Abstract
Aim
We investigated prolonged symptoms in children after COVID‐19, including the clinical characteristics and risk factors.
Methods
This multicentre retrospective study focused on 451 children under 18 years old who were diagnosed with symptomatic COVID‐19 between 14 March and 31 December 2020. Persistent symptoms were analysed with a telephone questionnaire by the attending physicians from 1 August to 30 September 2021. A control group of 98 with no history of COVID‐19, who were treated for other reasons, was also included.
Results
Most (82.0%) of the cases had mild infections that required outpatient care and 5.1% were admitted to the paediatric intensive care unit (PICU). We found that 18.4% had symptoms that lasted 4–12 weeks. There were also 14.6% who were symptomatic for longer than 12 weeks and the odds risks were higher for children aged 5 years or more (OR 3.0), hospitalised (OR 3.9), admitted to the PICU (OR 4.3) and with relatives who were symptomatic for 12 weeks or more (OR 2.8). The controls had similar percentages of prolonged symptoms, despite having no history of COVID‐19, especially those who were older than 5 years.
Conclusion
This study confirmed that a worrying percentage of children had prolonged symptoms after COVID‐19.
Keywords: children, multicentre study, pandemic, post‐COVID‐19, questionnaire
Abbreviations
- CI
confidence interval
- IQR
interquartile range
- MIS‐C
multisystem inflammatory syndrome
- NICE
National Institute for Health and Care Excellence
- OR
odds ratio
- PCR
polymerase chain reaction
- PICU
paediatric intensive care unit
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- WHO
World Health Organization
Key Notes.
There has been a lack of research about post‐COVID‐19 in children, which is defined as ongoing symptoms for more than 12 weeks.
This multicentre questionnaire focused on 451 children under 18 years who had COVID‐19 and found that 18.4% had symptoms that lasted 4–12 weeks and 14.6% had post‐COVID‐19.
The odds risks were higher for children aged 5 years and those who have been hospitalised and had relatives with post‐COVID‐19.
1. INTRODUCTION
Children tend to have a relatively mild and less severe course of COVID‐19 than adults and are less likely than them to require hospitalisation. 1 , 2 However, a small percentage experience serious symptoms during the acute phase. These are mainly pneumonia, but can also include multisystem inflammatory syndrome in children (MIS‐C). Many of these children need to be admitted to a paediatric intensive care unit (PICU). 3
Prolonged symptoms following the acute infection with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) may last longer than 4 or even 12 weeks and have been widely described in both adult 4 and paediatric populations. 5 , 6 , 7 The UK National Institute for Health and Care Excellence (NICE) published guidelines in December 2020 on long COVID. These categorised symptoms that lasted for 4–12 weeks, after SARS‐CoV‐2 were confirmed with microbiological testing, as ongoing COVID‐19. The guidelines further stated that if symptoms lasted more than 12 weeks they were considered to be prolonged COVID‐19. 8 Updated NICE guidelines were published in November 2021 and these used different terminology. 9 They used ongoing symptomatic COVID‐19 if the signs and symptoms of COVID‐19 lasted for 4–12 weeks and post‐COVID‐19 syndrome if they lasted for more than 12 weeks and were not explained by an alternative diagnosis. The World Health Organization (WHO) used a Delphi process and also defined the term post‐COVID‐19 in adults as symptoms that lasted for more than 12 weeks, with other diagnoses excluded. 10 This study uses the latest 2021 NICE and WHO terms, as the actual time categories are identical to those in place when this study was carried out in 2021. There has been a lack of data regarding the prevalence of post‐COVID‐19 in childhood and its characteristics. 11 Cases have ranged from 2% to 20% of children affected by SARS‐CoV‐2, possibly due to the methodology used. 12 , 13 , 14 , 15 , 16 Data published by the UK Office for National Statistics on 1 April 2021 estimated that 9.8% of children between 2 and 11 years of age and 13% of children between 12 and 16 years had symptoms 5 weeks after their SARS‐CoV‐2 infection was confirmed. After 12 weeks, 7.4% of the children aged between 2 and 11 years and 8.2% between 12 and 16 years continued to have symptoms. 17
Children with post‐COVID‐19 have reported that it has had a negative impact on their quality of life, similar to adults, and many have been unable to attend school. These symptoms may have initially gone unnoticed and not been attributed to the SARS‐CoV‐2 infection. 18 , 19 That is why early diagnoses and adequate follow‐up visits are important. 20 This is particularly crucial for paediatric patients, as they are at a sensitive stage of their emotional, physical and cognitive development. 11 The correct evaluation of the prevalence of post‐COVID‐19 in children is important when it comes to recommending vaccinations.
The main aim of this study was to determine the prevalence of prolonged symptoms after acute COVID‐19 at 4–12 weeks and more than 12 weeks in Spanish children with a confirmed SARS‐CoV‐2 infection. We also wanted to describe the clinical characteristics and identify possible risk factors in our population.
2. PATIENTS AND METHODS
A multicentre retrospective study was carried out at 3 university hospitals in Madrid. La Paz and Niño Jesús are the two largest hospitals in Madrid and during the first pandemic waves in 2020 they were responsible for any of the 1 million children in the region who needed to be admitted with COVID‐19. The Severo Ochoa hospital served a population of 60,000 children during the first pandemic wave, but only provided outpatient treatment. Telephone calls were made to the parents of children who were diagnosed with confirmed symptomatic COVID‐19 infections and were hospitalised, or attended any of the three hospitals, between 14 March and 31 December 2020. These calls were made by their attending physicians. The questionnaire on COVID‐19 symptoms was specifically designed for the study and this was used for the telephone calls, which were made between 1 August and 30 September 2021. The study was approved by the Ethics Committee of La Paz Hospital (PI‐4212) on the basis that we obtained consent from the parents during the telephone calls and that any data were incorporated into the patients' medical histories.
The inclusion criteria were children under 18 years old with a diagnosis of SARS‐CoV‐2 infection confirmed by polymerase chain reaction, or an antigen test or serology, between 14 March and 31 December with any symptoms related to the SARS‐CoV‐2 infection. The parents or legal guardians needed to agree to participate in the telephone questionnaire. We excluded any children with asymptomatic SARS‐CoV‐2 infections.
Post‐COVID‐19 was defined as when the subject had continuously presented symptoms, without recovering to their previous health status, for longer than 12 weeks. Ongoing symptomatic COVID‐19 was defined as symptoms that were present for 4–12 weeks. Although we used the 2020 NICE guidelines 8 in force when the study was carried out, the latest terminology is used to reflect the 2021 NICE and WHO guidelines, which cover exactly the same timelines and definitions. 9 , 10
A control group of inpatients and outpatients without a history of COVID‐19, who were treated by any of the three hospitals during the same period, namely 14 March to 31 December 2020, were randomly selected from the medical records. Their parents were contacted between 1 August and 30 September 2021. The children were either treated in the emergency room without being admitted or were hospitalised for an issue not related to COVID‐19. The children were chosen from patients with endocrinological, trauma or surgical pathology. The same questionnaire was used and carried out by telephone by the attending physicians who saw the controls.
2.1. Statistical analysis
A number of variables were collected by the questionnaire. The demographic data comprised the subject's date of birth, sex, the date of any COVID‐19 diagnosis and the onset of any symptoms. The variables related to those with a confirmed SARS‐CoV‐2 infection included the diagnostic method, clinical diagnosis, need for hospital admission and need for admission to the PICU. The parents were asked about the type of symptoms and how long they had lasted, the need for medical assistance and the type of assistance provided and any studies that were carried out. They were also asked about any comorbidities, the results of serology tests, whether any relatives also had COVID‐19 and whether the patients had a subjective feeling of having had their symptoms misinterpreted. It was generally only possible to assess some symptoms in children who were over 5 years of age, such as anosmia, ageusia, sadness, apathy or anxiety. The main variable was the presence of at least one symptom lasting longer than 12 weeks.
The statistical analysis was performed using SPSS, Version 21.0 (IBM Corp). Values were expressed as percentages for discrete and categorical variables and as medians and interquartile ranges (IQR) for continuous variables. The demographic, clinical and evolutionary characteristics were compared using the Mann‐Whitney U test, and Fisher's exact test, as appropriate. Odds ratios (OR) were calculated, with their 95% confidence intervals (CI). A p‐value of <0.05 was considered statistically significant.
3. RESULTS
We identified 668 children with COVID‐19 diagnoses and 150 controls treated for other reasons than COVID‐19 during the same period and contacted their parents. Of these, 451 and 98, respectively, agreed to complete the questionnaire. A flow chart of the patients and their clinical characteristics are shown in Figure 1. The median time between the acute episode and completing the survey from 1 August to 30 September 2021 was 351 days (IQR 330–471 days).
FIGURE 1.
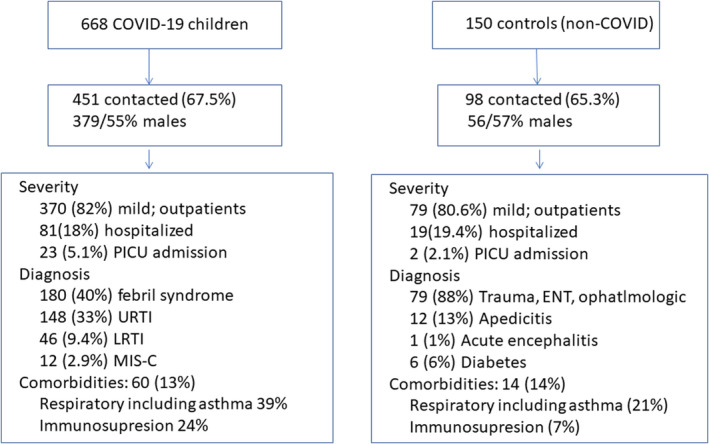
Flow chart of the cases and controls cohort
3.1. The COVID‐19 group
A total of 668 children under 18 years of age met the inclusion criteria for the COVID‐19 group and 451 (67.5%) of the parents could be contacted and agreed to participate. Just over half of the children (54.9%) were males and 82.0% had mild COVID‐19 and only required outpatient management. There were 23 children who required PICU admission (5.1%). The median age of the group with COVID‐19 was 4.0 years (interquartile range 1.0–10.5) and 52.9% were under 5 years old. Up to 60 children had comorbidities (13.3%) and respiratory chronic diseases or asthma and immunosuppression were the most frequent. The most common diagnosis related to COVID‐19 was febrile syndrome (39.9%), followed by upper respiratory tract infections (32.8%) and lower respiratory tract infections (9.4%), including pneumonia, bronchiolitis and asthma crisis. There were 13 patients (2.9%) with MIS‐C.
The symptoms and their duration are shown in Table 1. We found that 18.4% had at least one or more symptoms at 4–12 weeks and another 14.6% had symptoms at more than 12 weeks, namely post‐COVID‐19, which the parents associated with the SARS‐CoV‐2 infection. Most children with post‐COVID‐19 symptoms were 5 years of age or older (72.7%, OR 3.0, 95% CI 1.8–5.0). Some symptoms could not be evaluated in infants and young children, such as anosmia, ageusia, apathy or feeling sad, anxiety or concentration problems. Fever, rhinorrhoea and coughs were the most frequent symptoms and these generally had a short duration, with a median of <5 days.
TABLE 1.
Symptoms and duration in the total cohort of children with COVID‐19
| N (%) | Days of duration; median (IQR) | |
|---|---|---|
| Rhinorrhea | 212/451 (47%) | 3 (3–5) |
| Fever | 357/451 (79.1%) | 3 (2–4) |
| Cough | 218/451 (48.3%) | 4 (3–7) |
| Difficulty breathing | 93/451 (20.6%) | 4 (2.5–7) |
| Diarrhoea | 133/451 (29.5%) | 3 (2–5) |
| Vomits | 101/451 (22.4%) | 2 (1–3) |
| Abdominal pain | 90/433 (20.7%) | 3 (2–7) |
| Headache | 101/265 (38.1%) | 4 (3–10) |
| Loss of appetite | 204/451 (45.2%) | 5 (3–10) |
| Anosmia/ageusia | 33/220 (15%) | 15 (7.5–60) |
| Myalgia | 81/430 (18.8%) | 5 (3–15) |
| Asthenia | 155/337 (46%) | 8 (4–30) |
| Insomnia | 21/326 (6.4%) | 300 (52–422) |
| Concentration problems | 22/211 (10.4%) | 90 (15–253) |
| Apathy, feeling sad | 19/225 (8.4%) | 145 (97.5–298.7) |
| Anxiety | 24/225 (10.6%) | 205 (97.5–361.2) |
| Palpitations/tachycardia | 7/225 (3.1%) | 15 (3.2–30) |
| Dizziness | 15/225 (6.6%) | 4 (2–60) |
| Others a | 30/451 (6.6%) | 302.5 (112.5–272.5) |
Abbreviations: IQR, interquartile range; N, number of cases.
Other symptoms include mainly loss of hearing, memory loss, constipation and skin lesions.
The 66 children with post‐COVID‐19 symptoms for more than 12 weeks, required complementary tests in 43.9% of cases. The most frequent tests in these 66 children were blood tests (34.8%) and X‐rays (18.1%). They also underwent various other tests including spirometry and ultrasound. Almost half of those 66 patients (42.4%) also required specialist care.
The clinical characteristics of the children with symptoms for more than 12 weeks were compared with the rest of the patients and are shown in Table 2. A number of factors were significantly associated with post‐COVID‐19 and these were hospital admission (OR 3.9), need for PICU admission (OR 4.3) or a relative with symptoms for more than 12 weeks (OR 2.8). The post‐COVID‐19 group had longer hospital stays than those without this diagnosis (p = 0.047). Their symptoms were also different from other patients during acute COVID‐19, with a higher proportion of pneumonia or MIS‐C (p = 0.001). Comorbidities were not associated with symptoms lasting for more than 12 weeks, but symptoms such as dyspnoea, diarrhoea and abdominal pain were, even if they were short‐lived. Symptoms that were significantly associated with post‐COVID‐19 were headache, anosmia and ageusia, myalgia, asthenia, concentration difficulties insomnia, apathy or feeling sad, anxiety, palpitations and dizziness. A variety of prolonged symptoms were described in 5.9% of the children and those that stood out included hair loss, memory loss or skin lesions.
TABLE 2.
Comparison between children with persistent symptoms >12 weeks and the remaining cohort
|
Persistent‐COVID N = 66/451 (14.6%) |
No persistent symptoms N = 385/451 |
||
|---|---|---|---|
| Male gender | 40 (60%) | 208 (54%) | p = 0.35 |
| Admission | 26 (39.4%) | 55 (14.3%) | OR: 3.9, 95% CI (2.2–6.8) |
| Days of admission | 5.8 (SD 3.1) | 4.4 (SD 2.6) | p = 0.047 |
| PICU admission | 10 (15.2%) | 13 (3.9%) | OR: 4.3, 95% CI (1.8–10.4) |
| COVID−19 diagnosis | UTRI 24 (36%) | 124 (32%) | p = 0.001 |
| Febrile syndrome 16 (24%) | 164 (42%) | ||
| Pneumonia 11 (16.7%) | 15 (3.9%) | ||
| LRTI 1 (1,5%) | 19 (5%) | ||
| Gastroenteritis 5 (7.6%) | 31 (8%) | ||
| MIS‐C 6 (9%) | 7 (1.8%) | ||
| Comorbidity | 11 (16.7%) | 49 (12.7%) | p = 0.43 |
| Another family member with long COVID | 20 (36.4%) | 60 (16.9%) | OR: 2.8, 95% CI (1.5–5.2) |
| Rhinorrhoea | 26 (39.4%) | 186 (48.3%) | p = 0.185 |
| Fever | 53 (80.3%) | 306 (79.5%) | p = 0.345 |
| Cough | 39 (59%) | 177 (46%) | p = 0.051 |
| Dyspnoea | 32 (48.5%) | 60 (15.6%) | OR: 5.09, 95% CI (2.9–8.8) |
| Diarrhoea | 29 (44%) | 104 (27%) | OR: 2.1, 95% CI (1.2–3.6) |
| Vomits | 21 (31.8%) | 80 (20.8%) | p = 0.130 |
| Abdominal pain | 23/62 (37%) | 67/361 (18%) | OR: 2.6, 95% CI (1.5–4.7) |
| Loss of appetite | 36 (54.5%) | 168 (43.6%) | p = 0.100 |
| Headache | 32/55 (58.2%) | 69/310 (32.9%) | OR: 2.8, 95% CI (1.5–5.2) |
| Anosmia/ageusia | 17/54 (31.5) | 16/166 (9.6%) |
OR: 4.3, 95% CI (1.9–9.3) |
| Myalgia | 27/61 (44.3%) | 54/319 (15%) | OR: 4.4, 95% CI (2.5–8) |
| Asthenia | 44/60 (73.3%) | 111/277 (40%) | OR: 4.1, 95% CI (2.2–7.6) |
| Concentration problems | 16/52 (30.8%) | 6/159 (3.8%) | OR: 11.3, 95% CI (4.1–30.9) |
| Insomnia | 14/60 (23.3%) | 7/266 (2.6%) | OR: 11.2, 95% CI (4.3–24.4) |
| Apathy, sad feeling | 17/54 (31.5%) | 2/171 (1.2%) | OR: 28.8, 95% CI (8.5–178.3) |
| Anxiety | 22/55 (40%) | 2/170 (1.2%) | OR: 56, 95% CI (12.5–249.6) |
| Palpitations/tachycardia | 6/54 (11%) | 1/170 (0.6%) | OR: 21.1, 95% CI (2.4–179.7) |
| Dizziness | 8/54 (14.8%) | 7/171 (4.1%) | OR: 4.07, 95% CI (1.4–11.8) |
| >5 years old | 48 (72.7%) | 164 (42.6%) | OR: 3, 95% CI (1.8–5) |
| Age (years) | 8.7 (SD 5.3) | 5.4 (SD 5.2) | p = 0.001 |
Abbreviations: CI, confidence interval; LRTI, lower tract respiratory infection; OR, odds ratio; PICU, paediatric intensive care unit; SD, standard deviation; UTRI, upper tract respiratory infection.
Bold text indicates highlight significant results.
Post‐COVID‐19 symptoms for more than 12 weeks were more common in children aged 5 years of age or older (47.0%). They were compared with children under the age of 5 years. Any symptoms lasting 4–12 weeks were observed in 26.9% of the 212 children aged 5 years or older, compared to 10.9 of the 239 children under 5 years of age (p = 0.001). Symptoms that lasted longer than 12 weeks were observed in 22.6% and 7.5% of these age groups, respectively (p = 0.001). The diagnoses were slightly different, with more cases of MIS‐C and pneumonia in the children aged 5 years or older. However, the hospitalisation and PICU admission rates were not significantly different. The prevalence of the symptoms in the 2 age groups, and their duration, is shown in Figure 2.
FIGURE 2.
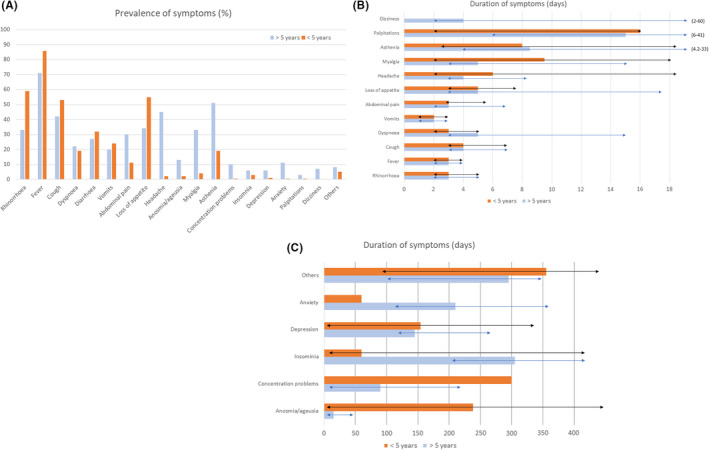
Prevalence (A) and duration (B and C) of symptoms in >5 years and ≤5 years children. Dark lines represent the interquartile range
Only 8.2% of the 451 children in the COVID‐19 group had 2 or more post‐COVID‐19 symptoms for more than 12 weeks (median 3, IQR 2–5). Of these 37 children, 70.3% had 1 or more affective or psychological symptoms and these were mainly accompanied by asthenia (40.5%), respiratory symptoms (29.7%), digestive symptoms (27.0%) or myalgia (24.3%) (Table 3 and S1). The post‐COVID‐19 symptoms of insomnia, concentration difficulties, apathy or sadness and anxiety persisted for a median of 90 days Figure 2.
TABLE 3.
Clinical data of children with more than 2 persistent symptoms >12 weeks
| N = 37/451 (8.2%) |
Days of duration of symptoms (SD) Median (IQR) |
|
|---|---|---|
| Male gender | 22 (59.5%) | |
| Admission | 17 (45.9%) | |
| PICU admission | 6 (37.8%) | |
| COVID−19 diagnosis | UTRI 14 (37.8%) | |
| Febrile syndrome 6 (16.2%) | ||
| Pneumonia 1 (2.7%) | ||
| LRTI 8 (21.6%) | ||
| Gastroenteritis 3 (8.1%) | ||
| MIS‐C 4 (10.8%) | ||
| Comorbidity | 9 (24.3%) | |
| Another family member with long COVID | 17 (45.9%) | |
| >5 years old | 28 (75.7%) | |
| Age (years) | 10.2. (IQR 6.8–13.4) | |
| Symptoms lasting more than 12 weeks | ||
| Dyspnoea | 21 (56.6%) |
120 (175) 10 (5–250) |
| Abdominal pain | 16 (43.2%) |
117 (167) 11 (3–247) |
| Loss of appetite | 22 (59.4%) |
123 (153) 45 (7–237) |
| Headache | 18 (48.6%) |
95 (123) 27 (6–160) |
| Anosmia/ageusia | 10 (27%) |
178 (208) 60 (27–469) |
| Myalgia | 18 (48.6%) |
83 (108) 30 (9–127) |
| Asthenia | 29 (78.3%) |
130 (131) 90 (40–180) |
| Concentration problems | 14 (37.8%) |
195 (143) 165 (90–296) |
| Insomnia | 11 (29.7%) |
380 (110) 380 (295–470) |
| Apathy, sad feeling | 17 (45.9%) |
225 (139) 180 (120–320) |
| Anxiety | 17 (45.9%) |
257 (144) 210 (120–410) |
| Others | 11 (29.7%) |
298 (143) 305 (150–450) |
Abbreviations: LRTI, lower tract respiratory infection; MIS‐C, multisystem inflammatory syndrome; PICU, paediatric intensive care unit; UTRI, upper tract respiratory infection.
Bold text indicates highlight significant results.
3.2. Control group
A total of 150 children under 18 years of age with no history of COVID‐19 formed the control group. Of these, 98 (65.3%) met the criteria and their parents agreed to participate: 80.6% were outpatients and 19.4% were hospitalised. Two were admitted to the PICU, one with newly diagnosed diabetes and one with encephalitis. Just over half (57.1%) were male and their median age was 7.8 years (IQR 4.1–10.3), with 28 (28.6%) under 5 years old. The clinical characteristics of the control group with the COVID‐19 patients are shown in Table 4 and S2.
TABLE 4.
Comparison between children with COVID‐19 and control group
|
COVID−19 N = 451 |
Control group N = 98 |
||
|---|---|---|---|
| Male gender | 248 (55%) | 56 (57%) | p = 0.761 |
| Admission | 81 (17.9%) | 19 (19.4%) | p = 0.690 |
| PICU admission | 23 (5.1%) | 2 (2%) | p = 0.131 |
| Comorbidity | 62 (13.7%) | 14 (14.3%) | p = 0.845 |
| Primary care need assistance | 95 (21.06%) | 8 (8.2%) |
p = 0.004 OR: 2.5, 95% CI (1.2–5.1) |
| Need specialised assistance | 51 (11.3%) | 16 (16.3%) | p = 0.152 |
| Symptoms more than 4 weeks | 83 (18.4%) | 29 (20.4%) | p = 0.597 |
| Persistent symptoms more than 12 weeks | 66 (14.6%) | 19 (19.4%) | p = 0.214 |
| >5 years old | 239 (53%) | 70 (71.4%) | p = 0.001 |
| Age (years) | 5.9 (SD 5.3) | 7.8 (SD 4.2) | p = 0.001 |
| Symptom more than 4 weeks >5 years old | 58/239 (24%) | 15/70 (21.4%) | p = 0.386 |
| Persistent symptoms more than 12 weeks >5 years old | 29/239 (20.5%) | 14/70 (20%) | p = 0.663 |
Significant results are marked in bold.
Abbreviations: CI, confidence interval; OR, odds ratio; PICU, pediatric intensive care unit; SD, standard deviation.
Although none of the controls had tested positive for SAR‐CoV‐2 before they were included in the study, we noted that the parents of 20 children (20.4%) reported symptoms that could have been attributed to COVID‐19 that lasted for 4–12 weeks. Another 19 children had symptoms that were characteristic of post‐COVID‐19 for longer than 12 weeks. These percentages were not significantly different from the children with confirmed COVID‐19. Given that the children in the control group were significantly older, at a median of 7.8 vs. 4.0 years, we analysed the percentages with persistent symptoms who were 5 or more years of age in both groups. There were no significant differences between the cases and controls (Table 4). However, symptoms that were present for 4–12 weeks and more than 12 weeks were significantly more common in children who were 5 years of age or older. The OR for children aged 5 years or over was 2.7 (95% CI 1.7–4.3) for 4–12 weeks when the reference category was children under 5 (25.8% vs. 11.3%, respectively). For symptoms that lasted more than 12 weeks, the OR for children aged 5 years or over was 3.1 (95% CI 1.8–5.3) when the reference category was children under 5 (22.3% vs. 8.3%, respectively).
To characterise the persistence of symptoms that indicated post‐COVID‐19, this specific sub‐group was compared with the other cases and controls who were 5 years of age or older (Table 5). The children in the post‐COVID‐19 group required significantly more complementary tests (p = 0.02) and the trend was for them to require more assistance from their primary care paediatrician (p = 0.056). Loss of appetite (p = 0.005), myalgia (p = 0.026) and asthenia (p = 0.001) were significantly associated with post‐COVID‐19, but the rest of the symptoms showed similar frequencies between the cases and controls. In children under 5 years of age, only loss of appetite was more frequent in the cases than controls with symptoms more than 12 weeks (47% vs. 0), with a tendency towards significance (p = 0.076).
TABLE 5.
Comparison between children >5 years old with at least one persistent symptom for more than 12 weeks in COVID‐19 and control group
|
COVID−19 N = 49 |
Control group N = 14 |
||
|---|---|---|---|
| Male gender | 28 (57.1%) | 7 (50%) | p = 0.635 |
| Admission | 20 (40.8%) | 5 (35.7%) | p = 0.731 |
| PICU admission | 20 (20.4%) | 0 | p = 0.064 |
| Comorbidity | 9 (18.4%) | 3 (21.4%) | p = 0.797 |
| Complementary test after acute episode | 26 (53.1%) | 1 (11.1%) |
p = 0.020 OR control group 0.77, 95% CI (0.61–0.96) |
| Primary care need assistance | 28 (57.1%) | 4 (28.6%) | p = 0.056 |
| Abdominal pain | 21 (42.9%) | 3 (33.38%) | p = 0.594 |
| Loss of appetite | 28 (57.1%) | 2 (14.3%) |
p = 0.005 OR: 5.4, 95% CI (1.3–22.4). |
| Headache | 31 (63.3%) | 5 (35.7%) | p = 0.063 |
| Myalgia | 27 (55.1%) | 1 (11.1%) |
p = 0.026 OR: 10.4, 95% CI (1.4–74) |
| Asthenia | 39 (79.6%) | 3 (21.4%) |
p = 0.001 OR: 7.3, 95% CI (2.2–23.4) |
| Concentration problems | 15 (31.3%) | 2 (14.3%) | p = 0.211 |
| Insomnia | 11 (22.4%) | 4 (28.6%) | p = 0.635 |
| Apathy, feeling sad | 15 (31.3%) | 2 (14.3%) | p = 0.211 |
| Anxiety | 22 (44.9%) | 8 (57.1%) | p = 0.418 |
| Palpitations/tachycardia | 6 (12.5%) | 1 (7.1%) | p = 0.577 |
| Dizziness | 8 (16.3%) | 1 (7.1%) | p = 0.386 |
| Other symptoms | 16 (34.8%) | 2 (14.3%) | p = 0.143 |
OR is shown when significant.
Abbreviations: CI, confidence interval; OR, odds ratio; PICU, paediatric intensive care unit; SD, standard deviation.
Bold text indicates highlight significant results.
4. DISCUSSION
Post‐COVID‐19 in children, which lasts for more than 12 weeks after testing positive for SARS‐CoV‐2, is still being studied and debated. However, there is still insufficient data to provide a definitive statement about their frequency and characteristics. Our multicentre study of 451 children with a confirmed, symptomatic SARS‐CoV‐2 infection, found that 18.4% of cases were symptomatic for 4–12 weeks and 14.6% had at least 1 symptom that lasted longer than 12 weeks. These are very high figures and a considerable cause for concern. Up to 8.2% of the children had 2 or more symptoms lasting longer than 12 weeks and this is probably the most relevant figure. The percentages were higher in our study than in a study from the United Kingdom 17 and in other studies, 14 , 16 possibly due to the difference in the methodologies used. Firstly, 18.4% of the COVID‐19 group in our study were hospitalised patients who suffered a more severe acute infection, with more prolonged symptoms, and asymptomatic children were not included. Secondly, we evaluated a significant number of nonorganic symptoms, such as anxiety, sadness and apathy, which are as important as the physical symptoms and may be very debilitating for children. Osmanov et al 15 carried out a study with a similar cohort and methodology in Russia and the percentages of prolonged symptoms in children were quite similar to our results. However, the authors did not include a control group in that study.
We may conclude that post‐COVID‐19 is more likely to affect children aged 5 years or more and it is particularly associated with the symptoms found in adults, such as loss of appetite, asthenia and myalgia. 5
Previous studies have shown that most children made a full recovery after COVID‐19 and that their symptoms lasted between 1 and 2 weeks. 12 In our series, most of the symptoms had a median duration of 3–5 days, with asthenia, anosmia and ageusia, and palpitations lasting about 2 weeks.
Our findings showed that post‐COVID‐19 was significantly associated with a number of factors, including children aged 5 years or older, the need for hospitalisation and PICU admissions. This suggests that the severity of the illness may have been an important risk factor. It is also worth noting that the presence of a family member with post‐COVID‐19 was associated with an increased risk of up to 2.8 times in children. This suggests that genetic factors could play a significant role, but the potential risk factors of difficult social situations cannot be ruled out. Comorbidities were not risk factors for post‐COVID‐19 symptoms. Remarkably, the percentage of children with symptoms at 4–12 and more than 12 weeks was quite similar.
A similar percentage of prolonged symptoms was observed when children with COVID‐19 were compared to a control group of children treated during the same period for problems other than COVID‐19. These included vague symptoms, such as anxiety, insomnia, tachycardia or dizziness. These symptoms may be related to social distancing measures, including full lockdowns, and not to the SARS‐CoV‐2 infection. However, this group of children had fewer characteristic symptoms, such as anosmia, ageusia, myalgia or headaches.
Our study had a number of strengths and limitations. One limitation was that most long‐lasting symptoms, such as anosmia, ageusia, concentration difficulties, apathy and anxiety, could only be assessed in older children. This could be considered a bias when being 5 or more years of age was considered a risk factor for post‐COVID‐19. Some of these symptoms cannot be attributed with certainty to the SARS‐CoV‐2 infection and could be partly related to such factors as the children's family situation, lockdowns and social restrictions, lack of social relationships and fear of the virus. This seems to be the most feasible theory given that children in the control group were treated for other reasons. It is also interesting that parents and children attributed some of their new symptoms to the SARS‐CoV‐2 infection, although they were not frequently associated with COVID‐19, such as hair loss, memory loss, constipation or skin lesions. These symptoms often lasted a median of 1 year. It is difficult to conclude that these symptoms were truly caused by the infection, as many features of this virus are not still well understood and causality cannot be ruled out. A number of symptoms were significantly associated with post‐COVID‐19 when the cases were compared with the controls. These included headaches, anosmia/ageusia, myalgia, asthenia, concentration problems, insomnia, apathy or feeling sad, anxiety, palpitations/tachycardia and dizziness. This suggests that these are symptoms due to the SARS‐CoV‐2 infection and that they may have an organic explanation. A study by Buonsenso et al showed lung perfusion defects and inflammation were observed in adolescents with post‐COVID‐19 and these may have had an organic aetiology that was linked to a previous SARS‐CoV‐2 infection. 21
Our study had other limitations. The response rates were not as high as we would have liked because we were not able to contact all the parents. The retrospective nature of the study could also have led to memory errors and the data that was obtained may not have been precise. In addition, the fact that some of the children were hospitalised may have determined the duration of some symptoms, especially respiratory issues. We excluded children with asymptomatic SARS‐CoV‐2 infections and this meant that we were unable to rule out whether symptoms developed after initial asymptomatic infection. The possible symptoms of post‐COVID‐19 in children under 5 years of age were difficult to assess.
One of the strengths of this study was the control group, which helped us to characterise the symptoms of post‐COVID‐19 in children. The number of children included in our study was high and it combined the level of severity often seen in paediatric SARS‐CoV‐2 cases with an important group of children with mild children symptoms. The study also included diagnoses made during the first and second waves of the pandemic, in Madrid hospitals that cared for paediatric inpatients and outpatients with COVID‐19. Our results are not necessarily generalisable to other areas and we do not know if other variants of SARS‐CoV‐2, such as Omicron, will give rise to similar prolonged symptoms or not.
5. CONCLUSION
Our study showed that children suffered prolonged symptoms after COVID‐19 and that these significantly affected their lives and required specific clinical attention. In our series, post‐COVID‐19 particularly affected children over 5 years of age and was associated with symptoms such as asthenia, loss of appetite and myalgia. There is a clear need for further research on post‐COVID‐19 in children.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Tab S1
Tab S2
Bergia M, Sanchez‐Marcos E, Gonzalez‐Haba B, et al. Comparative study shows that 1 in 7 Spanish children with COVID‐19 symptoms were still experiencing issues after 12 weeks. Acta Paediatr. 2022;111:1573–1582. doi: 10.1111/apa.16368
Funding information
No external funding.
REFERENCES
- 1. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID‐19 in children compared with adults in Shandong Province, China. Infection. 2020;48(3):445‐452. doi: 10.1007/s15010-020-01427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ISARIC Clinical Characterisation Group . COVID‐19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. 2021;49(5):889‐905. doi: 10.1007/s15010-021-01599-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tagarro A, Cobos‐Carrascosa E, Villaverde S, et al. Clinical spectrum of COVID‐19 and risk factors associated with severity in Spanish children [published online ahead of print, 2021 Nov 5]. Eur J Pediatr. 2021;181(3):1105‐1115. doi: 10.1007/s00431-021-04306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang DH, Roy DJ, Gu BJ, Hassett LC, McCoy RG. Postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection: a state‐of‐the‐art review. JACC Basic Transl Sci. 2021;6(9):796‐811. doi: 10.1016/j.jacbts.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ludvigsson JF. Case report and systematic review suggest that children may experience similar long‐term effects to adults after clinical COVID‐19. Acta Paediatr. 2021;110(3):914‐921. doi: 10.1111/apa.15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40(12):e482‐e487. doi: 10.1097/INF.0000000000003328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soriano JB, Ancochea J. On the new post COVID‐19 condition [published online ahead of print, 2021 Apr 16]. Sobre la nueva condición post COVID‐19 [published online ahead of print, 2021 Apr 16]. Arch Bronconeumol (Engl Ed). 2021;57(12):735‐736. doi: 10.1016/j.arbres.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19. London: National Institute for Health and Care Excellence (NICE); 2020. PMID: 33555768 Free Books & Documents. Review. [PubMed]
- 9. Updated NICE ‘long COVID’ guideline . By NICE, SIGN, royal college of general practitioners 12 November 2021). https://www.guidelines.co.uk/infection/nice‐long‐covid‐guideline/455728.article. Accessed 10 February 2022.
- 10. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post‐COVID‐19 Condition . A clinical case definition of post‐COVID‐19 condition by a Delphi consensus [published online ahead of print, 2021 Dec 21]. Lancet Infect Dis. 2022;22(4):e102‐e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munblit D, Sigfrid L, Warner JO. Setting priorities to address research gaps in long‐term COVID‐19 outcomes in children. JAMA Pediatr. 2021;175(11):1095‐1096. doi: 10.1001/jamapediatrics.2021.2281 [DOI] [PubMed] [Google Scholar]
- 12. Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school‐aged children tested for SARS‐CoV‐2 [published correction appears in Lancet Child Adolesc Health. 2021 Aug 31;:]. Lancet Child Adolesc Health. 2021;5(10):708‐718. doi: 10.1016/S2352-4642(21)00198-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208‐2211. doi: 10.1111/apa.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post‐acute COVID‐19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22‐e23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC global follow‐up protocol: a prospective cohort study [published online ahead of print, 2021 Jul 1]. Eur Respir J. 2021;59(2):2101341. doi: 10.1183/13993003.01341-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long‐term symptoms after SARS‐CoV‐2 infection in children and adolescents [published online ahead of print, 2021 Jul 15]. JAMA. 2021;326(9):869‐871. doi: 10.1001/jama.2021.11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Office for National Statistics, U. K. (1 de April de 2021) . Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK. United Kingdom. s.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021. Accessed 17 November 2021.
- 18. Nogueira López J, Grasa Lozano C, Ots Ruiz C, et al. Seguimiento telemático de COVID‐19: experiencia de un hospital terciario [Telemedicine follow‐ups for COVID‐19: Experience in a tertiary hospital] [published online ahead of print, 2020 Nov 2]. An Pediatr (Engl Ed). 2021;95(5):336‐344. doi: 10.1016/j.anpedi.2020.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nogueira López J, Grasa C, Calvo C, García L‐H. Long‐term symptoms of COVID‐19 in children. Acta Paediatr. 2021;110(7):2282‐2283. doi: 10.1111/apa.15849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buonsenso D, Fusco C, De Rose C, Valentini P, Vergari J. Long COVID in children: partnerships between families and paediatricians are a priority for better care. J Paediatr Child Health. 2022;58(1):201‐202. doi: 10.1111/jpc.15600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buonsenso D, Di Giuda D, Sigfrid L, et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post‐acute sequelae of SARS‐CoV‐2 infection. Lancet Child Adolesc Health. 2021;5(9):677‐680. doi: 10.1016/S2352-4642(21)00196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Tab S2


