A 51‐year‐old man presented with acute hypoxemic respiratory failure in the setting of SARS‐CoV‐2 infection requiring mechanical ventilation. A bronchoalveolar lavage (BAL) was performed on the 3rd postadmission day. ThinPrep of the BAL showed numerous scattered large atypical lymphoid cells with eccentric nuclei, vesicular chromatin, and prominent nucleoli as well as scattered mature plasmacytoid lymphocytes and plasma cells (Figure 1, panels A & B). The findings were worrisome for involvement by a large cell lymphoma or a plasma cell neoplasm. A morphologic review of the patient's peripheral blood smear revealed no atypical lymphocytes or plasma cells. Flow cytometric immunophenotyping was performed on the BAL fluid and revealed a large population of polytypic activated/plasmacytoid B cells that were positive for CD19, CD20, and CD38 and negative for CD138, CD56, CD5, and CD10. In addition, a smaller population of phenotypically unremarkable B cells and polytypic plasma cells was also detected (Figure 2, panels A–C). The patient was treated with remdesivir resulting in significant improvement in respiratory status. He was discharged on the 13th postadmission day. A repeat BAL performed 2 days before the discharge showed complete resolution of the atypical lymphoid and plasma cell proliferation.
FIGURE 1.
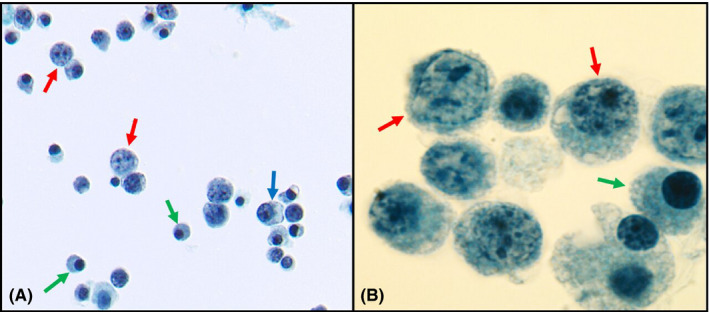
(A) BAL ThinPrep showing immunoblasts (red arrows), plasmacytoid lymphocytes (blue arrows), and mature plasma cells (green arrows) (Papanicolaou staining, magnification ×40). (B) Oil immersion image of BAL showing immunoblasts (red arrows) and plasma cells (green arrow) (Papanicolaou staining, magnification ×100)
FIGURE 2.
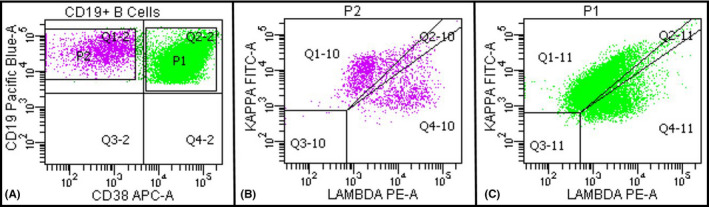
(A) Flow cytometric immunophenotyping performed on BAL fluid showing a large population of activated/plasmacytoid B cells that is CD19 and CD38 positive (green population) and a smaller population of phenotypically unremarkable B cells (lavender population). (B&C) Both populations show polytypic expression for kappa and lambda surface light chains
Profound proliferation of polytypic immunoblasts, plasmacytoid lymphocytes, and plasma cells can be seen in the peripheral blood of patients with viral infections (such as hepatitis, dengue fever, parvovirus B19, and Epstein–Barr virus), serum sickness, and autoimmune diseases. In rare instances, similar findings can be seen in the BAL fluid of patients with viral respiratory failure syndromes such as those caused by hantavirus and SARS‐CoV‐2. 1 , 2 , 3 Recognition of this association along with the use of flow cytometry is essential to avoid misdiagnosis as a B‐cell lymphoma with plasmacytic differentiation or a plasma cell neoplasm.
CONFLICT OF INTEREST
The author has no competing interests.
Tariq H. Marked polytypic proliferation of immunoblasts, plasmacytoid lymphocytes, and plasma cells in bronchoalveolar lavage fluid in SARS‐CoV‐2 infection. Int J Lab Hematol. 2022;00:1–2. doi: 10.1111/ijlh.13833
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs
REFERENCES
- 1. Giani M, Seminati D, Lucchini A, Foti G, Pagni F. Exuberant plasmocytosis in bronchoalveolar lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS‐CoV‐2 in Europe. J Thorac Oncol. 2020;15(5):e65‐e66. doi: 10.1016/j.jtho.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roig IL, Musher DM, Tweardy DJ. Severe pulmonary involvement in a case attributed to domestically acquired Seoul hantavirus in the United States. Clin Infect Dis. 2012;54(1):91‐94. doi: 10.1093/cid/cir748 [DOI] [PubMed] [Google Scholar]
- 3. Gelarden I, Nguyen J, Gao J, et al. Comprehensive evaluation of bronchoalveolar lavage from patients with severe COVID‐19 and correlation with clinical outcomes. Hum Pathol. 2021;113:92‐103. doi: 10.1016/j.humpath.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs


