Summary
The association between body mass index (BMI) and poor COVID‐19 outcomes in patients has been demonstrated across numerous studies. However, obesity‐related comorbidities have also been shown to be associated with poor outcomes. The purpose of this study was to determine whether BMI or obesity‐associated comorbidities contribute to elevated COVID‐19 severity in non‐elderly, hospitalized patients with elevated BMI (≥25 kg/m2). This was a single‐center, retrospective cohort study of 526 hospitalized, non‐elderly adult (aged 18–64) COVID‐19 patients with BMI ≥25 kg/m2 in suburban New York from March 6 to May 11, 2020. The Edmonton Obesity Staging System (EOSS) was used to quantify the severity of obesity‐related comorbidities. EOSS was compared with BMI in multivariable regression analyses to predict COVID‐19 outcomes. We found that higher EOSS scores were associated with poor outcomes after demographic adjustment, unlike BMI. Specifically, patients with increased EOSS scores had increased odds of acute kidney injury (adjusted odds ratio [aOR] = 6.40; 95% CI 3.71–11.05), intensive care unit admission (aOR = 10.71; 95% CI 3.23–35.51), mechanical ventilation (aOR = 3.10; 95% CI 2.01–4.78) and mortality (aOR = 5.05; 95% CI 1.83–13.90). Obesity‐related comorbidity burden as determined by EOSS was a better predictor of poor COVID‐19 outcomes relative to BMI, suggesting that comorbidity burden may be driving risk in those hospitalized with elevated BMI.
Keywords: BMI, comorbidities, COVID‐19, EOSS, obesity
What is already known about the subject?
Younger patients (<65 years old) with elevated BMI (≥25 kg/m2) are at increased risk of poor COVID‐19 outcomes when hospitalized
Comorbidities associated with obesity and BMI alone have both been shown to be independent predictors of severe COVID‐19 infection
It is unclear, however, in younger patients with elevated BMI (≥25 kg/m2), which contributes more to increased risk of severe COVID‐19 outcomes—BMI or obesity‐related comorbidities.
What this study adds?
In a cohort of hospitalized, non‐elderly (aged 18–64 years old), first‐surge COVID‐19 patients with a BMI in the overweight to obese range (≥25 kg/m2), increased obesity‐related comorbidity burden quantified by higher Edmonton Obesity Staging System (EOSS) scoring was associated with worse inpatient clinical outcomes, unlike BMI alone.
This direct comparison between obesity‐related comorbidity burden and BMI in patients with elevated BMI (≥25 kg/m2) known to be at risk of poor COVID‐19 outcomes suggests that this elevated risk is driven by obesity‐related comorbidity burden, not BMI.
This study is meaningful clinically as newly admitted patients with elevated BMI without obesity‐related comorbidities appear to have less severe outcomes than those with a higher obesity‐related comorbidity burden.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus causing the respiratory illness coronavirus disease 2019 (COVID‐19), has resulted in the deaths of close to a million individuals in the United States alone. Patients with obesity, defined as body mass index (BMI) ≥30 kg/m2, have been identified as being at higher risk for severe COVID‐19. 1 , 2 , 3 , 4 , 5 , 6 Specifically, studies have shown that BMI is an independent predictor of COVID‐19 severity 1 , 2 , 4 , 5 , 6 particularly in younger, non‐elderly patients. 2 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 However, obesity‐related comorbidities such as type 2 diabetes and hypertension have also been shown to be independent predictors of COVID‐19 severity when included in studies involving BMI. 6 , 9 , 12 , 15 , 16 , 17 , 18
In an effort to quantify the extent of weight‐related health impairment in patients with obesity, the Edmonton Obesity Staging System (EOSS) was developed in 2009 19 and has since been validated in multiple cohorts. 20 , 21 , 22 , 23 , 24 , 25 , 26 The EOSS is a five‐stage measure of obesity based on medical, psychological and/or functional complications of obesity. 19 It effectively serves as a surrogate measure of obesity‐related comorbidities and burden of disease. A comprehensive assessment of obesity‐related comorbidity burden such as the EOSS in COVID‐19 patients with elevated BMI would help clarify the interaction between BMI and obesity‐related comorbidity burden in driving poor COVID‐19 outcomes. The present study assessed the association between a modified version of EOSS and COVID‐19 outcomes compared with BMI alone in a cohort of hospitalized SARS‐CoV‐2 infected patients with elevated BMI (≥25 kg/m2) during the first surge of the pandemic in the spring of 2020. Better understanding of the interaction between BMI and obesity‐related comorbidity burden will be helpful to clinicians providing anticipatory guidance to patients with COVID‐19 who are known to be at increased risk of poor outcomes due to elevated BMI.
2. METHODS
Stony Brook University Hospital is a 600‐bed, tertiary care academic medical center on Long Island, NY. Patients hospitalized with laboratory‐confirmed SARS‐CoV‐2 infection from March 6, 2020, to May 11, 2020 were identified from the Stony Brook COVID‐19 Research Consortium Data Commons, 27 an integrated repository of clinical data from University Hospital. A confirmed case was defined as a positive result by reverse transcription quantitative polymerase chain reaction (RT‐qPCR) testing of nasopharyngeal samples using the Lyra SARS‐CoV 2 RT‐qPCR assay from Quidel. BMI was extracted from the Data Commons as a calculated value based on available heights and weights according to standard calculation (weight in kilograms divided by meters squared). All patient data analysed were de‐identified; thus, the study protocol was deemed not human subjects research by the Stony Brook University Institutional Review Board (IRB2020‐00447).
Baseline demographics included age, sex, ethnicity and race. Smoking status was also assessed. Patients were excluded if any of the following were met: (i) aged <18 years or ≥65 years, (ii) missing BMI or (iii) BMI <25 kg/m2 (Figure 1). Table S1 compares demographics of included and excluded patients. Physician‐made diagnoses were identified either through manual chart abstraction or ICD‐10 codes mapped to Clinical Classification Software (CCS) groups occurring 30 days before admission beginning January 1, 2017. Obesity‐related comorbidities were also inferred based on documented medications administered in the hospital or prescribed at discharge according to World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) classification system and outlined in Table S2. Specifically, if a patient had any one medication of a particular class either administered in the hospital or prescribed at discharge, that patient was classified as having been administered that medication class. Any medications routinely administered in the hospital were not included in the classification. All obesity‐related comorbidities identified in prior EOSS publications 20 , 21 , 22 , 23 , 24 that were available in the COVID‐19 Data Commons were used to classify patients according to EOSS stage using a modified staging system with three levels (stage 0, stage 1 and stage 2). Modified operational definitions of the three EOSS stages were based on previous studies. 20 , 21 , 22 , 23 , 24 A general overview of the classification scheme is outlined in Table 1. Patients were classified as EOSS stage 0/1, 2 or 3/4 based on fulfilling any one of 24, 46 or 30 criteria, respectively. A detailed overview of these criteria is outlined in Table S3. If a patient fulfilled any one of the criteria in an EOSS stage, the patient was classified in that EOSS stage. Furthermore, a total EOSS score was calculated for each patient via summation of all 84 categorical variables used to form criteria for stages outlined in Table S3. Patients were also classified according to WHO categories of BMI (overweight—BMI 25.00–29.99; class I & II—BMI 30.00–39.99; class III—BMI ≥40).
FIGURE 1.
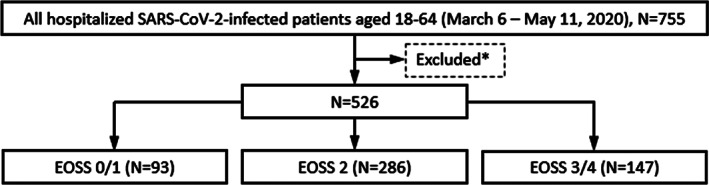
Flow chart of patients hospitalized with SARS‐CoV‐2 infection by EOSS stage. *Excluded patients include those with missing BMI (N = 127), BMI <25 (N = 102)
TABLE 1.
General overview of classification of patients with BMI ≥25 into EOSS stages based on obesity‐related comorbidities and disease burden
| EOSS 0/1 | EOSS 2 | EOSS 3/4 |
|---|---|---|
|
Absence of obesity‐related comorbidities with or without end‐organ damage or mild disease burden
|
Presence of obesity‐related comorbidities without end‐organ damage or moderate disease burden
|
Presence of obesity‐related comorbidities with end‐organ damage or severe disease burden
|
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; EOSS, Edmonton Obesity Staging System; ESRD, end stage renal disease; GERD, gastroesophageal reflux disease; HbA1C, (haemoglobin A1C); LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; HF, heart failure; HLD, hyperlipidaemia; HTN, hypertension; OA, osteoarthritis; RA, rheumatoid arthritis; MI, myocardial infarction; TIA, transient ischemic attack.
The primary outcome of this study was in‐hospital mortality. Secondary outcomes included acute kidney injury (AKI) defined as an increase in serum creatinine of 0.3 mg/dl within 48 h, intensive care unit (ICU) admission, length of hospitalization and necessity and duration of mechanical ventilation.
2.1. Statistical analysis
All results were expressed as median (interquartile range) for continuous variables and as frequency (percentage) for categorical variables. Continuous variables were compared between outcome groups with Kruskal–Wallis test and Dunn–Bonferroni post hoc pairwise tests. Mann–Whitney U test was used when only two groups were analysed. Categorical variables were compared with the chi‐squared or Fisher's exact test, where appropriate. For each outcome variable, six separate binary logistic regression models, each adjusted for age, sex, ethnicity, race and smoking status, were run with the following predictor variables included as follows: (1) BMI categories; (2) EOSS stage; (3) BMI categories+EOSS stage; (4) BMI value; (5) EOSS total score; and (6) BMI value+EOSS total score. Outcome variables included the following: mortality, AKI, ICU admission and requirement for mechanical ventilation. A p value of <.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics for Windows, version 27 (IBM Corp.).
3. RESULTS
3.1. Demographics
Table 2 includes demographic characteristics by EOSS stage. There were a total of 526 patients included (median age 51, IQR = 18–64; percentage male = 62%). Ninety‐three patients were classified as EOSS stage 0/1, 286 patients were classified as EOSS stage 2 and 147 patients were classified as EOSS stage 3/4. There was a significant difference in median age between the different EOSS stages (p < .001). EOSS stage 0/1 (median 40, IQR = 18–64) patients were significantly younger than both EOSS stage 2 (median 52, IQR = 21–64) and 3/4 (median 54, IQR = 18–64) patients (p < .001). There was no significant difference in median BMI between the different EOSS stages (p > .05). Furthermore, the percentage of patients classified according to WHO obesity class did not differ by EOSS stage (p > .05). The percentage of male patients differed by EOSS stage (p < .05). EOSS stage 0/1 (49%) was less likely to have male patients compared with both EOSS stage 2 (66%) and stage 3/4 (61%). The percentage of Hispanic or Latino patients differed by EOSS stage (p < .001). EOSS stage 0/1 (46%) and 2 (40%) had a higher percentage of Hispanic or Latino patients compared with EOSS stage 0/1 (19%). There was a significant relationship between race and EOSS stage (p < .001). EOSS stage 3/4 (15%) had a higher percentage of Black or African‐American patients compared with EOSS stage 2 (6%) or 0/1 (4%). EOSS stage 3/4 (48%) had a higher percentage of white patients compared with EOSS stage 2 (38%) or 0/1 (37%). The percentage of former or current smokers among patients differed by EOSS stage (p < .001). EOSS stage 0/1 (16%) and 2 (25%) were less likely to have current or former smokers compared with EOSS stage 3/4 (42%).
TABLE 2.
Demographic characteristics of hospitalized SARS‐CoV‐2‐infected patients with a BMI ≥25 by EOSS stage
| EOSS 0/1, N = 93 | EOSS 2, N = 286 | EOSS 3/4, N = 147 | p value | |
|---|---|---|---|---|
| Characteristics | ||||
| Median age, years (IQR) | 40.00 (18.00)a | 52.00 (15.00)b | 54.00 (13.00)b | <.001 |
| Median EOSS total score, (IQR) | 0 (0)a | 3.00 (3.00)b | 9.00 (9.00)c | <.001 |
| Median BMI, kg/m2 (IQR) | 30.83 (9.13) | 30.92 (8.06) | 30.48 (8.83) | .886 |
| WHO categories (BMI range) | .237 | |||
| Overweight (25.00–29.99) | 41 | 114 | 67 | |
| 44% | 40% | 46% | ||
| Class I and II (30.00–39.99) | 37 | 143 | 61 | |
| 40% | 50% | 41% | ||
| Class III (≥40) | 15 | 29 | 19 | |
| 16% | 10% | 13% | ||
| Sex | .0134 | |||
| Male | 46 | 190 | 90 | |
| 49% | 66% | 61% | ||
| Female | 47 | 96 | 57 | |
| 51% | 34% | 39% | ||
| Ethnicity | <.001 | |||
| Hispanic or Latino | 43 | 113 | 28 | |
| 46% | 40% | 19% | ||
| Not Hispanic or Latino | 50 | 173 | 119 | |
| 54% | 60% | 81% | ||
| Race | <.001 | |||
| Black or African‐American | 4 | 18 | 22 | |
| 4% | 6% | 15% | ||
| White | 34 | 109 | 71 | |
| 37% | 38% | 48% | ||
| Other | 55 | 159 | 54 | |
| 59% | 56% | 37% | ||
| Smoking status | <.001 | |||
| Former or Current | 15 | 69 | 60 | |
| 16% | 25% | 42% | ||
| Non‐smoker | 78 | 210 | 84 | |
| 84% | 75% | 58% | ||
Note: Groups with different superscripted letters are significantly different from each other by Dunn–Bonferonni's pairwise test.
Abbreviations: BMI, body mass index; EOSS, Edmonton Obesity Staging System; IQR, interquartile range; WHO, World Health Organization.
3.2. Clinical outcomes
Table 3 includes clinical outcomes by EOSS stage. The percentage of patients who died differed by EOSS stage (p < .01). There were fewer EOSS stage 0/1 (0%) patients who died compared with both EOSS stage 2 (3%) and stage 3/4 (7%) patients. There was a significant difference in median length of stay between the different EOSS stages (p < .001); EOSS stage 2 patients had a significantly longer length of stay compared with EOSS stage 0/1 patients (p = .00489). The percentage of patients with AKI differed by EOSS stage (p < .001); there were fewer EOSS stage 0/1 (0%) patients with AKI compared with both EOSS stage 2 (9%) and stage 3/4 (33%). The percentage of patients requiring ICU admission differed by EOSS stage (p < .001). There were fewer EOSS stage 0/1 (3%) patients requiring ICU admission compared with both EOSS stage 2 (27%) and stage 3/4 (34%) patients. The percentage of patients requiring mechanical ventilation differed by EOSS stage (p < .001). There were fewer EOSS stage 0/1 (0%) patients requiring mechanical ventilation compared with both EOSS stage 2 (19%) and stage 3/4 (26%) patients. There was no significant difference in median length of mechanical ventilation between EOSS stage 2 and 3/4 (p > .05).
TABLE 3.
Clinical outcomes of hospitalized SARS‐CoV‐2‐infected patients with a BMI ≥25 by EOSS stage
| Characteristic | EOSS 0/1, N = 93 | EOSS 1/2, N = 286 | EOSS 3/4, N = 147 | p value |
|---|---|---|---|---|
| Clinical outcomes | ||||
| Length of hospitalization, days | 6.00 (6.00)a | 8.00 (8.00)b | 7.00 (10.00)a,b | <.001 |
| AKI | 0 | 27 | 49 | <.001 |
| 0% | 9% | 33% | ||
| ICU admission | 3 | 77 | 50 | <.001 |
| 3% | 27% | 34% | ||
| Mechanical ventilation | 0 | 53 | 38 | <.001 |
| 0% | 19% | 26% | ||
| Length of mechanical ventilation, days | 10.00 (11.00) | 13.50 (10.00) | .149 | |
| Mortality | 0 | 8 | 11 | <.001 |
| 0% | 3% | 7% |
Note: Groups with different superscripted letters are significantly different from each other by Dunn–Bonferonni's pairwise test. Continuous dependent variables reported as median (IQR).
Abbreviations: AKI, acute kidney injury; BMI, body mass index; EOSS, Edmonton Obesity Staging System; ICU, intensive care unit; IQR, interquartile range.
3.3. Multivariable analyses
In Model 1, BMI category was not associated with an increase in the odds of poor COVID‐19 clinical outcomes (Table 4). However, in Model 2, higher EOSS stage was associated with poor COVID‐19 clinical outcomes (Table 4). When both BMI and EOSS stage were included in Model 3, higher EOSS stage remained associated with poor COVID‐19 clinical outcomes and BMI remained not significantly associated (Table 4). Specifically, in Model 3, for one stage higher in EOSS, there was an increase in the odds of AKI (aOR = 6.40; 95% CI, 3.71–11.05, p < .001), ICU admission (aOR = 10.71; 95% CI, 3.23–35.51 EOSS stage 1 versus 0, p < .001; aOR = 17.27; 95% CI, 5.02–59.38 EOSS stage 2 versus 0, p < .001), mechanical ventilation (aOR = 3.10; 95% CI, 2.01–4.78, p < .001) and mortality (aOR = 5.05; 95% CI, 1.83–13.90, p = .00174).
TABLE 4.
Multivariable analyses of hospitalized SARS‐CoV‐2‐infected patients with a BMI ≥25 with BMI and EOSS as categorical predictor variables
| AKI | ICU Admission | Mechanical Ventilation | Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p value | aOR | 95% CI | p value | aOR | 95% CI | p value | aOR | 95% CI | p value | |
| Model 4 | ||||||||||||
| BMI ≥40 | 0.66 | 0.27–1.63 | .368 | 0.64 | 0.30–1.37 | .252 | 0.79 | 0.34–1.84 | .583 | 2.91 | 0.60–14.16 | .185 |
| BMI 30.00–39.99 | 0.63 | 0.36–1.09 | .0959 | 0.99 | 0.64–1.53 | .953 | 0.92 | 0.56–1.53 | .752 | 2.15 | 0.64–7.19 | .213 |
| BMI 25.00–29.99 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| Model 5 | ||||||||||||
| EOSS 3/4 | 6.61 a | 3.83–11.40 a | <.001 a | 17.54 | 5.10–60.31 | <.001 | 3.10 a | 2.01–4.76 a | <.001 a | 4.40 a | 1.65–11.74 a | .00312 a |
| EOSS 2 | 6.61 a | 3.83–11.40 a | <.001 a | 10.86 | 3.28–36.01 | <.001 | 3.10 a | 2.01–4.76 a | <.001 a | 4.40 a | 1.65–11.74 a | .00312 a |
| EOSS 0/1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| Model 6 | ||||||||||||
| BMI ≥40 | 0.770 | 0.29–2.03 | .600 | 0.69 | 0.32–1.52 | .357 | 0.890 | 0.37–2.13 | .795 | 3.92 | 0.77–19.91 | .100 |
| BMI 30.00–39.99 | 0.73 | 0.41–1.32 | .297 | 1.00 | 0.64–1.57 | .994 | 1.020 | 0.610–1.72 | .933 | 2.70 | 0.79–9.26 | .115 |
| BMI 25.00–29.99 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| EOSS 3/4 | 6.40 a | 3.71–11.05 a | <.001 a | 17.270 | 5.02–59.38 | <.001 | 3.10 a | 2.01–4.78 a | <.001 a | 5.05 a | 1.83–13.90 a | .00174 a |
| EOSS 2 | 6.40 a | 3.71–11.05 a | <.001 a | 10.710 | 3.23–35.51 | <.001 | 3.10 a | 2.01–4.78 a | <.001 a | 5.05 a | 1.83–13.90 a | .00174 a |
| EOSS 0/1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
Note: Reference groups—Overweight (BMI 25.00–29.99) for WHO Categories of BMI and EOSS stage 0/1 for EOSS stage. Bolded aORs are statistically significant.
Abbreviations: AKI, acute kidney injury; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; EOSS, Edmonton Obesity Staging System; ICU, Intensive Care Unit.
EOSS stage is considered as a continuous predictor variable due to zero counts, with the reference group being the stage below each stage examined (i.e. reference group for EOSS stage 3/4 is EOSS stage 2 and reference group for EOSS stage 2 is EOSS stage 0/1).
In Model 4, BMI was not associated with poor COVID‐19 clinical outcomes (Table 5). However, in Model 5, the EOSS total score was associated with poor COVID‐19 clinical outcomes, but not mortality (Table 5). Lastly, when both BMI and the EOSS total score were included in Model 6, the EOSS total score remained associated with poor COVID‐19 clinical outcomes, but not BMI (Table 5). Specifically, for every unit increase in EOSS total score, there was an increase in the odds of AKI (aOR = 1.16; 95% CI, 1.11–1.22), ICU admission (aOR = 1.07; 95% CI, 1.03–1.11), mechanical ventilation (aOR = 1.08; 95% CI, 1.03–1.12), but not mortality (Table 5).
TABLE 5.
Multivariable analyses of hospitalized SARS‐CoV‐2‐infected patients with a BMI ≥25 with BMI and EOSS as continuous predictor variables
| Model 4 | Model 5 | Model 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | EOSS total score | BMI | EOSS total score | |||||||||
| aOR | 95% CI | p value | aOR | 95% CI | p value | aOR | 95% CI | p value | aOR | 95% CI | p value | |
| AKI | 0.98 | 0.94–1.03 | .449 | 1.16 | 1.11–1.22 | <.001 | 0.98 | 0.93–1.02 | .287 | 1.16 | 1.11–1.22 | <.001 |
| ICU Admission | 1.00 | 0.97–1.04 | .851 | 1.07 | 1.03–1.11 | .001 | 1.00 | 0.97–1.03 | .949 | 1.07 | 1.03–1.11 | .001 |
| Mechanical Ventilation | 1.00 | 0.97–1.04 | .729 | 1.08 | 1.03–1.12 | <.001 | 1.00 | 0.97–1.04 | .828 | 1.08 | 1.03–1.12 | <.001 |
| Mortality | 1.03 | 0.97–1.11 | .325 | 1.08 | 1.00–1.16 | .061 | 1.03 | 0.97–1.10 | .358 | 1.08 | 1.00–1.16 | .066 |
Note: Both BMI and the EOSS total score were treated as continuous variables in the three models. Bolded aORs are statistically significant.
Abbreviations: AKI, acute kidney injury; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; EOSS, Edmonton Obesity Staging System; ICU, intensive care unit.
4. DISCUSSION
This study demonstrates that in a cohort of hospitalized, non‐elderly, first‐surge COVID‐19 patients with a BMI in the overweight or obese range, obesity‐related comorbidity burden as measured by EOSS stage predicted poor clinical outcomes, unlike BMI alone. Specifically, our analysis shows that an increased EOSS stage was associated with longer hospitalization as well as higher rates of AKI, ICU admission, mechanical ventilation and death. Furthermore, the degree of obesity‐related comorbidity burden appears to be associated with the likelihood of poor clinical outcomes as EOSS total score was associated with poor inpatient outcome variables with the exception of mortality. Increased rates of AKI in this population may mediate the association between EOSS stage and poor clinical outcomes as AKI itself has been shown to be a strong predictor of in‐hospital mortality and other poor outcomes in COVID‐19. 27 , 28 Notably, no hospitalized patients with elevated BMI and no or minimal obesity‐related comorbidities (i.e. EOSS stage 0) died, required mechanical ventilation or developed AKI. This suggests that among patients with elevated BMI, who are known to be at elevated risk of poor COVID‐19 outcomes, those without comorbidities appear to be protected from deleterious clinical outcomes from COVID‐19 infection while hospitalized.
Our study corroborates recently published data from Mexico 29 utilizing EOSS to comprehensively assess obesity‐related comorbidity burden in COVID‐19 patients with elevated BMI. These analyses demonstrated that obesity‐related comorbidity burden, as assessed by EOSS, was associated with poor COVID‐19 outcomes, unlike BMI. Numerous studies have demonstrated an association between BMI and poor COVID‐19 outcomes 1 , 2 , 3 , 4 , 5 , 6 , 7 ; however, the degree to which obesity‐related comorbidities have been controlled for in these studies has been variable. Some have not controlled for any comorbidities 30 while most have only corrected for a select few, mainly comorbid diabetes and hypertension. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 9 , 12 , 31 , 32 , 33 , 34 , 35 Consistent with the results of the present study, the metabolic syndrome (the quartet of type 2 diabetes, hypertension, waist circumference and hyperlipidaemia), has been shown to be a better prognostic factor for COVID‐19 severity than its individual components including obesity. 16 However, this has been contrasted by other studies demonstrating that waist circumference alone is a better predictor relative to metabolic syndrome. 36 Despite this, other studies have also shown that the accrual of multimorbidity, 15 worse metabolic health, 37 and high comorbidity index 38 are associated with more severe COVID‐19 illness. However, these studies included all‐comers with BMI in the normal range and were not specific to patients with an elevated BMI.
Interestingly, both median BMI and the proportion of patients in each WHO obesity class did not differ by EOSS stage. This disassociation may reflect the inadequacy of BMI to predict metabolic health. 19 , 39 It may also reflect our real‐world cohort consisting of those presenting with an elevated BMI from suburban New York, which likely differs from previous study cohorts. 40 Furthermore, similar to other recently published cohorts of hospitalized COVID‐19 patients, 32 , 41 , 42 , 43 , 44 , 45 , 46 , 47 the present study did not demonstrate an association between BMI and poor COVID‐19 outcomes. This difference between earlier studies and more recent work may reflect better defined cohorts that enable a more nuanced analysis of the association between BMI and COVID‐19‐related outcomes such that the appropriate adjustments can be made for the effects of obesity‐related comorbidities. Future studies directly comparing BMI and obesity‐related comorbidities should be done in cohorts where BMI has been found to be a significant predictor of poor COVID‐19 outcomes. Moreover, alternative measures of obesity such as waist circumference, which has been shown to correlate with health outcomes, should be explored. It is likely we have captured “adiposity‐based chronic disease” 48 , 49 through EOSS staging, and it would be valuable to assess other measures of adiposity to better characterize the interactions between adiposity, comorbidity burden and COVID‐19 risk.
A strength of this study is our examination of a cohort during the first wave of COVID‐19 in early 2020 when outcomes were not impacted by now‐proven inpatient treatments including dexamethasone, remdesivir, tocilizumab and baricitinib. Moreover, inpatient clinical management in future cohorts would be based on lessons learned from the present study's cohort and others. This improvement 50 may have altered the relationship between obesity and COVID‐19 outcomes. Another strength of this study is the use of medications in addition to diagnostic codes to ensure a comprehensive and accurate staging of patients according to obesity‐related comorbidity burden via the EOSS. Lastly, similar results were obtained with both EOSS stage and total score in our multivariable analyses, which strengthens the internal validity of the present study.
There are several limitations of this study, many of which are trade‐offs for examining this cohort. Firstly, as with all retrospective observational designs, ascertainment of causality was limited in this study. Beyond this, almost 17% of hospitalized patients did not have a recorded BMI and were excluded. This may have resulted in selection bias, but it appears as though patients who were excluded did not differ demographically from those included (Table S1), which suggests that this bias was not limiting. We suspect that the urgency of hospitalization during the first wave of the pandemic contributed to a decrease in recorded BMI in the medical record.
Although classification of patients by EOSS staging resulted in a significant difference in age and sex between the EOSS stages, both of which are known risk factors for poor COVID‐19 outcomes, 6 , 27 , 38 multivariable analyses were adjusted for age and sex, which indicates that the significant associations found are independent of age and sex effects. Moreover, patients may have been misclassified as we tailored our classification scheme to the available data. For instance, EOSS stage 0/1 patients may have been misclassified secondary to a lack of health information available and could have had undiagnosed impairments in metabolic health. This was somewhat mitigated by our inclusion of medications, but nonetheless may have skewed the results. Another limitation of the present study was that not all aspects of the EOSS staging system 20 , 21 , 22 , 23 , 24 were able to be included (most notably—functional impairment secondary to obesity) as we were limited by the available data. Similarly, assessment of the correlation between laboratory data such as inflammatory markers and EOSS stage was not able to be done due to limited data availability. Similarly, we were unable to assess for socio‐economic status, which has been shown to be a risk factor for COVID‐19 severity and may be a mediator of the associations found in this study. 50 Lastly, our analysis does not pinpoint which particular obesity‐related comorbidities contributed most to the association between EOSS stage and poor COVID‐19 outcomes; future investigation with better characterized cohorts is needed in this regard.
In summary, we have demonstrated that among hospitalized, non‐elderly, first‐surge COVID‐19 patients with BMI in the overweight or obese range, obesity‐related comorbidities as measured by EOSS stage are associated with poor COVID‐19 outcomes. This association was significant after adjustment for BMI and suggests that impairment of health in patients with obesity is a better predictor of inpatient COVID‐19 outcomes than BMI alone. Future studies are needed to better understand this relationship so that accurate anticipatory guidance regarding risk can be given to patients with obesity who become infected and hospitalized.
CONFLICTS OF INTEREST
JDM reports receiving a research grant from Dexcom, Inc.; JDM reports receiving consulting fees as well as payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Medtronic Diabetes, Inc.; JDM reports serving on the scientific advisory board of MannKind, Inc. The other authors declared no conflicts of interest.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
MWT, VLG, HA and JDM conceived the analysis. MWT, VLG and WH completed analyses. All authors were involved in writing the paper and had final approval of the submitted and published versions. The authors thank everyone involved in the Stony Brook COVID‐19 Research Consortium's Data Commons for creating and curating the source of data for this study.
Tsoulis MW, Garcia VL, Hou W, Arcan C, Miller JD. Comparing body mass index and obesity‐related comorbidities as predictors in hospitalized COVID‐19 patients. Clinical Obesity. 2022;12(3):e12514. doi: 10.1111/cob.12514
REFERENCES
- 1. Anderson MR, Geleris J, Anderson DR, et al. Body mass index and risk for intubation or death in SARS‐CoV‐2 infection: a retrospective cohort study. Ann Intern Med. 2020;173(10):782‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breland JY, Wong MS, Steers WN, Yuan AH, Haderlein TP, Washington DL. Body mass index and risk for severe COVID‐19 among veterans health administration patients. Obesity. 2021;29(5):825‐828. [DOI] [PubMed] [Google Scholar]
- 3. Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID‐19: a systematic review and meta‐analysis. Metabolism: Clinical and Experimental. 2020;113:154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim TS, Roslin M, Wang JJ, Kane J, Hirsch JS, Kim EJ. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID‐19 in New York. Obesity. 2021;29(2):279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhasin A, Nam H, Yeh C, Lee J, Liebovitz D, Achenbach C. Is BMI higher in younger patients with COVID‐19? Association between BMI and COVID‐19 hospitalization by age. Obesity. 2020;28(10):1811‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonifazi M, Mei F, Skrami E, et al. Predictors of worse prognosis in young and middle‐aged adults hospitalized with COVID‐19 pneumonia: a multi‐center Italian study (COVID‐UNDER50). J Clin Med. 2021;10(6):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denova‐Gutiérrez E, Lopez‐Gatell H, Alomia‐Zegarra JL, et al. The Association of Obesity, type 2 diabetes, and hypertension with severe coronavirus disease 2019 on admission among Mexican patients. Obesity. 2020;28(10):1826‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eastment MC, Berry K, Locke E, et al. BMI and outcomes of SARS‐CoV‐2 among US veterans. Obesity. 2021;29(5):900‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity. 2020;28:1595‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman KE, Magder LS, Baghdadi JD, et al. Impact of sex and metabolic comorbidities on COVID‐19 mortality risk across age groups: 66,646 inpatients across 613 U.S. hospitals. Clin Infect Dis. 2020;73:e4113‐e4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svensson P, Hofmann R, Häbel H, Jernberg T, Nordberg P. Association between cardiometabolic disease and severe COVID‐19: a nationwide case‐control study of patients requiring invasive mechanical ventilation. BMJ Open. 2021;11(2):e044486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao M, Piernas C, Astbury NM, et al. Associations between body‐mass index and COVID‐19 severity in 6·9 million people in England: a prospective, community‐based, cohort study. Lancet Diab Endocrinol. 2021;9(6):350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drozd M, Pujades‐Rodriguez M, Lillie PJ, et al. Non‐communicable disease, sociodemographic factors, and risk of death from infection: a UKbiobank observational cohort study. The Lancet Infect Dis. 2021;21:1184‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lohia P, Kapur S, Benjaram S, Pandey A, Mir T, Seyoum B. Metabolic syndrome and clinical outcomes in patients infected with COVID‐19: does age, sex and race of the patient with metabolic syndrome matter? J Diabetes. 2021;13:420‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tchang BG, Askin G, Sahagun A, et al. The independent risk of obesity and diabetes and their interaction in COVID‐19: a retrospective cohort study. Obesity. 2021;29:971‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Treskova‐Schwarzbach M, Haas L, Reda S, et al. Pre‐existing health conditions and severe COVID‐19 outcomes: an umbrella review approach and meta‐analysis of global evidence. BMC Med. 2021;19(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes. 2009;33(3):289‐295. [DOI] [PubMed] [Google Scholar]
- 20. Atlantis E, Fahey P, Williams K, et al. Comparing the predictive ability of the Edmonton obesity staging system with the body mass index for use of health services and pharmacotherapies in Australian adults: a nationally representative cross‐sectional study. Clin Obes. 2020;10(4):e12368. [DOI] [PubMed] [Google Scholar]
- 21. Canning KL, Brown RE, Wharton S, Sharma AM, Kuk JL. Edmonton obesity staging system prevalence and association with weight loss in a publicly funded referral‐based obesity clinic. J Obes. 2015;2015:619734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiappetta S, Stier C, Weiner RA. The Edmonton obesity staging system predicts perioperative complications and procedure choice in obesity and metabolic surgery‐a German Nationwide register‐based cohort study (StuDoQ|MBE). Obes Surg. 2019;29(12):3791‐3799. [DOI] [PubMed] [Google Scholar]
- 23. Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population‐representative cohort of people with overweight and obesity. CMAJ: Canadian Med Assoc J. 2011;183(14):E1059‐E1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skulsky SL, Dang JT, Battiston A, et al. Higher Edmonton obesity staging system scores are associated with complications following laparoscopic roux‐en‐Y gastric bypass. Surg Endosc. 2019;34:3102‐3109. [DOI] [PubMed] [Google Scholar]
- 25. Bettini S, Quinto G, Neunhaeuserer D, et al. Edmonton obesity staging system: an improvement by cardiopulmonary exercise testing. Int J Obes. 2021;45:1949‐1957. [DOI] [PubMed] [Google Scholar]
- 26. Skulsky SL, Dang JT, Switzer NJ, Sharma AM, Karmali S, Birch DW. Higher Edmonton obesity staging system scores are independently associated with postoperative complications and mortality following bariatric surgery: an analysis of the MBSAQIP. Surg Endosc. 2020;34:3102‐3109. [DOI] [PubMed] [Google Scholar]
- 27. Stony Brook COVID‐19 Research Consortium . Geospatial distribution and predictors of mortality in hospitalized patients with COVID‐19: a cohort study. Open Forum Infect Dis. 2020;7(10):ofaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nadim MK, Forni LG, Mehta RL, et al. COVID‐19‐associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16(12):747‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez‐Flores M, Goicochea‐Turcott EW, Mancillas‐Adame L, et al. The utility of the Edmonton obesity staging system for the prediction of COVID‐19 outcomes: a multi‐Centre study. Int J Obes. 2022. doi: 10.1038/s41366-021-01017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID‐19‐related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death ‐ United States, march‐December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al‐Salameh A, Lanoix JP, Bennis Y, et al. The association between body mass index class and coronavirus disease 2019 outcomes. Int J Obes (Lond). 2021;45(3):700‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biscarini S, Colaneri M, Ludovisi S, et al. The obesity paradox: analysis from the SMAtteo COvid‐19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. 2020;30(11):1920‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goyal P, Ringel JB, Rajan M, et al. Obesity and COVID‐19 in New York City: a retrospective cohort study. Ann Intern Med. 2020;173(10):855‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gutierrez JP, Bertozzi SM. Non‐communicable diseases and inequalities increase risk of death among COVID‐19 patients in Mexico. PloS One. 2020;15(10):e0240394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pettit NN, MacKenzie EL, Ridgway JP, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID‐19. Obesity. 2020;28(10):1806‐1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Zelst CM, Janssen ML, Pouw N, Birnie E, Castro Cabezas M, Braunstahl GJ. Analyses of abdominal adiposity and metabolic syndrome as risk factors for respiratory distress in COVID‐19. BMJ Open Respir Res. 2020;7(e000792):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morys F, Dagher A. Poor metabolic health increases COVID‐19‐related mortality in the UKbiobank sample. Front Endocrinol. 2021;12:652765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ebinger JE, Achamallah N, Ji H, et al. Pre‐existing traits associated with Covid‐19 illness severity. PloS One. 2020;15(7):e0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640‐1649. [DOI] [PubMed] [Google Scholar]
- 40. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kooistra EJ, de Nooijer AH, Claassen WJ, et al. A higher BMI is not associated with a different immune response and disease course in critically ill COVID‐19 patients. Int J Obes. 2021;45(3):687‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nyabera A, Lakhdar S, Li M, Trandafirescu T, Ouedraogo TS. The association between BMI and inpatient mortality outcomes in older adults with COVID‐19. Cureus. 2020;12(10):e11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Randhawa G, Syed KA, Singh K, et al. The relationship between obesity, hemoglobin A1c and the severity of COVID‐19 at an urban tertiary care center in New York City: a retrospective cohort study. BMJ Open. 2021;11(1):e044526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolf M, Alladina J, Navarrete‐Welton A, et al. Obesity and critical illness in COVID‐19: respiratory pathophysiology. Obesity. 2021;29:870‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta‐analysis of obesity and COVID‐19 outcomes. Sci Rep. 2021;11(1):7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dana R, Bannay A, Bourst P, et al. Obesity and mortality in critically ill COVID‐19 patients with respiratory failure. Int J Obes. 2021;45(9):2028‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plataki M, Pan D, Goyal P, et al. Association of body mass index with morbidity in patients hospitalised with COVID‐19. BMJ Open Respir Res. 2021;8(1):e000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO Position Statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mechanick JI, Hurley DL, Garvey WT. Adiposity‐based chronic disease as a new diagnostic TERM: the AMERICAN association of clinical endocrinologists and AMERICAN college of ENDOCRINOLOGY POSITION STATEMENT. Endocr Pract. 2017;23(3):372‐378. [DOI] [PubMed] [Google Scholar]
- 50. Yeates EO, Nahmias J, Chinn J, et al. Improved outcomes over time for adult COVID‐19 patients with acute respiratory distress syndrome or acute respiratory failure. PloS One. 2021;16(6):e0253767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


