Abstract
Objectives
To clarify whether enuresis treatment was more effective during the stay‐home period for the coronavirus disease 2019 pandemic, when restrictions on activities enabled patients to concentrate on treatment.
Methods
We performed a retrospective, nonrandomized cohort study for monosymptomatic enuresis during the coronavirus disease 2019 pandemic (March–June 2020) and a 2‐year comparator period (March–June 2018 and March–June 2019). Primary outcome was treatment response, defined as a change in the number of wet nights per week within 6 months following enrollment. The time‐dependent occurrence of treatment response was evaluated with the Kaplan–Meier method and the log‐rank test. The Cox proportional hazards regression model was used to identify risk factors for treatment response. The range of appropriate sample sizes for this primary outcome was 39–48.
Results
Of our 41 enrolled patients, 28 (68%) were male and mean age was 8.8 years. The complete response rate was 73% during the coronavirus disease 2019 pandemic period and 27% during the comparator period. Log‐rank tests showed a higher cumulative incidence of complete response in the pandemic period (P = 0.020). Cox regression analysis identified treatment during the coronavirus disease 2019 pandemic (hazard ratio 2.533; 95% confidence interval 1.069–6.006) and dinner before 19:00 (hazard ratio 4.184; 95% confidence interval 1.56–11.252) as significantly associated with treatment response.
Conclusions
The rate of enuresis treatment response was uncommonly high during the stay‐home period for the coronavirus disease 2019 pandemic. Restrictions on daily life may provide opportunities to concentrate on treatments for chronic illnesses, leading to more success.
Keywords: activity restriction, chronic disease management, enuresis treatment, nocturnal enuresis, stay‐home period
Abbreviations & Acronyms
- ADHD
attention deficit hyperactivity disorder
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- CR
complete response
- HR
hazard ratio
- ICCS
International Children’s Continence Society
- PR
partial response
Introduction
Nocturnal enuresis affects at least 10% of all 6‐year‐olds. 1 , 2 , 3 , 4 , 5 , 6 , 7 This condition is reported to resolve in 15% of untreated patients 8 and 70% of treated patients 9 , 10 each year, but all treatments require patients and their families to adhere to the treatment regime with diligence. 1 , 11 Due to the disruptiveness of the recommended treatments, the drop‐out rate can be as high as 30%. 12 In practice, therefore, prior to the COVID‐19 pandemic, nocturnal enuresis outpatients had a treatment response rate of only 35–50% after 1 year. 13 , 14
In response to the COVID‐19 pandemic, the first state of emergency was declared on April 7, and although it was lifted on May 25, many Japanese children and parents started spending more time at home between March and May 2020. 15 This lifestyle change may have permitted families to focus more attention on treatments for nocturnal enuresis.
To date, very few papers have addressed chronic disease management during the COVID‐19 pandemic, 16 and no papers have reported on nocturnal enuresis management during the pandemic. To clarify whether enuresis treatments were more effective during the stay‐home period for the COVID‐19 pandemic than before, we gathered data from medical records for a retrospective cohort study.
Methods
Study design
By reviewing medical records, we performed a retrospective, nonrandomized cohort study on 41 patients seen at two facilities in Tokyo for monosymptomatic enuresis either during the stay‐home period of the COVID‐19 pandemic (March–June 2020) or during a comparator period consisting of the same season in each of the previous 2 years (March–June 2018 and March–June 2019). The two facilities were Keio University Hospital and the Saiwai Pediatric Clinic, the only clinic in Tachikawa where patients with neurodevelopmental disorders are referred to from the Child and Family Support Center as a child development support facility. At Keio University Hospital, the author treated patients with nocturnal enuresis, and at the Saiwai Pediatric Clinic, the author and two other pediatricians treated them during both periods.
The definition of enuresis was bedwetting at least once a month for more than 3 months in children 5 years of age or older. 17 The exclusion criteria were daytime incontinence and other lower urinary tract symptoms. 17 If the same patient had been seen more than once in the selected time periods, only the last visit was considered.
At both institutions, treatment for nocturnal enuresis was administered in accordance with contemporary guidelines. 1 , 11 , 18 , 19 At the first visit, we checked daytime lower urinary tract symptoms and, after ruling out the possibility of organ disease, we provided general lifestyle advice such as eating dinner earlier so as to have at least 2–3 h from dinner to bedtime, limiting water consumption in the evening, ensuring full urination before sleep, monitoring stool form, eliminating constipation, and rewarding children for dry nights. We also advised that using diapers at night is not a problem because convenience is important. If these steps had not resolved the issue after 2–4 weeks, we assessed each patient’s characteristics and lifestyle and determined whether to introduce desmopressin or alarm therapy. Patients take an oral tablet of 120 µg desmopressin every night, 30 min before bedtime. The alarm, which has a moisture sensor in the form of a diaper pad, has to be used every night.
Variables
The following data were collected: patient characteristics 20 (sex, age, after‐school activities, two‐income family, neurodevelopmental disorders, history of constipation, history of urinary tract infection, family history of enuresis, using diapers at night, timing of dinner, and bedtime), frequency of bedwetting, course of enuresis treatment (time since the first visit and use of desmopressin therapy, alarm therapy, or other therapy), regular use of other medications, examination time, frequency of checkups during the research periods, and history of relapse after prior enuresis treatment. Diagnoses and treatment histories related to autism spectrum disorder, ADHD, and learning disabilities were collected from electronic records.
Outcome measurements
Response to treatment was assessed by comparing the number of wet nights per week between enrollment and 6 months later. According to the ICCS guidelines, initial success categories were defined as follows: no response: <50% reduction in wet nights per week, PR: 50–99% reduction, CR: 100% reduction; long‐term success categories were defined as follows: relapse: more than one wet night per month, continued success: no relapse for 6 months after stopping treatment. 17
Statistical analysis and planned sample size
The primary outcome was initial success of enuresis treatment. We used the chi‐squared test, Fisher’s exact test, and Mann–Whitney U‐test to compare the proportions of patients with each background characteristic and risk ratios among the groups. We assessed the cumulative proportion of enuresis treatment response according to the Kaplan–Meier method and made comparisons using the log‐rank test. The Cox proportional hazards regression model was used to identify risk factors associated with enuresis treatment response by calculating the HRs with 95% CIs. The significance level was set at P < 0.05. All statistical analyses were performed using SPSS Statistics 27 (IBM, Armonk, NY, USA).
The planned sample size of 39–48 was calculated to achieve 80% power with a 0.05 significance level and 0–20% attrition 21 to detect the difference in initial success between the stay‐home and comparator periods assuming a 70% rate of response to the first choice treatment (nocturnal enuresis outpatients have a 50% response rate after 1 year, which presumably translates to 25% within a 6‐month treatment period).
Ethical approval
This study and the possibility of opting out were announced to the public on the hospital website. The study was approved by the Keio University School of Medicine Ethical Committee (No. 2020‐0349).
Results
Patient characteristics
Table 1 shows the characteristics of the 41 patients according to treatment period at enrollment; no patients met the exclusion criteria, and there were no dropouts during this study period. There were no significant differences in the characteristics of the two groups at the time of enrollment. The total number of nocturnal enuresis patients was 25 in 2018, 26 in 2019, and 15 in 2020; there were 15 patients during the pandemic period and 26 patients during the comparator period because the same patient was enrolled only in the last period. Among the 15 patients treated during the pandemic, the mean age was 9 years (range 5–12; 11 boys, four girls). Frequency of wet nights was 4.1/week (1–7/week), and 64.2% had a family history of enuresis. A total of 26.7% had a neurodevelopmental disorder (mostly mild autism spectrum disorder). One patient used methylphenidate hydrochloride for ADHD. Six patients used desmopressin, four used alarm therapy, and one used both. Ten patients (67%) started dinner before 19:00, the mean dinner–bedtime interval was 2.8 h, the proportion of patients participating in afterschool activities was 78.6%, but all were interrupted, the proportion of two‐income families was 66.7%, mean duration of prior treatment was 14 months (0–30 months), and two patients (13.3%) were on their first visit. Among the 26 patients treated during the comparator period, the mean age was 9 years (range 6–12; 17 boys, nine girls). Frequency of wet nights was 3.9/week (1–7/week), and 42.1% of patients had a family history of enuresis. A total of 39.8% had a neurodevelopmental disorder (mostly mild autism spectrum disorder). Two patients used methylphenidate hydrochloride for ADHD. Sixteen patients used desmopressin, one used alarm therapy, one used both, and one used oxybutynin hydrochloride. No patients started dinner before 19:00. Mean dinner–bedtime interval was 2.1 h, the proportion of patients participating in afterschool activities was 84.6%, the proportion of two‐income families was 66.7%, mean duration of prior treatment was 12.4 months (0–50 months), and nine patients (34.6%) were on their first visit.
Table 1.
Patient characteristics at enrollment
|
Comparator period: 2018 and 2019 n = 26 |
Pandemic period: 2020 n = 15 |
P‐value | |
|---|---|---|---|
| Age, mean ± SD, years | 8.92 ± 1.86 | 8.67 ± 1.66 | 0.622 |
| Male sex (%) | 17 (65.4) | 11 (73.3) | 0.734 |
| Wet nights/week, mean ± SD | 3.92 ± 2.35 | 4.07 ± 2.77 | 0.879 |
| Duration of treatment, mean ± SD, months | 12.4 ± 13.2 | 14.0 ± 10.0 | 0.389 |
| First visit patients (%) | 9 (34.6) | 2 (13.3) | 0.132 |
| Family history of enuresis (%) | 8/19 (42.1) | 9/14 (64.2) | 0.208 |
| Neurodevelopmental disorders (%) | 8 (39.8) | 4 (26.7) | 0.536 |
| Constipation (%) | 2 (7.7) | 0 (0) | 0.396 |
| Desmopressin therapy (%) | 9 (34.6) | 6 (40.0) | 0.730 |
| High‐dose desmopressin therapy | 0 | 1 | |
| Anticholinergic drug therapy | 1 | 0 | |
| Alarm therapy (%) | 1 (3.8) | 1 (6.7) | 0.604 |
| Desmopressin and alarm therapy (%) | 1 (3.8) | 1 (6.7) | 0.604 |
| Dinnertime, mean (range) | 19:28 (19:00–21:00) | 19:32 (18:00–21:00) | 0.903 |
| Dinner before 19:00 (%) | 0 (0) | 1 (6.7) | 0.437 |
| Bedtime, mean (range) | 21:38 (20:00–24:00) | 21:19 (20:00–22:00) | 0.366 |
| Interval between dinner and bedtime, h (range) | 2.17 (1–5) | 1.8 (1–3.5) | 0.351 |
| More than 3 h from dinner to bedtime (%) | 5/18 (28) | 2/14 (14) | 0.318 |
| Participation in afterschool activities (%) | 11 (84.6) | 11 (78.6) | 0.538 |
Table 2 shows the characteristics of the 41 patients according to treatment period during March and June. The treatment period groups differed significantly in dinner start time, likelihood of starting dinner before 19:00, dinner–bedtime interval, nighttime diaper use, and, naturally, likelihood of visiting during a state of emergency and/or participation in afterschool activities (P < 0.05). During the pandemic period, six patients used desmopressin, four used alarm therapy, and one used both. Ten patients (67%) started dinner before 19:00, the mean dinner–bedtime interval was 2.8 h, all afterschool activities were interrupted, and rate of nighttime diaper use was 60%. Mean examination time was 10.3 min and frequency of checkups was 2.5 times. During the comparator period, 16 patients used desmopressin, one used alarm therapy, one used both, and one used oxybutynin hydrochloride. No patients started dinner before 19:00, the mean dinner–bedtime interval was 2.1 h, and rate of nighttime diaper use was 91.3%. Mean examination time was 14.5 min and frequency of checkups was 2.3 times.
Table 2.
Patient characteristics during March and June
|
Comparator period: March–June 2018 and 2019 n = 26 |
Pandemic period: March–June 2020 n = 15 |
P‐value | |
|---|---|---|---|
| Desmopressin therapy (%) | 16 (61.5) | 6 (40.0) | 0.183 |
| High‐dose desmopressin therapy | 0 | 1 | |
| Anticholinergic drug therapy | 1 | 0 | |
| Alarm therapy (%) | 1 (3.8) | 4 (26.7) | 0.051 |
| Desmopressin and alarm therapy (%) | 1 (3.8) | 1 (6.7) | 0.604 |
| Examination time, mean ± SD, min | 14.5 ± 10.4 | 10.3 ± 6.5 | 0.242 |
| Frequency of checkups during the periods | 2.3 ± 0.7 | 2.5 ± 0.8 | 0.498 |
| Dinnertime, mean (range) | 19:21 (19:00–21:00) | 18:30 (18:00–20:00) | 0.001 |
| Dinner before 19:00 (%) | 0 (0) | 10 (67) | <0.001 |
| Bedtime, mean (range) | 21:30 (20:00–23:00) | 21:24 (20:00–23:00) | 0.331 |
| Interval between dinner and bedtime, h (range) | 2.08 (1–4) | 2.8 (2–4.5) | 0.030 |
| More than 3 h from dinner to bedtime (%) | 6/18 (33) | 9/14 (64) | 0.082 |
| Participation in afterschool activities (%) | 11 (84.6) | 0 (0) | <0.001 |
| Use of diapers at night (%) | 21/23 (91.3) | 9/15 (60.0) | 0.029 |
| Treatment during state of emergency (%) | 0 (0) | 14 (93.3) | <0.001 |
Treatment response
Among patients treated during the pandemic, within 6 months, 11 achieved CR (73%) and three achieved PR; thus, 14 out of 15 patients (93%) achieved a response of any kind, as Table 3 shows. Of four patients with neurodevelopmental disorders, three achieved a response of any kind. Eight of the 11 CR patients had continued success; two were lost to follow‐up. The remaining patient stopped alarm therapy and relapsed to three wet nights per month; general lifestyle advice reduced this to one wet night per month within 3 months.
Table 3.
Response to enuresis treatment after 6 months
|
Comparator period: March–June 2018 and 2019 n = 26 |
Pandemic period: March–June 2020 n = 15 |
Relative risk | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Total response (%) | 15 (58) | 14 (93) | 0.618 | 0.433–0.882 | 0.016 |
| PR (%) | 8 (31%) | 3 (20%) | |||
| CR (%) | 7 (27%) | 11 (73%) | 0.367 | 0.182–0.742 | 0.004 |
| Continued success (%) | 5/5 (100%)† | 8/9 (89%)‡ | |||
| No response (%) | 11 (42%) | 1 (7%) |
PR: 50–99% reduction. CR: 100% reduction. Continued success: no relapse in 6 months after cessation of treatment.
Two were lost to follow‐up.
Two were lost to follow‐up.
One patient who achieved PR during the pandemic had previously been treated with desmopressin, but only on the weekends because of extracurricular activities on weekday evenings. At that time he had six wet nights per week. During the stay‐home period, in contrast, the patient was able to take desmopressin every night, and his wet nights per week fell to three within 3 months and then to one within 6 months.
During the comparator period, seven patients achieved CR (27%) and eight achieved PR; thus, 15 out of 26 (58%) achieved a response of any kind. Of eight patients with neurodevelopmental disorders, seven achieved a response of any kind. Five out of seven CR patients had continued success; the other two were lost to follow‐up.
The response rate was significantly higher during the pandemic (CR 73%, PR 93%) than during the comparator period (CR 27%, PR 58%), even in a sensitivity analysis excluding first‐visit patients (pandemic: CR 77%, PR 92% vs comparator: CR 29%, PR 59%). Figure 1 shows the Kaplan–Meier curve for cumulative CR rates and total response rates according to treatment period. A log‐rank test confirmed a significantly higher response rate during the pandemic (P = 0.020, 0.003).
Fig. 1.
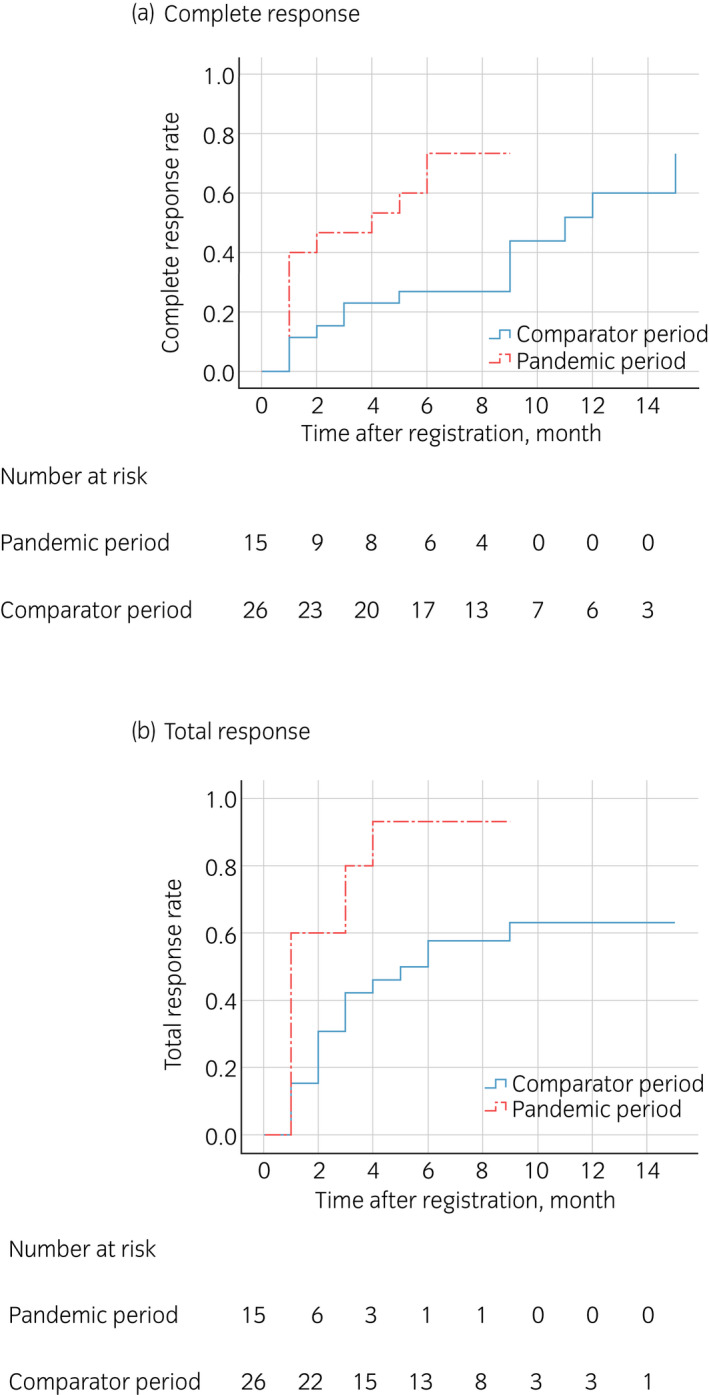
Kaplan–Meier curve for cumulative response to enuresis treatment during the COVID‐19 pandemic and the comparator period. (a) Log‐rank test revealed that the CR rate was significantly higher during the COVID‐19 pandemic period (P = 0.020). (b) Log‐rank test revealed that the total response rate was significantly higher during the COVID‐19 pandemic period (P = 0.003).
Risk factors associated with treatment effects
As Table 4 shows, treatment during the pandemic (HR 2.533; 95% CI 1.069–6.006), treatment during a state of emergency (HR 3.036; 95% CI 1.274–7.233) and dinner before 19:00 (HR 4.184; 95% CI 1.56–11.252) were significantly associated with both CR and total response. Frequency of wet nights at first visit was significantly associated only with CR, while nighttime diaper use and participation in afterschool activities were significantly associated only with total response.
Table 4.
Cox regression analysis of enuresis treatment response
| CR | Total response | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Treatment during COVID‐19 pandemic | 2.533 (1.069–6.006) | 0.035 | 2.597 (1.229–5.491) | 0.012 |
| Treatment during state of emergency | 3.036 (1.274–7.233) | 0.012 | 2.359 (1.116–4.985) | 0.025 |
| Dinner before 19:00 | 4.184 (1.556–11.252) | 0.005 | 3.045 (1.254–7.393) | 0.014 |
| Participation in afterschool activities | 0.456 (0.158–1.317) | 0.147 | 0.368 (0.138–0.976) | 0.045 |
| Dinnertime | 0.513 (0.244–1.079) | 0.078 | 0.561 (0.295–1.067) | 0.078 |
| Interval between dinner and bedtime | 1.267 (0.717–2.239) | 0.415 | 1.334 (0.809–2.201) | 0.259 |
| More than 3 h from dinner to bedtime | 2.088 (0.787–5.540) | 0.139 | 1.908 (0.829–4.392) | 0.129 |
| Number of wet nights/week | 0.746 (0.610–0.913) | 0.004 | 0.908 (0.785–1.052) | 0.199 |
| Desmopressin therapy | 0.946 (0.413–2.167) | 0.895 | 0.840 (0.409–1.724) | 0.634 |
| Alarm therapy | 1.967 (0.726–5.332) | 0.184 | 1.651 (0.630–4.332) | 0.308 |
| Use of diapers at night | 0.411 (0.162–1.042) | 0.061 | 0.396 (0.168–0.933) | 0.034 |
Discussion
This study demonstrated for the first time that enuresis treatment response rates were significantly higher during the COVID‐19 pandemic than they were during ordinary times, probably because the stay‐home policies in place during the pandemic allowed families to focus more of their attention on treatment. The factors that were significantly associated with treatment response were treatment during the COVID‐19 pandemic, treatment during the state of emergency, and dinner before 19:00.
The rate of CR to enuresis treatment was 73% during the COVID‐19 pandemic, in striking contrast to the 27% rate during the comparator period. Regardless of whether aggressive treatments such as desmopressin therapy and alarm therapy are used, the CR rate among all enuresis outpatients is reported to be 35–50% after 1 year of treatment; 13 , 14 therefore, the present study’s CR rate of 27% over the 6‐month comparator period is reasonable, as it represents approximately half of the 50% of patients expected to achieve a CR over 1 year.
According to previous reports on aggressive treatments, the CR and total response rates to desmopressin treatment are as high as 30% and 70%, respectively, but nearly 70% of patients who initially respond to desmopressin experience relapse upon interruption of treatment, and many patients find it necessary to skip medication to prevent water intoxication on days when evening activities prevent water restriction. 1 , 9 Alarm therapy, meanwhile, has a high CR rate of 70%, 10 but it also has a high withdrawal rate of 30%, 12 and cannot be introduced in the first place without strong motivation on the part of the patient and the family. 1 , 11 General lifestyle advice and behavioral therapy are less effective than desmopressin therapy and alarm therapy. 22
We speculate that the stay‐home policies in place during the pandemic allowed families to take time to focus more of their attention on treatment. 15 During the COVID‐19 pandemic, hospital and clinic visits to treat enuresis and other chronic pediatric diseases tended to be put off, yet at the same time, families tended to adhere more strictly to their treatment regimens. Thus, pandemic‐related policies may have indirectly enabled higher treatment effects.
Treatment during the COVID‐19 pandemic, treatment during the state of emergency, and dinner before 19:00 were significantly associated with response to treatment for nocturnal enuresis. It is likely that a larger proportion of families were able to comply with early dinnertime as recommended in the general lifestyle advice for nocturnal enuresis because extracurricular activities, in which approximately 80% of the children had previously been involved, were stopped, and because approximately 70% of two‐income households had at least one parent who switched to telework or stopped working during the pandemic. In addition, four patients took advantage of the stay‐home period to introduce alarm therapy, which led to CR as long as the therapy was continued. Some patients found it easier to comply with water restrictions because their extracurricular academic activities were stopped, which tended to make their treatment more effective. In this study, alarm therapy was a factor related to high response rate, although this trend was not significant because of our sample size.
This study has some limitations. First, because the number of patients was small, multivariate analysis could not be performed on the risk factors identified by univariate analysis, that is, treatment during the COVID‐19 pandemic, treatment during the state of emergency, and dinner before 19:00. It is reasonable, however, to assume that stay‐home policies led to higher compliance with enuresis treatments and earlier dinnertimes, which led, in turn, to lower frequencies of wet nights. We believe that the number of patients was appropriate for this study, as too many samples can be inappropriate for the proper evaluation of our primary outcome. A second limitation is that, since there were few new patients during the COVID‐19 pandemic period, the patients seen during this period are more likely to have been treated previously and to have improved over time. We believe, however, that our conclusions remain reasonable because, as described in the Results section, the mean durations of treatment were similar in the two periods, and we found similar results even in a subanalysis excluding all first‐visit patients from both periods. Third, many of these enrolled patients have neurodevelopmental disorders, which is reflective of the facilities they attended. The success rate of patients with nocturnal enuresis in the general population is unclear when applying the same analysis. Fourth, significantly more patients in the comparator period used diapers at night, suggesting a more severe patient population. However, since there was no difference in the frequency of wet nights between both periods, this is not always the case. Fifth, the total number of nocturnal enuresis patients decreased during the stay‐home period, so it is probable that only highly motivated patients visited. One limitation is a lack of clear data on compliance with therapy regimens and water restriction. To avoid these issues in future studies involving prospective multicenter joint research, including the functional bladder capacity and nocturnal urine volume as patient background and the compliance rate of water restriction, desmopressin therapy, and alarm therapy as indicators of treatment motivation is needed.
We confirmed a high rate of response to nocturnal enuresis treatment during the stay‐home period of the COVID‐19 pandemic. Situations that place restrictions on daily life, such as the COVID‐19 pandemic, can be good opportunities to focus on treatments for chronic illnesses and improve their success rates.
Author contributions
Takahisa Kimiya: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. Masayoshi Shinjoh: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. Hiroshi Asanuma: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. Tomohiro Inoguchi: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. Takao Takahashi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Conflict of interest
None declared.
Approval of the research protocol by an Institutional Reviewer Board
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Keio University School of Medicine Ethical Committee, Approval No. 2020‐0349.
Informed consent
N/A. This study and the possibility of opting out were announced to the public on the hospital website.
Registry and the Registration No. of the study/trial
N/A.
Animal studies
N/A.
Acknowledgment
We thank Akiko Miyata of the Saiwai Pediatric Clinic for providing us with data on enuresis patients.
References
- 1. Neveus T, Eggert P, Evans J et al. Evaluation of and treatment for monosymptomatic enuresis: a standardization document from the International Children’s Continence Society. J. Urol. 2010; 183: 441–7. [DOI] [PubMed] [Google Scholar]
- 2. Fergusson DM, Horwood LJ, Shannon FT. Factors related to the age of attainment of nocturnal bladder control: an 8‐year longitudinal study. Pediatrics 1986; 78: 884–90. [PubMed] [Google Scholar]
- 3. Bakker E, van Sprundel M, van der Auwera JC, van Gool JD, Wyndaele JJ. Voiding habits and wetting in a population of 4,332 Belgian schoolchildren aged between 10 and 14 years. Scand. J. Urol. Nephrol. 2002; 36: 354–62. [DOI] [PubMed] [Google Scholar]
- 4. Howe AC, Walker CE. Behavioral management of toilet training, enuresis, and encopresis. Pediatr. Clin. North Am. 1992; 39: 413–32. [DOI] [PubMed] [Google Scholar]
- 5. Yeung CK, Sreedhar B, Sihoe JD, Sit FK, Lau J. Differences in characteristics of nocturnal enuresis between children and adolescents: a critical appraisal from a large epidemiological study. BJU Int. 2006; 97: 1069–73. [DOI] [PubMed] [Google Scholar]
- 6. Srivastava S, Srivastava KL, Shingla S. Prevalence of monosymptomatic nocturnal enuresis and its correlates in school going children of Lucknow. Indian J. Pediatr. 2013; 80: 488–91. [DOI] [PubMed] [Google Scholar]
- 7. Kajiwara M, Inoue K, Kato M, Usui A, Kurihara M, Usui T. Nocturnal enuresis and overactive bladder in children: an epidemiological study. Int. J. Urol. 2006; 13: 36–41. [DOI] [PubMed] [Google Scholar]
- 8. Forsythe WI, Redmond A. Enuresis and spontaneous cure rate. Study of 1129 enuretics. Arch. Dis. Child. 1974; 49: 259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glazener CMA, Evans JHC. Desmopressin for nocturnal enuresis in children. Cochrane Database Syst. Rev. 2002; (3): CD002112. [DOI] [PubMed] [Google Scholar]
- 10. Caldwell PH, Codarini M, Stewart F, Hahn D, Sureshkumar P. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst. Rev. 2020; (5): CD002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Clinical Guideline Centre . Nocturnal enuresis: the management of bedwetting in children and young people. 2010. [Cited 15 Jun 2021.] Available from URL: https://www.nice.org.uk/guidance/cg111/evidence/full‐guideline‐136241965 [PubMed]
- 12. Evans J, Malmsten B, Maddocks A, Popli HS, Lottmann H; UK Study Group . Randomized comparison of long‐term desmopressin and alarm treatment for bedwetting. J. Pediatr. Urol. 2011; 7: 21–9. [DOI] [PubMed] [Google Scholar]
- 13. Akashi S. Trial of bed‐wetting score of the clinical observation in initial diagnosis and estimation of the therapy prognosis. J. Jpn. Soc. Enuresis 2009; 14: 29–33. [Google Scholar]
- 14. Akashi S. Comparison of three‐year treatment outcome of pediatric patients with monosymptomatic nocturnal enuresis (MNE) and non‐MNE: insight into the pathophysiology of MNE and non‐MNE. J. Jpn. Soc. Enuresis 2017; 22: 35–41. [Google Scholar]
- 15. Ministry of Internal Affairs and Communications, Japan . Information and communications in Japan. 2020. [Cited 15 Jun 2021.] Available from URL: https://www.soumu.go.jp/johotsusintokei/whitepaper/eng/WP2020/2020‐index.html
- 16. Brough HA, Kalayci O, Sediva A et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics – the 2020 COVID‐19 pandemic: a statement from the EAACI‐section on pediatrics. Pediatr. Allergy Immunol. 2020; 31: 442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PF, Bauer SB, Bower W et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016; 35: 471–81. [DOI] [PubMed] [Google Scholar]
- 18. Nevéus T, Fonseca E, Franco I et al. Management and treatment of nocturnal enuresis‐an updated standardization document from the International Children’s Continence Society. J. Pediatr. Urol. 2020; 16: 10–9. [DOI] [PubMed] [Google Scholar]
- 19. The Japanese Society on Enuresis . Clinical practice guidelines for Enuresis 2016. [Cited 15 Jun 2021.] Available from URL: https://minds.jcqhc.or.jp/docs/minds/nocturnal‐enuresis/nocturnal‐enuresis.pdf
- 20. Huang HM, Wei J, Sharma S et al. Prevalence and risk factors of nocturnal enuresis among children ages 5–12 years in Xi'an, China: a cross‐sectional study. BMC Pediatr. 2020; 20: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleiss JL, Tytun A, Ury HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics 1980; 36: 343–6. [PubMed] [Google Scholar]
- 22. Caldwell PH, Nankivell G, Sureshkumar P. Simple behavioural interventions for nocturnal enuresis in children. Cochrane Database Syst. Rev. 2013; (7): CD003637. [DOI] [PubMed] [Google Scholar]


