Dear Editor,
Aktas et al. published an interesting case of vitiligo after COVID‐19 vaccination. 1 Recently, similar cases of vitiligo have been reported after, 2 , 3 , 4 as well as other cutaneous reactions after COVID‐19 vaccination (both mRNA and ChAdOx1 vaccines) such as immune thrombocytopenic purpura and psoriatic flare‐up. 5 , 6 Even though no cutaneous adverse events were encountered in Phase 3 studies of mRNA vaccines, the chronology of the symptoms is very suggestive. We would like to report another case of vitiligo 3 days after COVID‐19 vaccine and to comment about possible theories that have been suggested to explain autoimmune phenomena and vitiligo following COVID 19 infection or vaccination.
A 60 year‐old woman presented to the Dermatology department with amelanotic macules and patches in her face and arms that have appeared in the last 3 weeks. Three days before the onset of the symptoms, she had received the first dose of Astrazeneca vaccine against COVID‐19 (ChAdOx1/AZD1222).
On physical examination, circumscribed depigmented patches were observed, affecting both cheeks (Figure 1), forehead and between the eyebrows, as well as both arms. The lesions became more clearly visible when examined under Wood's lamp (Figure 2).
FIGURE 1.
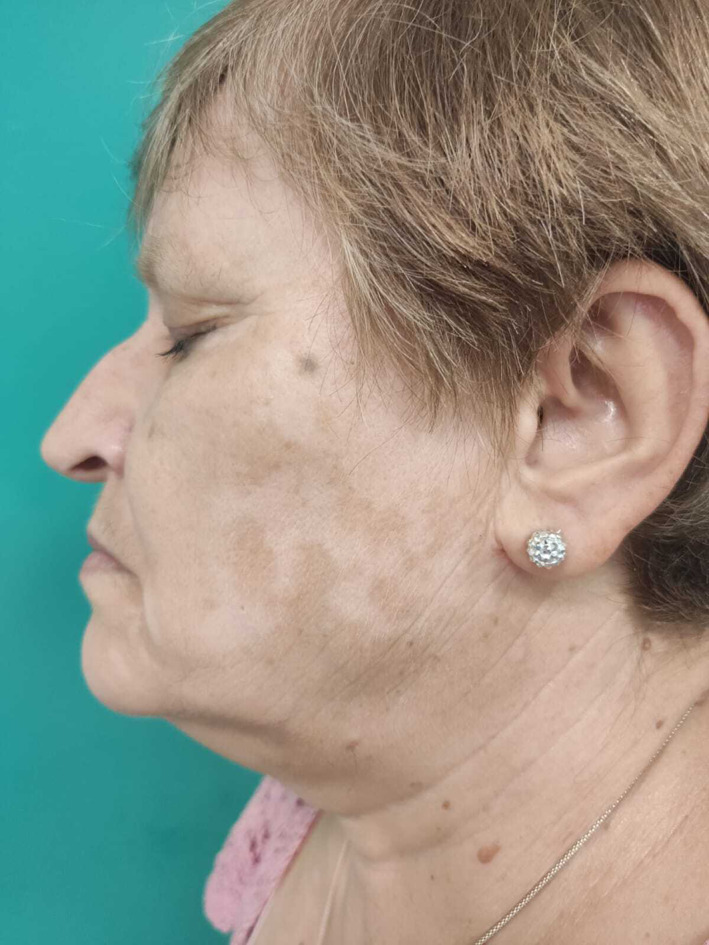
Well‐circumscribed depigmented patches in both cheeks, forehead and between eyebrows
FIGURE 2.

Depigmented patches were more noticeable when examined under Wood's lamp
Blood test including thyrotropin was normal. Antithyroid peroxidase and antithyroglobulin antibodies were negative. With the diagnosis of vitiligo, she initiated a treatment with tacrolimus ointment 0.1% twice a day.
Vitiligo is an autoimmune disease in which there is a progressive depigmentation due to the loss of melanocytes in epidermis. In cell stress conditions, oxygen free radicals cause cell damage. In this inflammatory microenvironment, specific neoantigens are generated by melanocytes, like HSP70i, HMGB1 and S100B, leading to activation of innate and adaptive immune response. Eventually, dendritic cells present antigens to T lymphocytes, leading to destruction of melanocytes. Various studies have proven the central role that HSP70i plays in the pathogenesis of vitiligo, since its overexpression is associated with greater activation of dendritic cells, and therefore an increased lymphocytic infiltration in depigmented areas. 7 , 8
A relation between COVID‐19 and autoimmune phenomena has been reported. The most accepted theory considers that molecular mimicry between antigenic epitopes of the virus and certain human proteins, such as heat shock proteins (HSP60 y 90), 9 , 10 could be the origin. In fact, these proteins have been associated with several autoimmune diseases triggered by COVID‐19 infection, like Guillain‐Barré syndrome, autoimmune bullous diseases 10 and some forms of vasculitis. Additionally, cross‐reactivity between SARS‐CoV‐2 and other tissular human proteins has been demonstrated, especially transglutaminase 2 and 3, ENA, myelin basic protein and even S100B. 11 These findings suggest that a similar mechanism could be the cause of autoimmune diseases triggered by covid‐19 vaccination. However, IgG antibodies generated against SARS‐CoV‐2 have not been shown to be able to recognize and react against heat shock proteins. 12
Another possible mechanism suggested by Abdullah et al. 13 posits that COVID‐19 infection would stimulate dendritic cells to produce massive amounts of IFN‐I, which also plays an important role in the pathogenesis of vitiligo. In the case of psoriatic flare‐up reactions, it has been suggested that the mRNA vaccines may cause a significant increase in IL‐6 production and recruitment of Th17 cells, 6 which not only participate in the pathogenesis of psoriasis, but also in many other autoimmune diseases.
In conclusion, we report another case of vitiligo after COVID‐19 vaccine in a patient with unremarkable medical history. Although mechanism remains unclear, given the suggestive time sequence following vaccination and the increasing number of cases reported, we believe these hypothesis deserve further investigation.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
INFORMED CONSENT
The patient in this manuscript has given written informed consent to publication of her case details and clinical pictures.
ACKNOWLEDGMENTS
No funding sources to declare.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Aktas H, Ertuğrul G. Vitiligo in a COVID‐19‐vaccinated patient with ulcerative colitis: coincidence? Clin Exp Dermatol. 2021;8:143‐144. doi: 10.1111/ced.14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciccarese G, Drago F, Boldrin S, Pattaro M, Parodi A. Sudden onset of vitiligo after COVID‐19 vaccine. Dermatol Ther. 2021;9:e15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaminetsky J, Rudikoff D. New‐onset vitiligo following mRNA‐1273 (Moderna) COVID‐19 vaccination. Clin Case Rep. 2021;9(9):e04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okan G, Vural P. Worsening of the vitiligo following the second dose of the BNT162B2 mRNA Covid‐19 vaccine. Dermatol Ther. 2021;21:e15280. [DOI] [PubMed] [Google Scholar]
- 5. Krajewski PK, Szepietowski JC. Immune thrombocytopenic purpura associated with COVID‐19 Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e626‐e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krajewski PK, Matusiak Ł, Szepietowski JC. Psoriasis flare‐up associated with second dose of Pfizer‐BioNTech BNT16B2b2 COVID‐19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e632‐e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosenson JA, Eby JM, Hernandez C, Caroline Le Poole I. A central role for inducible heat shock protein 70 in autoimmune vitiligo. Exp Dermatol. 2013;22(9):566‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara‐Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25(1):88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cappello F, Marino Gammazza A, Dieli F, deMacario EC, Macario AJL. Does SARS‐CoV‐2 trigger stress‐induced autoimmunity by molecular mimicry? A hypothesis. J Clin Med. 2020;9:2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kasperkiewicz M. Covid‐19, heat shock proteins, and autoimmune bullous diseases: a potential link deserving further attention. Cell Stress Chaperones. 2021;26:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mantej J, Bednarek M, Sitko K, Świętoń M, Tukaj S. Autoantibodies to heat shock protein 60, 70, and 90 are not altered in the anti‐SARS‐CoV‐2 IgG‐seropositive humans without or with mild symptoms. Cell Stress Chaperones. 2021;2:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdullah L, Awada B, Kurban M, Abbas O. Comment on 'Vitiligo in a COVID‐19‐vaccinated patient with ulcerative colitis: coincidence?': type I interferons as possible link between COVID‐19 vaccine and vitiligo. Clin Exp Dermatol. 2021;47:436‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


