Abstract
Animal trypanosomiasis (AT) is a parasitic disease with high socio-economic impact. Given the limited therapeutic options and problems of toxicity and drug resistance, this study assessed redirecting our previously identified antitrypanosomal nucleosides for the treatment of AT. Promising hits were identified with excellent in vitro activity across all important animal trypanosome species. Compound 7, an inosine analogue, and our previously described lead compound, 3′-deoxytubercidin (8), showed broad spectrum anti-AT activity, metabolic stability in the target host species and absence of toxicity, but with variable efficacy ranging from limited activity to full cure in mouse models of Trypanosoma congolense and T. vivax infection. Several compounds show promise against T. evansi (surra) and T. equiperdum (dourine). Given the preferred target product profile for a broad-spectrum compound against AT, this study emphasizes the need to include T. vivax in the screening cascade given its divergent susceptibility profile and provides a basis for lead optimization towards such broad spectrum anti-AT compound.
Keywords: Animal trypanosomiasis, Nucleoside analogues
Graphical abstract
1. Introduction
Trypanosomiasis is a neglected parasitic disease responsible for human and animal infections in Africa, Asia, South America and parts of Europe(Kennedy, 2013; Radwanska et al., 2018). Human African trypanosomiasis (HAT), mainly caused by T. b. gambiense and T. b. rhodesiense, is confined to a tsetse fly endemic region on the African continent due to its dependency on transmission by tsetse flies(Tirados et al., 2015). Animal trypanosomiasis (AT) is caused by a variety of species. T. b. brucei and T. congolense are transmitted by tsetse flies and cause infection of wild and domestic animals on the African continent(Truc et al., 2013). The transmission of T. vivax to the animal host includes a short developmental cycle in the anterior parts of the tsetse fly(Ooi et al., 2016), or mechanical transmission by tsetse flies and other blood sucking insects (horse flies: Tabanus spp.(Desquesnes and Dia, 2003) and stable flies: Stomoxys spp.)(Odeniran et al., 2019). The latter has contributed to the spread of T. vivax far outside the tsetse fly belt(Radwanska et al., 2018). A mechanical mode of transmission was also acquired by T. evansi explaining its spread outside the African continent; it is now endemic in Africa, Asia and South America, causing infection in many species including equines, bovines and camelids(Desquesnes et al., 2013). T. equiperdum is transmitted in equines during mating, without any vector involvement, and is prevalent in Africa, Asia, South America and parts of Europe(Gizaw et al., 2017).
Despite recent achievements in vaccine development for T. vivax(Autheman et al., 2021), global disease prevention through vaccination is not yet possible and AT control solely depends on vector control and chemotherapy. The drugs that are currently available to treat HAT have major limitations, including drug toxicity, parenteral administration, and the emergence of drug resistance(Babokhov et al., 2013; Baker et al., 2013; Sokolova et al., 2010; Wyllie et al., 2016). A new era in HAT treatment was ushered in with the global approval of fexinidazole, the first all-oral drug available (Mesu et al., 2018) and the positive clinical trial results for the one-day oral drug treatment with acoziborole(Dickie et al., 2020). Nonetheless, nifurtimox-eflornithine combination therapy remains the treatment of choice in severely ill patients and continued research remains important to achieve the goal of sleeping sickness elimination as set by the WHO.
For the treatment of AT, seven compounds are currently in use (diminazene aceturate, homidium bromide/chloride, isometamidium, pyrithidium bromide, quinapyramine, suramin and melarsomine dihydrochloride)(Giordani et al., 2016), however, each of these drugs has limitations of toxicity and increasing emergence of drug resistance in the field(Delespaux and de Koning, 2007; Geerts et al., 2001). Diminazene aceturate (DA) and isometamidium chloride (ISM) are mostly used, followed by suramin. In areas of high drug resistance, drugs are used sequentially alternating using compounds of distinct chemical classes (e.g. DA and ISM, called a sanative pair) or used at a higher dosage(Giordani et al., 2016; Leach and Roberts, 1981). Although in comparison, HAT burdens remain relatively low and are confined to African countries, the high prevalence of animal Trypanosoma species in domestic animals across different continents has a large socio-economic impact on communities via agricultural production and animal husbandry(Kasozi et al., 2021). It has been decades since the introduction of any new treatments for AT and the need for novel drug and/or vaccine candidates is now urgent.
African trypanosomes lack machinery for de novo purine synthesis and thus solely depend on the salvage of purines from the host environment(Berg et al., 2010). As purine uptake is essential for parasite replication, interfering with the involved pathways constitutes a logical strategy to find novel antitrypanosomal treatments. Our previous research contributed to the discovery of highly potent nucleoside analogues(Hulpia et al., 2019a, Hulpia et al., 2019b, Hulpia et al., 2020a), including 7-deaza adenosine analogues, 7-deaza inosine analogues, and 3′-deoxy-7-deaza adenosine analogues that represent interesting candidates for the treatment of late-stage HAT. Given the large socio-economic impact of AT, the present study evaluated the potential use of nucleoside analogues for the control of animal trypanosomiasis using in vitro drug sensitivity assays as well as animal (mouse) models.
2. Materials and methods
2.1. Ethics statement
The use of laboratory rodents was carried out in strict accordance with all mandatory guidelines (EU directives, including the Revised Directive, 2010/63/EU on the Protection of Animals used for Scientific Purposes that came into force on 01/01/2013, and the declaration of Helsinki in its latest version) and was approved by the Ethical Committee of the University of Antwerp, Belgium [UA-ECD 2019–26].
2.2. Animals and parasites
Female Swiss mice (8 weeks, ∼25 g) were purchased from Janvier (France). Food for laboratory rodents (Carfil, Arendonk, Belgium) and drinking water were available ad libitum. The animals were kept in quarantine for at least 5 days before infection and were randomly allocated to the experimental units.
In vitro drug assays were performed with bloodstream forms of several trypanosome species. T. congolense IL3000 and the diminazene-resistant clone 6C3 derived from it by in vitro exposure to increasing concentrations of the drug(Carruthers et al., 2021) were cultured in Dulbecco's minimum essential medium (MEM) supplemented with 25 mM HEPES, 26 mM NaHCO3, 5.6 mM D-glucose, 1 mM sodium pyruvate, 40 μM adenosine, 100 μM hypoxanthine, 16.5 μM thymidine, and 25 μM bathocuproinedisulfonic acid disodium salt. To this basal medium were added β-mercaptoethanol (0.0014% v/v), 1.6 mM glutamine, 10 units/mL penicillin, 0.1 mg/mL streptomycin, 20% goat serum (Gibco), and 5% Serum Plus (SAFC Biosciences)(Coustou et al., 2010).
T. evansi strain AnTat 3.3(Kageruka and Mortelmans, 1971) and T. equiperdum BoTat 1.1(Capbern et al., 1977) were cultured in Hirumi's Modified Iscove's (HMI-9) medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% heat inactivated Foetal Bovine Serum (FBS (PAA Laboratories Linz, Austria)), 14 μL/L β-mercaptoethanol (BDH, Dorset, United Kingdom), and 3.0 g/L NaHCO3 (Sigma) adjusted to pH 7.4. These parasites were kept at 37 °C in a humidified, 5% CO2 environment.
T. vivax (ILRAD700, kindly provided by dr. Nick Van Reet and Prof. Philippe Büscher of the Institute of Tropical Medicine, Antwerp) and T. congolense (TC13, kindly provided by Prof. Benoît Stijlemans of the Vrije Universiteit Brussel) were used for ex vivo and in vivo experiments.
2.3. Compounds
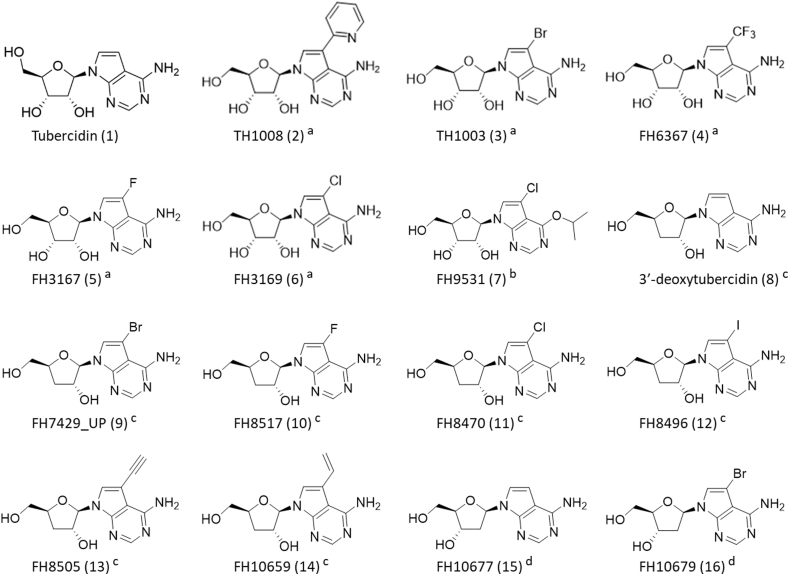
A range of tubercidin analogues (Fig. 1) was tested for in vitro and in vivo activity against the most pathogenic animal trypanosomes. Experimental details regarding the chemical synthesis have been described elsewhere(Hulpia et al., 2019a, 2019b, 2020a, 2020b; Mabille et al., 2021). Purity of all nucleoside analogues was >95%, as assayed via analytical LC/MS (UV-integration), of which the methods are as described before(Hulpia et al., 2020a). Analogues 5 and 6 were prepared as described in literature(Seela and Ming, 2007).
Fig. 1.
Overview of the nucleoside analogues used in this study. Compound name (compound code). a 7-deaza adenosine analogues. b Inosine analogues. c 3′-deoxy-7-deaza adenosine analogues. d 2′-deoxy-7-deaza adenosine analogues. Substitution of C-7 of tubercidin (1) with a pyridin-2-yl (TH1008), bromide (TH1003), trifluoromethyl (FH6367), fluoride (FH3167), or chloride (FH3169) group led to compounds 2–6. Compound 7 is a C6–O-alkylated 7-substituted 7-deazainosine analogue. Compound 8 is 3′-deoxytubercidin. Substitution of C-7 of 3′-deoxytubercidin with a bromide (FH7429_UP), fluoride (FH8517), chloride (FH8470), iodide (FH8496), propyn (FH8505), propene (FH10659) group led to compounds 9-14. Compounds 15 (FH10677) and 16 (FH10679) are 2′-deoxytubercidin analogues with a bromide substitution at C-7 for compound 16. Compound codes in original publication: (Tubercidin (1), TH1008 (13), TH1003 (31)) [(Hulpia et al., 2019a)], FH6367 (4) [(Mabille et al., 2021)], FH9531 (36) [(Hulpia et al., 2020a)], (3′-deoxytubercidin (9), FH7429_UP (10), FH10677 (11), FH10679 (12)) [(Hulpia et al., 2019b)], (FH8517 (7), FH8470 (8), FH8496 (9), FH8505 (12), FH10659 (16)) [(Hulpia et al., 2020b)].
2.4. Cytotoxicity assay on MRC-5 fibroblasts
MRC-5SV2 cells were cultured in MEM (Life Technologies) supplemented with l-glutamine, NaHCO3 and 5% heat-inactivated fetal bovine serum (hiFBS). To determine the in vitro cytotoxicity, cells were seeded at a concentration of 15,000 cells/well. Four-fold dilutions of the test compounds were added to the cells with a highest in-test concentration of 64 μM. After 72 h of drug exposure at 37 °C and 5% CO2, cell viability was determined by fluorescence reading (Tecan®, GENios) after a 4-h incubation with resazurin (Sigma Aldrich). The 50% cytotoxic concentration (CC50) was calculated for each of the compounds. The obtained cytotoxicity values were compared to those of the AT reference compounds: suramin (>64 μM, n = 3), diminazene aceturate (>64 μM, n = 1), isometamidium (22.74 μM, n = 1) and quinapyramine (>64 μM, n = 1).
2.5. In vitro drug susceptibility assays
T. b. brucei Squib 427(Kaiser et al., 2015) was cultured in HMI-9 culture medium supplemented with 10% hiFBS. To determine the in vitro susceptibility of T. b. brucei to the test compounds, cells were seeded at a concentration of 1.5 × 104 cells/well. Drug stock solutions were prepared in 100% DMSO. Four-fold dilutions of the test compounds were added to the cells with a highest in-test concentration of 64 μM. DMSO concentration in the wells never exceeded 1%. After 72 h of drug exposure at 37 °C and 5% CO2, cell viability was determined by fluorescence reading (Tecan®, GENios) after a 24-h incubation with resazurin (Sigma Aldrich) at a final concentration of 10 μg/mL. The 50% inhibitory concentration (IC50) was calculated for each of the compounds.
For T. evansi, T. equiperdum and T. congolense, the assays were performed in their respective media and temperatures as described above, in white 96-well plates (Greiner Bio-one, Frickenhausen, Germany), using either 11 (1 row) or 23-doubling dilutions (2 rows) as required to obtain a full sigmoid curve, starting at 100 μM drug concentration and leaving one well as drug-free control. To each well was added 100 μL of trypanosome culture adjusted to 2 × 104 cells/mL (T. equiperdum), 4 × 104 cells/mL (T. evansi) or 5 × 105 cells/mL (T. congolense). The cells were incubated with the drug dilutions for 48 h, followed by the addition of 20 μL of resazurin solution and a further incubation of 24 h. Fluorescence was determined using a FLUOstar Optima (BMG Labtech, Durham, NC, USA) at wavelengths of 544 nm for excitation and 590 nm for emission. The EC50 values for the drugs/test compounds were calculated by non-linear regression fitted to a sigmoidal dose-response curve with variable slope (Prism 8.0, GraphPad Software Inc., San Diego).
2.6. Ex vivo drug susceptibility assay for T. vivax
Donor mice were infected intraperitoneally with 104 T. vivax parasites derived from cryostabilates. At 5 days post-infection (dpi), blood was collected via cardiac puncture and parasites were isolated from the heparinized blood using the mini anion exchange centrifugation technique (mAECT) as used in the field for diagnosis of African trypanosomiasis(Buscher et al., 2009). Isolated parasites were seeded in HMI-9 culture medium supplemented with 10% hiFBS at a concentration of 105 parasites/well. Four-fold dilutions of the test compounds were added to the cells with a highest in-test concentration of 64 μM. After 24 h of drug exposure at 37 °C and 5% CO2, the viability was determined by fluorescence reading (Tecan®, GENios) after a 6- and 24-h incubation with resazurin (Sigma Aldrich) at a final concentration of 10 μg/mL. The 50% inhibitory concentration (IC50) was calculated for each compound by comparing cell viability of drug-treated wells to untreated control wells.
2.7. In vitro metabolic stability
The metabolic stability of selected hits was tested using human, bovine, horse and mouse liver microsomes (Corning) of phase-I (CYP450 and NADPH dependent enzymes) and phase-II (UGT enzymes) metabolism as described before (Hulpia et al., 2019a). In short, samples were collected after 0, 15, 30 and 60 min and the corresponding loss of parent compound was determined using ultra-performance liquid chromatography (UPLC) (Waters AquityTM) coupled with tandem quadrupole mass spectrometry (MS2) (Waters XevoTM), equipped with an electrospray ionization (ESI) interface and operated in multiple reaction monitoring (MRM) mode.
2.8. Exploratory in vivo acute toxicity evaluation
The test compounds were formulated at 6.25 mg/mL in 10% PEG400. Per compound, one uninfected mouse was treated twice daily for 5 consecutive days intraperitoneally at 50 mg/kg (3′-deoxytubercidin; 8) or 25 mg/kg (7, 9, 11 and 14). Body weight and general clinical appearance were monitored daily during the next 4 days for signs of toxicity.
2.9. Mouse model of AT
Female Swiss mice were randomly divided into groups of 3 animals and intraperitoneally infected with 104 T. congolense (TC13) or T. vivax (ILRAD700) derived from a heavily infected donor mouse. Analogues 7 and 8 were formulated in 10% PEG400 in water at 2 mg/mL, freshly prepared before each administration and administered by intraperitoneal injection for 5 days at 50 mg/kg (7) or 6.25 mg/kg (8) once a day. The reference drug diminazene aceturate was formulated in phosphate buffered saline at 2.5 mg/mL and administered intraperitoneally for 5 days at 10 mg/kg once a day. Treatment was initiated at day 3 post-infection when the parasitaemia reached 106/mL.
Parasitaemia was determined microscopically using an improved Neubauer haemocytometer, daily for the first 2 weeks, twice a week up to 30 dpi and once a week up to 60 dpi. At 60 dpi, mice were euthanized and 100 μL of blood was collected for RNA extraction using the QIAamp RNA blood mini kit (Qiagen) following the manufacturer's instructions prior to qPCR analysis to determine the presence of residual parasite burdens. Parasite levels were determined using quantitative real-time PCR targeting SL-RNA(Gonzalez-Andrade et al., 2014) with optimized assay conditions for T. congolense and T. vivax using the SensiFAST™ SYBR® Hi-ROX One-Step Kit (Table S1-2). RT-qPCR targeting the eukaryotic translation elongation factor 2 (Eef2), a mouse reference gene, was performed in parallel to confirm appropriate RNA extraction in all tested samples(Eissa et al., 2016).
3. Results
3.1. In vitro activity across a broad panel of animal trypanosome species
All nucleoside analogues were tested in vitro on MRC-5SV2 cells to evaluate the level of cytotoxicity/selectivity, and on T. b. brucei, T. evansi, T. equiperdum and T. congolense (IL3000 and the derived diminazene-resistant 6C3 strain). Activity against T. vivax was evaluated in an ex vivo assay (Table 1).
Table 1.
Evaluation of in vitro drug sensitivity of nucleoside analogues against animal trypanosomes. Cytotoxicity was evaluated against human lung fibroblasts (MRC-5SV2 cells). IC50 values are expressed in μM and represent the mean and SEM of at least two independent experiments each with 2 replicates.
| Compound | EC50 (μM) |
|||||||
|---|---|---|---|---|---|---|---|---|
| MRC-5 | T. b. brucei Squib 427 | T. vivax ILRAD700 | T. evansi AnTat 3.3 | T. equiperdum BoTat 1.1 | T. congolense IL3000 | T. congolense 6C3 | bSI | |
| 2 (TH1008) | 15.1 ± 4.1 | 0.31 ± 0.06 | 12.9 ± 4.4 | 0.04 ± 0.01 | 0.061 ± 0.005 | 0.17 ± 0.05 | 0.056 ± 0.013 | 1 − 378 |
| 3 (TH1003) | 12 ± 2 | 1.2 ± 0.3 | 0.74 ± 0.04 | 0.48 ± 0.06 | 0.085 ± 0.026 | 0.79 ± 0.14 | 0.52 ± 0.03 | 10 − 141 |
| 4 (FH6367) | 4.32 | 0.69 ± 0.11 | 0.82 ± 0.09 | 0.67 ± 0.11 | 0.19 ± 0.03 | 1.8 ± 0.4 | 0.89 ± 0.17 | 2 − 23 |
| 5 (FH3167) | 0.16 ± 0.03 | 0.035 ± 0.001 | 0.038 ± 0.006 | 0.18 ± 0.02 | 0.033 ± 0.007 | 0.45 ± 0.06 | 0.78 ± 0.10 | 0 − 5 |
| 6 (FH3169) | 13 ± 6 | 1.33 ± 0.34 | 0.53 ± 0.07 | 0.62 ± 0.06 | 0.12 ± 0.02 | 0.86 ± 0.12 | 0.75 ± 0.14 | 10 − 108 |
| 7 (FH9531)a | >64 | 0.09 ± 0.04 | 0.12 ± 0.01 | 0.20 ± 0.02 | 0.11 ± 0.00 | 4.04 ± 0.36 | 3.23 ± 0.36 | 16 − 711 |
| 8 (FH7429_D)a | >64 | 0.048 ± 0.009 | 3.48 ± 0.09 | 0.017 ± 0.002 | 0.003 ± 0.001 | 0.021 ± 0.001 | 0.019 ± 0.002 | 18 − 21333 |
| 9 (FH7429_UP) | 14.9 ± 3.4 | 0.0013 ± 0.0003 | 0.018 ± 0.003 | 0.0009 ± 0.0002 | 0.0005 ± 0.0001 | 0.0007 ± 0.0001 | 0.0005 ± 0.0001 | 828 − 29800 |
| 10 (FH8517) | 3.56 ± 0.8 | 0.002 ± 0.001 | 0.50 ± 0.01 | 0.004 ± 0.001 | 0.0005 ± 0.0001 | 0.0052 ± 0.0003 | 0.0031 ± 0.0003 | 7 − 7120 |
| 11 (FH8470)a | 9.9 ± 1.5 | 0.00210 ± 0.00004 | 0.096 ± 0.082 | 0.0018 ± 0.0002 | 0.0006 ± 0.0001 | 0.0012 ± 0.0002 | 0.00066 ± 0.00003 | 103 − 16500 |
| 12 (FH8496) | 3.4 ± 1.3 | 0.0085 ± 0.0004 | 0.056 ± 0.001 | 0.004 ± 0.001 | 0.002 ± 0.001 | 0.005 ± 0.001 | 0.0030 ± 0.0002 | 61 − 1700 |
| 13 (FH8505) | 1.29 ± 0.56 | 0.005 ± 0.002 | 0.159 ± 0.009 | 0.008 ± 0.002 | 0.003 ± 0.001 | 0.017 ± 0.002 | 0.008 ± 0.002 | 8 − 430 |
| 14 (FH10659)a | 25.6 ± 6.5 | 0.036 ± 0.005 | 1.57 ± 0.43 | 0.038 ± 0.002 | 0.011 ± 0.001 | 0.056 ± 0.004 | 0.040 ± 0.003 | 16 − 2327 |
| 15 (FH10677) | >64 | 48 ± 1 | >64 | >64 | 17.0 ± 2.3 | 43 ± 14 | 11.0 ± 2.3 | 1 − 6 |
| 16 (FH10679) | 6.1 ± 0.7 | 0.46 ± 0.08 | 4.3 ± 0.9 | 1.7 ± 0.5 | 0.067 ± 0.013 | 0.51 ± 0.09 | 0.31 ± 0.07 | 1 − 91 |
Compounds selected for in vivo analysis.
The selectivity index (SI) represents the CC50/IC50.
Of the C-7 substituted 7-deazaadenosine analogues (compounds 2-6), 2 was previously reported to have submicromolar activity against T. b. brucei in vitro (Hulpia et al., 2019a). The data in Table 1 shows that compounds 2-6 all exhibited (sub)micromolar activity against the entire panel of AT strains, except for compound 2, which was not active against T. vivax. These analogues showed various degrees of cytotoxicity on MRC-5SV2 cells, leading to variable SI values for the different species. A 7-trifluoromethyl (4) or fluoride (5) group resulted in particularly high host cytotoxicity. Compound 7, a C6–O-alkylated 7-substituted 7-deazainosine analogue, showed sub-micromolar activity against T. b. brucei (IC50 = 0.09 ± 0.04 μM), T. vivax (IC50 = 0.12 ± 0.01 μM), T. evansi (IC50 = 0.20 ± 0.02 μM) and T. equiperdum (IC50 = 0.11 ± 0.00 μM) and micromolar activity against both T. congolense strains (IC50 = 3.23–4.04 ± 0.36 μM) and very low toxicity against human cell lines.
3′-Deoxytubercidin (8)(Hulpia et al., 2019b) displayed sub-micromolar activity (IC50 = 0.003–0.048 ± 0.009 μM) against all animal trypanosomes examined, except T. vivax (IC50 of 3.48 ± 0.09 μM). The 3′-deoxytubercidin analogues 9-14 displayed potent activity against all AT strains, with selectivity indices up to 29800. 2′-Deoxytubercidin analogue 15, an isomer of 8, was inactive against all tested strains. Compound 16 exhibited (sub)micromolar activity, however accompanied by low micromolar host cell cytotoxicity. Based on the overall broad spectrum anti-AT activity and high selectivity, compounds 7, 8, 9, 11 and 14 were selected for further in vitro metabolic stability and in vivo analysis.
3.2. Explorative in vivo acute toxicity of selected anti-AT compounds
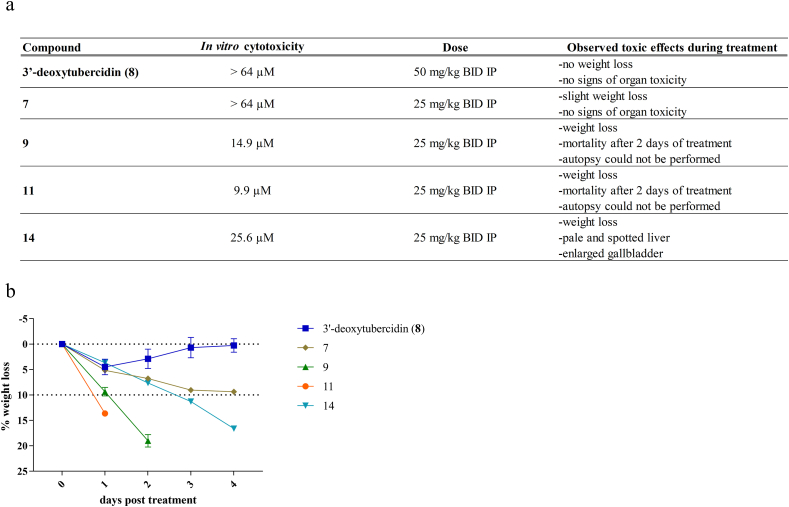
Given the observed in vitro cytotoxicity on MRC-5SV2 cells for some of the compounds, single mice were exposed to a high dose of either compound 7, 8, 9, 11, or 14, to assess toxicity prior to evaluating the in vivo efficacy of the compounds in various AT mouse models. Compound 8 showed no obvious signs of toxicity, as described previously(Hulpia et al., 2019b), at 5 daily doses of 50 mg/kg intraperitoneally (Fig. 2). For compounds 9(Hulpia et al., 2019b), 11 and 14 (5 × 25 mg/kg intraperitoneally), a drastic decrease in body weight was observed. This resulted in death for compounds 9 and 11 and in severe clinical pathology for compound 14. Treatment with compound 7 resulted in a minor weight loss within the acceptable range without observed organ toxicity (Fig. 2).
Fig. 2.
Pilot acute toxicity of selected nucleoside analogues in mice. (a) Overview of the toxic effects and (b) percentage weight loss observed after exposure to 3′-deoxytubercidin (8) (n = 3), compound 7 (n = 1), 9 (n = 3), 11 (n = 1) and 14 (n = 1).
3.3. In vitro metabolic stability of selected anti-AT compounds
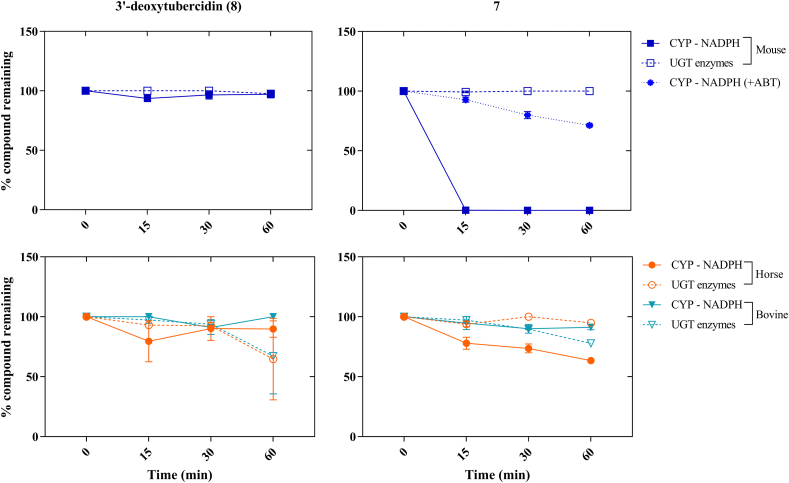
Compounds 7 and 8 were selected for further analysis. To evaluate their metabolic stability, both compounds were exposed to mouse, horse and bovine S9 liver microsomal fractions, followed by evaluating the percentage of parent compound remaining after incubation (Fig. 3). The results indicate that compound 7 is susceptible to Phase-I metabolism in mouse liver microsomes with a complete degradation of the parent compound within 15 min of incubation. However, acceptable levels of compound degradation (≥50% of parent compound remaining after 30 min) were observed in the target species, i.e. with horse and bovine microsomes. The Phase-I degradation of compound 7 in mice could partially be rescued by the addition of the non-selective CYP450 inhibitor 1-aminobenzotriazole (1-ABT) at 100 mg/kg, SID (semel in die, once a day) (Fig. 3), enabling the use of mice as a valid model for compound evaluation. Compound 8 was metabolically stable in all tested species warranting further in vivo evaluation. Based on the in vitro and in vivo results, compounds 7 and 8 were selected for further in vivo analysis against a range of animal trypanosome species.
Fig. 3.
In vitro metabolic stability (Phase-I and Phase-II) of nucleoside analogues (7 and 3′-deoxytubercidin (8)) using mouse, horse and bovine S9 microsomal fractions. The figures represent the percentage of remaining parent compound assayed at various time points of incubation (0-15-30-60 min). 1-ABT = 1-aminobenzotriazole.
3.4. In vivo efficacy of selected anti-AT compounds against T. vivax and T. congolense
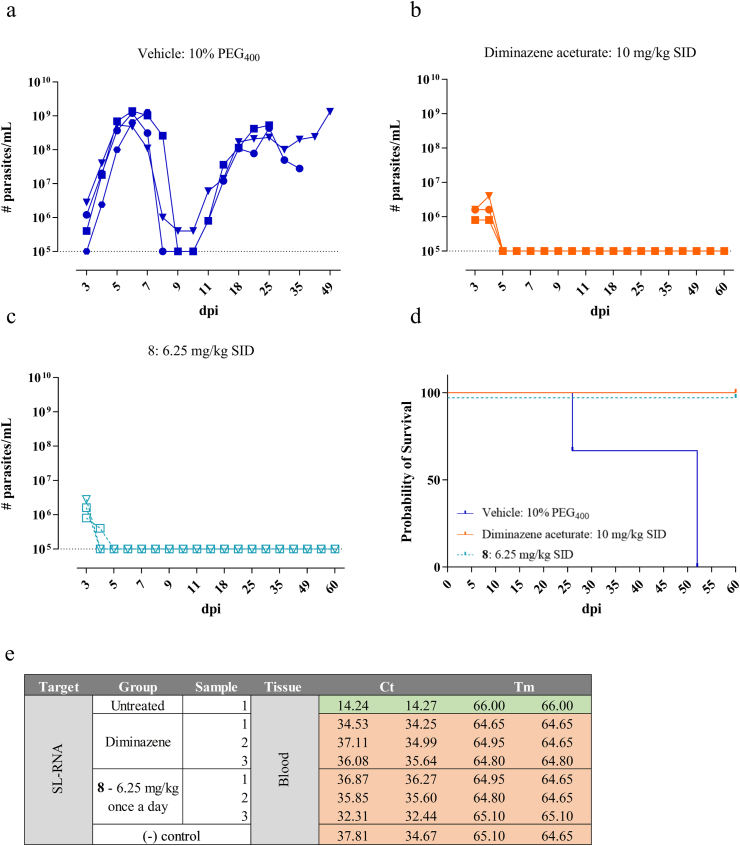
First, 8 was evaluated in an animal model of T. congolense. Intraperitoneal administration of 6.25 mg/kg once a day for 5 consecutive days resulted in a negative blood parasitaemia and survival of all treated animals up to the pre-set endpoint of 60 dpi (Fig. 4A–D). In two out of three mice, the parasitaemia dropped below the detection limit already after 1 treatment dose. All treated animals showed complete absence of residual parasite burdens in the blood, as evaluated by a highly sensitive spliced-leader RNA (SL-RNA) quantitative PCR (qPCR) detection method (Fig. 4E). However, similar treatment against T. vivax, resulted in parasite burdens and animal mortality equal to the vehicle-treated control group (Fig. 5).
Fig. 4.
In vivo activity of nucleoside analogue 8 (3′-deoxytubercidin) in a mouse model of T. congolense. (a) Vehicle group treated with 10% PEG400. (b) Reference drug diminazene aceturate administered at 10 mg/kg SID for 5 consecutive days. (c) Nucleoside analogue 3′-deoxytubercidin administered at 6.25 mg/kg SID for 5 consecutive days. (a–c): Blood parasitaemia in tail vein blood. Squares, triangles and circles represent the individual mice. The dotted line represents the detection limit of the counting chamber. (d) Survival analysis. The colours correspond to the different test groups depicted in a-c. (e) qPCR analysis of blood samples from surviving animals to probe for potential residual parasite levels. Cells in the table that are coloured in green are positive for the specific amplification product (SL-RNA). Cells coloured in red are negative for the specific amplification product. All samples tested positive for the presence of the mouse reference gene Eef2, demonstrating appropriate RNA extraction efficiency. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
In vivo activity of nucleoside analogue 8 (3′-deoxytubercidin) in a mouse model of T. vivax. (a) Vehicle group treated with 10% PEG400. (b) Reference drug diminazene aceturate administered at 10 mg/kg SID for 5 consecutive days. (c) Nucleoside analogue 3′-deoxytubercidin administered at 6.25 mg/kg SID for 5 consecutive days. (a–c): Blood parasitaemia in tail vein blood. Squares, triangles and circles represent the individual mice. The dotted line represents the detection limit of the counting chamber. (d) Survival analysis. The colours correspond to the different test groups depicted in a-c. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
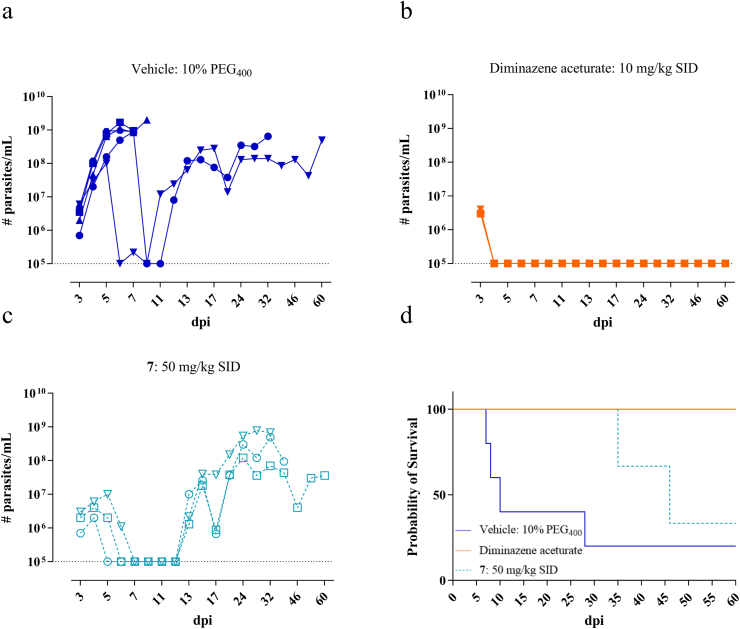
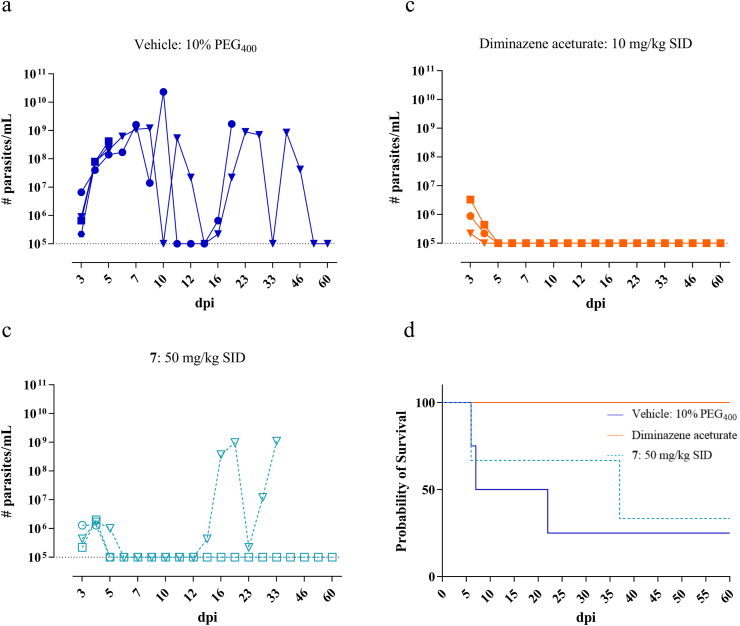
Treatment of T. congolense infected mice with compound 7 at 50 mg/kg, once a day, in combination with 1-ABT to overcome Phase-I metabolic degradation, resulted in parasitaemia levels below the detection limit. However, relapse occurred in all animals around 13 dpi resulting in animal death in part of the treated group (Fig. 6). Administration of compound 7 to T. vivax infected mice had variable results. One mouse died during the initial peak of infection while in two mice the parasitaemia dropped below the detection limit. One of them relapsed and died from infection around 37 dpi and the other survived the pre-set endpoint of 60 dpi without relapse (SL-RNA qPCR-negative in blood and spleen) (Fig. 7).
Fig. 6.
In vivo activity of nucleoside analogue 7 in a mouse model of T. congolense. (a) Vehicle group treated with 10% PEG400. (b) Reference drug diminazene aceturate administered at 10 mg/kg SID for 5 consecutive days. (c) Nucleoside analogue 7 administered at 50 mg/kg SID for 5 consecutive days. (a–c): Blood parasitaemia in tail vein blood. Squares, triangles and circles represent the individual mice. The dotted line represents the detection limit of the counting chamber. (d) Survival analysis. The colours correspond to the different test groups depicted in a-c. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7.
In vivo activity of nucleoside analogue 7 in a mouse model of T. vivax. (a) Vehicle group treated with 10% PEG400. (b) Reference drug diminazene aceturate administered at 10 mg/kg SID for 5 consecutive days. (c) Nucleoside analogue 7 administered at 50 mg/kg SID for 5 consecutive days. (a–c): Blood parasitaemia in tail vein blood. Squares, triangles and circles represent the individual mice. The dotted line represents the detection limit of the counting chamber. (d) Survival analysis. The colours correspond to the different test groups depicted in a-c. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
While drug discovery initiatives for HAT have stagnated with the recent implementation of fexinidazole(Lindner et al., 2020) and the Phase-II/III clinical progression of acoziborole, the treatment of animal trypanosomiasis still relies on drugs causing severe local reactions as well as systemic side-effects and the further spread of drug resistance(Chitanga et al., 2011; Richards et al., 2021). Based on the exclusive dependency of trypanosomes on purine salvage, we recently explored several series of nucleoside analogues, some of which are highly effective for the treatment of second-stage sleeping sickness(Hulpia et al., 2019b; Mabille et al., 2021). Given the current more pressing need for novel compounds for the treatment of AT, this study aimed at redirecting several promising nucleoside analogues for HAT towards animal trypanosomes.
The preferred target product profile (TPP) for trypanocidal drugs in animals describes a single treatment, active against the entire range of AT species,(GALVmed) and including parasite strains resistant against the existing drugs(Carruthers et al., 2021; Degneh et al., 2019). The in vitro susceptibility assays demonstrated a clear difference in drug-susceptibility of individual trypanosome species, in particular T. vivax which generally showed a lower susceptibility, indicating that T. vivax requires additional focus. This divergent susceptibility of T. vivax can be explained by the phylogeny of the Trypanosoma species, positioning T. vivax the most distant from the Trypanozoon cluster (including T. b. brucei spp., T. evansi and T. equiperdum)(Fraga et al., 2016). Due to a collaboration between laboratories, different assay conditions were used for the different AT strains which might have an impact on the obtained IC50 values. However, the main aim was not to compare the IC50 values between the different species but within one species to select the compounds with the most promising activity. Compounds with the overall highest potency across the different species were then selected for further in vivo evaluation.
Our lead compound 8 to treat late-stage sleeping sickness(Hulpia et al., 2019b), also showed promising activity against T. congolense in vivo, with total clearance of blood parasitaemia confirmed by RT-qPCR. However, compound 8 was less active against T. vivax as shown by the ex vivo results (IC50 = 3.48 ± 0.09 μM) and an in vivo mouse experiment, making compound 8 not compliant with the TPP for AT treatment. This emphasizes the importance of including T. vivax in the screening panel for the selection of broad spectrum anti-AT agents. While it is difficult to put an absolute value for an in vitro EC50 for progression to in vivo studies, the extent of the difference with the activity against the other trypanosome species (72 to >1000-fold) would seem to be incompatible with a broad anti-AT treatment. However, the requirement for broad spectrum anti-AT activity derives from the situation in sub-Saharan Africa, where it is almost always unknown which trypanosome species (T. b. brucei, T. b. rhodesiense, T. vivax, T. congolense) has infected a particular animal, and mixed infections are common(Giordani et al., 2016). With the non-tsetse transmitted animal trypanosomiases, e.g. surra and dourine, the trypanosome species is usually known by geographical region, host species and clinical symptoms. As T. vivax is limited to the tsetse belt and South America(Desquesnes, 2004; Garcia et al., 2014), compound 8 might still have very important use as treatment of T. evansi surra in Northern Africa, the Middle East and Asia, as well as the fatal equine disease dourine (T. equiperdum), for which there currently is no treatment.(OIE, 15/12/2020)
Based on the in vitro and ex vivo results, four compounds (7, 9, 11 and 14) showed promise and were selected for further analysis and confirmation of potency. The halogenated compounds (incl. compound 11) were found to display some cytotoxicity against MRC-5SV2 cells but maintained, nonetheless, an excellent selectivity index. Similar observations were made for the unsaturated carbon-based 7-substituents (incl. compound 14)(Hulpia et al., 2020b). Despite their excellent selectivity indices, however, compounds 9, 11 and 14 caused severe toxicity at 25 mg/kg twice a day intraperitoneally in a mouse, precluding further in vivo analysis. Compounds 8 and 9 were tested at a different time-point using 3 mice per group. Based on these results and the observed toxic effect of compound 9 in all three mice we decided that explorative evaluation in single mice (n = 1) is sufficiently indicative of toxicity within this chemical series. Treated mice showed hepatic injury as has been described for tubercidin(Kolassa et al., 1982). Previous studies on the co-administration of the nucleoside transport inhibitor NBMPR-P showed protection of mice from the hepatic and renal injury caused by tubercidin by limiting compound uptake in these organs(el Kouni et al., 1989). However, the basis of the in vivo toxicity remains to be clarified and progression of compounds 11 and 14 was not pursued further. Compound 7 did not cause toxicity but was unable to completely clear parasite burdens in all T. congolense and T. vivax infected mice, leading to post-treatment relapse. Based on observations in the in vitro assay system, this might be due to remaining Phase-I metabolism despite the co-administration of 1-ABT.
In summary, we conclude that nucleoside analogues hold promise for a broadly applicable treatment of animal trypanosomiasis provided further improvement on metabolic stability and absence of toxicity. Several compounds with promising profiles against surra and dourine were identified and could be developed further. Regardless, further drug discovery efforts will focus on activity against an extended panel of animal trypanosomes, with special attention for T. vivax given its divergent susceptibility profile.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Pim-Bart Feijens, An Matheeussen, Rik Hendrickx and Natascha Van Pelt for excellent technical assistance. LMPH is a partner of the Infla-Med Centre of Excellence (www.uantwerpen.be/infla-med). This work was supported by the Fonds Wetenschappelijk Onderzoek (www.fwo.be; grant numbers G033618N, G013118N), the University of Antwerp (www.uantwerpen.be; grant numbers TT-ZAPBOF 33049 and IOF-PoC 42404) and the Petroleum Technology Development Fund of Nigeria (PhD scholarship to M.A.U.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.05.001.
Contributor Information
Dorien Mabille, Email: dorien.mabille@uantwerpen.be.
Kayhan Ilbeigi, Email: kayhan.ilbeigi@uantwerpen.be.
Sarah Hendrickx, Email: sarah.hendrickx@uantwerpen.be.
Marzuq A. Ungogo, Email: 2226184U@student.gla.ac.uk.
Fabian Hulpia, Email: FHulpia@ITS.JNJ.com.
Cai Lin, Email: cai.lin@ugent.be.
Louis Maes, Email: louis.maes@uantwerpen.be.
Harry P. de Koning, Email: harry.de-koning@glasgow.ac.uk.
Serge Van Calenbergh, Email: serge.vancalenbergh@ugent.be.
Guy Caljon, Email: guy.caljon@uantwerpen.be.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Autheman D., Crosnier C., Clare S., Goulding D.A., Brandt C., Harcourt K., Tolley C., Galaway F., Khushu M., Ong H., Romero-Ramirez A., Duffy C.W., Jackson A.P., Wright G.J. An invariant Trypanosoma vivax vaccine antigen induces protective immunity. Nature. 2021;595:96–100. doi: 10.1038/s41586-021-03597-x. [DOI] [PubMed] [Google Scholar]
- Babokhov P., Sanyaolu A.O., Oyibo W.A., Fagbenro-Beyioku A.F., Iriemenam N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health. 2013;107:242–252. doi: 10.1179/2047773213Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., de Koning H.P., Maser P., Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M., Van der Veken P., Goeminne A., Haemers A., Augustyns K. Inhibitors of the purine salvage pathway: a valuable approach for antiprotozoal chemotherapy? Curr. Med. Chem. 2010;17:2456–2481. doi: 10.2174/092986710791556023. [DOI] [PubMed] [Google Scholar]
- Buscher P., Mumba Ngoyi D., Kabore J., Lejon V., Robays J., Jamonneau V., Bebronne N., Van der Veken W., Bieler S. Improved models of mini anion exchange centrifugation technique (mAECT) and modified single centrifugation (MSC) for sleeping sickness diagnosis and staging. PLoS Neglected Trop. Dis. 2009;3:e471. doi: 10.1371/journal.pntd.0000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. [Trypanosoma equiperdum: antigenic variations in experimental trypanosomiasis of rabbits] Exp. Parasitol. 1977;42:6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Carruthers L.V., Munday J.C., Ebiloma G.U., Steketee P., Jayaraman S., Campagnaro G.D., Ungogo M.A., Lemgruber L., Donachie A.M., Rowan T.G., Peter R., Morrison L.J., Barrett M.P., De Koning H.P. Diminazene resistance in Trypanosoma congolense is not caused by reduced transport capacity but associated with reduced mitochondrial membrane potential. Mol. Microbiol. 2021 doi: 10.1111/mmi.14733. [DOI] [PubMed] [Google Scholar]
- Chitanga S., Marcotty T., Namangala B., Van den Bossche P., Van Den Abbeele J., Delespaux V. High prevalence of drug resistance in animal trypanosomes without a history of drug exposure. PLoS Neglected Trop. Dis. 2011;5:e1454. doi: 10.1371/journal.pntd.0001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou V., Guegan F., Plazolles N., Baltz T. Complete in vitro life cycle of Trypanosoma congolense: development of genetic tools. PLoS Neglected Trop. Dis. 2010;4:e618. doi: 10.1371/journal.pntd.0000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degneh E., Ashenafi H., Kassa T., Kebede N., Shibeshi W., Asres K., Terefe G. Trypanocidal drug resistance: a threat to animal health and production in gidami district of kellem wollega zone, oromia regional state, western Ethiopia. Prev. Vet. Med. 2019;168:103–107. doi: 10.1016/j.prevetmed.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Delespaux V., de Koning H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updates. 2007;10:30–50. doi: 10.1016/j.drup.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Desquesnes M. France; Paris: 2004. Livestock Trypanosomoses and Their Vectors in Latin America. OIE, Paris, France: CIRAD-EMVT Publication; 174. ISBN 92-9044-634-X. OIE (World Organisation for Animal Health) [Google Scholar]
- Desquesnes M., Dargantes A., Lai D.H., Lun Z.R., Holzmuller P., Jittapalapong S. Trypanosoma evansi and surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res. Int. 2013;2013:321237. doi: 10.1155/2013/321237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquesnes M., Dia M.L. Trypanosoma vivax: mechanical transmission in cattle by one of the most common African tabanids, Atylotus agrestis. Exp. Parasitol. 2003;103:35–43. doi: 10.1016/s0014-4894(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Dickie E.A., Giordani F., Gould M.K., Maser P., Burri C., Mottram J.C., Rao S.P.S., Barrett M.P. New drugs for human African trypanosomiasis: a twenty first century success story. Trav. Med. Infect. Dis. 2020 doi: 10.3390/tropicalmed5010029. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa N., Hussein H., Wang H., Rabbi M.F., Bernstein C.N., Ghia J.E. Stability of reference genes for messenger RNA quantification by real-time PCR in mouse dextran sodium sulfate experimental colitis. PLoS One. 2016 doi: 10.1371/journal.pone.0156289. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni M.H., Diop D., O'Shea P., Carlisle R., Sommadossi J.P. Prevention of tubercidin host toxicity by nitrobenzylthioinosine 5'-monophosphate for the treatment of schistosomiasis. Antimicrob. Agents Chemother. 1989;33:824–827. doi: 10.1128/aac.33.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga J., Fernandez-Calienes A., Montalvo A.M., Maes I., Deborggraeve S., Buscher P., Dujardin J.C., Van der Auwera G. Phylogenetic analysis of the Trypanosoma genus based on the heat-shock protein 70 gene. Infect. Genet. Evol. 2016;43:165–172. doi: 10.1016/j.meegid.2016.05.016. [DOI] [PubMed] [Google Scholar]
- GALVmed, Target Product Profile - AAT.
- Garcia H.A., Rodrigues A.C., Rodrigues C.M., Bengaly Z., Minervino A.H., Riet-Correa F., Machado R.Z., Paiva F., Batista J.S., Neves L., Hamilton P.B., Teixeira M.M. Microsatellite analysis supports clonal propagation and reduced divergence of Trypanosoma vivax from asymptomatic to fatally infected livestock in South America compared to West Africa. Parasites Vectors. 2014;7:210. doi: 10.1186/1756-3305-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts S., Holmes P.H., Eisler M.C., Diall O. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 2001;17:25–28. doi: 10.1016/s1471-4922(00)01827-4. [DOI] [PubMed] [Google Scholar]
- Giordani F., Morrison L.J., Rowan T.G., De Koning H.P., Barrett M.P. The animal trypanosomiases and their chemotherapy: a review. Parasitology. 2016;143:1862–1889. doi: 10.1017/S0031182016001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizaw Y., Megersa M., Fayera T. Dourine: a neglected disease of equids. Trop. Anim. Health Prod. 2017;49:887–897. doi: 10.1007/s11250-017-1280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Andrade P., Camara M., Ilboudo H., Bucheton B., Jamonneau V., Deborggraeve S. Diagnosis of trypanosomatid infections: targeting the spliced leader RNA. J. Mol. Diagn. 2014;16:400–404. doi: 10.1016/j.jmoldx.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Hulpia F., Bouton J., Campagnaro G.D., Alfayez I.A., Mabille D., Maes L., de Koning H.P., Caljon G., Van Calenbergh S. C6-O-alkylated 7-deazainosine nucleoside analogues: discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 2020;188:112018. doi: 10.1016/j.ejmech.2019.112018. [DOI] [PubMed] [Google Scholar]
- Hulpia F., Campagnaro G.D., Alzahrani K.J., Alfayez I.A., Ungogo M.A., Mabille D., Maes L., de Koning H.P., Caljon G., Van Calenbergh S. Structure-activity relationship exploration of 3'-deoxy-7-deazapurine nucleoside analogues as anti-Trypanosoma brucei agents. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00105. [DOI] [PubMed] [Google Scholar]
- Hulpia F., Campagnaro G.D., Scortichini M., Van Hecke K., Maes L., de Koning H.P., Caljon G., Van Calenbergh S. Revisiting tubercidin against kinetoplastid parasites: aromatic substitutions at position 7 improve activity and reduce toxicity. Eur. J. Med. Chem. 2019;164:689–705. doi: 10.1016/j.ejmech.2018.12.050. [DOI] [PubMed] [Google Scholar]
- Hulpia F., Mabille D., Campagnaro G.D., Schumann G., Maes L., Roditi I., Hofer A., de Koning H.P., Caljon G., Van Calenbergh S. Combining tubercidin and cordycepin scaffolds results in highly active candidates to treat late-stage sleeping sickness. Nat. Commun. 2019;10:5564. doi: 10.1038/s41467-019-13522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageruka P., Mortelmans J. [Isolation of Trypanosoma evansi Steel 1885 from a capybara (Hydrochoerus hydrochoeris Lin.) imported to Belgium and preliminary study of its virulence] Ann. Soc. Belges. Med. Trop. Parasitol. Mycol. 1971;51:709–716. [PubMed] [Google Scholar]
- Kaiser M., Maes L., Tadoori L.P., Spangenberg T., Ioset J.R. Repurposing of the Open Access Malaria Box for kinetoplastid diseases identifies novel active scaffolds against trypanosomatids. J. Biomol. Screen. 2015;20:634–645. doi: 10.1177/1087057115569155. [DOI] [PubMed] [Google Scholar]
- Kasozi K.I., Zirintunda G., Ssempijja F., Buyinza B., Alzahrani K.J., Matama K., Nakimbugwe H.N., Alkazmi L., Onanyang D., Bogere P., Ochieng J.J., Islam S., Matovu W., Nalumenya D.P., Batiha G.E., Osuwat L.O., Abdelhamid M., Shen T., Omadang L., Welburn S.C. Epidemiology of trypanosomiasis in wildlife-implications for humans at the wildlife interface in Africa. Front. Vet. Sci. 2021;8:621699. doi: 10.3389/fvets.2021.621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness) Lancet Neurol. 2013;12:186–194. doi: 10.1016/S1474-4422(12)70296-X. [DOI] [PubMed] [Google Scholar]
- Kolassa N., Jakobs E.S., Buzzell G.R., Paterson A.R. Manipulation of toxicity and tissue distribution of tubercidin in mice by nitrobenzylthioinosine 5'-monophosphate. Biochem. Pharmacol. 1982;31:1863–1874. doi: 10.1016/0006-2952(82)90489-0. [DOI] [PubMed] [Google Scholar]
- Leach T.M., Roberts C.J. Present status of chemotherapy and chemoprophylaxis of animal trypanosomiasis in the Eastern hemisphere. Pharmacol. Ther. 1981;13:91–147. doi: 10.1016/0163-7258(81)90069-3. [DOI] [PubMed] [Google Scholar]
- Lindner A.K., Lejon V., Chappuis F., Seixas J., Kazumba L., Barrett M.P., Mwamba E., Erphas O., Akl E.A., Villanueva G., Bergman H., Simarro P., Kadima Ebeja A., Priotto G., Franco J.R. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. Lancet Infect. Dis. 2020;20:e38–e46. doi: 10.1016/S1473-3099(19)30612-7. [DOI] [PubMed] [Google Scholar]
- Mabille D., Cardoso Santos C., Hendrickx R., Claes M., Takac P., Clayton C., Hendrickx S., Hulpia F., Maes L., Van Calenbergh S., Caljon G. 4E Interacting protein as a potential novel drug target for nucleoside analogues in Trypanosoma brucei. Microorganisms. 2021;9:826. doi: 10.3390/microorganisms9040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesu V., Kalonji W.M., Bardonneau C., Mordt O.V., Blesson S., Simon F., Delhomme S., Bernhard S., Kuziena W., Lubaki J.F., Vuvu S.L., Ngima P.N., Mbembo H.M., Ilunga M., Bonama A.K., Heradi J.A., Solomo J.L.L., Mandula G., Badibabi L.K., Dama F.R., Lukula P.K., Tete D.N., Lumbala C., Scherrer B., Strub-Wourgaft N., Tarral A. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet. 2018;391:144–154. doi: 10.1016/S0140-6736(17)32758-7. [DOI] [PubMed] [Google Scholar]
- Odeniran P.O., Macleod E.T., Ademola I.O., Welburn S.C. Molecular identification of bloodmeal sources and trypanosomes in Glossina spp., Tabanus spp. and Stomoxys spp. trapped on cattle farm settlements in southwest Nigeria. Med. Vet. Entomol. 2019;33:269–281. doi: 10.1111/mve.12358. [DOI] [PubMed] [Google Scholar]
- OIE, 15/12/2020. OIE Technical Disease Card: Dourine.
- Ooi C.P., Schuster S., Cren-Travaille C., Bertiaux E., Cosson A., Goyard S., Perrot S., Rotureau B. The cyclical development of Trypanosoma vivax in the tsetse fly involves an asymmetric division. Front. Cell. Infect. Microbiol. 2016;6:115. doi: 10.3389/fcimb.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska M., Vereecke N., Deleeuw V., Pinto J., Magez S. Salivarian trypanosomosis: a review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Front. Immunol. 2018;9:2253. doi: 10.3389/fimmu.2018.02253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S., Morrison L.J., Torr S.J., Barrett M.P., Manangwa O., Mramba F., Auty H. Pharma to farmer: field challenges of optimizing trypanocide use in African animal trypanosomiasis. Trends Parasitol. 2021;37:831–843. doi: 10.1016/j.pt.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Seela F., Ming X. 7-Functionalized 7-deazapurine β-d and β-l-ribonucleosides related to tubercidin and 7-deazainosine: glycosylation of pyrrolo[2,3-d]pyrimidines with 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d or β-l-ribofuranose. Tetrahedron. 2007;63:9850–9861. [Google Scholar]
- Sokolova A.Y., Wyllie S., Patterson S., Oza S.L., Read K.D., Fairlamb A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010;54:2893–2900. doi: 10.1128/AAC.00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirados I., Esterhuizen J., Kovacic V., Mangwiro T.N., Vale G.A., Hastings I., Solano P., Lehane M.J., Torr S.J. Tsetse control and gambian sleeping sickness: implications for control strategy. PLoS Neglected Trop. Dis. 2015 doi: 10.1371/journal.pntd.0003822. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truc P., Buscher P., Cuny G., Gonzatti M.I., Jannin J., Joshi P., Juyal P., Lun Z.R., Mattioli R., Pays E., Simarro P.P., Teixeira M.M., Touratier L., Vincendeau P., Desquesnes M. Atypical human infections by animal trypanosomes. PLoS Neglected Trop. Dis. 2013;7:e2256. doi: 10.1371/journal.pntd.0002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S., Foth B.J., Kelner A., Sokolova A.Y., Berriman M., Fairlamb A.H. Nitroheterocyclic drug resistance mechanisms in Trypanosoma brucei. J. Antimicrob. Chemother. 2016;71:625–634. doi: 10.1093/jac/dkv376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.