Abstract
Non-cardia gastric cancer was significantly associated with Helicobacter pylori (H. pylori) infection. Reducing H. pylori prevalence was an important prevention strategy for non-cardia gastric cancer. However, national-level data on the H. pylori prevalence in non-cardia gastric cancer were limited in China. Therefore, we conducted this study to estimate the pooled prevalence of H. pylori in non-cardia gastric cancer in China. We searched PubMed, Embase, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan Fang, and VIP Database for Chinese Technical Periodicals for studies reporting H. pylori prevalence in non-cardia gastric cancer in China which were published before September 1, 2021. Pooled prevalence was calculated using a random-effect model. Subgroup analysis and meta-regression were used to explore the potential sources of heterogeneity. Egger’s test and funnel plot were used to assess publication bias. A total number of 55 studies with 5324 cases of non-cardia gastric cancer were included in this study. The pooled prevalence of H. pylori in non-cardia gastric cancer in China was 66.5% (95%CI: 62%-71%, I2=93.8%, P<0.0001). In subgroup analysis, a significant difference in the prevalence of H. pylori in non-cardia gastric cancer was noted when stratified by geographic region of China (P=0.0112). The highest H. pylori prevalence (78.9%, 95%CI: 69.9%-87.8%) was noted in Northwest China and the lowest (53.1%, 95%CI: 38.9%-67.3%) was in North China. In meta-regression, a significant association between H. pylori prevalence and geographic region was found, while type of sample, H. pylori testing method, diagnosis period, detection timing, type of study design, quality grade, publication year, and sample size were not associated with the prevalence of H. pylori in non-cardia gastric cancer (P>0.05). A large proportion of non-cardia gastric cancers were associated with H. pylori infection in China, emphasizing the possible benefits of H. pylori eradication for the prevention and control of non-cardia gastric cancer.
Keywords: prevalence, Helicobacter pylori, non-cardia gastric cancer, meta-analysis, China
Introduction
Gastric cancer is one of the most common malignant tumors. In 2020, it was estimated that there were 1.09 million new gastric cancer cases and 0.77 million deaths from gastric cancer all over the world. Among all the new gastric cancer cases, more than 40% occurred in China. Meanwhile, gastric cancer caused about 12.4% of all cancer-related deaths, making it the third leading cause of cancer-related deaths in China (1).
Gastric cancer can be classified into two categories according to anatomical subsites: cardia gastric cancer and non-cardia gastric cancer (2–4). Due to different epidemiological characteristics and distinct pathogeneses, cardia and non-cardia gastric cancer are treated as two different diseases. Non-cardia gastric cancer is more common than cardia gastric cancer. In 2018, non-cardia gastric cancer accounted for up to 82% (0.85/1.03 million) of all gastric cancer cases around the world (2, 5). Regarding pathogeneses, Helicobacter pylori (H. pylori) has been proven to be one of the most important risk factors for non-cardia gastric cancer, with approximately 90% of non-cardia gastric cancer cases attributable to H. pylori infection worldwide in 2018 (5, 6), whereas no association was found between cardia gastric cancer and H. pylori infection (7, 8).
Given the strong association between non-cardia gastric cancer and H. pylori infection, reducing H. pylori prevalence has been listed as an important primary prevention strategy for gastric cancer prevention (9–11). Reliable estimation of H. pylori prevalence in non-cardia gastric cancer may be essential to the control and prevention of non-cardia gastric cancer, policy-making, and health resource allocation. To date, lots of studies conducted in China have reported the prevalence of H. pylori in non-cardia gastric cancer. However, the prevalence varied greatly across studies (12, 13). To the best of our knowledge, there is no study pooling the prevalence of H. pylori in non-cardia gastric cancer at the national level. Therefore, the primary objective of this meta-analysis was to estimate the pooled prevalence of H. pylori in non-cardia gastric cancer in China. Additionally, we also explored potential causes of heterogeneity in the reported prevalence.
Materials and Methods
Data Sources and Searches Strategy
We conducted a systematic literature review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify Chinese and English language studies published before September 1, 2021, which examined the prevalence of H. pylori in non-cardia gastric cancer in China. Two investigators (XF and WY) independently searched the literature in the following English databases: PubMed, Embase, the Cochrane Library, and the following Chinese databases: China National Knowledge Infrastructure (CNKI), Wan Fang, and VIP Database for Chinese Technical Periodicals. The search terms included (“cardia”), and (“gastric” or “stomach”), and (“cancer” or “neoplasms”), and (“Helicobacter” or “pylori”), and (“China” or “Chinese”). Authors (XF and WY) independently reviewed the studies to identify eligible studies. The three authors (LY, XF, and WY) discussed inconsistencies to reach consensus. We also reviewed the reference lists of included articles to identify additional eligible studies.
Eligibility Criteria
We set the inclusion criteria as follows (1): studies reporting the prevalence of H. pylori from at least 10 cases of non-cardia gastric cancer, regardless of study design (2). Study site(s) located in China and Chinese participants were required (3). Studies were original studies published in English or Chinese language in any journal (4). H. pylori testing methods were clearly mentioned. We excluded studies which involved populations with special characteristics (e.g., recurrent cases) and studies with purposively selected cases (e.g., only advanced stage cases or metastatic cases). If several articles were based on the same research population, the one with the largest sample size was kept.
Data Extraction and Quality Assessment
Two authors (XF and WY) extracted the data independently, and the inconsistencies between the two authors were managed through discussion with third author (LY). We made a standardized data extraction sheet in Microsoft Excel to extract the following variables: title, first author, journal of publication, publication year, geographic location of study, study period, sex distribution, age of diagnosis (mean/median/range), type of sample, H. pylori testing method, detection timing, type of study design, sample size, the number of H. pylori-positive cases, and prevalence of H. pylori in non-cardia gastric cancer. When the required information could not be extracted directly from the article, we contacted the authors for relevant information at least two times.
The 11-item Cross-Sectional/Prevalence Study Quality Assessment Forms recommended by the Agency for Healthcare Research and Quality (AHRQ) were used to assess the methodological quality of included cross-sectional studies (14) and the Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of included case-control and cohort studies (15). The studies would then be classified as low quality, moderate quality, and high quality, if they had scores of 0-3, 4-7, and 8-11 for cross-sectional studies, and 0-3, 4-6, and 7-9 for case-control and cohort studies.
Data Analysis
Cochran’s Q test and I2 index were used to identify the heterogeneity across study. As the results showed significant heterogeneity, random-effect model was hence used to calculate the pooled prevalence of H. pylori in non-cardia gastric cancer and 95% confidence intervals (CI), weighted by DerSimonian-Laird model (16, 17).
We conducted subgroup analysis and meta-regression to explore the potential sources of heterogeneity (18). Subgroup analyses were carried out by the geographic region of China (Northwest, Northeast, Southwest, South Central, East, North, and not specified [NS]) (19), province, type of sample (breath, tissue, blood, and other), H. pylori testing method (14C urea breath test, immunohistochemical staining, Giemsa stain, Polymerase Chain Reaction [PCR], rapid urease test, Enzyme linked immunosorbent assay [ELISA], and other), diagnosis period (before 1999, 2000-2004, 2005-2009, 2010-2014, 2015-2019, and other), detection timing (before treatment and NS), type of study design (cross-sectional study, case-control study, and cohort study), and quality grade. Univariate meta-regression was performed based on the following variables: geographic region of China, type of sample, H. pylori testing method, diagnosis period, detection timing, type of study design, quality grade, publication year, and sample size.
We used Egger’s test and funnel plot to assess publication bias (20). Sensitivity analysis was conducted using leave-one-out method, which omitted one study at a time and re-conducted statistical analysis, in order to evaluate the influence of each omitted study on the pooled prevalence. All analyses were conducted using package “meta” in R 4.1.1, and statistical significance level was set as 0.05 for two-sided tests.
Results
Studies Selection
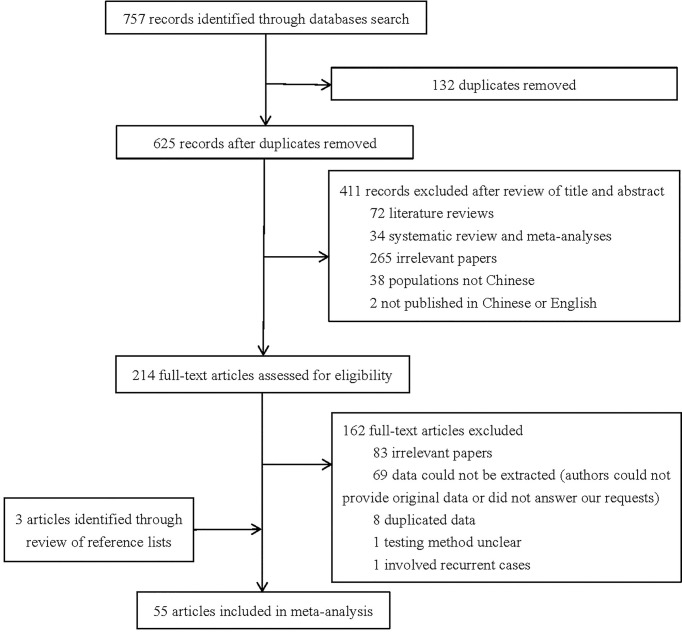
A total number of 757 studies were initially identified through literature search. We excluded 132 studies because of duplication. After examination of titles and abstracts, 411 studies were excluded as they did not meet the eligibility criteria. After full-text review, 162 studies were excluded for the following reasons (1): 83 studies were not relevant to our study (2); 69 studies had no available data (authors could not provide original data or did not answer our requests) (3); eight studies reused data included in other studies (4); one study did not mention testing method of H. pylori (5); one study involved recurrent cases. Through checking reference lists, three additional relevant studies were added. Ultimately, 55 studies with 5324 cases of non-cardia gastric cancer were enrolled in the final meta-analysis. The detailed process of studies selection is shown in Figure 1.
Figure 1.
Flow diagram of studies selection.
Characteristics of Included Studies
The detailed characteristics of the 55 included studies are summarized in Supplementary Table S1. The included studies were published between 1996 and 2020, and the sample size ranged from 10 to 343. Moreover, the majority of the studies were published in Chinese (44/55, 80%), and others were published in English. Of all the 55 studies, 17 were conducted in East China, 16 in South Central China, 10 in Northwest China, six in North China, three in Northeast China, one in Southwest China, and the geographic region of three studies was not specified. Regarding the type of sample, tissue was tested in 27 studies, blood in 18 studies, and breath in four studies. The most common H. pylori testing method was ELISA (n=15), followed by immunohistochemical staining (n=6), PCR (n=4), 14C urea breath test (n=4), Giemsa stain (n=3), and rapid urease test (n=3). Regarding type of study design, more than half (34/55, 61.8%) of included studies were cross-sectional studies. Additionally, about half of the studies (n=27) were rated as moderate quality, and 18 studies were regarded as high quality and 10 as low quality.
Prevalence of H. pylori
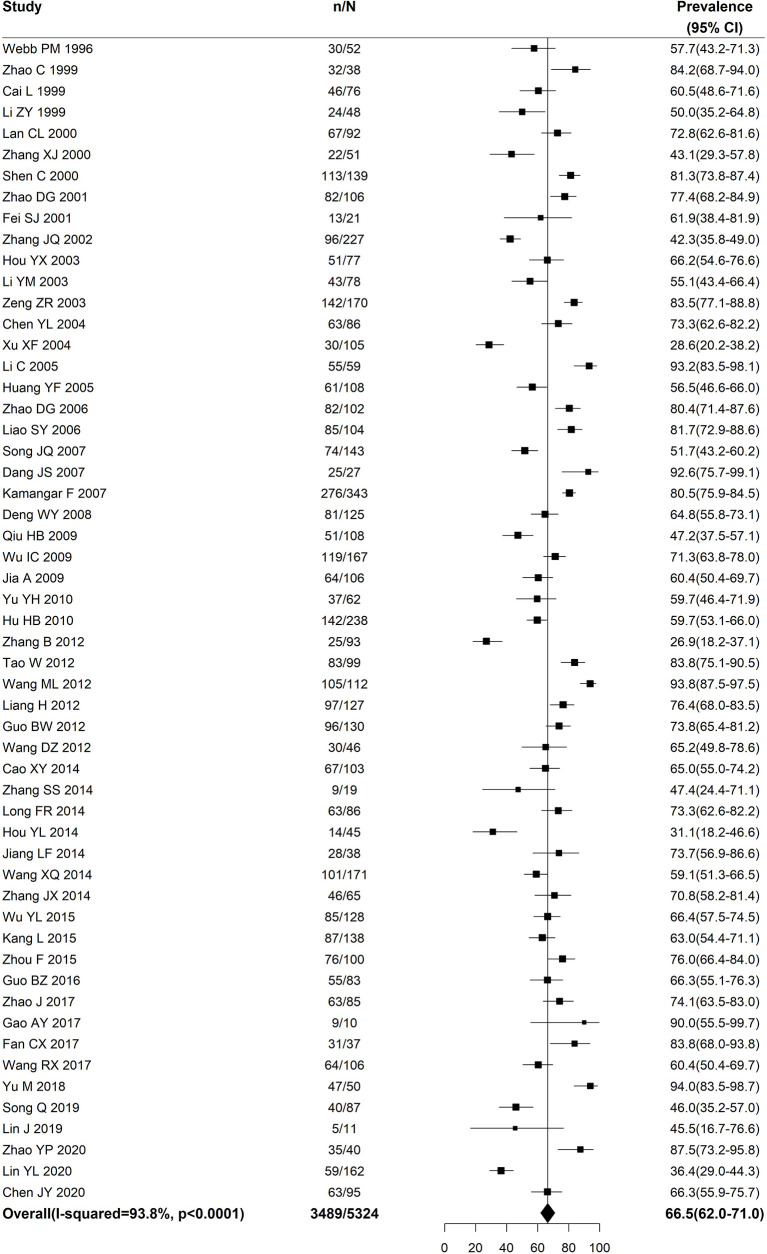
Figure 2 shows that the pooled prevalence of H. pylori in non-cardia gastric cancer in China was 66.5% (95%CI: 62%-71%). Statistical heterogeneity was observed among the included studies (I2=93.8%, P<0.0001).
Figure 2.
Forest plot for the pooled prevalence of Helicobacter pylori in non-cardia gastric cancer in China.
Subgroup Analysis
Table 1 shows the results of subgroup analysis. Significant differences in the prevalence of H. pylori in non-cardia gastric cancer was noted when stratified by geographic region of China (P=0.0112) and type of sample (P=0.0326). The geographic region with the highest prevalence of H. pylori was Northwest China (78.9%, 95%CI: 69.9%-87.8%) followed by Northeast China (74.3%, 95%CI: 61.9%-86.7%), and Southwest China (72.8%, 95%CI: 63.7%-81.9%), while the geographic region with the lowest prevalence was North China (53.1%, 95%CI: 38.9%-67.3%). Additionally, when stratified by province, the prevalence of H. pylori in non-cardia gastric cancer was also statistically different (P<0.0001). The top three provinces with the highest prevalence of H. pylori were Heilongjiang (90%, 95%CI: 71.4%-100%), Ningxia (89.2%, 95%CI: 79.5%-98.9%), and Shandong (84.2%, 95%CI: 72.6%-95.8%), whereas Beijing (43.1%, 95%CI: 29.5%-56.7%), Inner Mongolia (45.4%, 95%CI: 24.7%-66%), and Fujian (49.2%, 95%CI: 35.2%-63.3%) showed the lowest prevalence of H. pylori. The prevalence of H. pylori detected in breath (78.7%, 95%CI: 67.2%-90.2%) was significantly higher than that detected in tissue (67%, 95%CI: 60.7%-73.4%) and blood (64.1%, 95%CI: 54.8%-73.4%). The prevalence of H. pylori in non-cardia gastric cancer was generally similar for different H. pylori testing methods (P=0.4846), diagnosis periods (P=0.2623), detection timings (P=0.4719), types of study design, (P=0.6952), and quality grades (P=0.7712).
Table 1.
Prevalence of Helicobacter pylori in non-cardia gastric cancer by stratification variables.
| Studies | N | H. pylori positive cases | Pooled H. pylori prevalence(95% CI) | P value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2(%) | P value | ||||||
| Geographic region | 0.0112 | ||||||
| Northwest China | 10 | 880 | 657 | 78.9 (69.9; 87.8) | 92.9 | <0.0001 | |
| Northeast China | 3 | 198 | 139 | 74.3 (61.9; 86.7) | 66.6 | 0.0502 | |
| Southwest China | 1 | 92 | 67 | 72.8 (63.7; 81.9) | – | – | |
| South Central China | 16 | 1866 | 1307 | 69.9 (63.5; 76.4) | 90.2 | <0.0001 | |
| East China | 17 | 1543 | 911 | 62.2 (53.8; 70.6) | 93.4 | <0.0001 | |
| North China | 6 | 545 | 300 | 53.1 (38.9; 67.3) | 93.2 | <0.0001 | |
| NS | 3 | 200 | 108 | 51.2 (31.2; 71.2) | 87.6 | 0.0003 | |
| Province | <0.0001 | ||||||
| Heilongjiang | 1 | 10 | 9 | 90.0 (71.4; 100.0) | – | – | |
| Ningxia | 2 | 211 | 188 | 89.2 (79.5; 98.9) | 80.7 | 0.0227 | |
| Shandong | 1 | 38 | 32 | 84.2 (72.6; 95.8) | – | – | |
| Gansu | 2 | 123 | 94 | 77.9 (64.8; 83.4) | 46.3 | 0.1723 | |
| Shaanxi | 6 | 546 | 375 | 75.5 (61.7; 89.2) | 94.3 | <0.0001 | |
| Henan | 7 | 954 | 723 | 74.9 (70.3; 79.5) | 60.6 | 0.0186 | |
| Liaoning | 1 | 85 | 63 | 74.1 (64.8; 83.4) | – | – | |
| Chongqing | 1 | 92 | 67 | 72.8 (63.7; 81.9) | – | – | |
| Hebei | 2 | 176 | 126 | 71.5 (64.0; 79.0) | 13.8 | 0.2814 | |
| Taiwan | 1 | 167 | 119 | 71.3 (64.4; 78.1) | – | – | |
| Jiangsu | 7 | 397 | 294 | 70.7 (59.8; 81.6) | 77.2 | 0.0002 | |
| Guangdong | 5 | 453 | 304 | 67.5 (54.5; 80.4) | 90.5 | <0.0001 | |
| Hubei | 3 | 221 | 138 | 65.1 (35.8; 94.4) | 97.0 | <0.0001 | |
| Jilin | 1 | 103 | 67 | 65.1 (55.8; 74.3) | – | – | |
| Guangxi | 1 | 238 | 142 | 59.7 (53.4; 65.9) | – | – | |
| Shanghai | 3 | 365 | 189 | 57.5 (39.3; 75.7) | 93.1 | <0.0001 | |
| Fujian | 5 | 576 | 277 | 49.2 (35.2; 63.3) | 92.4 | <0.0001 | |
| Inner Mongolia | 3 | 318 | 152 | 45.4 (24.7; 66.0) | 94.2 | <0.0001 | |
| Beijing | 1 | 51 | 22 | 43.1 (29.5; 56.7) | – | – | |
| NS | 3 | 200 | 108 | 51.2 (31.2; 71.2) | 87.6 | 0.0003 | |
| Type of sample | 0.0326 | ||||||
| Breath | 4 | 278 | 204 | 78.7 (67.2; 90.2) | 83.2 | 0.0005 | |
| Tissue | 27 | 2452 | 1577 | 67.0 (60.7; 73.4) | 92.7 | <0.0001 | |
| Blood | 18 | 2036 | 1367 | 64.1 (54.8; 73.4) | 96.2 | <0.0001 | |
| Othera | 6 | 558 | 341 | 61.4 (57.3; 65.4) | 0.0 | 0.5004 | |
| H. pylori testing method | 0.4846 | ||||||
| 14C urea breath test | 4 | 278 | 204 | 78.7 (67.2; 90.2) | 83.2 | 0.0005 | |
| Immunohistochemical Staining | 6 | 355 | 250 | 71.1 (63.9; 78.3) | 56.9 | 0.0408 | |
| Giemsa stain | 3 | 412 | 242 | 66.4 (41.8; 91.0) | 97.4 | <0.0001 | |
| PCR method | 4 | 514 | 321 | 66.0 (50.5; 81.5) | 90.7 | <0.0001 | |
| Rapid urease test | 3 | 337 | 187 | 65.1 (32.8; 97.8) | 98.5 | <0.0001 | |
| ELISA | 15 | 1746 | 1138 | 62.4 (52.4; 72.4) | 96.0 | <0.0001 | |
| Otherb | 20 | 1682 | 1147 | 66.4 (59.8; 73.1) | 91.0 | <0.0001 | |
| Diagnosis period | 0.2623 | ||||||
| Before1999 | 6 | 488 | 338 | 64.7 (52.2; 77.2) | 87.7 | <0.0001 | |
| 2000-2004 | 3 | 299 | 154 | 52.7 (27.1; 78.3) | 95.9 | <0.0001 | |
| 2005-2009 | 3 | 390 | 236 | 60.6 (55.7; 65.4) | 0.0 | 0.7708 | |
| 2010-2014 | 7 | 591 | 410 | 69.5 (62.5; 76.5) | 73.3 | 0.0010 | |
| 2015-2019 | 4 | 188 | 127 | 69.8 (44.1; 95.5) | 95.4 | <0.0001 | |
| Otherc | 32 | 3368 | 2224 | 67.6 (61.4; 73.8) | 94.8 | <0.0001 | |
| Detection timing | 0.4719 | ||||||
| Before treatment | 19 | 1960 | 1192 | 64.1 (56.1; 72.2) | 93.9 | <0.0001 | |
| NS | 36 | 3364 | 2297 | 67.7 (62.2; 73.1) | 93.3 | <0.0001 | |
| Type of study design | 0.6952 | ||||||
| Cross-sectional study | 34 | 2698 | 1784 | 67.9 (62.3; 73.4) | 92.2 | <0.0001 | |
| Case-control study | 19 | 2231 | 1399 | 63.8 (55.5; 72.1) | 95.4 | <0.0001 | |
| Cohort study | 2 | 395 | 306 | 70.0 (47.8; 92.3) | 90.1 | 0.0015 | |
| Quality grade | 0.7712 | ||||||
| High | 18 | 2278 | 1480 | 64.1 (55.5; 72.8) | 95.8 | <0.0001 | |
| Moderate | 27 | 2222 | 1420 | 67.2 (61.2; 73.2) | 91.0 | <0.0001 | |
| Low | 10 | 824 | 589 | 68.9 (58.0; 79.7) | 93.3 | <0.0001 | |
H. pylori, Helicobacter pylori; CI, confidence interval; NS, not specific; PCR, polymerase chain reaction; ELISA, enzyme linked immunosorbent assay.
using several types of samples.
using other testing method or several testing methods.
The diagnosis period could not be divided into corresponding group or the diagnosis period was not clear.
Meta-Regression
The results of univariate meta-regression indicated that there was significant association between geographic region of China and the prevalence of H. pylori in non-cardia gastric cancer (Table 2). Compared with the prevalence in North China, the prevalence was higher in Northwest China (β=0.2570, 95%CI: 0.1002-0.4138, P=0.0013), Northeast China (β=0.2216, 95%CI: 0.0016-0.4417, P=0.0484), and South Central China (β=0.1663, 95%CI: 0.0202-0.3124, P=0.0257). The results of univariate meta-regression also revealed that type of sample, H. pylori testing method, diagnosis period, detection timing, type of study design, quality grade, publication year, and sample size were not significantly associated with the prevalence of H. pylori in non-cardia gastric cancer (P>0.05).
Table 2.
Assessing the effect of study variables on the pooled prevalence of Helicobacter pylori in non-cardia gastric cancer in China using univariable meta-regression analysis.
| Variable | β (95% CI) | SE | P value |
|---|---|---|---|
| Geographic region | |||
| Northwest China | 0.2570 (0.1002; 0.4138) | 0.0800 | 0.0013 |
| Southwest China | 0.1969 (-0.1293; 0.5232) | 0.1665 | 0.2368 |
| Northeast China | 0.2216 (0.0016; 0.4417) | 0.1123 | 0.0484 |
| South Central China | 0.1663 (0.0202; 0.3124) | 0.0745 | 0.0257 |
| East China | 0.0907 (-0.0552; 0.2366) | 0.0744 | 0.2233 |
| NS | -0.0185 (-0.2370; 0.2000) | 0.1115 | 0.8684 |
| North China | Reference | ||
| Type of sample | |||
| Breath | 0.1674 (-0.0479; 0.3826) | 0.1098 | 0.1275 |
| Tissue | 0.0488 (-0.1023; 0.1999) | 0.0771 | 0.5265 |
| Blood | 0.0218 (-0.1358; 0.1794) | 0.0804 | 0.7862 |
| Othera | Reference | ||
| H. pylori testing method | |||
| 14C urea breath test | 0.1268 (-0.0607; 0.3143) | 0.0957 | 0.1851 |
| Immunohistochemical Staining | 0.0415 (-0.1231; 0.2061) | 0.0840 | 0.6213 |
| Rapid urease test | -0.0101 (-0.2187; 0.1984) | 0.1064 | 0.9240 |
| Giemsa stain | 0.0012 (-0.2077; 0.2101) | 0.1066 | 0.9910 |
| PCR method | -0.0014 (-0.1877; 0.1848) | 0.0950 | 0.9879 |
| ELISA | -0.0374 (-0.1544; 0.0797) | 0.0597 | 0.5316 |
| Otherb | Reference | ||
| Diagnosis period | |||
| Otherc | -0.0355 (-0.2223; 0.1514) | 0.0954 | 0.7100 |
| Before1999 | -0.0667 (-0.2920; 0.1586) | 0.1150 | 0.5618 |
| 2000-2004 | -0.1847 (-0.4471; 0.0777) | 0.1339 | 0.1678 |
| 2005-2009 | -0.0952 (-0.3588; 0.1684) | 0.1345 | 0.4789 |
| 2010-2014 | -0.0265 (-0.2461; 0.1931) | 0.1120 | 0.8131 |
| 2015-2019 | Reference | ||
| Detection timing | |||
| Before treatment | -0.0352 (-0.1301; 0.0598) | 0.0484 | 0.4678 |
| NS | Reference | ||
| Type of study design | |||
| Cross-sectional study | -0.0210 (-0.2646; 0.2226) | 0.1243 | 0.8657 |
| Case-control study | -0.0609 (-0.3093; 0.1875) | 0.1267 | 0.6308 |
| Cohort study | Reference | ||
| Quality grade | |||
| High | -0.0471 (-0.1797; 0.0855) | 0.0677 | 0.4865 |
| Moderate | -0.0173 (-0.1430; 0.1085) | 0.0642 | 0.7875 |
| Low | Reference | ||
| Publication year | 0.0008 (-0.0068; 0.0078) | 0.0036 | 0.8243 |
| Sample size | -0.0002 (-0.0010; 0.0006) | 0.0004 | 0.6018 |
SE, standard error; CI, confidence interval; NS, not specific; PCR, polymerase chain reaction; ELISA, enzyme linked immunosorbent assay; H. pylori, Helicobacter pylori.
using several types of samples.
using other testing method or several testing methods.
The diagnosis period could not be divided into corresponding group or the diagnosis period was not clear.
Publication Bias and Sensitivity Analysis
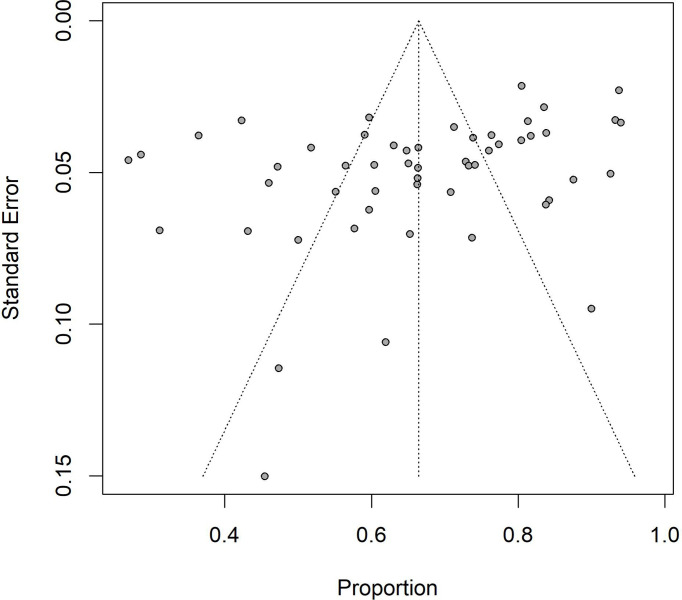
The Egger’s test for funnel plot (Figure 3) asymmetry was significant (t=-3.01, P=0.0040), indicating that there was obvious publication bias in all studies. The results of sensitivity analysis indicated that the lowest prevalence of H. pylori in non-cardia gastric cancer was 65.92% (95%CI: 0.6146-0.7038) when the Wang ML et al. study was omitted, and the highest prevalence was 67.25% (95%CI: 0.6292-0.7157) when the Zhang B study was omitted. The omission of studies did not significantly modify the pooled prevalence (Supplementary Figure S1).
Figure 3.
Funnel plot for the pooled prevalence of Helicobacter pylori in non-cardia gastric cancer in China.
Discussion
Based on 5324 non-cardia gastric cancer patients from 55 studies, which covered more than half of provinces and autonomous regions in China, we conducted a meta-analysis to estimate the prevalence of H. pylori in non-cardia gastric cancer patients at the national level for the first time. We reported a comprehensive estimate of H. pylori prevalence in non-cardia gastric cancer patients in China at 66.5% (95%CI: 62%-71%). Moreover, substantial geographic variations were noted in the prevalence of H. pylori, which was the highest in Northwest China and the lowest in North China.
The results of our study indicated that the pooled prevalence of H. pylori in non-cardia gastric cancer was 66.5% (95%CI: 62%-71%) in China. The prevalence of H. pylori in non-cardia gastric cancer in different countries varied greatly, such as 38.5% in the United States, 57% in Spain, 79.5% in South Korea, and 91.8% in Japan (4, 21–23). This difference might be explained by the following reasons: socio-economic status, dietary habits, and racial disparities. Suerbaum S et al. have mentioned that there was a strong inverse correlation between H. pylori prevalence and socio-economic status. Populations with lower socio-economic status were more likely to be infected with H. pylori (24, 25). Lower social status was commonly accompanied by crowded living conditions and poor hygienic conditions, which might increase the risk of acquisition and transmission of H. pylori (24, 26). Several previous studies have revealed that the consumption of high-salt foods (such as pickles and preserved products) might be associated with an increased chance of H. pylori infection (27–29). Tsugane S et al. speculated that a high-salt diet might induce gastric mucosa damage and destroy mucosal barrier. These changes in gastric mucosa might lead to an increase in H. pylori infection (29). Data based on National Health and Nutrition Examination Surveys of the United States have also shown that racial disparities played a certain role in the prevalence of H. pylori. Compared with H. pylori prevalence in whites, the prevalence in African Americans was higher (30).
Several previous studies, especially in Japan, reported that the H. pylori prevalence in gastric cancer was as high as 99% when strict criteria were used to diagnose H. pylori infection (31–33). Some other studies in Japan and South Korea used multiple testing methods to diagnose H. pylori infection, which also showed that the H. pylori prevalence in gastric cancer patients was above 90% (34–36). The H. pylori prevalence of these studies was much higher than the pooled H. pylori prevalence in our study. There were several possible reasons for this difference. First, these previous studies used strict criteria or multiple testing methods to diagnose H. pylori infection, which could greatly increase the sensitivity of detection. Moreover, most participants of these studies were cases of gastric cancer, while the participants of our study were non-cardia gastric cancer. Finally, most of these studies were conducted in Japan or South Korea. The H. pylori prevalence varied in different countries and regions.
The results of our study indicated a significant heterogeneity in the prevalence of H. pylori in non-cardia gastric cancer across different geographical regions in China. The highest H. pylori prevalence was noted in Northwest China at 78.9%, which was consistent with the highest incidence of gastric cancer in this region reported by the 2016 Chinese Cancer Registry Annual Report (37). Meanwhile, in line with our findings showing that the lowest H. pylori prevalence in non-cardia gastric cancer was noted in North China, Zhang WD et al. have also mentioned that the lowest H. pylori prevalence among the general population was in North China (38). The socio-economic status might be the main factor accounting for this geographic region difference. Unbalanced socio-economic development in different regions would lead to differences in living environment, education level, hygiene conditions, as well as water source (26, 39–41). The combined effect of the above factors may have resulted in the variation of H. pylori prevalence in different geographic regions.
The prevalence of H. pylori in non-cardia gastric cancer also showed significant variations across different provinces, and the highest H. pylori prevalence was noted in Heilongjiang Province at 90%. However, the estimate of Heilongjiang Province was based on only 10 cases from one study, leading to a wide range of its 95% CI. Furthermore, there were another seven provinces where the estimates of H. pylori prevalence were also based on only one study with the sample size ranging from 38 to 238. At the provincial level, part of the estimates in our study were estimated in the basis of a relatively small sample size, which might result in unstable results. Therefore, the results should be interpreted with caution, and additional studies with a larger sample size are needed to confirm our findings in the future.
The prevalence of H. pylori in the general population and non-cardia gastric cancer cases from different geographic regions was not completely consistent. From January 2002 to June 2004, Zhang WD et al. conducted an epidemiological survey on the H. pylori prevalence among 26,341 general people from 19 provinces and autonomous regions in China. The study showed that the H. pylori prevalence in the general population from different geographic regions ranged from high to low as: 66.26% in Central China, 59.16% in Eastern China, 58.27% in Western China, 50.08% in Southern China, and 46.84% in Northern China. In terms of provinces, the H. pylori prevalence among general population in Tibet was the highest at 84.62%, while the H. pylori prevalence in Guangdong was the lowest at 42.01% (38). Our results indicated that the geographic region with the lowest H. pylori prevalence in non-cardia gastric cancer cases was noted in North China, which was in line with the previous survey conducted by Zhang WD et al. The possible reason for the difference in the distribution of H. pylori between general population and cases of non-cardia gastric cancer was that non-cardia gastric cancer was caused by a combination of various risk factors including H. pylori infection, dietary habits, ethnicity, smoking, radiation exposure, family history, etc. H. pylori infection is one of the most important factors, but not the only one (42).
Type of sample might have an influence on the estimates of prevalence of H. pylori in non-cardia gastric cancer. The results of subgroup analysis revealed significant heterogeneity across different types of samples, with the highest prevalence of H. pylori detected in breath (78.7%). However, the results of meta-regression indicated that the differences in H. pylori prevalence among different sample types were not significant. The disparity in results might be attributed to the small number of the studies included. This meta-analysis only included four studies with 278 cases that detected the H. pylori prevalence in breath. Spineli LM et al. have mentioned that in order to obtain robust results, at least 10 studies should be included for each covariate in meta-regression implementation. In order to further confirm our finding, more studies detecting the H. pylori prevalence in breath will be needed in the future (43). In line with our findings, Liao YQ et al. also mentioned that the prevalence of H. pylori detected in breath would be higher than that in other samples. This might be partly because H. pylori infection detected in breath could reflect the H. pylori infection status in the whole gastrointestinal tract. Other pathogenic microorganisms in the gastrointestinal tract that could produce urease might cause false positive in the detection, which might increase the positive rate of H. pylori (44, 45).
The results of subgroup analysis and meta-regression both showed that the prevalence of H. pylori in non-cardia gastric cancer was not significantly associated with different H. pylori testing methods. This finding was in agreement with a recent study estimating the prevalence of H. pylori in cases with gastrointestinal diseases other than gastric cancer, which showed that the H. pylori prevalence detected by different H. pylori testing methods were generally similar (46). In our study, all H. pylori detected in breath was tested using 14C urea breath test. However, type of sample was significantly associated with H. pylori prevalence, while the relationship between testing method and H. pylori prevalence was not significant. Spineli LM et al. have revealed that when the subgroups contained fewer studies, the subgroup analysis could be underpowered to test the relationship between variables (43). Borenstein M et al. also indicated that one of the key factors driving the precision of subgroup analysis was the number of studies (47). Compared with type of sample, H. pylori testing method contained more subgroups, resulting in less studies in each subgroup. This may be the possible reason that the results of the above two subgroup analyses were different.
The studies included in our meta-analysis used several single testing methods to detect H. pylori infection among non-cardia gastric cancer cases. However, several guidelines revealed that one single testing method could not be considered as the gold standard for H. pylori detection (48–50). These commonly used testing methods all have some disadvantages. For example, IgG would remain in the blood for months or years even after H. pylori was eradicated. As such, antibody-based tests (e.g., ELISA) could not distinguish between current and past infections (51, 52). Moreover, ELISA is less accurate than 14C urea breath test and the cut-off values need a local validation (48, 53). As for 14C urea breath test, several factors such as atrophy, bismuth, proton pump inhibitor (PPI), and antibiotics may lead to false-negative, and it also should be validated locally (48, 54). Several guidelines have recommended the combination of single testing methods (e.g., combination of a validated serology and urea breath test), which could improve the accuracy of the detection (48–50). However, in our meta-analysis, most included studies (43/55, 78.2%) used a single testing method to detect H. pylori, which might lead to bias in the pooled H. pylori prevalence among non-cardia gastric cancer cases in China. Because H. pylori infection tends to clear as non-cardia gastric cancer progresses and the detection of past H. pylori infection is difficult, especially in retrospective studies (19/55, 34.5%), the pooled H. pylori prevalence in Chinese non-cardia gastric cancer cases may be underestimated (35, 55, 56).
The results of our study also suggested that there was no correlation between the diagnosis period and the prevalence of H. pylori in non-cardia gastric cancer. Previous meta-analysis conducted by Qiao J et al. showed that the prevalence of H. pylori in cases with gastric cancer in China declined from 1996 to 2015 (57). However, no decreasing trend of H. pylori prevalence in non-cardia gastric cancer was observed in our study. This difference might be attributed to the different study population. The previous study included cases with gastric cancer, while the included cases in our study were limited as cases with non-cardia gastric cancer.
The “test and treat” strategy for H. pylori infection has been shown to be cost-effective in some western countries (e.g., the United Kingdom and the United States) (58, 59). Japan has included H. pylori eradication therapy into the coverage of national medical insurance, becoming the first country to implement universal H. pylori “test and treat” strategy worldwide (60). Even with high prevalence of H. pylori infection and gastric cancer, whether to implement the “test and treat” strategy for H. pylori infection among the general population in China was still controversial. Several factors such as cost-effectiveness, personal willingness, usage of antibiotic, gastric cancer incidence, and prevalence of H. pylori infection would affect the implementation of this strategy (59, 61). Our study pooled the H. pylori prevalence in non-cardia gastric cancer and explored the influence factors of pooled prevalence, which might have certain public health significance in providing evidence for the control and prevention of non-cardia gastric cancer, as well as for policy making and health resource allocation.
The strengths of this meta-analysis included the following items. To the best of our knowledge, this was the first meta-analysis that estimated the pooled prevalence of H. pylori in non-cardia gastric cancer in China. An overview of current studies about H. pylori prevalence in non-cardia gastric cancer conducted in China was presented in our study. Moreover, our study has included a large number of studies. Based on a large sample size of 5324 cases from 55 studies, we were able to obtain a relatively robust estimate of the pooled H. pylori prevalence in non-cardia gastric cancer.
There were several limitations in our meta-analysis. Even though subgroup analysis and meta-regression were performed to minimize the heterogeneity across the included studies, significant heterogeneity still could be observed in subgroup analysis. The factors included in our study could not well explain the heterogeneity, which might affect the generalizability of our results. Moreover, some important factors (e.g., dietary habit, drinking, and gender) could not be extracted from the included studies, which might have potential influence on the heterogeneity. Another limitation was that some estimates in our study (e.g., H. pylori prevalence in different provinces) were calculated based on the small number of cases. Therefore, these results should be interpreted with caution, and more studies are needed to further confirm these results in the future. Another limitation of our study was that single testing methods used in the included studies for detecting H. pylori infection all had some limitations, and the results could not be completely accurate, which may lead to a certain bias in the pooled H. pylori prevalence. Finally, significant publication bias may result in an underestimation of pooled H. pylori prevalence.
Conclusions
This meta-analysis presented an overview of H. pylori prevalence in non-cardia gastric cancer in China. In conclusion, our study estimated the pooled prevalence of H. pylori in non-cardia gastric cancer was 66.5% (95%CI: 62%-71%) in China. Variation in H. pylori prevalence across different geographical regions was statistically significant, with the highest H. pylori prevalence (78.9%) in Northwest China and the lowest (53.1%) in North China. Type of sample might be associated with H. pylori prevalence, and further studies are needed to confirm this finding. A large proportion of non-cardia gastric cancers was associated with H. pylori infection, emphasizing the potential benefits of H. pylori eradication for reducing the disease burden of non-cardia gastric cancer. Our study might have certain public health significance in providing evidence for the control and prevention of non-cardia gastric cancer, as well as for policy making and health resource allocation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Study concepts: FH; Study design: FH; Data acquisition: FX and YW; Quality control of data and algorithms: YL and ZW; Data analysis and interpretation: YL and DL; Manuscript preparation: YL, FX, and YW; Manuscript editing: YL; Manuscript review: ZW, DL, and FH. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Doctoral Start-up Foundation of Guizhou Medical University [No. J (2020)65], National Natural Science Foundation of China Incubation Program, Guizhou Medical University [No. 20NSP061], the First-Class Discipline Construction Project in Guizhou Province Public Health and Preventive Medicine [No.2017 (85)], Foundation for the Establishment of Postdoctoral Mobile Station in Public Health and Preventive Medicine, Guizhou Medical University [41202020204], and Guizhou Basic Research (Science and Technology Fund) Project [ZK (2022) General 373]. All funding parties did not have any role in the design of the study or in the explanation of the data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.850389/full#supplementary-material
Abbreviations
H. pylori, Helicobacter pylori; CNKI, China National Knowledge Infrastructure; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; AHRQ, Agency for Healthcare Research and Quality; NOS, Newcastle-Ottawa Scale; CI, confidence interval; PCR, Polymerase Chain Reaction; ELISA, Enzyme linked immunosorbent assay; SE, standard error; PPI, proton pump inhibitor.
References
- 1. IARC . Globocan 2020. Available at: https://gco.iarc.fr/today/home (Accessed November 8, 2021).
- 2. Arnold M, Ferlay J, van Berge Henegouwen MI. Global Burden of Oesophageal and Gastric Cancer by Histology and Subsite in 2018. Gut (2020) 69(9):1564–71. doi: 10.1136/gutjnl-2020-321600 [DOI] [PubMed] [Google Scholar]
- 3. Kharazmi E, Babaei M, Fallah M, Chen T, Sundquist K, Hemminki K. Importance of Tumor Location and Histology in Familial Risk of Upper Gastrointestinal Cancers: A Nationwide Cohort Study. Clin Epidemiol (2018) 10:1169–79. doi: 10.2147/clep.s168152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter Pylori Infection and Environmental Factors in Non-Cardia Gastric Cancer in Japan. Gastric Cancer (2004) 7(1):46–53. doi: 10.1007/s10120-004-0268-5 [DOI] [PubMed] [Google Scholar]
- 5. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob Health (2020) 8(2):e180–e90. doi: 10.1016/s2214-109x(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 6. Ang TL, Fock KM. Clinical Epidemiology of Gastric Cancer. Singapore Med J (2014) 55(12):621–8. doi: 10.11622/smedj.2014174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing Risks of Gastric Cardia and Noncardia Gastric Adenocarcinomas Associated With Helicobacter Pylori Seropositivity. J Natl Cancer Inst (2006) 98(20):1445–52. doi: 10.1093/jnci/djj393 [DOI] [PubMed] [Google Scholar]
- 8. Hansen S, Melby KK, Aase S, Jellum E, Vollset SE. Helicobacter Pylori Infection and Risk of Cardia Cancer and non-Cardia Gastric Cancer. A Nested Case-control Study. Scand J Gastroenterol (1999) 34(4):353–60. doi: 10.1080/003655299750026353 [DOI] [PubMed] [Google Scholar]
- 9. Park JY, von Karsa L, Herrero R. Prevention Strategies for Gastric Cancer: A Global Perspective. Clin Endosc (2014) 47(6):478–89. doi: 10.5946/ce.2014.47.6.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn HJ, Lee DS. Helicobacter Pylori in Gastric Carcinogenesis. World J Gastrointest Oncol (2015) 7(12):455–65. doi: 10.4251/wjgo.v7.i12.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiso M, Yoshihara M, Ito M, Inoue K, Kato K, Nakajima S, et al. Characteristics of Gastric Cancer in Negative Test of Serum Anti-Helicobacter Pylori Antibody and Pepsinogen Test: A Multicenter Study. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2017) 20(5):764–71. doi: 10.1007/s10120-016-0682-5 [DOI] [PubMed] [Google Scholar]
- 12. Zhang B. Roles of SNP Rs4072037 in the MUC1 Gene in Helicobacter Pylori Infection and Noncardia Gastric Cancer Risk in Baotou Han Population [Master]. Baotou: : Baotou Medical College; (2012). [Google Scholar]
- 13. Yu M, Wang B, Zhang YF, Tian YL, Liu JN, Li JH. Relationship Between Precancerous Lesion and Gastric Cancer and Helicobacter Pylori Infection. Shaanxi Med J (2018) 47(11):1424–6. doi. 10.3969/j.issn.1000-7377.2018.11.017 [DOI] [Google Scholar]
- 14. Bindman AB. The Agency for Healthcare Research and Quality and the Development of a Learning Health Care System. JAMA Intern Med (2017) 177(7):909–10. doi: 10.1001/jamainternmed.2017.2589 [DOI] [PubMed] [Google Scholar]
- 15. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations Between Socioeconomic Status and Chronic Kidney Disease: A Meta-Analysis. J Epidemiol Community Health (2018) 72(4):270–9. doi: 10.1136/jech-2017-209815 [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18. Lau J, Ioannidis JP, Schmid CH. Quantitative Synthesis in Systematic Reviews. Ann Intern Med (1997) 127(9):820–6. doi: 10.7326/0003-4819-127-9-199711010-00008 [DOI] [PubMed] [Google Scholar]
- 19. Song P, Wang H, Theodoratou E, Chan KY, Rudan I. The National and Subnational Prevalence of Cataract and Cataract Blindness in China: A Systematic Review and Meta-Analysis. J Global Health (2018) 8(1):10804. doi: 10.7189/jogh.08-010804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen TH, Mallepally N, Hammad T, Liu Y, Thrift AP, El-Serag HB, et al. Prevalence of Helicobacter Pylori Positive Non-Cardia Gastric Adenocarcinoma Is Low and Decreasing in a US Population. Dig Dis Sci(2020) 65(8):2403–11. doi: 10.1007/s10620-019-05955-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huerta JM, Chirlaque MD, Molina AJ, Amiano P, Martín V, Fernández-Villa T, et al. Physical Activity Domains and Risk of Gastric Adenocarcinoma in the MCC-Spain Case-Control Study. PloS One (2017) 12(7):1–16. doi: 10.1371/journal.pone.0179731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong EJ, Lee JY, Bae SE, Park YS, Choi KD, Song HJ. Characteristics of non-Cardia Gastric Cancer With a High Serum Anti-Helicobacter Pylori IgG Titer and its Association With Diffuse-Type Histology. PloS One (2018) 13(4):1–11. doi: 10.1371/journal.pone.0195264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malaty HM, Graham DY. Importance of Childhood Socioeconomic Status on the Current Prevalence of Helicobacter Pylori Infection. Gut (1994) 35(6):742–5. doi: 10.1136/gut.35.6.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suerbaum S, Michetti P. Helicobacter Pylori Infection. New Engl J Med (2002) 347(15):1175–86. doi: 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 26. Kayali S, Manfredi M, Gaiani F, Bianchi L, Bizzarri B, Leandro G, et al. Helicobacter Pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta bio-med Atenei Parmensis (2018) 89(8-s):72–6. doi: 10.23750/abm.v89i8-S.7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang TZ, Zhang TM, Zhao DD, Tang GY. Meta-Analysis of Factors Relative to Helicobacter pylori Infection in China. In: World Chin J Dig. Beijing China: (2009). doi. 17 (15):1582–9. doi: 10.3969/j.issn.1009-3079.2009.15.021 [DOI] [Google Scholar]
- 28. Xue JH, Yu HP. The Prevalence of Helicobacter Pylori Infection and the Research Progress of Dangerous Lifestyle. Health Educ Health Promotion (2021) 16(04):378–82. [Google Scholar]
- 29. Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K. Salty Food Intake and Risk of Helicobacter Pylori Infection. Japanese J Cancer Res Gann (1994) 85(5):474–8. doi: 10.1111/j.1349-7006.1994.tb02382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varga MG, Butt J. Racial Differences in Helicobacter Pylori CagA Sero-Prevalence in a Consortium of Adult Cohorts in the United States. Cancer Epidemiol Biomarkers Prev (2020) 29(10):2084–92. doi: 10.1158/1055-9965.epi-20-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kato S, Matsukura N, Tsukada K, Matsuda N, Mizoshita T, Tsukamoto T, et al. Helicobacter Pylori Infection-Negative Gastric Cancer in Japanese Hospital Patients: Incidence and Pathological Characteristics. Cancer Sci (2007) 98(6):790–4. doi: 10.1111/j.1349-7006.2007.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter Pylori-Negative Gastric Cancer: Characteristics and Endoscopic Findings. Digestive Endoscopy Off J Japan Gastroenterological Endoscopy Soc (2015) 27(5):551–61. doi: 10.1111/den.12471 [DOI] [PubMed] [Google Scholar]
- 33. Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low Prevalence of Helicobacter Pylori-Negative Gastric Cancer Among Japanese. Helicobacter (2011) 16(6):415–9. doi: 10.1111/j.1523-5378.2011.00889.x [DOI] [PubMed] [Google Scholar]
- 34. Kwak HW, Choi IJ, Cho SJ, Lee JY, Kim CG, Kook MC, et al. Characteristics of Gastric Cancer According to Helicobacter Pylori Infection Status. J Gastroenterol Hepatol (2014) 29(9):1671–7. doi: 10.1111/jgh.12605 [DOI] [PubMed] [Google Scholar]
- 35. Morais S, Peleteiro B, Araújo N, Malekzadeh R, Ye W, Plymoth A, et al. Identifying the Profile of Helicobacter Pylori-Negative Gastric Cancers: A Case-Only Analysis Within the Stomach Cancer Pooling (StoP) Project. Cancer epidemiol Biomarkers Prev Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2022) 31(1):200–9. doi: 10.1158/1055-9965.epi-21-0402 [DOI] [PubMed] [Google Scholar]
- 36. Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, et al. Helicobacter Pylori-Negative Gastric Cancer in South Korea: Incidence and Clinicopathologic Characteristics. Helicobacter (2011) 16(5):382–8. doi: 10.1111/j.1523-5378.2011.00859.x [DOI] [PubMed] [Google Scholar]
- 37. He J, Chen WQ. 2016 Chinese Cancer Registry Annual Report. Beijing: Tsinghua University Press: (2017). pp. 110–4. [Google Scholar]
- 38. Zhang WD, Hu FL, Xiao SD, Xu ZM. Prevalence of Helicobacter Pylori Infection in China. Modern Digestion Intervention (2010) 15(5):265–70. doi: 10.1111/jgh.12605 [DOI] [Google Scholar]
- 39. Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter Pylori Infection Worldwide: A Systematic Review of Studies With National Coverage. Digestive Dis Sci (2014) 59(8):1698–709. doi: 10.1007/s10620-014-3063-0 [DOI] [PubMed] [Google Scholar]
- 40. Yang L, Zhou L, Zhang YH, Lei JJ. Epidemiological Investigation and Multivariate Analysis of Helicobacter Pylori in the Middle-Aged and Elderly People of Buyi, Miao and Han Nationalities in Zhenning County, Guizhou Province. Chin J Gastroenterol Hepatol (2009) 18(02):131–4. doi: 10.3969/j.issn.1006-5709.2009.02.013 [DOI] [Google Scholar]
- 41. Bellack NR, Koehoorn MW, MacNab YC, Morshed MG. A Conceptual Model of Water's Role as a Reservoir in Helicobacter Pylori Transmission: A Review of the Evidence. Epidemiol infect (2006) 134(3):439–49. doi: 10.1017/s0950268806006005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akbari A, Ashtari S, Tabaiean SP, Mehrdad-Majd H, Farsi F, Shojaee S, et al. Overview of Epidemiological Characteristics, Clinical Features, and Risk Factors of Gastric Cancer in Asia-Pacific Region. Asia-Pacific J Clin Oncol (2022). doi: 10.1111/ajco.13654 [DOI] [PubMed] [Google Scholar]
- 43. Spineli LM, Pandis N. Problems and Pitfalls in Subgroup Analysis and Meta-Regression. Am J orthodontics dentofacial orthopedics Off Publ Am Assoc Orthodontists its constituent soc Am Board Orthodontics (2020) 158(6):901–4. doi: 10.1016/j.ajodo.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 44. Liao YQ, Wang LL, Guo X, Duan YF. Biological Characteristics of Helicobacter Pylori and Laboratory Detection of its Infection. Chin J Clin Lab Mgt (Electronic Edition) (2020) 8(02):71–7.doi 0.3877/cma.j.issn.2095-5820.2020.02.002 [Google Scholar]
- 45. Alzoubi H, Al-Mnayyis A. The Use of (13)C-Urea Breath Test for Non-Invasive Diagnosis of Helicobacter Pylori Infection in Comparison to Endoscopy and Stool Antigen Test. Diagnostics (Basel)(2020) 10(7):448. doi: 10.3390/diagnostics10070448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao JG. Three Helicobacter Pylori Detection Methods for Diagnosis of Helicobacter Pylori Infection Comparison of Effects. Med J Chin People's Health (2021) 33(18):117–8. doi 10.3969/j.issn.1672-0369.2021.18.047 [DOI] [Google Scholar]
- 47. Borenstein M, Hedges L, Higgins J, Rothstein H. Notes on Subgroup Analyses and Meta-Regression. Introduction to Meta-Analysis(2021) .p:213–9. doi: 10.1002/9781119558378.ch23 [DOI] [Google Scholar]
- 48. Miftahussurur M, Yamaoka Y. Diagnostic Methods of Helicobacter Pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. BioMed Res Int (2016) 2016:4819423. doi: 10.1155/2016/4819423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, et al. Guidelines for the Management of Helicobacter Pylori Infection in Japan: 2009 Revised Edition. Helicobacter (2010) 15(1):1–20. doi: 10.1111/j.1523-5378.2009.00738.x [DOI] [PubMed] [Google Scholar]
- 50. Chey WD, Wong BC. American College of Gastroenterology Guideline on the Management of Helicobacter Pylori Infection. Am J Gastroenterol (2007) 102(8):1808–25. doi: 10.1111/j.1572-0241.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 51. Kosunen TU, Seppälä K, Sarna S, Sipponen P. Diagnostic Value of Decreasing IgG, IgA, and IgM Antibody Titres After Eradication of. Helicobacter pylori Lancet (London England) (1992) 339(8798):893–5. doi: 10.1016/0140-6736(92)90929-w [DOI] [PubMed] [Google Scholar]
- 52. Kim JH, Cheung DY. Must-Have Knowledge About the Helicobacter Pylori-Negative Gastric Cancer. Gut liver (2016) 10(2):157–9. doi: 10.5009/gnl16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burucoa C, Delchier JC, Courillon-Mallet A, de Korwin JD, Mégraud F, Zerbib F, et al. Comparative Evaluation of 29 Commercial Helicobacter Pylori Serological Kits. Helicobacter (2013) 18(3):169–79. doi: 10.1111/hel.12030 [DOI] [PubMed] [Google Scholar]
- 54. Jonaitis LV, Kiudelis G, Kupcinskas L. Evaluation of a Novel 14C-Urea Breath Test "Heliprobe" in Diagnosis of Helicobacter Pylori Infection. Med (Kaunas Lithuania) (2007) 43(1):32–5. doi: 10.3390/medicina43010004 [DOI] [PubMed] [Google Scholar]
- 55. Peleteiro B, Lunet N, Barros R, La Vecchia C, Barros H. Factors Contributing to the Underestimation of Helicobacter Pylori-Associated Gastric Cancer Risk in a High-Prevalence Population. Cancer causes control CCC (2010) 21(8):1257–64. doi: 10.1007/s10552-010-9553-2 [DOI] [PubMed] [Google Scholar]
- 56. Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter Pylori Infection a Necessary Condition for Noncardia Gastric Cancer? Am J Epidemiol (2004) 159(3):252–8. doi: 10.1093/aje/kwh039 [DOI] [PubMed] [Google Scholar]
- 57. Qiao J. The Characteristics of Positive Rate of Helicobacter pylori in Patients With Gastric Cancer in the Past 20 Years: A Meta-Analysis [Master] Taiyuan: Shanxi Medical University: (2016). [Google Scholar]
- 58. Lansdorp-Vogelaar I, Sharp L. Cost-Effectiveness of Screening and Treating Helicobacter Pylori for Gastric Cancer Prevention. Best Pract Res Clin Gastroenterol (2013) 27(6):933–47. doi: 10.1016/j.bpg.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen Q, Liang X, Long X, Yu L, Liu W, Lu H. Cost-Effectiveness Analysis of Screen-and-Treat Strategy in Asymptomatic Chinese for Preventing Helicobacter Pylori-Associated Diseases. Helicobacter (2019) 24(2):e12563. doi: 10.1111/hel.12563 [DOI] [PubMed] [Google Scholar]
- 60. Hiroi S, Sugano K, Tanaka S, Kawakami K. Impact of Health Insurance Coverage for Helicobacter Pylori Gastritis on the Trends in Eradication Therapy in Japan: Retrospective Observational Study and Simulation Study Based on Real-World Data. BMJ Open (2017) 7(7):e015855. doi: 10.1136/bmjopen-2017-015855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth Chinese National Consensus Report on the Management of Helicobacter Pylori Infection. Helicobacter (2018) 23(2):e12475. doi: 10.1111/hel.12475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.