICU post‐delivery | 29 (11.0%) | 20 (3.5%) | 3.43 (1.83–6.52) | 0.001 |
| Level 2 | 17 (6.5%) | 20 (3.5%) | ||
| Level 3 | 12 (4.6%) | 0 | ||
| Mechanical ventilation | 8 (3.0%) | 0 | ∞ (3.8 to ∞) | 0.001 |
| ICU stay; days | 5.0 (2.0–9.0 [1.0–59.0]) | 1.0 (1.0–2.0 [1.0–2.0]) | 4.0 (1.5–6.5) | 0.003 |
| Hospital stay; days | 3.0 (2.0–5.0 [0.0–74.0]) | 2.0 (1.0–3.0 [0.0–10.0]) | 1.0 (0.5–1.5) | 0.001 |
Effect sizes are odds ratio or median difference with 95%CI.
Discussion
To the best of our knowledge, this is one of the largest observational multicentre studies focusing specifically on analgesic and anaesthetic interventions in parturients who tested positive for SARS‐CoV‐2 antenatally [13, 20, 21]. We also believe it to be the first that investigates UK practice in parturients infected with SARS‐CoV‐2.
The labour epidural analgesia rate in SARS‐CoV‐2‐positive parturients in the north‐west of England was 20.4%. This mirrors the mean epidural analgesia rate of 21% reported in the UK National Obstetric Anaesthesia Database analysis [22]. Considered in the context of this existing evidence, our study suggests that COVID‐19 neither hampered nor accelerated the uptake of epidural labour analgesia in our region of the UK [23]. Our study was not designed to ascertain the reasons why uptake of neuraxial labour analgesia in the north‐west of England was not higher, contrary to recommendations [9, 10, 11]. Factors such as parturient choice; easy availability of non‐neuraxial analgesics; presence of maternal symptoms such as fever; significant thrombocytopenia (1.2% in our study); shortage of personal protective equipment during the early onset of the pandemic; and healthcare professionals' anxiety and apprehension of contracting SARS‐CoV‐2 while performing an epidural could have contributed to this finding [13, 24, 25].
Nitrous oxide and parenteral opioids are well‐established non‐neuraxial labour analgesia techniques in the UK. Pre‐pandemic, almost 60% of parturients used nitrous oxide, while nearly 25% of parturients utilised parenteral opioids during labour [26, 27]. We found similar rates of nitrous oxide usage and higher rates of parenteral opioid usage in our study, which may be reflective of the high incidence of asymptomatic infection. Further, delivery of nitrous oxide/oxygen by demand valve was not deemed to be an aerosol generating procedure as per the UK guidelines and this technique could be readily administered by midwifery staff without modification to the ‘droplet precautions’ personal protective equipment [9].
Remifentanil patient‐controlled analgesia has been suggested as an alternative labour analgesic to neuraxial techniques in SARS‐CoV‐2‐positive parturients, providing that maternal oxygen saturations are >95% [9]. To our knowledge, our study is one of the first to highlight a remifentanil patient‐controlled analgesia utilisation rate of 3.8% for labour analgesia in this group.
The regional anaesthesia rate of 94.2% for caesarean birth in our study is not surprising since most guidelines and societies encouraged the use of regional anaesthesia in this cohort [9, 10, 11]. Spinal anaesthesia was the most frequently utilised (85%) anaesthetic technique for caesarean delivery. The reasons for using a spinal anaesthetic over an epidural may include a lower‐risk of conversion to a GA (and thus avoidance of an aerosol generating procedure) and a superior quality of sensory and motor block.
The overall GA rate for caesarean birth in women who tested positive for SARS‐CoV‐2 was 5.8% in our study. This is lower than the mean GA rate of 8.75% reported pre‐pandemic in the UK National Obstetric Anaesthesia Database analysis [22], but higher than our previously reported rate of 3.7% in hospitals in north‐west England in the early stages of the pandemic [12]. Our current data, collected over a longer time period and with more participating hospitals and over 10 times as many parturients, indicate that the GA rate increased in this cohort as the pandemic progressed. Factors that may have contributed to this finding may include a higher proportion of symptomatic parturients presenting for caesarean birth, a better understanding of the risks associated with so‐called aerosol generating procedures [28, 29] and better availability of personal protective equipment allowing anaesthetists to take appropriate precautions during a GA. The most common indications for administering a GA among symptomatic parturients in our study were clinical urgency (e.g. category‐1 caesarean), escalating oxygen requirements and thrombocytopenia. Failed neuraxial anaesthesia was a contributory factor for conversion to GA in asymptomatic parturients.
Our findings indicate that parturients with symptomatic COVID‐19 in this region are more likely to be of Asian ethnicity, have lower lymphocyte and platelet counts and are more likely to experience preterm delivery. These findings align with multiple reviews reporting maternal outcomes in parturients with SARS‐CoV‐2 infection [1, 4, 5, 8]. The higher caesarean birth rate in our study, especially in symptomatic parturients, corroborates the findings of the published UK Obstetric Surveillance System (UKOSS) studies which reported caesarean birth rates varying from 40 to 60% in COVID‐19‐affected parturients [4, 8].
Significant morbidity was experienced by parturients with symptomatic COVID‐19 during the peripartum period. Our overall ICU (level 2 or level 3) utilisation rate during the peripartum period was 8.4%, which is similar to that quoted by the international study by Villar et al. (8.4%) [5] and the UK‐based UKOSS studies (9–10%) [4, 8]. Higher mechanical ventilation rates and increased duration of ICU and hospital stay have been consistently reported in symptomatic parturients [5, 6, 7, 8]. We observed a preterm birth rate of 16% in our study, which may have contributed to the overall NICU admission rate of 13.9% and a neonatal tracheal intubation rate of 2.5%. No differences were noticed in stillbirth or in hospital neonatal mortality rates between symptomatic and asymptomatic patients in our study. Again, this is in line with the published evidence from UKOSS studies [4, 8].
Comparing our data with other studies, we note some differences with practices in other countries. Most strikingly, labour epidural utilisation rates ranged from 47% to 80% in parturients with SARS‐CoV‐2 infection in France, Europe and the USA [13, 20, 21]. In these regions, however, the usual uptake of epidural in women during labour (not affected by SARS‐CoV‐2) is 60–80%, much higher than the UK, so higher rates of labour epidural analgesia are unsurprising [13, 21, 22, 30]. Studies from Europe and the USA have reported that <2% of SARS‐CoV‐2‐positive parturients utilised parenteral opioids for labour analgesia [13, 21], and no parturients utilised nitrous oxide for pain relief in the study from the USA [13]; however, nitrous oxide is uncommonly used for labour analgesia in the USA [26, 31]. The view that parenteral opioids may worsen respiratory symptoms in COVID‐19 parturients may also have been a contributory factor to low parenteral opioid usage in these studies. The available literature suggests that labour analgesia regimens observed in SARS‐CoV‐2‐positive parturients across different countries may simply reflect the usual national and regional pre‐pandemic trends. Our study findings seem to support this assumption when viewed in combination with pre‐pandemic UK data [22]. The regional anaesthesia rate for caesarean birth in our study was higher than reported in studies from the USA (91.3%) [13], Europe (75%) and France (72%) [20, 21], and comparable with a small single‐centre study from Turkey (95%) [32]. Accordingly, the GA rate for caesarean section was lower in our sample than in other studies [13, 20, 21]. Notably, parturients with symptomatic COVID‐19 (primarily respiratory failure) were quoted to have a higher rate of GA for caesarean section in the studies from the USA [13, 20].
Data from our study emphasise that parturients with symptomatic SARS‐CoV‐2 infection, especially those needing respiratory support pre‐delivery, should be counselled by the multidisciplinary team on the maternal and fetal risks associated with COVID‐19. The decision‐making including the mode of delivery along with labour analgesia and anaesthetic choices should form part of this individualised discussion, taking into account patient preferences, cardiorespiratory symptoms and the risks and benefits posed by each anaesthetic and analgesic technique.
Our study has a number of limitations. The lack of SARS‐CoV‐2‐negative controls limits the inferences that can be drawn. The findings in this study come from a limited number of consultant‐led maternity units in the north‐west of England and these may not necessarily be reflected nationally or in the community setting. The retrospective nature of the study makes it prone to selection and information bias. Routine testing was introduced into maternity units from May 2020, hence some women who were symptomatic but untested may have not been studied. Finally, our study did not examine maternal interventions such as the use of steroids, immunomodulatory therapies or vaccination in symptomatic or asymptomatic parturients, all of which might have influenced maternal outcomes.
Overall, we conclude that, among parturients in the north‐west of England who tested positive for the SARS‐CoV‐2 virus, non‐neuraxial analgesic regimens were commonly utilised for labour. Neuraxial blockade was employed for the majority of caesarean births, though its utilisation rate for labour analgesia appeared to follow national pre‐pandemic trends. Significant maternal morbidity characterised by higher preterm births, higher caesarean birth rates and higher peripartum critical care admission rates are common in symptomatic parturients with COVID‐19.
Supporting information
Appendix S1. Group of Obstetric Anaesthetists of Lancashire, Greater Manchester and Mersey (GOAL GM) Study Writing Group and Collaborators.
Table S1. Secondary outcomes: indications for caesarean birth in COVID‐19‐positive/negative parturients.
Table S2. Secondary outcomes: neonatal outcomes in COVID‐19‐positive parturients.
Acknowledgements
The authors thank B. Hammond, Research Nurse and M. Milner, Co‐ordinator/Data Manager at Burnley General Teaching Hospital; A. Keegan, Nurse Practitioner at North Manchester General Hospital; and S. Thomas and S. Hammond, Research Midwives, and M Walker, Governance and Risk Management Administrator at Wythenshawe Hospital for their valuable assistance with data retrieval. MC has editorial board roles with the European Journal of Anaesthesiology, British Journal of Anaesthesia and International Journal of Obstetric Anesthesia. CS is the Executive Editor of Anaesthesia Reports. No external funding or other competing interests declared.
Contributor Information
K. Bhatia, Email: kailash.bhatia@mft.nhs.uk.
Group of Obstetric Anaesthetists of Lancashire, Greater Manchester and Mersey Study Collaborators:
K. Bhatia, M. Columb, E. Eslam, C. Shelton, J. Lie, S. Leach, O. Froud, D. Verma, P. Sturgess, A. Sawyerr, J Desai, N Gould, S Kumari, U Sen, P. Verma, P. Kamath, A. Koirala, S. Kimber‐Craig, J. Eccles, A. Bewlay, M Radwan, J. Johal, H Bennett, E Thompson, M. Hulgur, J. Christian, and A. Aiyad
References
- 1. Gurol‐Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection at the time of birth in England: national cohort study. American Journal of Obstetrics and Gynecology 2021; 225: 522.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Toro F, Gjoka M, Di Lorenzo D, et al. Impact of COVID‐19 on maternal and neonatal outcomes: a systematic review and meta‐analysis. Clinical Microbiology and Infection 2021; 27: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinical Medicine 2020; 25: 100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population‐based cohort study. British Medical Journal 2020; 369: m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19 infection. The INTERCOVID multinational cohort study. Journal of the American Medical Association Paediatrics 2021; 175: 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. British Medical Journal 2020; 370: m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID‐19 have increased composite morbidity compared to non‐pregnant matched controls. American Journal of Obstetrics and Gynecology 2021; 224: 510.e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vousden N, Bunch K, Morris E, et al. The incidence, characteristics birth and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS‐CoV‐2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021; 16: e0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bampoe S, Odor PM, Lucas DN. Novel coronavirus SARS‐CoV‐2 and COVID‐19. Practice recommendations for obstetric anaesthesia: what we have learned thus far. International Journal of Obstetric Anesthesia 2020; 43: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer ME, Bernstein K, Dinges E, et al. Obstetric anesthesia during the COVID‐19 pandemic. Anesthesia and Analgesia. 2020; 131: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uppal V, Sondekoppam R, Landau R, El‐Boghdadly K, Narouze S, Kalagara HKP. Neuraxial anaesthesia and peripheral nerve blocks during the COVID‐19 pandemic: a literature review and practice recommendations. Anaesthesia 2020; 75: 1350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatia K, Columb M, Bewlay A, et al. The effect of COVID‐19 on general anaesthesia rates for caesarean section. A cross sectional analysis of six hospitals in the north‐west of England. Anaesthesia 2021; 76: 312–9. [DOI] [PubMed] [Google Scholar]
- 13. Katz D, Bateman BT, Kjaer K, et al. The Society for Obstetric Anesthesia and Perinatology (SOAP) COVID‐19 Registry: an analysis of outcomes among pregnant women delivering during the initial SARS‐CoV‐2 outbreak in the United States. Anesthesia and Analgesia 2021; 133: 462–73. [DOI] [PubMed] [Google Scholar]
- 14. Mccallum AR, Broom MA, Litchfield KN, Shaw M, Kearns RJ. The effect of COVID‐19 on general anaesthesia rates for caesarean section. International Journal of Obstetric Anesthesia 2021; 47: 103188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherwood O, Lee J, Dean A, et al. P.65 Anaesthetic considerations and outcomes in 90 pregnant women with COVID‐19: a prospective observational study. International Journal of Obstetric Anesthesia 2021; 46: 103063. [Google Scholar]
- 16. NMPA Project Team . National maternity and perinatal audit: clinical report 2019. Based on births in NHS maternity services between 1st April 2016 and 31st March 2017. Royal College of Obstetricians and Gynaecologists. 2019. https://maternityaudit.org.uk/pages/reports (accessed 01/01/ 2021).
- 17. National Institute for Health and Care Excellence . Caesarean section: clinical guideline [CG132], 2011. www.nice.org.uk/guidance/cg132 (accessed 01/02/2021). [PubMed]
- 18. Royal College of Anaesthetists . Guidelines for the Provision of Anaesthesia Services for an Obstetric Population 2020. https://rcoa.ac.uk/gpas/chapter‐9#chapter‐7 (accessed 5/10/ 2021).
- 19. Royal College of Obstetricians and Gynaecologists . Classification of Urgency of Caesarean Section. A Continuum of Risk. Good Practice No 11. 2010. https://www.rcog.org.uk/globalassets/documents/guidelines/goodpractice11classificationofurgency.pdf (accessed 01/08/2021).
- 20. Keita H, James A, Bouvet L, et al. Clinical, obstetrical and anaesthesia outcomes in pregnant women during the first COVID‐19 surge in France: a prospective multicentre observational cohort study. Anaesthesia Critical Care and Pain Medicine 2021; 40: 100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioscovich A, Guasch E, Brogly N, et al. Peripartum anesthetic management of women with SARS‐CoV‐2 infection in eight medical centers across three European countries: prospective cohort observation study. Journal of Maternal‐Fetal and Neonatal Medicine 2021: 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Bamber JH, Lucas DN, Plaat F, Russell R. Obstetric anaesthetic practice in the UK: a descriptive analysis of the National Obstetric Anaesthetic Database 2009–14. British Journal of Anaesthesia 2020; 125: 580–7. [DOI] [PubMed] [Google Scholar]
- 23. Bamber JH, Lucas DN. COVID‐19 and access to labour epidural analgesia in UKhospitals. Anaesthesia 2020; 75: 1119–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehrotra P, Malani P, Yadav P. Personal protective equipment shortages during COVID‐19: supply chain‐related causes and mitigation strategies. Journal of the American Medical Association Health Forum. 2020; 1: e200553. [DOI] [PubMed] [Google Scholar]
- 25. Magoon R, Choudhary N, Saxena K. Labour analgesia in COVID‐19 positive parturients: Points to ponder! Trends in Anaesthesia and Critical Care. 2021; 38: 16–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rooks JP. Nitrous oxide for pain in labor – why not in the United States? Birth 2007; 34: 3–5. [DOI] [PubMed] [Google Scholar]
- 27. Redshaw M, Henderson J. Safely Delivered: A National Survey of Women's Experience of Maternity Care. Oxford: National Perinatal Epidemiology Unit, 2014. https://www.npeu.ox.ac.uk/assets/downloads/reports/Safely%20delivered%20NMS%202014.pdf (accessed 18/11/2021). [Google Scholar]
- 28. Shrimpton AJ, Brown JM, Gregson FKA, et al. Quantitative evaluation of aerosol generation during manual facemask ventilation. Anaesthesia 2022; 77: 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown J, Gregson FKA, Shrimpton AJ. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia 2021; 76: 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butwick AJ, Bentley J, Wong CA, Snowden JM, Sun E, Guo N. United States state‐level variation in the use of neuraxial analgesia during labor for pregnant women. Journal of the American Medical Association Network Open 2018;1:e186567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aaronson J, Abramovitz S, Smiley R, Tangel V, Landau R. A survey of intravenous remifentanil use for labor analgesia at academic medical centers in the United States. Anesthesia and Analgesia 2017; 124: 1208–10. [DOI] [PubMed] [Google Scholar]
- 32. Karasu D, Kilicarslan N, Ozgunay SE, Gurbuz H. Our anesthesia experiences in COVID‐19 positive patients delivering by cesarean section: A retrospective single-center cohort study. Journal of Obstetrics and Gynaecology Research 2021; 47 : 2659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Group of Obstetric Anaesthetists of Lancashire, Greater Manchester and Mersey (GOAL GM) Study Writing Group and Collaborators.
Table S1. Secondary outcomes: indications for caesarean birth in COVID‐19‐positive/negative parturients.
Table S2. Secondary outcomes: neonatal outcomes in COVID‐19‐positive parturients.


