Abstract
Background
The risk of thrombosis increases in infectious diseases, yet observational studies from single centers have shown a decrease in admission of acute ischemic stroke patients during the COVID‐19 pandemic. To investigate unselected stroke admission rates we performed a nationwide study in Denmark.
Methods
We extracted information from Danish national health registries. The following mutually exclusive time periods were compared to the year before the lockdown: (1) first national lockdown, (2) gradual reopening, (3) few restrictions, (4) regional lockdown, and (5) second national lockdown.
Results
Generally, admission rates were unchanged during the pandemic. In the unadjusted data, we observed a small decrease in the admission rate for all strokes under the first lockdown (incidence rate ratio: 0.93, confidence interval [CI]: 0.87–0.99) and a slight increase during the periods with gradual reopening, few restrictions, and the regional lockdown driven by ischemic strokes. We found no change in the rate of severe strokes, mild strokes, or 30‐day mortality. An exception was the higher mortality for all strokes during the first lockdown (risk ratio: crude 1.30 [CI: 1.03–1.59]; adjusted 1.17 [CI: 0.93–1.47]). The quality of care remained unchanged.
Conclusion
Stroke admission rates remained largely unchanged during the pandemic, while an increased short‐term mortality rate in patients admitted with stroke observed during the first lockdown was seen, probably reflecting that the more frail patients constituted a higher proportion of admitted patients at the beginning of the pandemic.
Keywords: COVID‐19, incidence, nationwide, stroke risk
The rate of stroke admissions during the COVID‐19 pandemic remained largely unchanged in Denmark. Higher mortality was only seen in the first period. Quality of treatment remained unchanged.

BACKGROUND
COVID‐19 has filled emergency departments around the world and it was feared that hospitals would also be burdened by diseases possibly elicited by the infection. By means of mechanisms such as systemic hypercoagulability, endothelial injury, and infection‐related atrial fibrillation [1, 2], it was observed that COVID‐19 increased the risk of stroke by 30% [3]. However, observations from single hospitals from around the world reported no increase in the admission rate for stroke in the wake of COVID‐19, but rather a decrease [4, 5].
We undertook a nationwide study in Denmark in which we compared the admission rate and 30‐day mortality for all patients with stroke (ischemic and hemorrhagic) and for transient ischemic attack (TIA) seeking medical attention. Admission rate and mortality were compared for the year before the outbreak (baseline) and then for various subsequent time periods during which COVID‐19 was endemic. We also examined the quality of care by comparing a composite of quality indicators in the various periods in relation to the baseline period.
METHODS
In Denmark there is equal, unrestricted, and tax‐funded access to acute care. All acute stroke and TIA patients are evaluated in public hospitals where it is mandatory to report to the Danish Stroke Registry (DSR). The DSR contains structured data that are collected prospectively and on a nationwide basis. It is estimated that more than 80% of all strokes are hospitalized at stroke units and registration has been found to be complete for 97% of cases [6].
We included all acute (onset <7 days prior to admission) stroke events, both ischemic and hemorrhagic, as well as TIAs. Events for which the date of hospital admission was missing were excluded together with recurrent strokes in order to ensure independency between observations (see Figure 1).
FIGURE 1.
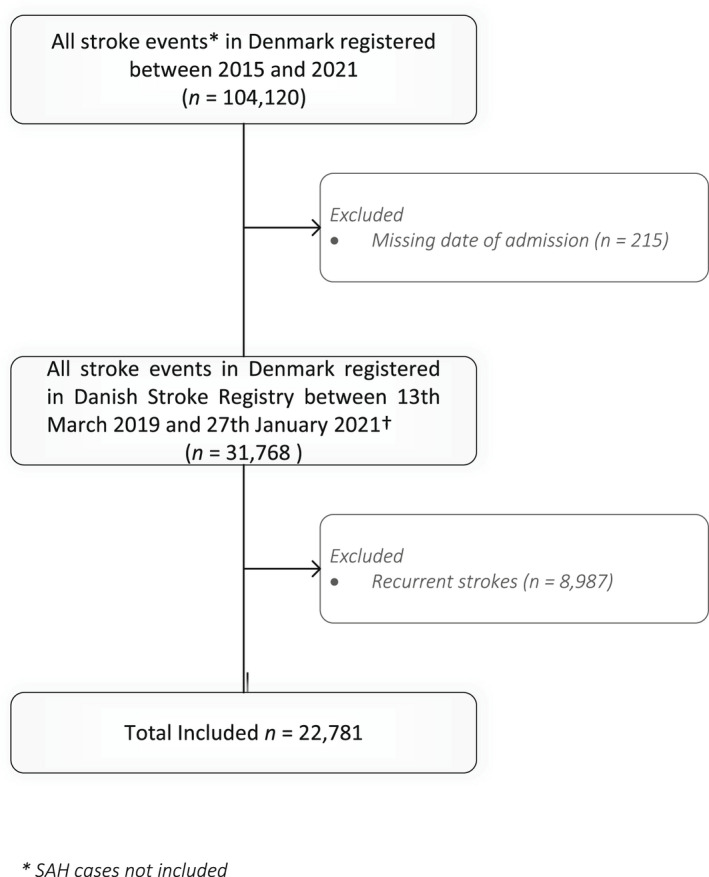
Flow chart describing patients included in the study
The time periods were divided as follows: “Baseline” was defined as 13 March 2019–10 March 2020 (the year prior to the lockdown); “First national lockdown” as 11 March–15 April 2020; “Gradual reopening” as 16 April–8 June 2020; “Few restrictions” as 9 June–30 September 2020; “Regional lockdown” as 1 October–15 December 2020; and finally “Second national lockdown” as 16 December 2020–27 January 2021. The first vaccine arrived on 27 December 2020 and did not affect admission numbers.
Incidence rate was defined as
The population size was defined as the number of people in Denmark on 1 January for the relevant year according to Statistics Denmark (for 2019: 5,806,000 and for 2020: 5,823,000). The incidence rate was measured as rate of cases per 1000 person‐years. The incidence rate ratio (IRR) was the incidence rate for a specific period in relation to the baseline incidence rate.
Stroke severity was measured by the Scandinavian Stroke Scale (SSS). A mild stroke was defined as an SSS score between 30 and 58, moderate stroke as a score between 15 and 29, and severe stroke as a score between 0 and 14.
The quality of care performance measures were as follows: reperfusion treatment, stroke unit door‐to‐needle time (≤45 min), first hospital door‐to‐groin puncture (≤3 h), admission to a stroke unit (≤24 h after hospital admission), neuroimaging obtained (<6 h from arrival), start of anti‐platelet treatment for eligible patients with ischemic stroke/TIA (within 4 h after brain scan), oral anticoagulation treatment <14 days from stroke onset for eligible patients with atrial fibrillation, assessment by physiotherapist/occupational therapist (≤ second day of admission), out of bed orders (day of admission), nutrition screening (≤ second day of admission), indirect and direct dysphagia assessment (day of admission), imaging of carotid arteries (<4 days from ischemic stroke onset), and carotid surgery/stent (<14 days from stroke onset in patients deemed eligible for surgery). The composite measure reflects the proportion of fulfilled performance measures among all eligible performance measures for the individual patient.
The 95% confidence intervals (CIs) for crude risk, incidence rates, and IRRs were calculated using standard methods. Crude risk ratios and their CIs were derived using Poisson regression. The risk ratios (RR) for mortality were adjusted using weighted Poisson regression. The adjusting variables were age, sex and SSS score. All analyses were conducted using Stata Version 16 (StataCorp LLC).
Ethical approval is not required for register‐based studies in Denmark. Data can be accessed through the Danish Health Data Authority and Statistics Denmark for researchers at authorized institutions.
RESULTS
During the study period 22,781 patients were admitted with stroke/TIA. The median age was 73.3 years and 55.1% were male. The prevalence of comorbidities (diabetes, hypertension, atrial fibrillation, prior myocardial infarction, and peripheral arterial disease) was unchanged in the study periods (Table 1.) We only observed small changes in admission rates during the time periods (Figure 2 and Table 2). Compared to baseline (the year before COVID‐19 where the incidence rate for a stroke admission was 2.09 per 1000 person‐years), the admission rate for all strokes was slightly decreased during the first national lockdown with an IRR of 0.93 (CI: 0.87–0.99) and then slightly elevated in the subsequent time periods. This was driven mainly by an increase in the admission of patients with ischemic stroke in the time period of few restrictions (IRR: 1.08 [CI: 1.01–1.14]) and gradual reopening (IRR: 1.05 [CI: 1.01–1.10]).
TABLE 1.
Distribution of age, sex and comorbidities in the study periods
| Baseline | First national lockdown | Gradual reopening | Few restrictions | Regional lockdown | Second national lockdown | |
|---|---|---|---|---|---|---|
| (N = 11,950) | (N = 1075) | (N = 1901) | (N = 3903) | (N = 2593) | (N = 1359) | |
| Age (years) | 73.3 (62.8–81) | 73.4 (64.4–81) | 73.5 (63.7–80.8) | 72.9 (62.3–80.5) | 73.7 (63.8–80.7) | 74.6 (64.4–81.8) |
| Sex (proportion males) | 54.8% (6550) | 55.0% (591) | 55.0% (1046) | 55.9% (2180) | 54.8% (1422) | 56.3% (765) |
| Diabetes | 14.2% (1661) | 15.8% (166) | 13.8% (257) | 13.4% (512) | 14.0% (357) | 15.6% (209) |
| Hypertension | 54.3% (6466) | 57.1% (612) | 53.4% (1012) | 53.5% (2074) | 55.1% (1423) | 55.3% (747) |
| Atrial fibrillation | 16.1% (1917) | 15.9% (170) | 16.7% (316) | 14.9% (578) | 16.6% (429) | 16.1% (218) |
| Prior myocardial infarction | 6.1% (723) | 5.0% (53) | 6.4% (120) | 5.6% (219) | 5.2% (133) | 6.1% (82) |
| Peripheral arterial disease | 3.7% (430) | 2.6% (28) | 3.2% (59) | 3.4% (129) | 3.7% (94) | 4.0% (54) |
FIGURE 2.
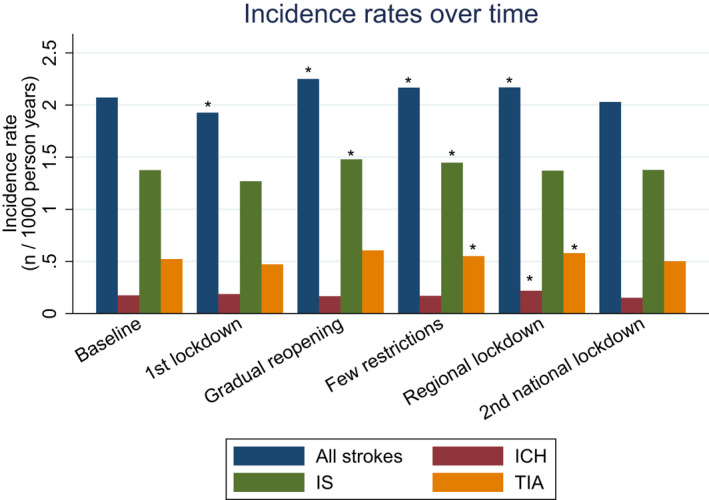
Incidence rates per 1000 person‐years for all strokes (blue bars), ischemic strokes (IS) (green bars), intracerebral hemorrhages (ICH) (red bars) and transient ischemic attacks (TIA) (yellow bars) for the different time periods. Asterisk symbols (*) indicate a significant difference compared with baseline [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Incidence rate ratios (crude)
| Type of stroke | Time period | Incidence rate ratios |
|---|---|---|
| All | First national lockdown: baseline | 0.93 (0.87–0.99) |
| Gradual reopening: baseline | 1.05 (1.01–1.08) | |
| Few restrictions: baseline | 1.09 (1.03–1.14) | |
| Regional lockdown: baseline | 1.05 (1.00–1.09) | |
| Second national lockdown: baseline | 0.98 (0.93–1.04) | |
| Hemorrhagic stroke | First national lockdown: baseline | 1.07 (0.88–1.31) |
| Gradual reopening: baseline | 0.97 (0.86–1.11) | |
| Few restrictions: baseline | 0.96 (0.80–1.14) | |
| Regional lockdown: baseline | 1.26 (1.10–1.44) | |
| Second national lockdown: baseline | 0.87 (0.71–1.07) | |
| Ischemic stroke | First national lockdown: baseline | 0.92 (0.85–1.00) |
| Gradual reopening: baseline | 1.05 (1.01–1.10) | |
| Few restrictions: baseline | 1.08 (1.01–1.14) | |
| Regional lockdown: baseline | 1.00 (0.95–1.05) | |
| Second national lockdown: baseline | 1.00 (0.94–1.07) | |
| Transient ischemic attack | First national lockdown: baseline | 0.90 (0.80–1.02) |
| Gradual reopening: baseline | 1.06 (0.98–1.13) | |
| Few restrictions: baseline | 1.16 (1.05–1.27) | |
| Regional lockdown: baseline | 1.11 (1.02–1.21) | |
| Second national lockdown: baseline | 0.96 (0.86–1.08) |
For TIA, we observed no change in admission during the lockdown but a slight increase during the reopening (few restrictions) (IRR: 1.16 [CI: 1.05–1.27]) and during regional lockdown (IRR: 1.10 [CI: 1.02–1.20]). For intracerebral hemorrhage (ICH), we saw no change in admission rates during the time periods. Risk ratio of mortality (crude numbers) was increased for all strokes during the first national lockdown (IRR: 1.28 [CI: 1.03–1.59]), but this risk estimate was attenuated after adjustment for age, sex, and stroke severity (IRR: 1.17 [CI: 0.93–1.47]). Risk ratio of mortality for all other time periods was unchanged (Table 3). Table S1 shows these data together with details on the severity of the strokes; of note, stroke severity proportions were unchanged during the time periods interrogated. We could not detect any difference in the quality‐of‐care performance measures for stroke patients during any of the time periods, for example, the rate of eligible patients offered thrombolysis <45 min after onset fluctuated between 81.1% and 86.4% and did not change significantly during the period (p = 0.49, Chi square test.)
TABLE 3.
Mortality risk ratios
| Stroke type | Time period | Thirty day mortality (Crude) | Thirty day mortality (weighted) |
|---|---|---|---|
| All | 1st national lockdown: baseline | 1.28 (1.03–1.59) | 1.17 (0.93–1.47) |
| All | Gradual reopening: baseline | 0.92 (0.75–1.12) | 1.01 (0.81–1.24) |
| All | Few restrictions: baseline | 0.90 (0.78–1.04) | 0.98 (0.84–1.15) |
| All | Regional lockdown: baseline | 1.03 (0.88–1.22) | 1.01 (0.85–1.21) |
| All | Second national lockdown: baseline | 1.15 (0.93–1.41) | 1.12 (0.91–1.38) |
| Intracerebral hemorrhage | 1st national lockdown: baseline | 1.04 (0.72–1.51) | 1.01 (0.67–1.52) |
| Intracerebral hemorrhage | Gradual reopening: baseline | 1.03 (0.74–1.43) | 1.17 (0.83–1.65) |
| Intracerebral hemorrhage | Few restrictions: baseline | 1.16 (0.92–1.45) | 1.17 (0.92–1.49) |
| Intracerebral hemorrhage | Regional lockdown: baseline | 1.12 (0.88–1.43) | 0.98 (0.75–1.29) |
| Intracerebral hemorrhage | Second national lockdown: baseline | 1.21 (0.86–1.70) | 1.23 (0.86–1.75) |
| Ischemic stroke | 1st National lockdown: baseline | 1.29 (0.99–1.68) | 1.22 (0.93–1.60) |
| Ischemic stroke | Gradual reopening: baseline | 0.93 (0.73–1.18) | 0.96 (0.74–1.23) |
| Ischemic stroke | Few restrictions: baseline | 0.80 (0.66–0.97) | 0.91 (0.75–1.10) |
| Ischemic stroke | Regional lockdown: baseline | 0.94 (0.76–1.17) | 0.98 (0.79–1.22) |
| Ischemic stroke | Second national lockdown: baseline | 1.15 (0.89–1.47) | 1.11 (0.86–1.42) |
DISCUSSION
The rate of stroke admissions based on data from the nationwide stroke register only showed minor changes during the first year of the COVID‐19 epidemic compared to the year before the outbreak. Stroke admission rates decreased by 7% during lockdown and increased by 5–7% in the period of gradual reopening and the period with few restrictions and these changes were driven by ischemic stroke. Admission rates for ICH were unchanged throughout the interrogated periods. This could indicate that the hospital admission rates for ischemic stroke and TIA were more sensitive to the direct and indirect effects of the COVID‐19 pandemic compared with ICH.
The overall quality of stroke care as judged by our composite of several performance measures of care remained unchanged during the periods examined. The 30‐day mortality rate for all strokes was higher during the period immediately after lockdown. At the beginning of the pandemic the increased mortality probably reflects the frailest patients dying. In support of this explanation we observed that the mortality rate estimate was attenuated after adjustment for age, for example. We observed a slight increase in the number of TIA patients in the period of few restrictions and regional lockdown indicating that patients with mild symptoms did not appear to avoid hospitalization.
Soon after the COVID‐19 epidemic began in December 2019, the first reports of neurological disease including stroke associated with the infection appeared [7]. That infectious diseases increase the risk of stroke has been observed in many studies. Sepsis increases the risk of ischemic stroke by a factor of 28 [8]. In an observational study, the risk of stroke was reported to be increased by a factor of 25 in patients with a laboratory‐confirmed diagnosis of Streptococcus pneumoniae infection and by a factor of 10 after an influenza infection [9].
Studies of patients afflicted with COVID‐19 also indicate an increased stroke risk. In a study in New York, the risk of stroke after COVID‐19 was seen to be eight times higher than the risk of stroke after an influenza infection [10]. In Sweden, where COVID‐19 was more prevalent than in Denmark at that time, the odds ratio for stroke increased by 3.63 in the weeks following COVID‐19 infection [11]. These numbers conflict with reports from large university hospitals. In the Ontario province in Canada, a 20% decrease in the numbers of stroke codes activations in the emergency department was seen, but there was no change in the number of treated strokes. This was believed to be due to patients being fearful of exposure to the virus [12]. The decrease of 20% was also seen in urban centers in Berlin [13] and Tokyo [14]. In a single‐center report from England, the decrease in admissions was 40% but, as in Ontario, the rate of reperfusion treatment remained the same, suggesting that the decrease was driven primarily by patients with mild strokes who “stayed at home” [15].
A comprehensive stroke center in New York reported a concerning case series of young patients with COVID‐19 and large vessel occlusion stroke [16]. This inspired researchers behind a software program that evaluates scans of patients with large vessel occlusion from 856 hospitals to analyze their data. They could not confirm the suspicion of an increased risk of large vessel occlusion since they found a significant decrease of 39% in patients being evaluated for large vessel occlusion in March 2020 [17]. A clinical database in the US, again driven mostly by academic centers, examined monthly discharges with the diagnosis of ischemic stroke and found decreasing numbers in March and April 2020. Numbers returned to pre‐pandemic levels by July 2020 [18]. In a multinational study involving 457 stroke centers from 70 countries, a decrease of 11.5% in the admission rate and a decrease of 13.2% in thrombolysis treatment were observed [19].
International data generally found decreased admission rates for patients with ischemic stroke. In comparison with our national data, the admission rate decreased mildly. The hospitals participating in the international studies were typically large teaching hospitals that likely also admitted many patients with COVID‐19; the resulting burden of care may have affected the hospitals' ability to admit stroke patients at pre‐COVID‐19 rates. The decreased stroke rate in these large centers might reflect the decompensation of a local health care system. In Denmark, decreased stroke admission rates were also observed in the regions that were most severely affected [20]. Our results reflect admission of stroke for an entire country and carry a lower risk of uncertainty regarding flow on a hospital level. A study in Switzerland [21] also found decreasing national admission rates, but not to the same extent as the single‐center studies, which suggests that admission rates are attenuated when looking at national data compared with single‐center data.
A strength of our study is that this nationwide assessment includes stroke units and comprehensive and non‐comprehensive centers with and without reperfusion treatment. The paradox of the missing patients has been explained by patients staying at home due to fear of contracting COVID‐19 in hospital. Also, recognition of stroke symptoms often depends on a bystander [22] and during this period of social distancing the lack of bystanders might also explain the lower stroke hospitalization rate in some settings. However, we did not find that the milder affected patients avoided admission since our rate of TIA patients was also unchanged and, if anything, slightly increased in the time periods of few restrictions and the regional lockdown.
This prompted us to consider alternative explanations. Other infections have been outcompeted by COVID‐19 or almost eliminated by the restrictions following the pandemic [23], for example, influenza which also has a “stroke potential” [10]. The risk of stroke caused by COVID‐19 might have been counterbalanced by a lower risk of other infections or triggers of stroke so that stroke risk ended up as a zero‐sum game in the pandemic. It has also been questioned whether the pandemic has contributed positively to a healthy lifestyle; but sadly, if anything, it has been reported that it has resulted in decreased physical activity [24], a moderate decline in mental health [25] and possibly a widening of the health‐related socioeconomic gap [26].
Further, we speculate whether some patients have a stroke after they contract the virus (i.e., that they are in‐hospital cases of stroke). The delay from onset of COVID‐19 symptoms to stroke is 9–10 days [27, 28], so it is conceivable that patients with a severe COVID‐19 infection have a stroke after being admitted to an intensive care unit. In this scenario it is conceivable that some strokes are overlooked due to competing severe illness, or because the patient dies before a stroke diagnosis can be confidently established. The latter is not an unlikely scenario, as the odds ratio for mortality in a COVID‐19 patient that has a stroke is 5.6 [3].
This might also be the main limitation of our study. Even though we have a high completeness of data at a national level, we cannot guarantee detection of stroke in patients severely affected by COVID‐19. It is possible that registration was affected in the time periods interrogated when hospitals were at their busiest. Also, our data describe admission for stroke/TIA on a cohort level with no information on COVID‐19 infection status. Following individual patients, on a national level, with a positive test and their future risk of stroke would be an important future research goal to improve the current understanding of the stroke potential of COVID‐19.
CONCLUSION
On a national level, the admission rate for stroke and TIA decreased by 7% during the first lockdown and increased by 5–7% in the subsequent time periods during the COVID‐19 epidemic. These changes suggest only a minor influence of the pandemic on stroke hospitalization.
CONFLICT OF INTEREST
This study was supported by a research grant from Lundbeck Foundation. C.Z.S. is supported by a research grant from Novo Nordisk Foundation and Health Research Foundation of Central Denmark Region.
AUTHOR CONTRIBUTIONS
Claus Ziegler Simonsen:Data curation (equal); Writing – original draft (lead). Rolf A. Blauenfeldt: Funding acquisition (equal); Writing – review & editing (equal). Jakob N. Hedegaard: Data curation (equal); Formal analysis (lead). Christina Kruuse: Writing – review & editing (equal). David Gaist: Writing – review & editing (equal). Troels Wienecke: Writing – review & editing (equal). Boris Modrau: Writing – review & editing (equal). Søren Paaske Johnsen: Conceptualization (equal); Supervision (equal); Writing – review & editing (equal). Grethe Andersen: Conceptualization (equal); Funding acquisition (equal).
Supporting information
Simonsen CZ, Blauenfeldt RA, Hedegaard JN, et al. COVID‐19 did not result in increased hospitalization for stroke and transient ischemic attack: A nationwide study. Eur J Neurol. 2022;29:2269–2274. doi: 10.1111/ene.15350
DATA AVAILABILITY STATEMENT
Data can be accessed through the Danish Health Data Authority and Statistics Denmark by researchers at authorized institutions.
REFERENCES
- 1. Bahouth MN, Venkatesan A. Acute viral illnesses and ischemic stroke: pathophysiological considerations in the era of the COVID‐19 pandemic. Stroke. 2021;52:1885‐1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aghayari Sheikh Neshin S, Shahjouei S, Koza E, et al. Stroke in SARS‐CoV‐2 infection: a pictorial overview of the pathoetiology. Front Cardiovasc Med. 2021;8:649922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katsanos AH, Palaiodimou L, Zand R, et al. The impact of SARS‐CoV‐2 on stroke epidemiology and care: a meta‐analysis. Ann Neurol. 2021;89:380‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bekelis K, Missios S, Ahmad J, et al. Ischemic stroke occurs less frequently in patients with COVID‐19: a multicenter cross‐sectional study. Stroke. 2020;51:3570‐3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butt JH, Fosbøl EL, Østergaard L, et al. Effect of COVID‐19 on first‐time acute stroke and transient ischemic attack admission rates and prognosis in Denmark: a nationwide cohort study. Circulation. 2020;142:1227‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wildenschild C, Mehnert F, Thomsen RW, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2014;6:27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MS. Risk of acute stroke after hospitalization for sepsis: a case‐crossover study. Stroke. 2017;48:574‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohland J, Warren‐Gash C, Blackburn R, et al. Acute myocardial infarctions and stroke triggered by laboratory‐confirmed respiratory infections in Denmark, 2010 to 2016. Euro Surveill. 2020;25:1900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID‐19) vs patients with influenza. JAMA Neurol. 2020;77:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsoularis I, Fonseca‐Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID‐19 in Sweden: a self‐controlled case series and matched cohort study. Lancet. 2021;398:599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bres Bullrich M, Fridman S, Mandzia JL, et al. COVID‐19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020;47:693‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erdur H, Siegerink B, Leithner C, et al. Stroke admissions, stroke severity, and treatment rates in urban and rural areas during the COVID‐19 pandemic. Front Neurol. 2020;11:607193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ota T, Shiokawa Y, Hirano T. Impact of COVID‐19 on stroke admissions and the medical care system in the Tokyo metropolitan area. Front Neurol. 2020;11:601652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Padmanabhan N, Natarajan I, Gunston R, Raseta M, Roffe C. Impact of COVID‐19 on stroke admissions, treatments, and outcomes at a comprehensive stroke centre in the United Kingdom. Neurol Sci. 2021;42:15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid‐19 on stroke evaluation in the United States. N Engl J Med. 2020;383:400‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Havenon A, Ney JP, Callaghan B, et al. Characteristics and outcomes among US patients hospitalized for ischemic stroke before vs during the COVID‐19 pandemic. JAMA Network Open. 2021;4:e2110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nogueira RG, Qureshi MM, Abdalkader M, et al. Global impact of COVID‐19 on stroke care and IV thrombolysis. Neurology. 2021;96:e2824‐e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drenck N, Grundtvig J, Christensen T, et al. Stroke admissions and revascularization treatments in Denmark during COVID‐19. Acta Neurol Scand. 2021;145(2):160‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Marchis GM, Wright PR, Michel P, et al. Association of the COVID‐19 outbreak with acute stroke care in Switzerland. Eur J Neurol. 2022;29:724‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iversen AB, Blauenfeldt RA, Johnsen SP, et al. Understanding the seriousness of a stroke is essential for appropriate help‐seeking and early arrival at a stroke centre: a cross‐sectional study of stroke patients and their bystanders. Eur Stroke J. 2020;5:351‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID‐19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25:2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salman D, Beaney T, E Robb C, et al. Impact of social restrictions during the COVID‐19 pandemic on the physical activity levels of adults aged 50–92 years: a baseline survey of the CHARIOT COVID‐19 Rapid Response prospective cohort study. BMJ Open. 2021;11:e050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. García‐Esquinas E, Ortolá R, Gine‐Vázquez I, et al. Changes in health behaviors, mental and physical health among older adults under severe lockdown restrictions during the COVID‐19 pandemic in Spain. Int J Environ Res Public Health. 2021;18:7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen J, Strandberg‐Larsen K, Gerds T, et al. Risk of major cardiovascular events according to educational level before and after the initial COVID‐19 public lockdown: a nationwide study. J Epidemiol Community Health. 2021;75:829‐835. [DOI] [PubMed] [Google Scholar]
- 27. Tan YK, Goh C, Leow AST, et al. COVID‐19 and ischemic stroke: a systematic review and meta‐summary of the literature. J Thromb Thrombolysis. 2020;50:587‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valencia‐Enciso N, Ortiz‐Pereira M, Zafra‐Sierra MP, Espinel‐Gómez L, Bayona H. Time of stroke onset in coronavirus disease 2019 patients around the globe: a systematic review and analysis. J Stroke Cerebrovasc Dis. 2020;29:105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be accessed through the Danish Health Data Authority and Statistics Denmark by researchers at authorized institutions.


