Abstract
Background
Male patients ages 12–17 years have an elevated risk of mRNA vaccination‐associated myo/pericarditis. A risk‐benefit analysis of first and second doses of mRNA vaccination in adolescent boys by health status and history of SARS‐CoV‐2 infection has not been performed.
Methods
Using the Vaccine Adverse Event Reporting System (VAERS), we identified BNT162b2 [Pfizer‐BioNTech] myo/pericarditis occurrence according to CDC criteria. Main outcomes were as follows: 1) post‐vaccination myo/pericarditis crude incidence in adolescents aged 12–15 and 16–17; and 2) two risk‐benefit analyses by age, sex, comorbidity, variant and history of infection.
Results
Cases of myo/pericarditis (n = 253) included 129 after dose 1 and 124 after dose 2; 86.9% were hospitalized. Incidence per million after dose two in male patients aged 12–15 and 16–17 was 162.2 and 93.0, respectively. Weighing post‐vaccination myo/pericarditis against COVID‐19 hospitalization during delta, our risk‐benefit analysis suggests that among 12–17‐year‐olds, two‐dose vaccination was uniformly favourable only in nonimmune girls with a comorbidity. In boys with prior infection and no comorbidities, even one dose carried more risk than benefit according to international estimates. In the setting of omicron, one dose may be protective in nonimmune children, but dose two does not appear to confer additional benefit at a population level.
Conclusions
Our findings strongly support individualized paediatric COVID‐19 vaccination strategies which weigh protection against severe disease vs. risks of vaccine‐associated myo/pericarditis. Research is needed into the nature and implications of this adverse effect as well as immunization strategies which reduce harms in this overall low‐risk cohort.
Keywords: COVID‐19, drug‐related adverse reactions, myocarditis, paediatrics, SARS‐CoV‐2, vaccination
1. INTRODUCTION
The Pfizer‐BioNTech BNT162b2 mRNA vaccine has demonstrated safety and real‐world effectiveness in preventing severe disease and death from COVID‐19, including among adolescents. Concerns about vaccination‐related myo/pericarditis in young men were initially raised in Israel, with rates between 1/3000 and 1/6000. 1 In the United States, the first Centers for Disease Control and Prevention (CDC) report 2 identified a rate of 1/44,640 for ages 12–15 and 1/29,400 for ages 16–17. 2 Recent international data 3 , 4 , 5 in adolescent male patients after vaccine dose two have found myopericarditis rates of 1/6600 (ages 16–19) in Israel, 3 1/7400 (ages 12–17) in Ontario 4 and 1/2700 (ages 12–17) in Hong Kong. 5 In the US, recent estimates for dose two incidence among young male patients have been reported by Kaiser Permanente (1/2650), 6 the FDA (1/5600 to 1/5000) 7 , 8 and the CDC (1/22,000 to 1/14,200). 9
To our knowledge, a comprehensive risk‐benefit analysis which considers a child's risk of COVID‐19 hospitalization in the setting of underlying medical conditions and history of SARS‐CoV‐2 infection has not been undertaken. Our study has two aims: (1) stratify post‐mRNA vaccination myo/pericarditis occurrence by age and vaccination dose within the US 12–17‐year‐old population to complement the CDC’s 2 , 9 , 10 and FDA’s 7 , 8 rates; and (2) perform a two‐part risk‐benefit analysis weighing the benefits of one and two doses of vaccination in adolescents to prevent COVID‐19 hospitalization against the risks of vaccination‐associated myo/pericarditis stratified by age, sex, prior infection history, variant and medical comorbidity status. We report our findings in the context of international rates of post‐BNT162b2 vaccination myo/pericarditis in this age group.
2. METHODS
2.1. Aim 1: Stratify post‐mRNA vaccination myo/pericarditis occurrence by sex, age and vaccination dose
2.1.1. Search methodology
We searched the Vaccine Adverse Event Reporting System (VAERS) system, 11 an open access passive reporting system in the United States, for reports processed from 1 January 2021 to 18 June 2021 with symptom codes for ‘myocarditis’, ‘pericarditis’, ‘myopericarditis’ or ‘chest pain’ for children aged 12–17 years. Reports were required to meet the CDC working definition for probable acute myocarditis 2 as defined in Appendix S1: new or worsening symptoms plus at least one abnormal laboratory or clinical finding (e.g. elevated troponin; electrocardiogram (EKG), echocardiogram (ECHO), or cardiac MRI (cMRI)) consistent with myo/pericarditis. Exclusions were made for lack of objective laboratory findings or suspected viral myo/pericarditis. VAERS entries included product name and vaccination dose number. Cases and hospitalizations with an unknown dose number were assigned to dose one or dose two in the same proportions as the known doses (15% and 85%, respectively).
2.1.2. Crude reporting rates
To estimate a rate per million for doses one and two, our denominators included all children with at least one dose of BNT162b2 vaccination and children with two doses of BNT162b2, respectively, as of 11 June 2021 12 to accommodate reporting lag and a pre‐defined minimum 7‐day risk window; we divided total persons in each vaccinated group by two to create sex‐specific denominators for our rates. Confidence intervals were constructed for these rates using the Poisson distribution.
2.2. Aim 2: Risk‐benefit analysis of one versus two doses of mRNA COVID‐19 vaccination
2.2.1. Relative risk of hospitalization in the presence of comorbidities
To estimate hospitalization risk, we constructed risk ratios for children with and without comorbidities among those hospitalized, and in the general population. (Appendix S4) Approximately, 70% of children hospitalized for COVID‐19 have one or more medical comorbidities, a ratio of 2.33:1. 13 , 14 , 15 A literature review for the prevalence of chronic conditions in the population found that approximately 33% of children in this age group have one or more comorbidities, a ratio of 1:2. 16 , 17 , 18 Using these estimates of comorbidity prevalence among children admitted for COVID‐19 and in the population, our analysis considers that children with at least one medical comorbidity have 4.8 times the likelihood of COVID‐19 hospitalization as those without comorbidities.
2.2.2. Infection hospitalization rate
The overall infection hospitalization rate (IHR) for children was estimated using an age‐specific international serological study which reported the IHR for children aged 10–19 years to be 0.22%. 19 Given the relative risks above, we computed the IHR for children with (y) and without (x) comorbidities as follows:
2.33*(0.22%) = 0.513% = y, hospitalization rate with comorbidities.
0.49*(0.22%) = 0.108% = x, hospitalization rate with no comorbidities.
y = 0.513% x = 0.108%
We also used a second set of IHR estimates from Germany among children ages 12–17 with and without comorbidities 20 : hospitalizations requiring therapeutic intervention for COVID‐19 (which eliminates incidental hospitalizations) were 0.147% and 0.042% of infections, respectively.
The estimated reduction in severity (IHR) for omicron relative to delta is 66%. 21
2.2.3. Background myo/pericarditis
The expected background myo/pericarditis rate 22 was calculated for boys and girls separately over the course of 7 days, consistent with the CDC window. 2 , 9 , 10 The expected rate of background myo/pericarditis was thus 2.1/million cases per week in boys and 1.4/million in girls.
2.3. Risk‐benefit analyses
Our risk‐benefit analysis for children age 12–17 was conducted using two methods: 1) estimate hospitalizations prevented with dose one and dose two during the delta and omicron waves, in the setting of previous infection and stratified by comorbidity; and 2) 120‐day cumulative COVID‐19 hospitalizations per 100,000 population.
To compute the estimated COVID‐19 hospitalizations prevented by doses one and two, we referenced publications which met the following criteria: 1) BNT162b2‐specific vaccine effectiveness against hospitalization (VEH) estimates; 2) stratified by partial and full vaccination; 3) with strata‐specific rates for children or young adults; and 4) during a time of delta and omicron variant predominance. The delta VEH ranges used in our analysis were 84.5 23 to 91.1% 24 for dose one and 81.0 25 to 93.0% 15 for dose two. Finnish data suggest equivalent VEH rates for dose one (89.8%) and dose two (90.2%) in this age group. 26 The UK and US VEH estimates during omicron were 58% 27 to 73% 28 for dose 1 and 44% 27 to 64% 28 for dose 2, respectively. The estimated range of protection against hospitalizations conferred by a history of infection is 87.8% 29 during omicron to 97.2% 30 during delta.
In the first IHR analysis, relative risks (RRs) are stratified by sex, age, comorbidity, prior infection history, variant and vaccination dose number comparing the risk of vaccine‐associated myo/pericarditis with the benefit of hospitalizations prevented after partial and full vaccination and in the context of international myo/pericarditis estimates. In the second 120‐day analysis, the cumulative hospitalization rates are presented at low, moderate and high incidence, such as during the delta and omicron waves. We also present hospitalization rates adjusted for the estimated 40% of hospitalizations with COVID‐19 as an incidental finding. 31 , 32 , 33 , 34
Methods for both approaches are further described in Appendix S5.
Data were analyzed using Microsoft PowerBI, StataIC and Microsoft Excel.
3. RESULTS
A total of 276 reports met our initial search criteria; of these, 22 cases were excluded. (Appendix S2) Of the 253 myo/pericarditis cases included, 23 were female patient and 230 were male patient. Interactive data visualizations and full VAERS case notes for all included and excluded cases are available at this link: https://bit.ly/Krug‐MyoPerdicarditis.
Peak troponin values were recorded in 208 reports; median troponin values for boys ages 12–15 and 16–17 were 4.5 ng/mL and 9.9 ng/mL, respectively. For girls, median troponins were 0.8 ng/mL and 7.0 ng/mL. Of the 253 included cases, 252 recorded receiving BNT162b2 (although mRNA‐1273 is not approved for <18 years) and in 37, the dose number was unknown.
Figure 1A and Table 1 report the myo/pericarditis cases by age, sex and dose number. For boys aged 12–15, the rate per million after dose two was 162.2/million or 1/6200. Among boys aged 16–17, our estimate was 93.0/million or 1/10,800. For girls 12–15 and 16–17 years old, our rates following dose two were 13.0/million and 12.5/million, respectively. Our identified post‐vaccination myo/pericarditis rates are compared with international estimates in Figure 2.
FIGURE 1.
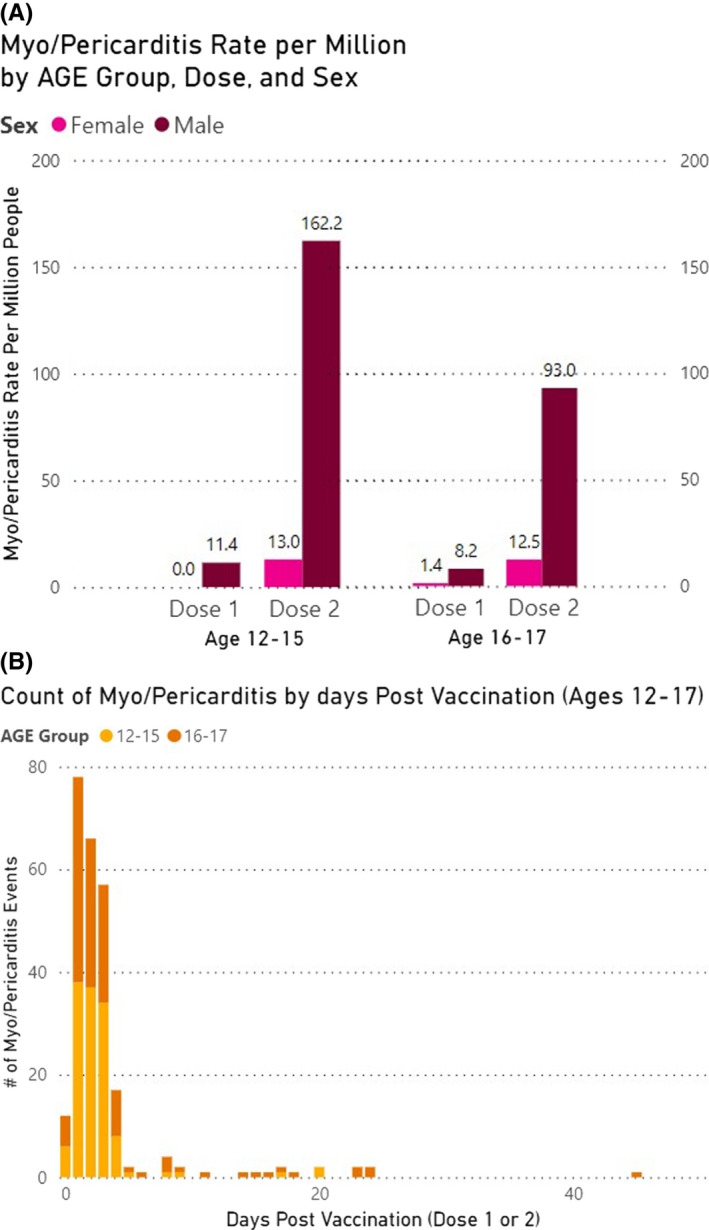
(A) Myo/pericarditis rates per million adolescents following Pfizer‐BioNTech [BNT162b2] mRNA vaccination doses 1 and 2, by age and sex. (B) Myo/pericarditis cases by days after BNT162b2 vaccination and by age
TABLE 1.
Myo/pericarditis occurrence per million adolescents following BNT162b2 vaccination doses 1 and 2, by age and sex
| Age and Dose | Number Vaccinated* as of 6/11/21 | Females | Males | ||||
|---|---|---|---|---|---|---|---|
| Expected | Observed | Myo/pericarditis per Million Vaccinated (95% CI) | Expected | Observed | Myo/pericarditis per Million Vaccinated (95% CI) | ||
| 12–15 | |||||||
| Dose 1 | 3,669,373 | 1.35 | 0 | 0.0 (0.0‐0.2) | 2.12 | 21 | 11.4 (7.09, 17.5) |
| Dose 2 | 1,233,021 | 1.35 | 8 | 13.0 (5.6, 25.6) | 2.12 | 100 | 162.2 (132.0, 197.3) |
| 16–17 | |||||||
| Dose 1 | 2,943,756 | 1.35 | 2 | 1.4 (0.2, 4.9) | 2.12 | 12 | 8.2 (4.21, 14.2) |
| Dose 2 | 2,085,725 | 1.35 | 13 | 12.5 (6.6, 21.3) | 2.12 | 97 | 93.0 (75.4, 113.5) |
| 12–17 | |||||||
| Dose 1 | 6,613,129 | 1.35 | 2 | 0.6 (0.1, 2.2) | 2.12 | 33 | 10.0 (6.9, 14.0) |
| Dose 2 | 3,318,746 | 1.35 | 21 | 12.7 (7.8, 19.3) | 2.12 | 197 | 118.7 (102.7, 136.5) |
Number vaccinated is divided by two to compute male and female rates per million.
FIGURE 2.
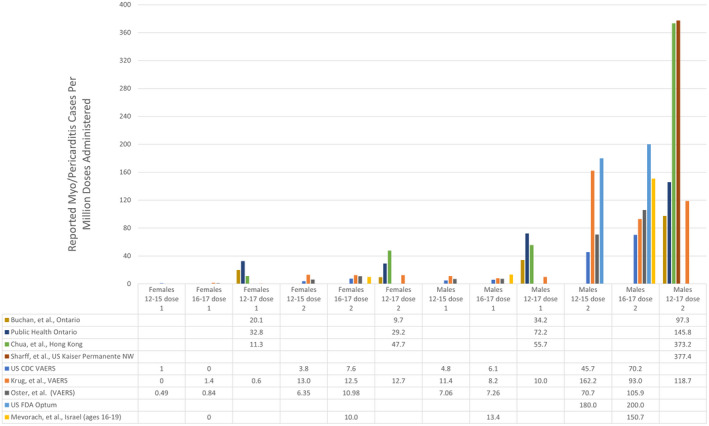
Comparison of VAERS‐derived estimates of myo/pericarditis occurrence to international rates of BNT162b2 vaccine‐associated myo/pericarditis
The myo/pericarditis cases in our investigation occurred a median of 2.0 days following vaccination (Figure 1B), and 91.9% occurred within 5 days. The hospitalization rate in our reports was 220/253 (86.9%) overall, with 111/129 (86.0%) in the 12–15‐year‐old cohort and 109/124 (87.9%) in the 16–17‐year‐old cohort.
3.1. Risk‐benefit analyses
3.1.1. Method 1
The relative risks (RR) of one dose of BNT162b2 vaccination compared to COVID‐19 hospitalizations prevented are presented in Table 2 and Figure 3A by sex, comorbidity, prior infection, variant and reported international vaccine‐associated myo/pericarditis rates following dose one. Using the two IHR 19 , 20 and omicron and delta VEH estimates 23−31, the benefit of one vaccination dose appears to outweigh the risk of myo/pericarditis in nonimmune boys and girls even during omicron and at the highest estimated myo/pericarditis rates (RRs 0.82 and 0.41 for boys and girls without comorbidities, respectively, Table 2). The benefits of one dose to prevent omicron and delta hospitalizations in boys with and without comorbidities are displayed in Figure 3A.
TABLE 2.
Relative risk (RR) of vaccine‐associated myo/pericarditis after BNT162b2 dose one compared to COVID‐19 hospitalizations prevented stratified by comorbidity status, history of infection and variant.
| Males | Dose 1 myo/pericarditis per million | Relative Risk of Dose 1 compared with hospitalizations prevented in those with medical comorbidities | Relative Risk of Dose 1 compared with hospitalizations saved in those without medical comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Prior Infection | Prior Infection | No Prior Infection | Prior Infection | ||||||
| Omicron | Delta | Omicron | Delta | Omicron | Delta | Omicron | Delta | ||
| 281.4 | 1242.2 | 34.3 | 34.8 | 80.4 | 354.9 | 9.8 | 9.9 | ||
| CDC (12‐15) VAERS | 4.8 | 0.02 | 0.00 | 0.14 | 0.14 | 0.06 | 0.01 | 0.49 | 0.48 |
| CDC (16‐17) VAERS | 6.1 | 0.02 | 0.00 | 0.18 | 0.18 | 0.08 | 0.02 | 0.62 | 0.61 |
| Oster, et al. (12‐15) VAERS | 7.1 | 0.03 | 0.01 | 0.21 | 0.20 | 0.09 | 0.02 | 0.72 | 0.71 |
| Oster, et al. (16‐17) VAERS | 7.3 | 0.03 | 0.01 | 0.21 | 0.21 | 0.09 | 0.02 | 0.74 | 0.73 |
| Krug, et al. (16‐17) VAERS | 8.2 | 0.03 | 0.01 | 0.24 | 0.24 | 0.10 | 0.02 | 0.84 | 0.83 |
| Krug, et al. (12‐15) VAERS | 11.4 | 0.04 | 0.01 | 0.33 | 0.33 | 0.14 | 0.03 | 1.16 | 1.15 |
| Mevorach, et al. (16‐19) | 13.4 | 0.05 | 0.01 | 0.39 | 0.39 | 0.17 | 0.04 | 1.37 | 1.35 |
| Buchan, et al. (12‐17) | 34.2 | 0.12 | 0.03 | 1.00 | 0.98 | 0.43 | 0.10 | 3.49 | 3.44 |
| Chua, et al. (12‐17) | 55.7 | 0.20 | 0.04 | 1.62 | 1.60 | 0.69 | 0.16 | 5.68 | 5.61 |
| Public Health Ontario (12‐17) | 66.3 | 0.24 | 0.05 | 1.93 | 1.91 | 0.82 | 0.19 | 6.76 | 6.67 |
| Females | |||||||||
| CDC (16‐17) VAERS | 0.0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Krug, et al. (12‐15) VAERS | 0.0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mevorach, et al. (16‐19) | 0.0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oster, et al. (12‐15) VAERS | 0.5 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.05 | 0.05 |
| Oster, et al. (16‐17) VAERS | 0.8 | 0.00 | 0.00 | 0.02 | 0.02 | 0.01 | 0.00 | 0.09 | 0.08 |
| CDC (12‐15) VAERS | 1.0 | 0.00 | 0.00 | 0.03 | 0.03 | 0.01 | 0.00 | 0.10 | 0.10 |
| Krug, et al. (16‐17) VAERS | 1.4 | 0.00 | 0.00 | 0.04 | 0.04 | 0.02 | 0.00 | 0.14 | 0.14 |
| Chua, et al. (12‐17) | 11.3 | 0.04 | 0.01 | 0.33 | 0.32 | 0.14 | 0.03 | 1.15 | 1.14 |
| Buchan, et al. (12‐17) | 20.1 | 0.07 | 0.02 | 0.59 | 0.58 | 0.25 | 0.06 | 2.05 | 2.02 |
| Public Health Ontario (12‐17) | 33.2 | 0.12 | 0.03 | 0.97 | 0.95 | 0.41 | 0.09 | 3.39 | 3.34 |
FIGURE 3.
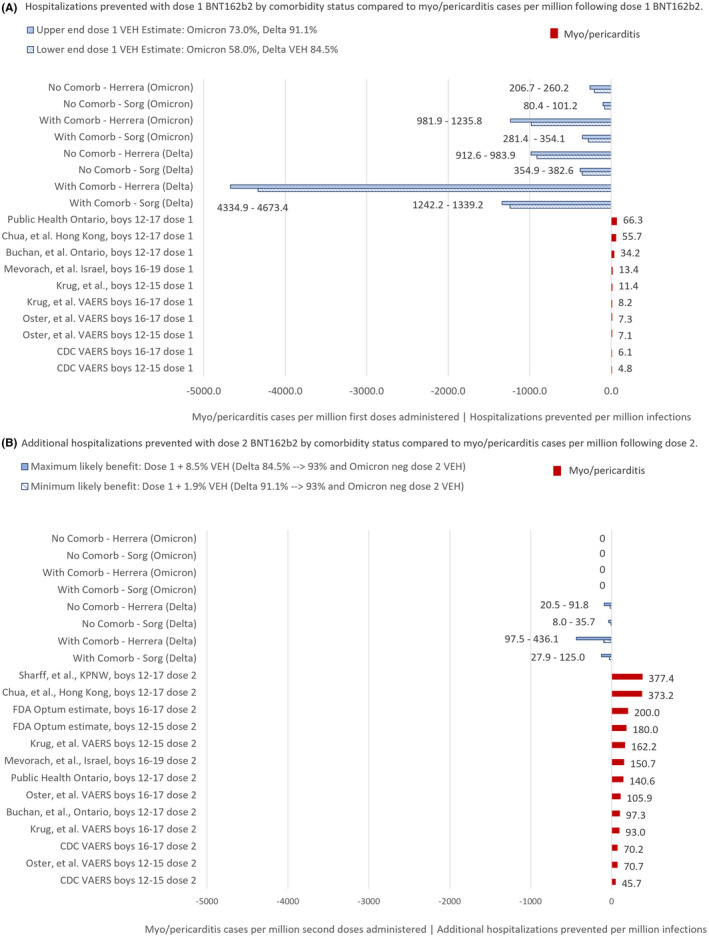
(A,B) COVID‐19 hospitalizations per million infections compared with myo/pericarditis rates per million BNT162b2 vaccine doses* in boys 12–17, stratified by vaccination dose, estimated infection hospitalization risk (IHR), comorbidity status and variant. *Hospitalizations prevented with dose two are based on IHR estimates by Herrera, et al. 19 and Sorg, et al. 20 and two range of vaccine effectiveness against hospitalization (VEH) estimates:1) minimum benefit conferred by increasing protection 1.9% from 91.1% 24 (upper end of dose 1 VEH range) to 93.0% 26 (upper end of dose 2 VEH range); and2) maximum benefit conferred by increasing protection 8.5% from 84.5% 23 (lower end of dose 1 VEH range) to 93.0% 26 (upper end of dose 2 VEH range)
The RR of a second dose of BNT162b2 vaccination compared to additional COVID‐19 hospitalizations prevented are displayed in Table 3 and Figure 3B. For girls, the benefits of a second dose appear to outweigh the risks of myo/pericarditis during delta, but the protective effect of a second dose for any child during omicron is uncertain given the lower VEH with dose two compared to dose one (VEH 44% 27 vs 58% 27 ). For boys with a medical comorbidity, our analysis suggests that the benefits of a second dose may outweigh the risks depending on the VEH of the first and second doses and the severity of the variant. With a higher first dose VEH (91.1% 24 ) in the setting of delta variant, the RR of myo/pericarditis outweighed the marginal benefit of a second dose by up to 5.81 and 3.33 times for 12–15‐ and 16–17‐year‐old boys, respectively, according to our estimates. (Appendix S5, Tables S2A and S2B) When compared to higher FDA 7 , 8 and international estimates 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 36 , 37 of myo/pericarditis, the risks clearly outweighed the incremental benefits during delta. In the setting of omicron, there is currently no evidence of additional benefit of a second dose for any child.
TABLE 3.
Relative risk (RR) of vaccine‐associated myo/pericarditis after BNT162b2 dose two compared to additional hospitalizations prevented stratified by comorbidity status, history of infection and variant.
| Males | Dose 2 myo/pericarditis per million | Relative Risk of Dose 2 compared with hospitalizations prevented in those with medical comorbidities | Relative Risk of Dose 2 compared with hospitalizations saved in those without medical comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Prior Infection | Prior Infection | No Prior Infection | Prior Infection | ||||||
| Omicron | Delta | Omicron | Delta | Omicron | Delta | Omicron | Delta | ||
| ‐67.9 | 125.0 | ‐8.3 | 3.5 | ‐19.4 | 35.7 | ‐2.4 | 1.0 | ||
| CDC (12‐15) VAERS | 45.7 | ND | 0.37 | ND | 13.06 | ND | 1.28 | ND | 45.72 |
| CDC (16‐17) VAERS | 70.2 | ND | 0.56 | ND | 20.07 | ND | 1.97 | ND | 70.23 |
| Oster, et al. (12‐15) VAERS | 70.7 | ND | 0.57 | ND | 20.22 | ND | 1.98 | ND | 70.76 |
| Krug, et al. (16‐17) VAERS | 93.0 | ND | 0.74 | ND | 26.58 | ND | 2.61 | ND | 93.04 |
| Buchan, et al. (12‐17) | 97.3 | ND | 0.78 | ND | 27.81 | ND | 2.73 | ND | 97.34 |
| Oster, et al. (16‐17) VAERS | 105.9 | ND | 0.85 | ND | 30.26 | ND | 2.97 | ND | 105.90 |
| Public Health Ontario (12‐17) | 140.6 | ND | 1.13 | ND | 40.19 | ND | 3.94 | ND | 140.66 |
| Mevorach, et al. (16‐19) | 150.7 | ND | 1.21 | ND | 43.07 | ND | 4.22 | ND | 150.76 |
| Krug, et al. (12‐15) VAERS | 162.2 | ND | 1.30 | ND | 46.36 | ND | 4.54 | ND | 162.26 |
| FDA (12‐15) | 180.0 | ND | 1.44 | ND | 51.45 | ND | 5.04 | ND | 180.07 |
| FDA (16‐17) | 200.0 | ND | 1.60 | ND | 57.17 | ND | 5.60 | ND | 200.08 |
| Chua, et al. (12‐17) | 373.2 | ND | 2.99 | ND | 106.67 | ND | 10.45 | ND | 373.35 |
| Sharff, et al. (12‐17) | 377.4 | ND | 3.02 | ND | 107.87 | ND | 10.57 | ND | 377.55 |
| Females | |||||||||
| CDC (12‐15) VAERS | 3.8 | ND | 0.03 | ND | 1.09 | ND | 0.11 | ND | 3.80 |
| Oster, et al. (12‐15) VAERS | 6.4 | ND | 0.05 | ND | 1.82 | ND | 0.18 | ND | 6.35 |
| CDC (16‐17) VAERS | 7.6 | ND | 0.06 | ND | 2.17 | ND | 0.21 | ND | 7.60 |
| Buchan, et al. (12‐17) | 9.7 | ND | 0.08 | ND | 2.77 | ND | 0.27 | ND | 9.70 |
| Mevorach, et al. (16‐19) | 10.0 | ND | 0.08 | ND | 2.86 | ND | 0.28 | ND | 10.00 |
| Oster, et al. (16‐17) VAERS | 11.0 | ND | 0.09 | ND | 3.14 | ND | 0.31 | ND | 10.98 |
| Krug, et al. (16‐17) VAERS | 12.5 | ND | 0.10 | ND | 3.57 | ND | 0.35 | ND | 12.51 |
| Krug, et al. (12‐15) VAERS | 13.0 | ND | 0.10 | ND | 3.72 | ND | 0.36 | ND | 13.01 |
| Public Health Ontario (12‐17) | 27.1 | ND | 0.22 | ND | 7.75 | ND | 0.76 | ND | 27.11 |
| Chua, et al. (12‐17) | 47.7 | ND | 0.38 | ND | 13.63 | ND | 1.34 | ND | 47.72 |
For boys without a medical comorbidity, our analysis suggests that the risks of a second dose exceed the benefits of additional hospitalizations prevented during both delta and omicron. For example, the RR of a second dose during delta may have been up to 2.61–4.54 times greater than the hospitalizations prevented using our estimates, and 1.98–2.97 times greater using the most recent myo/pericarditis estimate 10 from the CDC VAERS analysis. (Table 3) The RRs for these boys may be up to 10.5 times greater when compared to the highest US and international estimates. 5 , 6 (Table 3) For girls without a comorbidity, the RR may have been as low as 0.18 according to the CDC’s 10 most recent VAERS rate but up to 6 times greater according to the highest US and international estimates and a high first dose VEH (Appendix S5 Table S2B). 5 , 6
Among boys with a history of prior infection and no comorbidities, the risks of myo/pericarditis after even dose one appear to outweigh the benefits in both delta and omicron. (Table 2 and Figure 4) Taking into account rates reported by the FDA, 7 , 8 Israel, 1 , 3 , 36 Ontario, 4 , 37 Hong Kong 5 and Kaiser Permanente, 6 the RR of the first dose may be more than 6 times the risk of COVID‐19 hospitalization. Furthermore, if considering approximately 40% of paediatric hospital admissions for COVID may be incidental positives, 31 , 32 , 33 , 34 the risk‐benefit analysis would be even less in favour of a first dose in boys with no underlying medical conditions.
FIGURE 4.
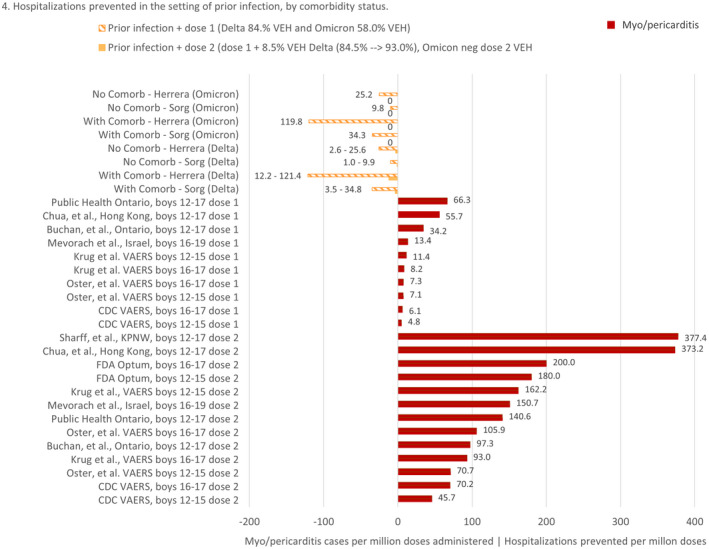
Risk‐benefit analysis comparing additional hospitalizations prevented by dose one and dose two vaccination among children with a history of prior infection vs. vaccine‐associated myo/pericarditis following BNT162b2 in boys 12–17, stratified by vaccination dose, comorbidity status and variant
3.1.2. Method 2
Figure 5 presents the cumulative risks of COVID‐19 hospitalization during a 120‐day window for boys with and without a medical comorbidity. Even at times of high hospitalization rates, such as during delta and omicron, the myo/pericarditis hospitalization rate after the second vaccination dose for boys 12–15 is 2.8 times higher than their 120‐day COVID‐19 hospitalization risk. In 16–17‐year‐old boys without comorbidities, the risk of Covid‐19 hospitalization is 1.6 times higher than their post‐dose two vaccination myo/pericarditis risk according to our estimates and international estimates of myo/pericarditis risk exceed even the highest hospitalization risk by 6.5 times. 5 , 6 Boys with at least one comorbidity and all girls appear to have a favorable benefit‐risk ratio from vaccination during times of moderate to very high disease prevalence according to our estimates, but with this method we do not stratify for history of infection or consider the benefits of one dose alone. To be conservative, our estimate does not take into account the fact that the incidental hospitalization rate may have risen with the omicron variant.
FIGURE 5.
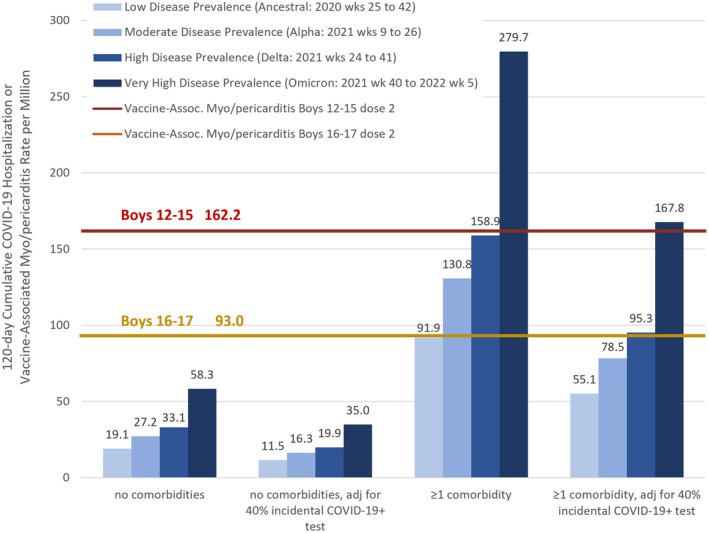
Risk‐benefit analysis for second dose of BNT162b2 vaccine‐associated myo/pericarditis vs. 120‐day COVID‐19 hospitalization risk for boys ages 12–17, without and with adjustment for 40% of pediatric hospitalizations having an incidental COVID‐19+ positive test, 31 , 32 , 33 , 34 in the setting of disease incidence, comorbidity and variant.
4. DISCUSSION
The main finding of this study was a total of 253 cases of myo/pericarditis identified over the study period giving estimated rates of 162.2/million and 93.0/million post‐Pfizer‐BioNTech BNT162b2 vaccination dose two for the 12–15‐ and 16–17‐year‐old boys, respectively. Although these estimates are higher than the CDC’s 2 , 9 , 10 they are lower than the FDA’s 7 , 8 and numerous international estimates. 1 , 3 , 4 , 5 , 6 , 36 , 37 (Figure 2).
We used a case‐finding method in VAERS which included the symptom ‘chest pain’ to identify adolescents for review of troponins, EKG/ECG and ECHO findings. We maintained the specificity of our analysis by requiring the same objective findings of cardiac injury used by the CDC to identify probable cases (Appendix S1) of myo/pericarditis and excluded cases without sufficient objective evidence or where other aetiology of the myo/pericarditis could not be excluded.
Our results are consistent with other studies 1 , 3 , 4 , 5 , 6 , 9 , 10 , 36 , 37 finding the risk of myo/pericarditis depends heavily on sex and age. In our analysis, boys 12–17 had a post‐vaccination myo/pericarditis risk tenfold higher than girls.
Our identified cases of myo/pericarditis had a hospitalization rate of 87%. The highest troponin elevations were seen in the 16–17‐year‐olds, both boys and girls. In the setting of cardiac symptoms, children with elevated troponin levels have a high likelihood of cardiac disease. 39 Of note, the threshold for normal levels in children may be even lower than the 0.1 ng/mL used in adults. 39
4.1. Risk‐benefit analyses
Our first risk‐benefit analysis considered the sex of the child as well as multiple scenarios including the setting of previous infection, with and without medical comorbidities, delta and omicron variants, and just one instead of two doses of vaccination. For adolescent boys without medical comorbidities, their risk of post‐vaccination dose two myo/pericarditis exceeded their risk of COVID‐19 hospitalization during delta after one dose of vaccination. During omicron, the additional benefit of the second dose cannot be estimated due to the reduced VEH with dose two compared to dose one. Our risk‐benefit analysis also does not favour the second dose, or even one dose, in all boys 12–17 with a history of infection. However, clinicians are cautioned to consider the specific risks associated with the child's health circumstances in their guidance. In girls with or without medical comorbidities, our risk‐benefit analysis does not favour two doses if they have a history of SARS‐CoV‐2 infection. By some estimates, even a first dose after previous infection is not favourable for girls 12–17 without comorbidities.
The protective effects of previous infection in children against hospitalization are not fully elucidated, but data from Qatar, 29 Israel, 30 the United Kingdom 35 and the US 38 suggest previous infection provides at least equivalent protection against hospitalization, although durability and duration of these are unclear. Seroprevalence in some regions of the United States exceeded 80%, 40 and the CDC has estimated at least 40% among children ages 12–17 41 prior to the omicron wave and this is expected to have risen dramatically over the winter of 2021–2022.
In our second risk‐benefit analysis we, like the CDC, used a 120‐day COVID‐19 hospitalization rate as a meaningful comparator to vaccination‐related risks. According to our VAERS estimates, the myo/pericarditis risk for a 12–15‐year‐old boy without a comorbidity receiving his second dose of the vaccine is 2.8x higher than his 120‐day risk of hospitalization even without adjusting for 40% 31 , 32 , 33 , 34 incidental hospitalizations. For older boys, the risk of myo/pericarditis is 1.6x their cumulative 120‐day hospitalizations. For those with medical comorbidities, the 120‐day COVID hospitalization rates are higher than their rates of myo/pericarditis during times of moderate to high incidence if not adjusting for a possible 40% overestimate of hospitalization rates. 31 , 32 , 33 , 34 (Figure 5) During times of very high incidence, such as the omicron wave, the 120‐day risk of COVID‐19 hospitalization for boys with a medical comorbidity is 1.7x‐3x higher than their risk of vaccine‐associated myo/pericarditis and is approximately equivalent to their post‐vaccination myo/pericarditis risk after adjustment for incidental admissions. It is important to note that incidental hospitalization rates are expected to have risen even in unvaccinated adolescents during omicron because of decreased intrinsic virulence. Figure 5 displays how a surge in disease incidence can drive hospitalizations up even if the severity of disease is lower.
4.2. Severity of vaccination‐associated myo/pericarditis
Beyond a hospitalization rate of 87%, we are not able to provide follow‐up data on the severity of included myo/pericarditis cases. One report 42 of 15 adolescents hospitalized with post‐vaccination myo/pericarditis indicated one had an abnormal echocardiogram on follow‐up and four had ongoing symptoms post‐discharge. Another study 43 found 16/23 (70%) of male patients with vaccination‐associated myo/pericarditis had resolution of symptoms within a week. Three additional case series 45 , 46 , 47 reported 14/18 cases (79%) had late gadolinium enhancement (LGE), which signifies myocardial fibrosis and is associated with ventricular arrhythmias and adverse cardiac outcomes. 48 Mevorach, et al. 3 described four patients with severely reduced left ventricular ejection fraction and one death attributed to vaccine‐related myocarditis. Witberg, et al. 36 deemed 76% of myocarditis cases to be ‘mild’, 22% as ‘intermediate’ and one patient suffered cardiogenic shock. Finally, the CDC recently reported 10 that 96% of the 813 cases of myocarditis cases reviewed through August 2021 were hospitalized; of these, 87% had resolution of symptoms upon discharge. An update on cases through 6 October 2021 by the CDC provided 3‐month follow‐up data on adolescents 12–17 with vaccine‐associated myo/pericarditis: 50% had new or worsening symptoms, 40% were still symptomatic and 50% were on activity restrictions. 44 The implications of myo/pericarditis may be greater in athletic boys who would be restricted from sports for 3–6 months following a diagnosis. Some have argued that vaccination of children without comorbidities is not ethical until more is learned about the frequency and severity of side effects. 49
COVID‐19 has been found to result in symptomatic myo/pericarditis in 0.3% of collegiate athletes, 50 , 51 but its rate in children post‐COVID‐19 infection has not been well described. Two studies 52 , 53 have relied on small denominators to estimate COVID‐19‐associated myo/pericarditis. A recent Oxford study 54 found an increased risk of myo/pericarditis among male patients <40 years in the 1–28 days after each dose of BNT162b2. The risk of vaccine‐associated myo/pericarditis after the first dose of BNT162b2 in this age group was comparable to post‐SARS‐CoV‐2 diagnosis, but the second and third vaccine doses were associated with higher rates of myo/pericarditis in this demographic than following COVID‐19. The specific rate of post‐COVID‐19 myo/pericarditis in 12–17‐year‐olds has still not been adequately estimated.
COVID‐19 has adverse effects in children beyond hospitalization and myo/pericarditis. In the United States, there have been nearly 1000 paediatric deaths 55 and approximately 6,900 56 MIS‐C cases. Although the incidence of MIS‐C declined in the delta wave, 55 it is uncertain whether this trend will continue with new variants. Our analysis compares two rare adverse outcomes: hospitalization due to COVID‐19 and myo/pericarditis following vaccination. Both conditions require further research to describe the long‐term prognosis given that only half of the myo/pericarditis cases reviewed by the CDC had recovered by 90 days 44 and, similarly, a recent systematic review and meta‐analysis found that most prolonged symptoms after SARS‐CoV‐2 infection occur with similar frequency among children who have had COVID‐19 compared with controls. 57 When looking specifically at COVID‐19 in children without medical comorbidities, Germany has reported an infection‐fatality rate of 0/3.2 million, 20 which should also be used to help inform vaccination policy when considering the myo/pericarditis described in this article. Furthermore, the vaccination's benefits in transmission prevention may be quite limited as no difference in household transmission from vaccinated vs. unvaccinated was detected for the delta variant. 58
4.3. Limitations
A concern about a passive reporting system such as VAERS is the risk of over‐ascertainment. 59 , 60 To address this concern, we aligned our inclusion criteria with the CDC’s case definition and excluded cases with other possible myo/pericarditis aetiologies and have publicly shared our included and excluded cases (https://bit.ly/Krug‐MyoPerdicarditis). We chose not to subtract the close‐to‐negligible background myo/pericarditis rate 22 as we excluded cases of myo/pericarditis with another possible aetiology but have provided the expected rates for comparison.
Our group's VAERS‐based rates may have exceeded those reported by the CDC and approached those of international estimates due to our expanded symptom search criteria (though we required objective evidence of cardiac damage consistent with myo/pericarditis and verified by a cardiologist, JM in the acknowledgements, Appendix S2B) and our inclusion of cases with unknown vaccination dose number. In our sample, approximately 15% of cases had an unknown dose number, similar to the CDC’s reports. 2 , 9 , 10 , 44 We allocated these cases using the proportion of reports with known dose number.
Our analysis only describes rates associated with the Pfizer‐BioNTech vaccine. Recent reports from the CDC, 2 , 9 , 10 , 44 Canada, 4 , 37 and Nordic 61 , 62 , 63 countries suggest a two‐ to fivefold higher rate of post‐vaccination myo/pericarditis for Moderna compared to Pfizer‐BioNTech. In several European countries, Moderna use among young male patients has been paused. 61 , 62 , 63 Our sample only includes 23 cases in girls, which is a limitation of this data set, but still, taken in the context of other international estimates and expected background rates, suggests a real but smaller‐in‐ magnitude safety signal in them. Given low numbers in other existing databases, international collaboration on presentation and prognosis among girls would be a useful contribution to this research.
Multiple arguments indicate our rates are not an overestimate. Firstly, we report rates lower than those reported by the FDA 7 , 8 and other U.S. and international estimates 1 , 3 , 4 , 5 , 6 , 36 , 37 as shown in Figures 2 and 4. Furthermore, the reports reviewed for this study were of children with myo/pericarditis presenting with cardiac symptoms, most of whom were admitted to the hospital. The authors of the large Israeli study 3 which found a rate higher than ours similarly suspected their study provided an underestimate of the true incidence. VAERS has also historically provided an underestimate of vaccine safety signals, detecting up to 76% of post‐vaccination anaphylaxis cases. 59 , 60
Another potential concern is the lack of confirmation with the reporting clinician. While we recognize this as a limitation, we also point to the fact that in multiple CDC analyses, approximately 90% of the myo/pericarditis reports in VAERS were confirmed. 2 , 9 , 10 , 44 These data, combined with the clinical notes in VAERS (symptoms plus ECG, ECHO and troponin abnormalities), suggest that myo/pericarditis is an adverse event amenable to rapid administrative review in VAERS and would likely approach a minimum rate of vaccine‐associated myo/pericarditis.
The reduced virulence of the omicron variant 21 , 27 , 28 , 64 for children (66% reduction in hospitalization risk compared to delta 21 ) combined with a degradation in VEH due to immune evasion (58% 27 dose 1 vs 84.5% 23 –91.1% 24 ) appear to diminish the vaccination benefits described in this report. Omicron‐wave relative risks (Table 2) favour one dose of vaccination according to most estimates except for children with a history of prior infection. No additional benefit for the second dose (Table 3) is apparent at this time (VEH 44% 27 vs 81.0% 25 –93% 15 ), but as paediatric hospitalization rates are published, this assessment must be re‐evaluated according to vaccination status and history of prior infection.
This analysis also has multiple uncertainties. First, the efficacy of one vs. two doses of vaccination in children is extrapolated from sparse data on children and young adults. The duration of protection against hospitalization conferred by each dose is not known for children, thus it is possible that a one‐shot strategy to minimize harms for adolescent boys without comorbidities and without a history of immunity from infection might need to consider a booster based on changing individual health considerations and future variants. Second, VEH likely varies based on comorbidity status, which was not taken into account in our analysis. Third, the estimated IHR has implicit uncertainties due to unknown false‐negative and ‐positive rates. Fourth, we restricted our risk comparisons to hospitalizations; the analysis did not account for other benefits of vaccination, such as the transient prevention of infection, nor does it include other vaccine‐associated adverse events. Fifth, we acknowledge that we cannot be 100% certain that all myo/pericarditis cases were contained in our vaccinated denominators. Sixth, our estimate of additional hospitalizations prevented by one or two doses of vaccination in the setting of previous infection was only theoretical; there is currently no evidence of additional protection of vaccination against hospitalization among children who have already been infected. Finally, the estimated risk differential between children with and without comorbidities may lead to either under‐ or overestimates; indeed, children with certain comorbidities may be at much higher risk than the average risk for a child with a comorbidity. Even the hospitalization rates from Germany 20 for children with comorbidities will provide an under‐ or overestimate of risk depending on the child's particular health condition/s. This highlights the appropriateness of individualized vaccination approaches for children against SARS‐CoV‐2. This is especially true for children with a history of SARS‐CoV‐2 infection, which appears to provide equivalent and, by most estimates, superior immunity to full vaccination against hospitalization according to data from Qatar, 29 Israel, 30 the United Kingdom 35 , 65 and the United States. 38
Little is known about risk factors for developing post‐vaccine myo/pericarditis beyond sex, age and dose number. However, those with a history of infection have been found to be at around a fourfold increased risk of post‐vaccination myo/pericarditis. 66
Given the vaccination‐related harms outlined in this article, the options of one dose, a lower dose, 67 , 68 , 69 , 70 , 71 increased interval between doses 70 , 71 or no vaccination in the setting of previous infection should be studied and considered in the context of individual health risks. Additional research is urgently needed: 1) to further elucidate the aetiology, long‐term sequelae and rates of this post‐vaccination cardiac condition; and 2) to determine the optimal vaccination strategy for children based on history of prior infection, severity of the predominant variant and individual health status to minimize overall harm.
CONFLICT OF INTEREST
We declare no conflict of interest.
AUTHOR CONTRIBUTION
TH, AK and JS designed the study and the approach, TH led the project overall and is guarantor. Contributions are as follows: design, TH, AK, JS; data curation: TH, AK, JS analysis, TH, AK, JS; information governance: JS, TH, AK; methodology: TH, AK, JS; project administration: TH, AK, JS; resources: JS, AK; software: JS, AK supervision: TH; writing (original draft): TH, AK. All authors participated in the decision to submit. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge John Mandrola, MD, for reviewing the included and excluded myo/pericarditis cases and providing valuable insight in terms of the prognosis and severity of the described cardiac injuries. We would also like to thank Cathrine Axfors, MD, PhD, for providing useful feedback on the methodology and discussion. Finally, we would like to thank Bradley Pollock, MPH, PhD, for providing useful feedback on the methodology. Finally, the authors wish to dedicate this analysis to AE and his mother EJE.
Krug A, Stevenson J, Høeg TB. BNT162b2 Vaccine‐Associated Myo/Pericarditis in Adolescents: A Stratified Risk‐Benefit Analysis. Eur J Clin Invest. 2022;52:e13759. doi: 10.1111/eci.13759
DATA AVAILABILITY STATEMENT
Data were linked, stored and analyzed within the App PowerBI platform (https://bit.ly/Krug‐MyoPerdicarditis). Data include anonymized VAERS data.
REFERENCES
- 1. Israel reports link between rare cases of heart inflammation and COVID‐19 vaccination in young men. Science. Published June 1, 2021. Available at https://www.science.org/content/article/israel‐reports‐link‐between‐rare‐cases‐heart‐inflammation‐and‐covid‐19‐vaccination Accessed November 26, 2021
- 2. Shimabukuro T. COVID‐19 Vaccine Safety Updates. Centers for Disease Control and Prevention. Advisory Committee for Immunization Practices. [ACIP Workgroup Presentation] ACIP Meeting. Atlanta, GA, United States. https://www.fda.gov/media/150054/download Accessed December 11, 2021; 2021, June 10.
- 3. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid‐19 in Israel. N Eng J Med. 2021;385(23):2140‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Weekly summary: adverse events following immunization (AEFIs) for COVID‐19 in Ontario: December 13, 2020 to October 3, 2021. Toronto, ON: Queen’s Printer for Ontario;. 2021. Available at https://www.publichealthontario.ca/‐/media/documents/ncov/epi/covid‐19‐aefi‐report.pdf?sc_lang=en accessed November 24, 2021
- 5. Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung EC‐K. Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination. Clin Infect Dis. 2021;989:2021. doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharff KA, Dancoes DM, Longueil JL, et al. Risk of Myopericarditis following COVID‐19 mRNA vaccination in a Large Integrated Health System: A Comparison of Completeness and Timeliness of Two Methods. medRxiv [Preprint] Available at https://www.medrxiv.org/content/ 10.1101/2021.12.21.21268209v1. accessed on January 29, 2022. [DOI] [PMC free article] [PubMed]
- 7. Food and Drug Administration. Summary for regulatory action. Page 24: 2021. Accessed November 26 https://www.fda.gov/media/151733/download
- 8. Food and Drug Administration . FDA Briefing Document EUA amendment request for Pfizer‐BioNTech COVID‐19 Vaccine for use in children 5 through 11 years of age. Vaccines Related Biological Products Advisory Committee, October 26, 2021. Available at https://www.fda.gov/media/153447/download Page 13 Accessed November 14, 2021
- 9. Su JR COVID‐19 vaccine safety updates: Primary series in children and adolescents ages 5‐11 and 12‐15 years, and booster doses in adolescents ages 16‐24 years 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2022‐01‐05/02‐COVID‐Su‐508.pdf Accessed February 10, 2022
- 10. Oster ME, Shay DK, Su JR, et al. Myocarditis Cases Reported After mRNA‐Based COVID‐19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022;327(4):331‐340. doi: 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United States Department of Health and Human Services , Department of Health and Human Services (DHHS) , Public Health Service (PHS) , Food and Drug Administration (FDA) / Centers for Disease Control (CDC), Vaccine Adverse Event Reporting System (VAERS). Last Friday, CDC Wonder Online Database.; 1990. Available at: vaers.hhs.gov.
- 12. Centers for Disease Control and Prevention . Vaccination Demographics Trends. https://covid.cdc.gov/covid‐data‐tracker/#vaccination‐demographics‐trends Accessed November 24, 2021
- 13. Havers FP, Whitaker M, Self JL, et al. Hospitalization of Adolescents Aged 12–17 Years with Laboratory‐Confirmed COVID‐19 ‐ COVID‐NET, 14 States, March 1, 2020–April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:851‐857. doi: 10.15585/mmwr.mm7023e1external [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. COVID‐NET: COVID‐19‐Associated Hospitalization Surveillance Network, Centers for Disease Control and Prevention. https://gis.cdc.gov/grasp/covidnet/covid19_3.html: Accessed on November 26, 2021
- 15. Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of Pfizer‐BioNTech mRNA Vaccination Against COVID‐19 Hospitalization Among Persons Aged 12–18 Years — United States, June–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1483‐1488. doi: 10.15585/mmwr.mm7042e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E‐Stats. Available at https://www.cdc.gov/nchs/data/hestat/obesity‐child‐17‐18/obesity‐child.htm Accessed on November 26, 2021.
- 17. National Survey of Children’s Health . NSCH 2018 19: Number of Current or Lifelong Health Conditions, Nationwide, Age in 3 Groups. Available at https://www.cdc.gov/healthyschools/chronicconditions.htm Accessed November 26, 2021
- 18. Miller GF, Coffield E, Leroy Z, Wallin R. Prevalence and Costs of Five Chronic Conditions in Children. J Sch Nurs. 2016;32(5):357‐364. doi: 10.1177/1059840516641190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrera‐Esposito D, De Los CG . Age‐specific rate of severe and critical SARS‐CoV‐2 infections estimated with multi‐country seroprevalence studies. medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2021.07.29.21261282v2.full.pdf Accessed November 14, 2021 [DOI] [PMC free article] [PubMed]
- 20. Sorg AL, Hufnagel M, Doenhardt M, et al. Risk of Hospitalization, severe disease, and mortality due to COVID‐19 and PIMS‐TS in children with SARS‐CoV‐2 infection in Germany. medRxiv [preprint]. 2021;. doi: 10.1101/2021.11.30.21267048. Accessed on December 8. [DOI]
- 21. Wang L, Berger NA, Kaelber DC, et al. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2022.01.12.22269179v1.full.pdf Accessed on January 24, 2022. [DOI]
- 22. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid‐19 vaccines in eight countries: multinational network cohort study. BMJ. 2021;373: doi: 10.1136/bmj.n1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Powell AA, Kirsebom F, Stowe J, McOwat K, Saliba V, Ramsay ME, Lopez‐Bernal J, Andrews N, Ladhani SN. Adolescent vaccination with BNT162b2 (Comirnaty, Pfizer‐BioNTech) vaccine and effectiveness of the first dose against COVID‐19: national test‐negative case‐control study. England. Table S6. 2021;. medRxiv [Preprint] Available at https://www.medrxiv.org/content/ 10.1101/2021.12.10.21267408v1.supplementary-material accessed on December 22. [DOI]
- 24. Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Chand M, Brown K, Ladhani SN, Ramsay M, Bernal JL. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID‐19 in the UK. medRxiv. Table S4. Available at medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2021.09.15.21263583v2 Accessed on December 22, 2021. [DOI]
- 25. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID‐19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet. 2021;398(10309):1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finnish Institute for Health and Welfare . Effectiveness of corona vaccinations in Finland ‐ THL publishes a new set of open data material for use by the media and citizens. Available at https://thl.fi/en/web/thlfi‐en/‐/effectiveness‐of‐corona‐vaccinations‐in‐finland‐thl‐publishes‐a‐new‐set‐of‐open‐data‐material‐for‐use‐by‐the‐media‐and‐citizens?redirect=%2Fen%2Fweb%2Finfectious‐diseases‐and‐vaccinations Accessed December 11, 2021
- 27. SARS‐CoV‐2 variants of concern and variants under investigation in England Technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC‐21NOV‐01 (B.1.1.529). United Kingdom Health Security Agency. Technical Briefing 34. Published on January 14, 2022. Accessed at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1046853/technical‐briefing‐34‐14‐january‐2022.pdf on January 18, 2022
- 28. Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS‐CoV‐2 variant in southern California. Available at medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2022.01.11.22269045v1 Accessed on January 12, 2022. [DOI]
- 29. Altarawneh H, Chemaitelly H, Tang P, et al. Protection afforded by prior infection against SARS‐CoV‐2 reinfection with the Omicron variant. medRxiv [Preprint] Available at https://www.medrxiv.org/content/ 10.1101/2022.01.05.22268782v1.full Accessed on January 12, 2022. [DOI]
- 30. Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben‐Tov A, Cohen D, Muhsen K, Chodick G, Patalon T. Comparing SARS‐CoV‐2 natural immunity to vaccine‐induced immunity: reinfections versus breakthrough infections. Preprint medRxiv [Preprint]. 2021;. doi: 10.1101/2021.08.24.21262415. Accessed November 24. [DOI]
- 31. Webb NE, Osburn TS. Characteristics of Hospitalized Children Positive for SARSCoV‐2: Experience of a Large Center. Hosp Pediatr. 2021;11(8):e133‐e141. doi: 10.1542/hpeds.2021-005919 [DOI] [PubMed] [Google Scholar]
- 32. Kushner LE. “For COVID” or “With COVID”: Classification of SARS‐CoV‐2. Hospitalizations in Children. Hosp Pediatr 2021;11(8):e151‐e156. doi: 10.1542/hpeds.2021-006001 [DOI] [PubMed] [Google Scholar]
- 33. Drouin O, Hepburn CM, Farrar DS, et al. Characteristics of children admitted to hospital with acute SARS‐CoV‐2 infection in Canada in 2020. Canadian Med Assoc J. 2021;193(38):E1483‐E1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Havers FP, Whitaker M, Self JL, et al. Hospitalization of Adolescents Aged 12–17 Years with Laboratory‐Confirmed COVID‐19 — COVID‐NET, 14 States, March 1, 2020–April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:851‐857. doi: 10.15585/mmwr.mm7023e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coronavirus (COVID‐19) Infection Survey, characteristics of people testing positive for COVID‐19, UK:17. Office of National Statistics; November 2021. Accessed at https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveycharacteristicsofpeopletestingpositiveforcovid19uk/17november2021 on November 24, 2021
- 36. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid‐19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385(23):2132‐2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2021.12.02.21267156v1.full.pdf Accessed on December 10, 2021. [DOI] [PMC free article] [PubMed]
- 38. León TM, Dorabawila V, Nelson L, et al. COVID‐19 Cases and Hospitalizations by COVID‐19 Vaccination Status and Previous COVID‐19 Diagnosis — California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:125‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abrams JY, Oster ME, Godfred‐Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS‐C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Covidestim – COVID‐19 nowcasting project. Accessed at https://covidestim.org/ on November 24, 2021
- 41. Havers F. Epidemiology of COVID‐19 in Children Aged 5‐11 Years. VRBAC October 26, 2021. Accessed at https://www.fda.gov/media/153508/download on November 24, 2021
- 42. Dionne A, Sperotto F, Chamberlain S, et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID‐19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021;6(12):1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montgomery J, Ryan M, Engler R, et al. Myocarditis Following Immunization With mRNA COVID‐19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6(10):1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oster M. mRNA COVID‐19 Vaccine‐Associated Myocarditis. Vaccines and Related Biologics (VRBAC) Meeting. October 26, 2021. Available at Vaccines and Related Biological Products Advisory Committee October 26, 2021 Meeting Presentation‐ Vaccine Associated Myocarditis (fda.gov) Accessed on November 27, 2021.
- 45. Marshall M, Ferguson ID, Lewis P, et al. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer‐BioNTech COVID‐19 Vaccination. Pediatrics. 2021;148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 46. Levin D, Shimon G, Fadlon‐Derai M, et al. Myocarditis following COVID‐19 vaccination ‐ A case series. Vaccine. 2021;39(42):6195‐6200. doi: 10.1016/j.vaccine.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis Temporally Associated With COVID‐19 Vaccination. Circulation. 2021;144(6):502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greulich S, Seitz A, Müller KAL, et al. Predictors of Mortality in Patients With Biopsy‐Proven Viral Myocarditis: 10‐Year Outcome Data. J Am Heart Assoc. 2020;9(16):1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gur‐Arie R, Kraaijeveld SR, Jamrozik E. An ethical analysis of vaccinating children against COVID‐19: benefits, risks, and issues of global health equity [version 1; peer review: 1 approved, 1 approved with reservations]. Wellcome Open Res. 2021;6:252. [Google Scholar]
- 50. de Schoor V. Myocardial fibrosis in Athletes. Mayo Clin Proceedings. 2016;91(11):1617‐1631. [DOI] [PubMed] [Google Scholar]
- 51. Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS‐CoV‐2 Infection. JAMA Cardiol. 2021;6(9):1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singer ME, Taub IB, Kaelber DC. Risk of Myocarditis from COVID‐19 Infection in People Under Age 20: A Population‐Based Analysis. medRxiv [Preprint] https://www.medrxiv.org/content/ 10.1101/2021.07.23.21260998v1.full [DOI]
- 53. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association Between COVID‐19 and Myocarditis Using Hospital‐Based Administrative Data — United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228‐1232. doi: 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patone M, Mei XW, Handunnethi L, Dixon S, Zaccardi F, et al. Risk of myocarditis following sequential COVID‐19 vaccinations by age and sex. medRxiv [Preprint]. 2021;. doi: 10.1101/2021.12.23.21268276. Accessed on December 26. [DOI]
- 55. National Center for Health Statistics (NCHS) Provisional COVID‐19 Deaths: Focus on Ages 0‐18 Years. Available at https://data.cdc.gov/NCHS/Provisional‐COVID‐19‐Deaths‐Focus‐on‐Ages‐0‐18‐Yea/nr4s‐juj3 Accessed on December 28, 2021
- 56. Health Department‐Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS‐C) in the United States. https://covid.cdc.gov/covid‐data‐tracker/#mis‐national‐surveillance Accessed December 28, 2021
- 57. Benhood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS‐CoV‐2 infection among children and young people: a meta‐analysis of controlled and uncontrolled studies. J Infect. 2021;2021:11. doi: 10.1016/j.jinf.2021.11.011. Accessed on December 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS‐CoV‐2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398‐4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miller ER, McNeil MM, Moro PL, Duffy J, Su JR. The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain‐Barré syndrome. Vaccine. 2020;38(47):7458‐7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Myokarditt etter koronavaksinasjon i Norge. Accessed at https://www.fhi.no/nyheter/2021/myokarditt‐etter‐koronavaksinasjon‐i‐norge/ on November 24, 2021
- 62.Iceland Joins Nordic Peers in Halting Moderna Covid Vaccinations https://www.bloomberg.com/news/articles/2021‐10‐08/iceland‐joins‐nordic‐peers‐in‐halting‐moderna‐covid‐vaccinations Accessed October 14, 2021
- 63. FDA defends Moderna's COVID‐19 vaccine after three Nordic countries pause the shots’ use in young people due to rare heart inflammation concerns. https://www.dailymail.co.uk/health/article‐10080769/FDA‐defends‐Modernas‐COVID‐19‐vaccine‐three‐Nordic‐countries‐pause‐shots‐use.html Accessed October 14, 2021.
- 64. The Genotype to Phenotype Japan (G2P‐Japan) Consortium. Attenuated fusogenicity and pathogenicity of SARS‐CoV‐2 Omicron variant. [Preprint]. Available at https://drive.google.com/file/d/1rhCazFav1pokFKmsZI5_oqIeH9ofFckR/view Accessed on December 26, 2021
- 65. Hall V, Foulkes S, Insalata F, Saei A, Kirwan P, SIREN Study Group . Effectiveness and durability of protection against future SARS‐CoV‐2 infection 2 conferred by COVID‐19 vaccination and previous infection; findings from the UK 3 SIREN prospective cohort study of healthcare workers March 2020 to September 2021. medRxiv [Preprint]. https://www.medrxiv.org/content/ 10.1101/2021.11.29.21267006v1.full.pdf Accessed on December 28, 2021. [DOI]
- 66. Barda N, Dagan N, Ben‐Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid‐19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385(12):1078‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. French health authority advises against Moderna COVID‐19 vaccine for under 30s. Reuters. November 9, 2021. Accessed at https://www.reuters.com/business/healthcare‐pharmaceuticals/french‐health‐authority‐advises‐against‐moderna‐covid‐19‐vaccine‐under‐30s‐2021‐11‐09/ on November 24, 2021
- 68. Joint Committee on Vaccination and Immunisation. United Kingdom. JCVI statement on COVID‐19 vaccination of children aged 12. to 15 years: 3 September 2021 https://www.gov.uk/government/publications/jcvi‐statement‐september‐2021‐covid‐19‐vaccination‐of‐children‐aged‐12‐to‐15‐years/jcvi‐statement‐on‐covid‐19‐vaccination‐of‐children‐aged‐12‐to‐15‐years‐3‐september‐2021 Accessed on October 14, 2021
- 69. Covid: Single jab recommended for 12 to 15‐year‐olds by UK's top doctors. BBC News. https://www.bbc.com/news/health‐58547659 Accessed on October 14, 2021.
- 70. CORONAVIRUS/Committee advises 2nd vaccine dose for 12‐17 age group, booster shots. November 12, 2021. Accessed at https://focustaiwan.tw/society/202111290015 on December 4, 2021
- 71. Vaccination of Children and Adolescents. Norwegian Institute of Public Health. Available at https://ww w.fhi.no/en/id/vaccines/coronavirus‐immunisation‐programme/coronavirus‐vaccine/#vaccination‐of‐children‐and‐adolescents Accessed on December 28, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data were linked, stored and analyzed within the App PowerBI platform (https://bit.ly/Krug‐MyoPerdicarditis). Data include anonymized VAERS data.


