Summary
Background and Aim
To investigate and quantify the risks of AKI and ALI associated with remdesivir use, given the underlying diseases of SARS‐CoV‐2 infection.
Methods
This self‐controlled case series (SCCS) study was conducted using electronic hospital records between 23 January 2020 and 31 January 2021 as retrieved from the Hong Kong Hospital Authority which manages all laboratory‐confirmed COVID‐19 cases in Hong Kong. Outcomes of AKI and ALI were defined using the KDIGO Guideline and Asia Pacific Association of Study of Liver consensus guidelines. Incidence rate ratios (IRR) for AKI and ALI following the administration of remdesivir (exposure) in comparison to a non‐exposure period were estimated using the conditional Poisson regression models.
Results
Of 860 COVID‐19 patients administered remdesivir during hospitalisation, 334 (38.8%) and 137 (15.9%) had incident ALI and AKI, respectively. Compared with the baseline period, both ALI and AKI risks were increased significantly during the pre‐exposure period (ALI: IRR = 6.169, 95% CI = 4.549–8.365; AKI: IRR = 7.074, 95% CI = 3.763–13.298) and remained elevated during remdesivir treatment. Compared to the pre‐exposure period, risks of ALI and AKI were not significantly higher in the first 2 days of remdesivir initiation (ALI: IRR = 1.261, 95% CI = 0.915–1.737; AKI: IRR = 1.261, 95% CI = 0.889–1.789) and between days 2 and 5 of remdesivir treatment (ALI: IRR = 1.087, 95% CI = 0.793–1.489; AKI: IRR = 1.152, 95% CI = 0.821–1.616).
Conclusion
The increased risks of AKI and ALI associated with intravenous remdesivir treatment for COVID‐19 may be due to the underlying SARS‐CoV‐2 infection. The risks of AKI and ALI were elevated in the pre‐exposure period, yet no such increased risks were observed following remdesivir initiation when compared to the pre‐exposure period.
Keywords: acute kidney injury, acute liver injury, case series, COVID‐19, remdesivir

1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has posed an unprecedented challenge to nearly all governments worldwide, which are trying desperately to control the infection and mortality rate by means of vaccination and a variety of treatments. The pathogenesis of SARS‐CoV‐2 infection has been well‐described. 1 Spike protein of coronaviruses binds with the receptor angiotensin‐converting enzyme 2 (ACE2) expressed in alveolar cells, thereby promoting viral entry and utilising host cell machinery for replication with viral RNA‐dependent RNA polymerase (RdRp). 2 , 3 Meanwhile, podocytes and proximal tubular cells in the kidney also express high levels of ACE2, which smay contribute to the development of acute kidney injury (AKI) upon SARS‐CoV‐2 infection. 3 While a remarkable drop in kidney function indicates the onset of acute tubular injury, the situation is often mild. 4 Another possible injury mechanism involves the immune system that triggers inflammation and immune cell infiltration, which play a critical role in tubular injury and thrombi. 4 A similar mechanism mediated by immune response and thrombosis could also be responsible for hepatocytes injury. 5 Meanwhile, hepatic injury is also noticed alongside elevated levels of liver enzymes, such as aspartate transaminase (AST) and alanine transaminase (ALT). 6 In addition, ACE2 is expressed at the highest level in cholangiocytes, followed by hepatocytes based on RNA sequencing data. 7 Therefore, hepatotoxicity is directly linked to viral infection despite variation in expression level. 8
Remdesivir is an effective pharmaceutical option targeting the infection pathway and subsequent immune responses. It is a broad‐spectrum antiviral monophosphoramidate prodrug that is metabolised in the liver to form remdesivir triphosphate; the metabolite is a nucleotide analogue that competes with ATP and interferes with RdRp activity, so viral RNA replication ceases to operate. 9 , 10 , 11 In this regard, this drug could trigger mitochondrial injury as it inhibits mammalian DNA and RNA polymerases. 9 , 11 , 12 , 13 , 14 , 15 This may lead to increased aminotransferase level in liver and mitochondrial injury in renal tubular cells, although action in the kidney may only occur with long‐term treatment. 9 , 12 , 15 In addition, CYP3A4, which metabolises remdesivir in the liver, and hepatocytes transporters are susceptible to drug interactions with other agents, thus potentially causing liver damage. 11 Product label from FDA and EMA include increased transaminase level, bilirubin and creatinine as clinical implications, while the increase in liver enzymes is highlighted by FDA as a possible adverse side effect. 16 , 17 Despite these possible injurious mechanisms, the previous usage of remdesivir treating MER and EVD demonstrates a safe profile without significant renal adverse events. 9 , 18 Although cases of AKI and increased aminotransferase level have been reported for treating COVID‐19, even among healthy volunteers, many randomised controlled studies have demonstrated limited adverse events with an acceptable safety profile. 9 , 11 , 12 , 13 , 14 , 15 , 19 , 20 , 21
In brief, kidney and liver injury are reported shortly after remdesivir initiation in case studies, 9 , 22 , 23 , 24 but the exact injury mechanisms remain to be defined and investigated. Controlled trials may find remdesivir to be generally tolerable, 25 , 26 , 27 yet its safety data on AKI and acute liver injury (ALI) in the post‐marketing real‐world setting have not been published so far. With patients serving as their own control, this self‐controlled case series (SCCS) study aims to estimate the risks of AKI and ALI with reference to remdesivir initiation among hospitalised COVID‐19 patients who also had incident AKI or ALI.
2. METHODS
2.1. Data source and study population
We analysed all patients with COVID‐19 diagnosis, defined by positive polymerase chain reaction (PCR) test for SARS‐CoV‐2 infection, in the Hong Kong Special Administrative Region, China for the study period between 23 January 2020 and 31 January 2021 using SCCS method. According to local government policies, all patients with laboratory‐confirmed COVID‐19 would be admitted to public hospitals for clinical management and isolation purposes, regardless of their disease severity. Electronic medical records of patients hospitalised with COVID‐19 were retrieved from the Hong Kong Hospital Authority, a statutory body that manages all public hospitals and their ambulatory clinics in Hong Kong. Data from the Hospital Authority has been validated and utilised for drug safety 28 and pharmaco‐epidemiological studies of drug treatments for COVID‐19. 29 , 30
2.2. Exposure and study outcomes
Patients who had initiated remdesivir during their hospitalisation for COVID‐19 were included in the current analysis if they had incident ALI or AKI. Remdesivir is one of the treatment options for patients hospitalised with COVID‐19 in Hong Kong. 31 The recommended dosage is 200 mg once for the first day, and 100 mg once daily for the next 4 days or until hospital discharge. 32 Remdesivir is suggested to be used for COVID‐19 patients with severe but non‐critical disease (oxygen saturation <94% on room air); and against routine use in critical cases such as admission to an intensive care unit (ICU), requiring the initiation of high‐flow nasal oxygen, mechanical ventilation or extracorporeal membrane oxygenation (ECMO). 31
ALI was defined as satisfying at least one of the following conditions 33 : (i) increase in ALT was over two times the upper limit of normal (ULN); (ii) increase in AST was over two times the ULN; (iii) increase in total bilirubin was over two times the ULN; or (iv) the international normalised ratio (INR) was over 1.7. According to the Asia Pacific Association of Study of Liver consensus guidelines, 34 the ULN of ALT, AST and total bilirubin were defined as 40 U/L, 40 U/L, and 19 μmol/L, respectively. AKI was defined as satisfying at least one of the following conditions: (i) increase in serum creatinine (SCr) by 0.3 mg/dL within 48 h; (ii) increase in SCr to 1.5 times of baseline, which was known or presumed to have occurred within the week prior, according to the KDIGO Clinical Practice Guideline for AKI. 35 Definition of ALI and AKI referred to serum abnormality at any point during the observation period.
2.3. Self‐controlled case series
The SCCS was used to investigate the association of remdesivir use for COVID‐19 treatment and the risk of ALI or AKI. The SCCS study design relies on comparisons within individuals who have experienced both the outcome and exposure of interest, with participants serving as their own control. 36 Incidence rate ratios (IRRs) are derived by comparing the rate of events during periods of medication exposure with the rate during all other observed time periods (ie, without medication). The major advantage of SCCS lies in its ability to control for the fixed confounders and time‐invariant confounding that possibly vary between individuals (namely socioeconomic factors, and genetic factors). 37
2.4. Study assumptions
In SCCS, there were three key assumptions such that the study would provide valid and unbiased estimates. 37 First, recurrent events of ALI and AKI among remdesivir users were assumed to be independent. If the events were dependent, it would be possible for the first event to increase the risk of a future event, 37 so the only first incident event was studied. Second, the occurrence of an event must not alter the probability of subsequent exposure. Therefore, pre‐exposure period was included to resolve the problem that the occurrence of ALI and AKI may temporarily alter the probability of remdesivir initiation. Third, there must be no censoring by the outcome of interest. It is inadmissible for SCCS analyses to censor exposure by the outcome since the exposure history would then be event‐dependent and violate another SCCS assumption. This would produce bias in an unpredictable direction. When a risk period is censored by patient’s death, the incidence rate would be estimated to be higher. If death occurs during the baseline period, IRR would be biassed downwards. On the other hand, death during the exposed period would bias IRR upwards. This is a case of event‐dependent observation periods (Figure S1), which violates the assumption of SCCS, and requires an extended version of SCCS which is adjusted for censoring by applying a weighting according to the duration from the event to the end of observation. 38
2.5. Exposure and risk periods
Study exposure was the initiation of remdesivir treatment in patients hospitalised with COVID‐19. Patients were censored on the following dates: date of hospital discharge, death or the end of the observation period (30 April 2021), whichever occurred the earliest. The risk periods were patient time divided into six mutually exclusive risk windows: (i) the baseline period covered that from hospital admission to 3 days before treatment initiation, and more than 5 days after treatment to the end of the observation period, which would be used as reference for comparison; and the exposure‐related risk periods were defined as (ii) pre‐exposure period (1–2 days before treatment initiation), (iii) first 2 days (days 0–1) on remdesivir initiation, (iv) days 2–5 on treatment, (v) more than 5 days on drug use to the end of treatment (applicable to patients on an extended treatment course only), and (vi) wash‐out period (within 5 days after treatment). The pre‐exposure period, which was designed to evaluate any increased incidences of ALI or AKI before the initiation of remdesivir, would help prevent any temporary changes in probability of exposure. 37 Figure 1 illustrates the schema of SCCS and describes the six risk periods in this observational study.
FIGURE 1.
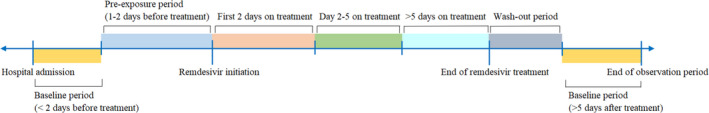
Study schema and definitions of treatment periods. The study was divided into six separate periods: Pre‐exposure period, first 2 days on treatment, day 2–5 on treatment, more than 5 days on drug use to the end of treatment, wash‐out period and more than 5 days after treatment to the end of the observation period
2.6. Statistical analysis
Baseline characteristics of remdesivir users who had incident ALI or AKI during the observation period were described in this SCCS study. The association between remdesivir use and ALI or AKI during different risk periods were estimated by comparing the rates of event occurrence. Incidence rates, in terms of events per 10,000 person‐days, of ALI and AKI over the remdesivir treatment period were calculated. Incidence rate ratios (IRRs) and their corresponding 95% confidence intervals (CIs) of events for different risk periods compared with the baseline period were estimated using a conditional Poisson regression model with an offset for the length of the risk period. Age is not adjusted for in the analysis given the short hospitalisation period of each COVID‐19 patient.
To test the credibility and robustness of the main results, sensitivity analyses were conducted to compare the IRRs between different risk periods of (i) removing patients who died during hospitalisation, as death cases within hospitalisation could raise an issue where the exposure that might have otherwise occurred after the event would never be known 37 ; (ii) patients with at least 5 days use of remdesivir; (iii) extending the observation period to 30 April 2021 for discharged cases; (iv) removing those with events at the day of remdesivir initiation; (v) removing those re‐initiating remdesivir after discontinuation; and varying definitions of ALI: (vi) ALT or AST >5× ULN or alkaline phosphatase (ALP) >2× ULN confirmed on at least 2 consecutive blood draws in patients with previously normal values; (vii) any elevation of ALT, ALP or AST, associated with (a) increased total bilirubin [≥2.5 mg/dL], in absence of prior diagnosis of liver disease, Gilbert’s syndrome or evidence of hemolysis or (b) coagulopathy with INR >1.5 in absence of coumadin therapy or known vitamin K deficiency, (viii) add ALP to define ALI using Drug‐Induced Liver Injury Network definition. 39
To determine the effects in different scenarios, eight subgroup analyses were also performed in this study. Patients were allocated into the following subgroups: (i) age ≤60 years; (ii) age >60 years; (iii) those who presented with WHO Clinical Progression Scale score ≤4 on remdesivir initiation; (iv) those who presented with WHO Clinical Progression Scale score ≥5 on remdesivir initiation; (v) those who had remdesivir discontinued; (vi) those who had interferon‐β‐1b; (vii) those who had ribavirin; and (vii) those who had dexamethasone.
All statistical analyses were performed with the stata version SE 17.0 (StataCorp LLC) and R, and the R code was adapted in an SCCS approach in this study. 40 A two‐sided significance level of 5% was used in all statistical analyses.
3. RESULTS
Among 10,412 patients hospitalised with COVID‐19 between 23 January 2020 and 31 January 2021, 860 of them were administered intravenous remdesivir as in‐patient treatment (Figure 2). There were 334 (38.8%) remdesivir users who had incident ALI (Grade 3: 61; Grade 4: 7), and 137 (15.9%) who had incident AKI during hospitalisation. Incidence rates of ALI and AKI among hospitalised COVID‐19 patients were 154 and 39 per 10,000 person‐days respectively. Distributions of the timing of remdesivir initiation, and that of the incident and recurrent outcomes by the day since remdesivir initiation are plotted in Figures S2 and S3, respectively. Baseline characteristics of remdesivir users who had incident ALI or AKI are listed in Table 1. Among remdesivir users with ALI and AKI, there were 34 (10.2%) and 46 (33.6%) deaths during the observation period, including 27 and 36 deaths that occurred during the baseline period, respectively. 93 (27.8%) and 18 (13.1%) patients required the discontinuation of remdesivir due to incident ALI and AKI, respectively.
FIGURE 2.
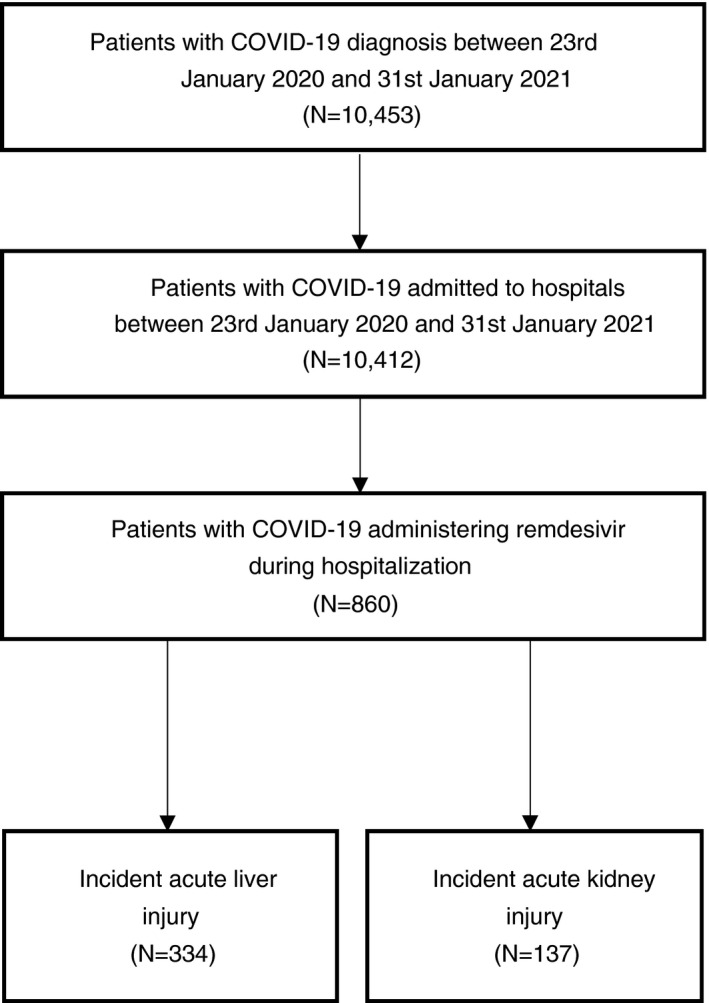
Flowchart of inclusion and exclusion of hospitalised COVID‐19 patients administering remdesivir between 23 January 2020 and 31 January 2021 in Hong Kong SAR, China. Patients with COVID‐19 were admitted to hospitals between 23 January 2020 and 31 January 2021 (N = 10,412). Patients with COVID‐19 were administered with remdesivir during hospitalisation (N = 860)
TABLE 1.
Baseline characteristics of hospitalised patients with COVID‐19 initiating remdesivir users who had an incident acute liver injury or acute kidney injury
| Baseline characteristics | Remdesivir users | |||
|---|---|---|---|---|
| Acute liver injury (n = 334) | Acute kidney injury (n = 137) | |||
| N/Mean | %/SD | N/Mean | %/SD | |
| Age (years) a | 61.5 | 14.4 | 69.4 | 12.8 |
| ≤65 | 198 | 59.3% | 44 | 32.1% |
| >65 | 136 | 40.7% | 93 | 67.9% |
| Sex | ||||
| Male | 216 | 64.7% | 85 | 62.0% |
| Female | 118 | 35.3% | 52 | 38.0% |
| Pre‐existing comorbidities | ||||
| Charlson’s Index a , b | 3.9 | 2.3 | 6.1 | 2.4 |
| 0–4 | 221 | 66.2% | 33 | 24.1% |
| 5–6 | 74 | 22.2% | 49 | 35.8% |
| 7–15 | 39 | 11.7% | 55 | 40.2% |
| Chronic heart disease | 35 | 10.5% | 35 | 25.5% |
| Chronic kidney disease | 39 | 11.7% | 62 | 45.3% |
| Chronic lung disease | 47 | 14.1% | 24 | 17.5% |
| Diabetes mellitus | 137 | 41.0% | 104 | 75.9% |
| Hypertension | 197 | 59.0% | 124 | 90.5% |
| Chronic liver disease | 48 | 14.4% | 24 | 17.5% |
| Hepatitis | 2 | 0.6% | 0 | 0.0% |
| Cirrhosis | 3 | 0.9% | 1 | 0.7% |
| Hepatocellular carcinoma | 0 | 0.0% | 1 | 0.7% |
| Malignancy | 7 | 2.1% | 6 | 4.4% |
| Vitamin D deficiency | 16 | 4.8% | 14 | 10.2% |
| Long‐term medications | ||||
| ACEI/ARB | 82 | 24.6% | 66 | 48.2% |
| Anticoagulant | 197 | 59.0% | 111 | 81.0% |
| Antiplatelet | 44 | 13.2% | 44 | 32.1% |
| Lipid‐lowering agent | 118 | 35.3% | 86 | 62.8% |
| NSAID | 56 | 16.8% | 52 | 38.0% |
| Treatment performed prior to baseline | ||||
| Remdesivir | 334 | 100.0% | 137 | 100.0% |
| Time from admission to remdesivir initiation (days) a | 4.2 | 3.4 | 4.1 | 4.1 |
| Cumulative dosage of remdesivir (mg) a | 598.5 | 238.2 | 632.1 | 280.2 |
| Duration of use of remdesivir (days) a | 4.4 | 2 | 4.8 | 2.2 |
| Other antimicrobials | 237 | 71.0% | 112 | 81.8% |
| Antivirals | 160 | 47.9% | 67 | 48.9% |
| Ribavirin | 128 | 38.3% | 45 | 32.8% |
| Lopinavir‐ritonavir | 46 | 13.8% | 27 | 19.7% |
| Antibiotics | 184 | 55.1% | 100 | 73.0% |
| Immunomodulators | 305 | 91.3% | 127 | 92.7% |
| Dexamethasone | 240 | 71.9% | 111 | 81.0% |
| Time from admission to dexamethasone initiation, days a | 4.0 | 3.6 | 3.3 | 3.8 |
| Administration route of dexamethasone | ||||
| Oral | 50 | 18.1% | 25 | 20.0% |
| Intravenous injection | 226 | 81.9% | 100 | 80.0% |
| Dosage of dexamethasone | ||||
| Up to 6 mg daily | 106 | 38.4% | 40 | 32.0% |
| More than 6 mg daily | 170 | 61.6% | 85 | 68.0% |
| Cumulative dosage of dexamethasone (mg) a | 70.1 | 88.5 | 92.3 | 112.5 |
| Duration of use of dexamethasone (days) a | 10.1 | 12.9 | 13.5 | 16.4 |
| Other systemic steroid | 11 | 3.3% | 11 | 8.0% |
| Interferon‐β‐1b | 228 | 68.3% | 92 | 67.2% |
| Baricitinib | 5 | 1.5% | 2 | 1.5% |
| Tocilizumab | 18 | 5.4% | 10 | 7.3% |
| Paracetamol | 309 | 92.5% | 121 | 88.3% |
| ECMO | 2 | 0.6% | 2 | 1.5% |
| Dialysis | 4 | 1.2% | 5 | 3.6% |
| ICU admission | 101 | 30.2% | 81 | 59.1% |
| Admission via emergency department | 154 | 46.1% | 81 | 59.1% |
| Clinical severity by WHO Clinical Progression Scale | ||||
| WHO Clinical Progression Scale Score (range 0–10) a | 4.9 | 1.2 | 5.6 | 1.3 |
| No oxygen therapy (Score 4) | 200 | 59.9% | 46 | 33.6% |
| Supplemental oxygen without ventilation (Score 5–6) | 110 | 32.9% | 72 | 52.6% |
| Mechanical ventilation (Score 7–9) | 24 | 7.2% | 19 | 13.9% |
| Laboratory parameters [normal range] a | ||||
| White blood cell, ×109/L [3.7–9.2 × 109/L] | 5.8 | 2.7 | 6.8 | 3.7 |
| Neutrophil, ×109/L [1.7–5.8 × 109/L] | 4.3 | 2.6 | 5.3 | 3.5 |
| Lymphocyte, ×109/L [1.0–3.1 × 109/L] | 1.0 | 0.5 | 0.9 | 0.6 |
| Platelet, ×109/L [145–370 × 109/L] | 178.5 | 61.5 | 184.0 | 71.7 |
| Lactate dehydrogenase, U/L [110–210 U/L] | 353.5 | 160.9 | 372.9 | 182.3 |
| Creatine kinase, U/L [26–192 U/L] | 329.0 | 629.0 | 345.8 | 637.0 |
| Total bilirubin, μmol/L [5–27 μmol/L] | 10.2 | 8.1 | 10.2 | 9.4 |
| C‐reactive protein, mg/L [<5 mg/L] | 61.6 | 56.3 | 72.7 | 64.9 |
| Cycle threshold value, cycle | 22.5 | 5.2 | 20.8 | 4.7 |
| eGFR, ml/min/1.73m2 [>90 ml/min/1.73m2] | 103.6 | 58.4 | 91.1 | 88.6 |
| ALT, U/L [<46.5 U/L] | 51.3 | 36.0 | 35.1 | 23.1 |
| AST, U/L | 73.0 | 119.4 | 43.0 | 117.7 |
| ALP, U/L [30–120 U/L] | 70.7 | 32.2 | 73.0 | 32.2 |
| R score | 2.1 | 1.7 | 1.3 | 4.2 |
| INR [<1.1] | 1.1 | 0.4 | 1.1 | 0.6 |
| Haemoglobin g/dL [13.4–17.1 g/dL] | 13.5 | 1.7 | 12.9 | 2.0 |
Abbreviations: ACEI, Angiotensin converting enzyme inhibitor; ALP, Alkaline phosphatase; ALT, Alanine transaminase; ARB, Angiotensin receptor blockers; AST, Aspartate transaminase; ECMO, Extracorporeal membrane oxygenation; eGFR, Estimated glomerular filtration rate; ICU, intensive care unit; INR, international normalised ratio; NSAID, nonsteroidal anti‐inflammatory drugs; R score, (ALT/ULN)/(ALP/ULN); SD, standard deviation; ULN, upper limit of normal.
Age, Charlson Index, clinical severity, cumulative dosage, duration of use of dosage, time from admission to remdesivir and dexamethasone initiation, and laboratory parameters on admission are presented in mean ± SD.
The calculation of Charlson Index does not include Acquired Immune Deficiency Syndrome (AIDS).
Table 2 shows the incidence rates of remdesivir users who had ALI and AKI in different observation periods, and the IRRs of ALI and AKI in each risk period compared to the baseline period and pre‐exposure period, respectively. The mean duration of the observation period was 30.7 and 40.0 days for remdesivir users with ALI and AKI. Patients with ALI had a mean of 4.5 days for remdesivir treatment, while that for patients with AKI was 4.7 days. Compared with the baseline period, ALI risk increased significantly during the pre‐exposure period (IRR = 6.169, 95% CI = 4.549–8.365), remained elevated during first 2 days of remdesivir treatment (IRR = 7.778, 95% CI = 5.973–10.130), 2–5 days of treatment (IRR = 6.702, 95% CI = 5.193–8.650), and >5 days after remdesivir initiation (IRR = 4.902, 95% CI = 2.353–10.214). Compared to the pre‐exposure period, the risk of ALI was not significantly higher during remdesivir treatment periods. Similarly, there was an increased risk of AKI during pre‐exposure period (IRR = 7.074, 95% CI = 3.763–13.298) compared with that of the baseline period. Such elevated risk sustained during first 2 days of remdesivir treatment (IRR = 8.227, 95% CI = 5.064–13.364), subsequent days 2–5 (IRR = 5.922, 95% CI = 3.705–9.467) and >5 days of remdesivir treatment (IRR = 6.185, 95% CI = 2.483–15.408). When compared to the pre‐exposure period, AKI risk was not significantly higher during the remdesivir treatment periods.
TABLE 2.
Comparison of risks of acute liver injury and acute kidney injury between different risk periods
| Outcomes | Events | Rate | Incidence rate (events/10,000 person‐days) | 95% CI | Person‐days | Baseline period as reference | Pre‐exposure period as reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | p‐value | IRR | 95% CI | p‐value | ||||||
| Acute liver injury (N = 334) | |||||||||||
| Baseline period | 183 | 40.1% | 642 | 552, 742 | 2850 | Reference | 0.162 | 0.120, 0.220 | <0.001 | ||
| Pre‐exposure period | 67 | 22.0% | 1370 | 1062, 1740 | 489 | 6.169 | 4.549, 8.365 | <0.001 | Reference | ||
| Day 0–1 on drug initiation | 103 | 30.8% | 1829 | 1493, 2219 | 563 | 7.778 | 5.973, 10.130 | <0.001 | 1.261 | 0.915, 1.737 | 0.156 |
| Day 2–5 on drug treatment | 121 | 41.9% | 1909 | 1584, 2280 | 634 | 6.702 | 5.193, 8.650 | <0.001 | 1.087 | 0.793, 1.489 | 0.606 |
| Day >5 on drug treatment | 9 | 25.0% | 882 | 403, 1675 | 102 | 4.902 | 2.353, 10.214 | <0.001 | 0.795 | 0.370, 1.707 | 0.556 |
| Wash‐out period | 186 | 55.7% | 1670 | 1438, 1928 | 1114 | 3.134 | 2.497, 3.932 | <0.001 | 0.508 | 0.380, 0.679 | <0.001 |
| Acute kidney injury (N = 137) | |||||||||||
| Baseline period | 85 | 45.9% | 445 | 356, 551 | 1909 | Reference | 0.180 | 0.130, 0.249 | <0.001 | ||
| Pre‐exposure period | 13 | 11.5% | 714 | 380, 1221 | 182 | 7.074 | 3.763, 13.298 | <0.001 | Reference | ||
| Day 0–1 on drug initiation | 27 | 19.7% | 1080 | 712, 1571 | 250 | 8.227 | 5.064, 13.364 | <0.001 | 1.261 | 0.889, 1.789 | 0.194 |
| Day 2–5 on drug treatment | 30 | 24.8% | 1038 | 700, 1482 | 289 | 5.922 | 3.705, 9.467 | <0.001 | 1.152 | 0.821, 1.616 | 0.412 |
| Day >5 on drug treatment | 6 | 28.6% | 938 | 344, 2041 | 64 | 6.185 | 2.483, 15.408 | <0.001 | 0.843 | 0.405, 1.758 | 0.649 |
| Wash‐out period | 45 | 32.8% | 794 | 579, 1062 | 567 | 2.904 | 1.927, 4.377 | <0.001 | 0.548 | 0.400, 0.750 | <0.001 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
Similar results were found in the sensitivity (Table S1) and subgroup (Table S2) analyses. Results of the subgroup analyses were generally comparable to those of the main analysis, where increased risks of ALI and AKI were consistently observed during the pre‐exposure period and remdesivir treatment, and not significantly higher during remdesivir treatment when compared with the pre‐exposure period.
4. DISCUSSION
This current study investigates the safety of remdesivir treatment initiation of hospitalised COVID‐19 patients in terms of AKI and ALI. The result does not suggest a significant association of remdesivir initiation with the risk of AKI and ALI. The increased risks of ALI and AKI after intravenous remdesivir treatment for COVID‐19 may be due to the underlying SARS‐CoV‐2 infection. The risks of ALI and AKI were elevated in the pre‐exposure and treatment periods compared with baseline, yet no such increased risks were observed following remdesivir initiation when compared to the pre‐exposure period.
Approximately 7% of the remdesivir recipients developed AKI, which was the most common adverse event for drug discontinuation, 41 but this incidence rate was not significant. Meanwhile, no severe nephrotoxicity was found in a retrospective review of 5‐day remdesivir treatment with 15 days follow‐up period, but 10.5% of the patients still showed at least 10 ml/min/1.73m2 decrease in eGFR, which was comparable to data reported in the randomised controlled trial. 15 Similarly, remdesivir did not lead to significant AKI risk at the end of treatment or 2 days after treatment completion even in patients with impaired eGFR of less than 30 ml/min/1.73m2. 42 These results were consistent with our finding that remdesivir initiation was not associated with an increased risk of AKI when compared to pre‐exposure period. Meanwhile, based on the pharmacokinetics of remdesivir and its short administration duration, remdesivir was considered safe for patients with impaired renal function, and the benefits of its use may outweigh the risk. 9 Remdesivir was also generally tolerated in kidney transplant patients as AKI was reported in 27% of them where half of them have been diagnosed AKI before administration, at which the peak of serum creatinine was detected, and they retained baseline function 3 days after initiation towards at the end of treatment. 43 Therefore, remdesivir was not responsible for the injury, although randomised controlled trials were necessary to compare the incidence of AKI, especially under the challenge presented by the similar renal complications of COVID‐19 and remdesivir. 43 This study was also consistent with our finding of insignificant risk of AKI after remdesivir initiation compared to pre‐exposure. A similar finding was reported in solid‐organ transplant recipients whose elevation in GFR or hepatic enzyme was insignificant compared with other antiviral drugs. 44 However, analysis of the WHO Safety database with other pharmaceutical treatments highlighted a 20‐fold increased risk of acute renal failure, suggesting a disproportionality signal of remdesivir nephrotoxicity. 14 This elevated risk could be caused by the concurring SARS‐CoV‐2 infection while our result illustrated an insignificant increased risk on AKI and ALI compared to the pre‐exposure period. While some illustrated a direct cause of remdesivir initiation to AKI, most believed that AKI was multifactorial, with drug‐induced AKI being one of the contributors. 9 , 14 , 15 , 45 The literature demonstrated an elevated risk of AKI after remdesivir treatment for COVID‐19 patients, but the risk had no significant difference compared with AKI risk before COVID‐19 hospitalisation.
Meanwhile, 25% and 33% of patients had elevated AST and ALT level, respectively; patients generally suffered from different degrees of elevation in liver enzymes, but only a maximum of 6% of the population would have grade 3 elevation or above. 15 A similar finding was reported when comparing remdesivir with other treatments of COVID‐19: serum AST and ALT levels were significantly higher in the remdesivir group and the risk of hepatic impairment increased. 46 , 47 Some case studies suggested causality of hepatotoxicity as AST and ALT levels were elevated or peaked immediately after the initiation of remdesivir, but the situation ameliorated afterwards. 22 , 48 , 49 In addition, the disproportionately high reporting of aminotransferase elevation compared with other COVID‐19 treatment options suggested drug‐induced liver injury. 50
Nonetheless, some studies did not associate remdesivir with liver injury as the difference of clinical measurements was insignificant between treatment and placebo groups. 51 Most patients with kidney transplants also showed no significant hepatoxicity with stable liver function throughout the study period, despite its small sample size. 43 Successful disease management with remdesivir was also reported in liver transplants patients as the bilirubin and aminotransferase levels did not elevate during the period, or such elevation was insignificant. 44 , 52 A case of remdesivir initiation immediately post‐liver transplant showed near‐complete recovery after 3 months, but it is unclear if remdesivir directly caused the elevated liver enzymes following the transplant. 53 The patient had increased CT value only when the remdesivir was initiated and had negative PCR result within a month. 53 These studies demonstrated an inconsistent result regarding the risk of hepatotoxicity after remdesivir initiation, but mild transient elevation of liver enzymes was still described. 51 , 54 SARS‐CoV‐2 infection per se could lead to raised AST and ALT levels, through a variety of mechanisms including cytokine storm, hypoxic injury and vascular thrombosis. 5 , 6 , 8 Therefore, the elevation of liver enzymes during the course of COVID‐19 was not solely attributable to the administration of remdesivir. If the potential benefits of remdesivir initiation would outweigh the risks, this antiviral treatment should not be precluded. 53 , 55
Using a population‐based cohort of COVID‐19 patients, this study has evaluated the safety of remdesivir treatment during hospitalisation. Although our results did not suggest treatment toxicity in terms of AKI and ALI, several key limitations have to be addressed. First, unmeasured or residual confounding could remain and influence the findings due to the observational nature of this study, although any fixed confounders were controlled for in the SCCS study design. Second, the majority of the admitted patients were on long‐term anticoagulant medication, likely being prescribed under pre‐existing conditions, and were treated with interferon‐β‐1b at baseline as part of the effective triple combination therapy for COVID‐19, 56 hence our results would not be applicable to other patient populations. Lastly, any combined or synergistic effects of remdesivir with other concomitant medications such as baricitinib and tocilizumab were not explored given their limited use in this patient cohort. However, concomitant medications were unlikely to affect the results because of the within‐patient comparison nature of SCCS.
Remdesivir initiation in treating COVID‐19 did not significantly increase the risks of AKI and ALI when compared to the pre‐exposure period. Although most adverse events were mild and severe adverse events were rare, the cautious use of remdesivir was still recommended with close monitoring of kidney and liver functions. The challenge of assessing the safety of remdesivir lay in its similar laboratory measures with COVID‐19. Therefore, impaired kidney and liver functions should not be solely evaluated as a contraindication to remdesivir use. 15 Our findings would also suggest that the increased risks of AKI and ALI were attributed to the persisting manifestation of SARS‐CoV‐2 infection. However, should the clinical condition worsen or an acute liver or kidney injury develop after remdesivir initiation, discontinuation of remdesivir may be necessary and other treatment options should be explored.
AUTHORSHIP
Guarantor of the article: Carlos K. H. Wong.
Author contributions: C.K.H.W. reviewed the literature, designed statistical analysis, conducted analyses, and wrote the manuscript. C.H.A. and W.Y.C. reviewed the literature, contributed to the interpretation of the analysis, and wrote the manuscript. C.H.A. conducted analyses. Y.L.M. and S.L.L. contributed to the clinical input, and interpretation of the analysis. X.X., E.H.Y.L. and B.J.C. contributed to the interpretation of the analysis. M.C. wrote the manuscript. K.K.C.M. and K.T.K.L. contributed to the interpretation of the analysis, critically reviewed and revised the manuscript. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
ETHICS APPROVAL AND INFORMED CONSENT
The study protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Reference No. UW 20–493). Given the extraordinary nature of the COVID‐19 pandemic, individual patient informed consent was not required for this retrospective cohort study using anonymized data.
TRANSPARENCY STATEMENT
The manuscript’s guarantor affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Supporting information
Tables S1‐S2
Figures S1‐S3
ACKNOWLEDGEMENTS
We thank the Hospital Authority for data provision.
Declaration of personal interests: B.J.C. consults for Roche, Sanofi Pasteur, GSK and Moderna. The authors report no other potential conflicts of interest.
Declaration of funding interests: This study was funded in full by the Health and Medical Research Fund, The Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, China, grant number COVID190210. The funders did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Wong CK, Au IC, Cheng WY, Man KK, Lau KT, Mak LY, et al. Remdesivir use and risks of acute kidney injury and acute liver injury among patients hospitalised with COVID‐19: a self‐controlled case series study. Aliment Pharmacol Ther. 2022;56:121–130. 10.1111/apt.16894
Carlos K. H. Wong, Ivan C. H. Au and Wing Yiu Cheng contributed equally as co‐first author.
The Handling Editor for this article was Dr Rohit Loomba, and it was accepted for publication after full peer‐review.
Funding information We received financial support from the Health and Medical Research Fund, The Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, China (grant no. COVID190210). The funders did not have any role in design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study were provided by the Hong Kong Hospital Authority. Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1. Wu JT, Leung K, Lam TTY, Ni MY, Wong CKH, Peiris JSM, et al. Nowcasting epidemics of novel pathogens: lessons from COVID‐19. Nat Med. 2021;27(3):388–95. [DOI] [PubMed] [Google Scholar]
- 2. Ferner RE, Aronson JK. Remdesivir in Covid‐19. BMJ. 2020;369:m1610. [DOI] [PubMed] [Google Scholar]
- 3. Beyerstedt S, Casaro EB, Rangel EB. COVID‐19: angiotensin‐converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS‐CoV‐2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID‐19‐associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marjot T, Webb GJ, AS B, Moon AM, Stamataki Z, Wong VW, et al. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirola CJ, Sookoian S. SARS‐CoV‐2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID‐19. Liver Int. 2020;40(8):2038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73(4):807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, et al. Remdesivir in patients with acute or chronic kidney disease and COVID‐19. J Am Soc Nephrol. 2020;31(7):1384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remdesivir . LiverTox: clinical and research information on drug‐induced liver injury. Bethesda (MD) 2012. [PubMed]
- 12. Rahimi MM, Jahantabi E, Lotfi B, Forouzesh M, Valizadeh R, Farshid S. Renal and liver injury following the treatment of COVID‐19 by remdesivir. Journal of Nephropathology. 2021;10(2):1–4. [Google Scholar]
- 13. Zhai G, Li M, Wang Y, Wu J. Drug‐induced liver disturbance during the treatment of COVID‐19. Front Pharmacol. 2021;12(2149):719308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerard AO, Laurain A, Fresse A, Parassol N, Muzzone M, Rocher F, et al. Remdesivir and acute renal failure: a potential safety signal from disproportionality analysis of the WHO safety database. Clin Pharmacol Ther. 2021;109(4):1021–4. [DOI] [PubMed] [Google Scholar]
- 15. van Laar SA, de Boer MGJ, Gombert‐Handoko KB, Guchelaar HJ, Zwaveling J. Liver and kidney function in patients with Covid‐19 treated with remdesivir. Br J Clin Pharmacol. 2021;87(11):4450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. U.S. Food and Drug Administration . Highlights of prescribing information 2020, October 22. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf.
- 17. Package leaflet: Information for the patient 2021. Updated 2021 May. Available from: https://www.medicines.org.uk/emc/files/pil.11597.pdf.
- 18. Nili A, Farbod A, Neishabouri A, Mozafarihashjin M, Tavakolpour S, Mahmoudi H. Remdesivir: a beacon of hope from Ebola virus disease to COVID‐19. Rev Med Virol 2020;30(6):e2133, 1, 13. [DOI] [PubMed] [Google Scholar]
- 19. Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug remdesivir: an update. Biomed Pharmacother. 2020;130:110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carothers C, Birrer K, Vo M. Acetylcysteine for the treatment of suspected Remdesivir‐associated acute liver failure in COVID‐19: a case series. Pharmacotherapy. 2020;40(11):1166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid‐19 in outpatients. N Engl J Med. 2022;386(4):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, et al. Liver injury in remdesivir‐treated COVID‐19 patients. Hepatol Int. 2020;14(5):881–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thakare S, Gandhi C, Modi T, Bose S, Deb S, Saxena N, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021;6(1):206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gevers S, Welink J, van Nieuwkoop C. Remdesivir in COVID‐19 patients with impaired renal function. J Am Soc Nephrol. 2021;32(2):518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324(11):1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ader F, Bouscambert‐Duchamp M, Hites M, Peiffer‐Smadja N, Poissy J, Belhadi D, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID‐19 (DisCoVeRy): a phase 3, randomised, controlled, open‐label trial. Lancet Infect Dis. 2022;22(2):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Man KKC, Coghill D, Chan EW, Lau WCY, Hollis C, Liddle E, et al. Association of Risk of suicide attempts with methylphenidate treatment. JAMA Psychiat. 2017;74(10):1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong CKH, Lau KTK, Au ICH, Xiong X, Lau EHY, Cowling BJ. Clinical improvement, outcomes, antiviral activity, and costs associated with early treatment with remdesivir for patients with coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2021. 10.1093/cid/ciab631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong CKH, Lau KTK, Au ICH, Xiong X, Chung MSH, Lau EHY, Cowling BJ. Optimal timing of Remdesivir initiation in hospitalized patients with coronavirus disease 2019 (COVID‐19) administered with dexamethasone. Clin Infect Dis. 2021. 10.1093/cid/ciab728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hospital Authority: communication kit – coronavirus disease 2019 (COVID‐19) formerly named novel coronavirus (nCoV). Version 7.12, 2021.
- 32. Therapeutic Management of Hospitalized Adults with COVID‐19 (2021).
- 33. Yip TC‐F, Lui GC‐Y, Wong VW‐S, Chow VC‐Y, Ho TH‐Y, Li TC‐M, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID‐19. Gut. 2021;70(4):733–42. [DOI] [PubMed] [Google Scholar]
- 34. Devarbhavi H, Aithal G, Treeprasertsuk S, Takikawa H, Mao Y, Shasthry SM, et al. Drug‐induced liver injury: Asia Pacific Association of Study of liver consensus guidelines. Hepatol Int. 2021;15(2):258–82. [DOI] [PubMed] [Google Scholar]
- 35. Haskell R. https://www.nursingcenter.com/ncblog/january‐2020/acute‐kidney‐injury‐and‐chronic‐kidney‐disease
- 36. Whitaker HJ, Paddy Farrington C, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med. 2006;25(10):1768–97. [DOI] [PubMed] [Google Scholar]
- 37. Petersen I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 38. Farrington P, Whitaker H, Weldeselassie YG. Self‐controlled case series studies: a modelling guide with R. Chapman & Hall/CRC biostatistics series. Boca Raton, Florida: CRC Press; 2018. [Google Scholar]
- 39. Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug‐induced liver injury network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heather J, Whitaker CPF, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med. 2006;2005(25):1768–97. [DOI] [PubMed] [Google Scholar]
- 41. Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID‐19. BMC Nephrol. 2021;22(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2021;65(2):e02290–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buxeda A, Arias‐Cabrales C, Perez‐Saez MJ, Cacho J, Cabello Pelegrin S, Melilli E, et al. Use and safety of Remdesivir in kidney transplant recipients with COVID‐19. Kidney Int Rep. 2021;6(9):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shafiekhani M, Shahabinezhad F, Niknam T, Tara SA, Haem E, Mardani P, et al. Evaluation of the therapeutic regimen in COVID‐19 in transplant patients: where do immunomodulatory and antivirals stand? Virol J. 2021;18(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le MP, Le Beller C, Le Hingrat Q, Jaquet P, Wicky PH, Bunel V, et al. Reply to Yan and Muller, "Captisol and GS‐704277, but not GS‐441524, are credible mediators of Remdesivir’s nephrotoxicity". Antimicrob Agents Chemother. 2020;64(12):e01937–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghosh CK, Hasan SA, Dey S. Remdesivir induced liver injury and severe COVID‐19 infection. Am J Int Med. 2020;8(6):285–8. [Google Scholar]
- 47. Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of Remdesivir for coronavirus 2019. Clin Gastroenterol Hepatol. 2020;18(12):2835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabers AJ, Williams AL, Farley TM. Use of remdesivir in the presence of elevated LFTs for the treatment of severe COVID‐19 infection. BMJ Case Rep. 2020;13(10):e239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGrowder DA, Miller F, Anderson Cross M, Anderson‐Jackson L, Bryan S, Dilworth L. Abnormal liver biochemistry tests and acute liver injury in COVID‐19 patients: current evidence and potential pathogenesis. Diseases. 2021;9(3):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh A, Kamath A. Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 Using real‐world data. Expert Opin Drug Saf. 2021;20(12):1559–64. [DOI] [PubMed] [Google Scholar]
- 51. Li Y, Cai H, Rajabalee N, Au X, Friedenberg F, Wallach S. S1027 hepatotoxicity of Remdesivir for COVID‐19: systematic review and meta‐analysis. Am College Gastroenterol. 2020;115:S523. [Google Scholar]
- 52. Jamir I, Lohia P, Pande RK, Setia R, Singhal AK, Chaudhary A. Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID‐19 pneumonia. Ann Hepatobil Pancreat Surg. 2020;24(4):526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yohanathan L, Campioli CC, Mousa OY, Watt K, Friedman DZP, Shah V, et al. Liver transplantation for acute liver failure in a SARS‐CoV‐2 PCR‐positive patient. Am J Transplant. 2021;21(8):2890–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre‐clinical data, and emerging clinical experience for COVID‐19. Pharmacotherapy. 2020;40(7):659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu X, Sun L, Guo Z, Wu C, Yu X, Li J. Management of COVID‐19 patients with chronic liver diseases and liver transplants. Ann Hepatol. 2022;27(1):100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hung IF‐N, Lung K‐C, Tso EY‐K, Liu R, Chung TW‐H, Chu M‐Y, et al. Triple combination of interferon beta‐1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Figures S1‐S3
Data Availability Statement
The data that support the findings of this study were provided by the Hong Kong Hospital Authority. Restrictions apply to the availability of these data, which were used under license for this study.


