Abstract
Background and purpose
Cognitive dysfunction has been observed following recovery from COVID‐19. To the best of our knowledge, however, no study has assessed the progression of cognitive impairment after 1 year. The aim was to assess cognitive functioning at 1 year from hospital discharge, and eventual associations with specific clinical variables.
Methods
Seventy‐six patients (aged 22–74 years) who had been hospitalized for COVID‐19 were recruited. Patients received neuropsychological assessments at 5 (n = 76) and 12 months (n = 53) from hospital discharge.
Results
Over half (63.2%) of the patients had deficits in at least one test at 5 months. Compared to the assessment at 5 months, verbal memory, attention and processing speed improved significantly after 1 year (all p < 0.05), whereas visuospatial memory did not (all p > 0.500). The most affected domains after 1 year were processing speed (28.3%) and long‐term visuospatial (18.1%) and verbal (15.1%) memory. Lower PaO2/FiO2 ratios in the acute phase were associated with worse verbal long‐term memory (p = 0.029) and visuospatial learning (p = 0.041) at 5 months. Worse visuospatial long‐term memory at 5 months was associated with hyposmia (p = 0.020) and dysgeusia (p = 0.037).
Conclusion
Our study expands the results from previous studies showing that cognitive impairment can still be observed after 1 year. Patients with severe COVID‐19 should receive periodic cognitive follow‐up evaluations, as cognitive deficits in recovered patients could have social and occupational implications.
Keywords: COVID‐19, cognition, long‐COVID, neuropsychological evaluation
Cognitive impairment can still be observed in almost 50% of formerly hospitalized patients with COVID‐19 at 1 year from respiratory clinical recovery. Clinicians should be aware that the ratio of arterial oxygen partial pressure to fractional inspired oxygen and hyposmia/dysgeusia might represent risk factors for the development of cognitive impairment in COVID‐19.
INTRODUCTION
Neurological complications are present in patients with coronavirus disease 2019 (COVID‐19) [1, 2, 3, 4]. Amongst such complications, cognitive dysfunction following clinical recovery from acute respiratory symptoms has also been reported [5, 6, 7, 8, 9, 10, 11, 12, 13]. These studies, however, present a significant degree of heterogeneity in terms of sample size, sociodemographic and clinical characteristics of the patients, assessment methods and follow‐up duration.
As a result, many studies have found cognitive deficits in the months following hospitalization for COVID‐19 to be frequent [5, 6, 10, 11], whilst some others have observed significantly lower rates of cognitive impairment [8, 13]. This heterogeneity is also evident when one examines the qualitative profile of cognitive impairment in patients with COVID‐19, with some studies reporting deficits of executive functions, attention and processing speed, as well as memory problems [5, 10, 12], whilst others report verbal memory deficits to be the predominant feature [6, 11] and others report significant language impairment [11, 13]. Although somewhat contrasting, these studies show that persisting cognitive deficits can be frequently found in patients with COVID‐19.
It is also important to assess whether different clinical aspects of COVID‐19 are associated with a greater risk of long‐term cognitive impairment, as this could allow clinicians to predict which patients might be at an increased risk of developing such complications. As is known, COVID‐19 is a disease which presents multiple pathological mechanisms that could lead to cognitive impairment [14, 15, 16]. In addition to prolonged respiratory distress, which can cause hypoxia‐related brain injury [17, 18], hyperinflammation also plays a significant role in determining the severity and mortality of COVID‐19 [19, 20]. The activation of a systemic inflammation pathway could produce aberrant stress responses which could in turn damage the central nervous system (CNS) [21], thus suggesting that the aetiology of COVID‐19‐related cognitive dysfunction might be both inflammatory and not inflammatory [17]. For this reason, clinical indicators of respiratory function, hyperinflammation and coagulopathy, as well as potential indices of viral access to the CNS, should be assessed as potential predictors of cognitive impairment in COVID‐19 patients.
To date, no study has assessed the progression of cognitive impairment after 1 year from hospital discharge. Detailing the progression of cognitive impairment is key, as it represents an important indicator of functional recovery. Indeed, whilst most COVID‐19 patients might show a good clinical recovery in the months following hospitalization, persisting cognitive symptoms can impact negatively on their quality of life.
Our aim was to assess the prevalence of cognitive impairment in COVID‐19 patients after 1 year from hospital discharge. Potential clinical predictors of cognitive impairment were also evaluated.
METHODS
Seventy‐six patients (age [mean ± SD] 56.24 ± 12.08, 56 males) who had been hospitalized for COVID‐19 between February and April 2020 in the ASST Santi Paolo E Carlo University Hospitals in Milan, Italy, were recruited. Patients were included if they had been hospitalized for COVID‐19, were aged between 18 and 75 and were not diagnosed with cognitive impairment before COVID‐19.
Neuropsychological assessments at 5 months (T1, 5.4 ± 1.5 months) and at 12 months (T2, 11.92 ± 1.46 months) from hospital discharge were conducted. Of the 76 patients who were assessed at T1, 53 returned for the 1‐year follow up and 23 dropped out (Figure 1).
FIGURE 1.
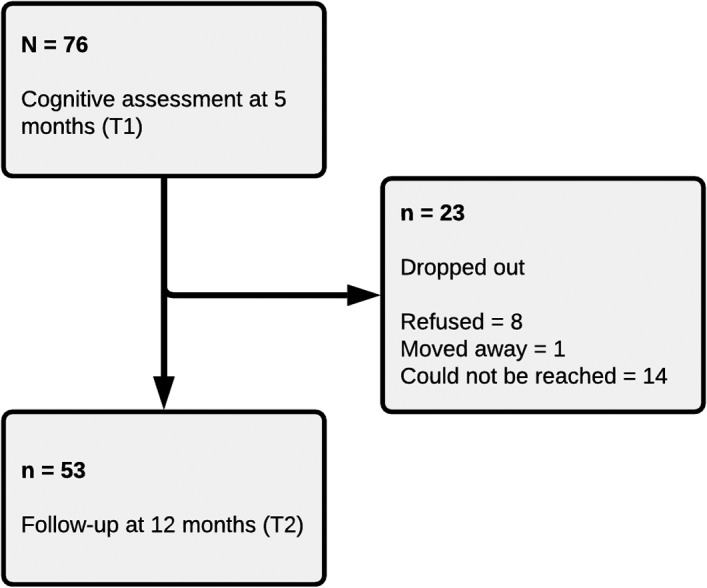
Flow chart of the study
Clinical data such as type and duration of oxygen therapy, hospitalization duration, comorbidities and viral clearance time, defined as the number of days elapsed between the first positive and second consecutive negative severe acute respiratory coronavirus 2 (SARS‐CoV‐2) nasopharyngeal swabs, were retrospectively collected. Anosmia/dysgeusia during and/or after hospitalization for COVID‐19 was assessed clinically by asking patients (in the form of yes/no questions).
Other clinical data were collected: (i) to assess hypoxia, the ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) (P/F ratio) was measured during hospitalization, as well as peripheral oxygen saturation (SpO2) level upon hospital arrival; (ii) as inflammatory biomarkers, C‐reactive protein blood levels upon hospital arrival and during hospitalization were noted; (iii) to assess hypercoagulability, D‐dimer values were measured upon hospital arrival and during hospitalization; (iv) to assess systemic alterations and organ damage, serum levels of creatinine, alanine transaminase and aspartate transaminase were noted upon hospital arrival and during hospitalization.
The primary aim was to assess cognitive function at 1 year from hospital discharge, compared to 5 months. A secondary aim was to evaluate associations with clinical factors (e.g., respiratory distress, inflammation, smell/taste alterations).
Neuropsychological assessment
Patients were screened using the Montreal Cognitive Assessment, a screening test for global cognitive functioning, in order to exclude those with global cognitive decline [22].
Cognitive functioning was assessed using the Brief Repeatable Battery of Neuropsychological Tests (BRB‐NT) [23]. The BRB‐NT includes tests which assess different cognitive functions (verbal and visuospatial long‐term memory, attention, working memory, processing speed, language). The Serial Recall Test (SRT) is a test of verbal memory composed of three subscores: (i) long‐term storage (SRT‐LTS), an index of efficiency in verbal long‐term memory storage processes; (ii) consistent long‐term retrieval (SRT‐CLTR), which reflects the consistency of unaided retrieval from verbal long‐term memory storage; (iii) delayed recall (SRT‐D), an index of long‐term verbal recall ability. The Spatial Recall Test (SPART) evaluates visuospatial memory and produces two subscores: (i) SPART, a measure of visuospatial learning; (ii) SPART‐D, a measure of delayed visuospatial recall. The Symbol‐Digit Modalities Test (SDMT) is a measure of processing speed and visual attention. The Paced Serial Additions Test (PASAT) evaluates working memory and attention and consists of two tests, one in which numbers are presented with an interval of 3 s (PASAT‐3) and one with an interval of 2 s (PASAT‐2). Lastly, the Word List Generation (WLG) test represents an index of language functioning and specifically of semantic verbal fluency.
At the time of cognitive testing, patients were also evaluated with Beck’s Depression Inventory–II (BDI‐II) [24] in order to assess the presence of depressive symptoms. The BDI‐II allows for a categorization of the severity of depressive symptoms, which can be defined as none or minimal (score ≤13), mild (score 14–19), moderate (score 20–28) or severe (score ≥29) [25].
Data analysis
All analyses were performed with SPSS 26.0 (IBM, 2019). All tests were two‐sided, and a p value ≤0.05 was considered significant. Normality of distribution of the data was assessed by analysing skewness and kurtosis [26].
Descriptive analyses were conducted for demographic and clinical data [23]. Differences in performance to the eight subscales of the BRB‐NT between T1 and T2 were assessed using a paired samples t test. As suggested by Armstrong [27], multiple comparisons were not corrected for in order to minimize the risk of a type II error and because the eight neuropsychological scores represent different cognitive processes and functions.
The potential predictors of cognitive performance were examined through a series of hierarchical multiple linear regression analyses. Our dependent variables were the raw scores to the eight tests of the BRB‐NT. As independent variables, in block 1 four sociodemographic and clinical predictors were entered, namely age, sex, years of education and the time interval between hospital discharge and neuropsychological assessment. In block 2, the lowest P/F ratio observed in each patient during their hospital stay was entered. As effect sizes, the partial correlation coefficient r for each independent variable and the adjusted percentage of explained variance (R 2) for each block were reported.
Associations between hyposmia, dysgeusia and BRB‐NT subscale scores were assessed using analyses of covariance (ANCOVAs), with hyposmia or dysgeusia as independent variables (present vs. absent), BRB‐NT test scores as dependent variables, and age, sex, education and discharge–assessment interval as covariates. As a measure of effect size, η p 2 values are reported.
Standard protocol approvals, registrations and patient consents
The study was approved by the Institutional Ethics Committee of the San Paolo Hospital of Milan (CogCov study: Reg. no. 2020/ST/105) and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent to participate in this study.
RESULTS
Study population
Our study population (see Table 1) consisted predominantly of male patients (n = 56, 73.7%); mean age was 56.24 ± 12.08 (mean ± SD) and the majority of our participants (n = 54, 70.2%) had at least 13 years of education. On average, patients were hospitalized for 12 days (11.97 ± 7.48), and the mean viral clearance time was about 1 month (33.59 ± 9.79 days).
TABLE 1.
Sociodemographic and clinical characteristics of the 76 hospitalized patients affected by COVID‐19
| N (valid %) or mean (SD) | ||
|---|---|---|
| T1 (N = 76) | T2 (N = 53) | |
| Sex | ||
| Females | 20 (26.3%) | 15 (28.3%) |
| Males | 56 (73.7%) | 38 (71.7%) |
| Age | 56.24 (12.08) | 58.51 (10.29) |
| Education (years) | ||
| ≤8 | 22 (28.9%) | 17 (32.1%) |
| 9–13 | 33 (43.4%) | 22 (41.5%) |
| >13 | 21 (27.6%) | 14 (26.4%) |
| Hospitalization duration (days) | 11.97 (7.48) | 11.94 (7.07) |
| Viral clearance time (days) | 33.59 (9.79) | 34.02 (10.09) |
| Oxygen therapy | ||
| None | 8 (10.5%) | 6 (11.3%) |
| Low‐flow | 35 (46.1%) | 24 (45.3%) |
| NIV | 26 (34.2%) | 16 (30.2%) |
| Intubation | 7 (9.2%) | 7 (13.2%) |
| Hyposmia | ||
| No | 41 (55.4%) | 48 (90.6%) |
| Yes | 33 (44.6%) | 5 (9.4%) |
| Dysgeusia | ||
| No | 38 (51.4%) | 46 (86.8%) |
| Yes | 36 (48.6%) | 7 (13.2%) |
Abbreviation: NIV, non‐invasive ventilation.
The P/F values were available for 69 patients. Of these, 33 (48.5%) experienced no acute respiratory distress syndrome (ARDS) (P/F ratio >300), 18 (26.5%) experienced mild ARDS (P/F ratio 200–300), 15 (19.7%) experienced moderate ARDS (P/F ratio 100–200) and two patients experienced severe ARDS (P/F ratio <100) [28].
At T1, about half of our patients reported hyposmia (n = 33, 44.6%) or dysgeusia (n = 36, 48.6%), and 32 (n = 42.1%) reported the occurrence of both symptoms. Subjective cognitive complaints were reported by 31 (41.3%) patients at the time of the first assessment. Out of the 53 patients who were assessed at T2, five (9.4%) still reported persisting hyposmia, whilst seven (13.2%) reported persisting dysgeusia. Subjective cognitive deficits were still reported by 29 (54.7%) patients.
The prevalence of depressive symptoms at T1 was low (mean BDI‐II score 9.59 ± 9.46), with 74.7% of patients reporting no or minimal symptoms, 11.8% mild symptoms and 6.6% moderate symptoms, whilst 6.6% reported severe symptoms. At T2, four (7.5%) patients had mild depressive symptoms, five (9.4%) had moderate depressive symptoms and five (9.4%) had severe depressive symptoms; the remaining 39 (73.6%) reported no/minimal depressive symptoms.
The patients who dropped out after T1 tended to be younger (51.00 ± 14.35 years vs. 58.51 ± 10.29 years, p = 0.030), but there were no other significant differences in terms of male/female ratio (p = 0.777), education (p = 0.780), type of oxygen therapy (p = 0.938), hospitalization duration (p = 0.938) or viral clearance time (p = 0.592).
Cognitive functioning
At T1, more than half (n = 48, 63.2%) of patients had deficits in at least one test, 31 (40.8%) in at least two tests and 18 (23.7%) in three or more tests. The most affected domain was processing speed (SDMT, n = 31, 40.8% of patients), followed by long‐term verbal memory (SRT‐D, n = 20, 26.3%; SRT‐LTS, n = 13, 17.1%; SRT‐CLTR, n = 15, 19.7%) and long‐term visuospatial memory (SPART‐D, n = 14, 18.2%).
At T2, 26 (49.1%) still showed deficits in at least one cognitive test, 17 (32.1%) showed deficits in at least two tests and seven (13.2%) showed deficits in three or more tests. Given that patients who recover are usually less willing to undergo follow‐up examinations, it is possible that the observed rate of cognitive impairment after 1 year might represent an overestimation of the real rate of cognitive impairment. Assuming that all patients who were lost to follow‐up had returned to normal cognition, the prevalence of cognitive impairment would be 34.1%.
Processing speed remained the most frequently affected domain (SDMT, n = 15, 28.3%), followed by long‐term visuospatial (SPART‐D, n = 10, 18.9%) and verbal (SRT‐D, n = 8, 15.1%) memory. Detailed results of the neuropsychological assessments are reported in Table 2.
TABLE 2.
Results of the cognitive assessments at 5 (T1) and 12 (T2) months from discharge
| Test | Test score (mean ± SD) | t(52) | p | Cut‐off | Percentage below the normative cut‐off | ||
|---|---|---|---|---|---|---|---|
| T1 (N = 76) | T2 (N = 53) | T1 (N = 76) | T2 (N = 53) | ||||
| SRT‐LTS | 35.64 ± 13.77 | 40.62 ± 14.20 | 2.966 | 0.005 | >23.3 | 17.1% | 7.5% |
| SRT‐CLTR | 27.75 ± 13.06 | 31.49 ± 14.61 | 2.259 | 0.028 | >15.5 | 19.7% | 5.7% |
| SRT‐D | 6.92 ± 2.66 | 7.45 ± 2.43 | 2.038 | 0.047 | >4.9 | 26.3% | 15.1% |
| SPART | 17.75 ± 5.01 | 18.21 ± 5.12 | 0.579 | 0.565 | >12.7 | 14.5% | 15.1% |
| SPART‐D | 5.66 ± 2.07 | 5.87 ± 2.07 | 0.648 | 0.520 | >3.6 | 18.2% | 18.9% |
| SDMT | 38.81 ± 9.88 | 42.85 ± 10.14 | 4.891 | <0.001 | >37.9 | 40.8% | 28.3% |
| PASAT‐3 | 41.66 ± 11.98 | 44.94 ± 11.72 | 2.961 | 0.005 | >28.4 | 14.5% | 7.5% |
| PASAT2 | 30.81 ± 9.36 | 33.15 ± 10.82 | 2.331 | 0.024 | >17.1 | 3.9% | 5.7% |
| WLG | 24.75 ± 4.69 | 25.36 ± 5.10 | 0.985 | 0.329 | >17.0 | 5.3% | 5.7% |
p values reflect the statistical significance of differences (paired t test) between scores at T1 and T2 for the 53 patients who received the follow‐up examination at T2.
Bold indicates statistically significant (p<0.05) results.
Abbreviations: PASAT, Paced Auditory Serial Addition Test; SDMT, Symbol‐Digit Modalities Test; SPART, Spatial Recall Test; SPART‐D, Spatial Recall Test (delayed recall); SRT‐CLTR, Serial Recall Test Consistent Long‐Term Retrieval; SRT‐D, Serial Recall Test (delayed recall); SRT‐LTS, Serial Recall Test Long‐Term Storage; WLG, Word List Generation.
Compared to T1 (5 months), a significant improvement in cognitive performance was observed for all tests of verbal memory (SRT‐LTS, p = 0.005; SRT‐CLTR, p = 0.028; SRT‐D, p = 0.047) and attention/processing speed (SDMT, p < 0.001; PASAT‐3, p = 0.005; PASAT‐2, p = 0.024). It should be noted that, although PASAT‐2 mean scores improved significantly, the percentage of patients with scores below the norm increased slightly, possibly for the reason outlined above (i.e., patients who recovered to normal cognition may have been less motivated to return for the follow‐up assessment). No significant improvements for tests of visuospatial learning (SPART, p = 0.565), visuospatial delayed recall (SPART‐D, p = 0.520) and verbal fluency (WLG, p = 0.329) were observed.
Associations between clinical variables and cognitive functioning
At T1, block 2 of the regression models reached significance only for SRT‐LTS and SPART subscales. As for SRT‐LTS, at block 1 sociodemographic and clinical variables contributed significantly to the regression model (see Table 3 for F values, df and p values), accounting for 19.5% of the variation in the dependent variable. Introducing the P/F ratio explained an additional 4.7% of the variation in SRT‐LTS and this change in R 2 was significant (F change [1, 63] = 4.993; p = 0.029). As for SPART, at block 1 sociodemographic and clinical variables contributed significantly to the regression model and explained up to 11.4% of the variation in the dependent variable. Introducing the P/F ratio explained an additional 4.3% of the variation in SPART and this change in R 2 was significant (F change [1, 65] = 4.332; p = 0.041). In both cases, a greater P/F ratio during hospital stay was a significant positive predictor of cognitive performance in the months following hospital discharge, whilst controlling for all other variables in the model (see Table 3).
TABLE 3.
Unstandardized B, standard errors, standardized beta, t values, p values and partial r correlation coefficients in the sample of 76 hospitalized patients affected by COVID‐19
| B | SE | Beta | t | p | Partial r | ||
|---|---|---|---|---|---|---|---|
| Predictors of SRT‐LTS | |||||||
| Block 1 | F(4, 64) = 5.123, p = 0.001; adjusted R 2 = 0.195 | ||||||
| Intercept | 60.016 | 11.877 | 5.053 | <0.001 | |||
| Sex | −2.059 | 3.940 | −0.060 | −0.523 | 0.603 | −0.065 | |
| Age | −0.509 | 0.148 | −0.409 | −3.446 | 0.001 | −0.396 | |
| Education | 0.738 | 0.511 | 0.172 | 1.443 | 0.154 | 0.177 | |
| Discharge–assessment interval | −0.015 | 0.039 | −0.044 | −0.374 | 0.710 | −0.047 | |
| Block 2 | F(1, 63) = 5.353, p < 0.001; adjusted R 2 = 0.242 | ||||||
| Intercept | 37.137 | 15.415 | 2.409 | 0.019 | |||
| Sex | −1.095 | 3.847 | −0.032 | −0.285 | 0.777 | −0.036 | |
| Age | −0.427 | 0.148 | −0.342 | −2.883 | 0.005 | −0.341 | |
| Education | 0.693 | 0.496 | 0.162 | 1.396 | 0.167 | 0.173 | |
| Discharge–assessment interval | 0.024 | 0.042 | 0.071 | 0.575 | 0.568 | 0.072 | |
| P/F ratio | 0.044 | 0.020 | 0.277 | 2.235 | 0.029 | 0.271 | |
| Predictors of SPART | |||||||
| Block 1 | F(4, 65) = 3.220, p = 0.018; adjusted R 2 = 0.114 | ||||||
| Intercept | 14.995 | 4.282 | 3.502 | 0.001 | |||
| Sex | 1.653 | 1.408 | 0.139 | 1.174 | 0.245 | 0.144 | |
| Age | −0.095 | 0.053 | −0.225 | −1.794 | 0.077 | −0.217 | |
| Education | 0.287 | 0.179 | 0.200 | 1.604 | 0.114 | 0.195 | |
| Discharge–assessment interval | 0.024 | 0.014 | 0.206 | 1.699 | 0.094 | 0.206 | |
| Block 2 | F(1, 63) = 3.574, p = 0.007; adjusted R 2 = 0.157 | ||||||
| Intercept | 7.304 | 5.576 | 1.310 | 0.195 | |||
| Sex | 1.941 | 1.380 | 0.163 | 1.407 | 0.164 | 0.173 | |
| Age | −0.068 | 0.053 | −0.161 | −1.280 | 0.205 | −0.158 | |
| Education | 0.280 | 0.174 | 0.195 | 1.608 | 0.113 | 0.197 | |
| Discharge–assessment interval | 0.037 | 0.015 | 0.316 | 2.438 | 0.018 | 0.291 | |
| P/F ratio | 0.015 | 0.007 | 0.269 | 2.081 | 0.041 | 0.252 | |
Abbreviations: P/F, partial oxygen pressure/fractional inspired oxygen; SPART, Spatial Recall Test; SRT‐LTS, Serial Recall Test Long‐Term Storage.
At T2, block 1 was significant for all cognitive tests with the exception of SPART‐D (p = 0.620). The P/F ratio was added in block 2 but was not found to be a significant predictor of performance in any cognitive test at T2 (see Table S1).
After controlling for age, sex, education and discharge–assessment interval, worse long‐term visuospatial memory performance was observed at T1 (SPART‐D score) in patients who reported hyposmia (F[1, 68] = 5.65; p = 0.020; η p 2 = 0.077) or dysgeusia (F[1, 68] = 4.50; p = 0.037; η p 2 = 0.062). There were no significant differences in other cognitive functions (all p > 0.070; see Table S2). Cognitive performance at T2 was not significantly different between patients who had ARDS and those who did not (see Table S3).
Bivariate analyses were conducted to assess the relationships between other clinical variables and cognitive performance at T1. After controlling for age, sex, education and discharge–assessment interval, an inverse correlation was observed between serum alanine transaminase levels during hospitalization and long‐term verbal memory performance (SRT‐D, r = −0.294; p = 0.014). BDI‐II scores did not correlate with cognitive performance. Detailed results are reported in Table S4.
DISCUSSION
The long‐term cognitive profile of recovered patients with COVID‐19 was assessed by neuropsychological assessments after 1 year from discharge. To the best of our knowledge, this is the first study to report cognitive data at 1 year from clinical recovery from COVID‐19.
More than half of our patients had deficits in at least one cognitive test after about 5 months from hospital discharge. Attention/processing speed was the most frequently impaired domain, followed by long‐term verbal and visuospatial memory. Whilst attention/processing speed remained the most frequently affected domains, the prevalence of long‐term verbal memory deficits decreased significantly after 1 year, whereas no significant change was observed in visuospatial learning and delayed recall.
Our study also expands the results from previous studies on cognitive functioning in the first months following clinical recovery and shows that, whilst attention/processing speed and long‐term verbal memory deficits represent the dominant feature of cognitive impairment in the post‐acute phase, they tend to improve as time progresses, whereas visuospatial memory deficits do not. Miskowiak et al. [10] reported similar rates of cognitive impairment at 4 months from hospital discharge, observing that verbal memory and executive function were the most affected domains. Méndez et al. [11] reported deficits in at least one cognitive function in 58.7% of patients at 4 months from clinical recovery, verbal memory being the most affected domain. Nevertheless, other studies have reported lower rates of cognitive impairment [8, 13, 29]. The heterogeneity of results from different studies probably reflects sociodemographic, clinical and methodological differences. Indeed, Mattioli et al. [8] studied healthcare workers who had recovered from mild COVID‐19 and were younger than our sample, reporting the presence of cognitive deficits in 30%. Almeria et al. [13] evaluated patients with severe COVID‐19, but it should be noted that the mean age of the patients included in their study was also significantly lower than that of our sample.
A recent meta‐analysis which included data from 43 studies reported a pooled proportion of cognitive impairment of 22% (95% confidence interval 17%–28%). Crucially, the prevalence of cognitive impairment rose to 36% (95% confidence interval 27%–46%) when only the studies which performed objective neuropsychological assessments were considered (n = 12), highlighting the importance of performing formal neuropsychological assessments as mild cognitive deficits are historically often underreported [30]. Notably, whilst hospitalized patients tended to present higher rates of long‐term cognitive impairment (30%) compared to non‐hospitalized patients (20%), this difference was not significant (p = 0.096) [29]. However, this meta‐analysis did not further evaluate the prevalence of cognitive impairment across the different cognitive domains and did not differentiate between studies using a complete neuropsychological assessment and those that only used brief global cognition screening tests, probably due to the small number of published studies with formal neuropsychological testing. Future meta‐analyses should aim to provide a more detailed cognitive profile of these patients, as our results suggest that not all cognitive domains and functions are affected equally.
When the impact of clinical variables was assessed, it was found that worse P/F ratios were associated with worse long‐term memory and visuospatial learning, whilst hyposmia and dysgeusia were associated with worse visuospatial long‐term memory. Neither the P/F ratio nor hyposmia/dysgeusia were associated with cognitive dysfunction after 1 year, indicating that clinical illness variables affect the profile of cognitive impairment in the short and medium term but not in the long term. Overall, these results suggest that different—but not necessarily mutually exclusive—pathological processes may underlie cognitive dysfunction in recovered COVID‐19 patients, and that time from clinical recovery also plays a significant role. Respiratory distress, which leads to hypoxaemia and hypoxia, can cause long‐lasting cognitive impairment [17, 31] and brain abnormalities [32]. Prior to the current pandemic, studies on the cognitive sequelae of patients hospitalized for ARDS have highlighted substantial cognitive deficits at the time of discharge, with slow but evident improvement in the longer term [33, 34]. Recent studies found that hospitalization in the intensive care unit for severe COVID‐19‐related ARDS increases the risk of cognitive impairment [35, 36]. It is less clear, however, whether a distinct cognitive profile could be associated with ARDS. Indeed, some have found memory impairment to be predominant [18], whilst others reported a greater impact on executive functioning [31].
Our study expands upon this evidence, showing that clinical indices such as P/F ratio values during hospitalization can predict memory performance. The specific association between memory deficits and respiratory distress could reflect a direct consequence of hypoxic damage, considering the known susceptibility of the hippocampus to hypoxia [37]. This hypothesis is supported by the fact that the P/F ratio selectively predicted the scores in two tests of memory which reflect in particular the integrity of memory encoding and storage processes, which are classically ascribed to the hippocampus and other medial temporal lobe areas [38, 39]. However, other factors such as cytokine‐related hyperinflammation, coagulopathy, blood–brain barrier breakdown and production of anti‐neuronal antibodies [16, 20, 40] might also play a significant role.
Additionally, our data expand the findings of a study which reported an association between hyposmia and cognitive impairment at 6 months [9]. The authors did not conduct detailed neuropsychological assessments and therefore could not establish whether hyposmia was associated with an impairment of specific cognitive functions. The association between smell and taste alterations and memory deficits observed in our study might suggest a direct involvement of the olfactory tract and the entorhinal cortex, which is anatomically and functionally associated with the hippocampus [41]. Nevertheless, the direct relationship between hyposmia and cognitive dysfunction has not yet been fully understood. In the context of COVID‐19, smell/taste alterations have been hypothesized to reflect viral access of SARS‐CoV‐2 to the CNS via trans‐synaptic olfactory pathways [42]. Although studies have demonstrated that SARS‐CoV‐2 can access both brainstem [43] and brain [16, 44], the specific contribution of either viral neurotropism or inflammatory response to the aetiology of brain alterations observed in these patients is still debated.
Our work has some key limitations, such as the lack of baseline data on cognitive function, that stem from the fact that adult participants were assessed who did not report cognitive problems prior to having COVID‐19 and therefore had never required a cognitive assessment. A control group was also lacking, a limitation shared by most studies published on this topic to date [6, 9, 11, 13, 35], reflecting the consequences of the ongoing pandemic, which limits the possibility of recruiting healthy participants for in‐person hospital assessments. Finally, due to missing data at T2, results could not be generalized but, as appropriate analyses can eliminate or reduce bias, studies with larger sample sizes are needed in order to confirm our results.
In conclusion, cognitive impairment can still be observed in almost 50% of formerly hospitalized patients with COVID‐19 at 1 year from respiratory clinical recovery. The prevalence of cognitive impairment decreased at 1 year from hospitalization, indicating that many patients recovered during the course of 1 year. Clinical variables such as P/F ratio and hyposmia or dysgeusia were associated with long‐term memory performance in the months following hospitalization, but not at 1 year. Clinicians should be aware that P/F ratio and hyposmia/dysgeusia might represent risk factors for the development of cognitive impairment in COVID‐19.
It is therefore suggested that patients who had more severe forms of COVID‐19, as indicated by lower P/F ratio values (<300), should undergo a cognitive assessment following clinical recovery and receive periodic follow‐up evaluations. Cognitive deficits in recovered patients with COVID‐19 could carry significant social and occupational implications, in particular for younger patients who might struggle to regain premorbid levels of performance in work‐related tasks and thus experience increased mental fatigue and stress.
CONFLICT OF INTEREST
All authors report no competing interests.
AUTHOR CONTRIBUTIONS
Roberta Ferrucci: Conceptualization (equal); methodology (equal); project administration (equal); writing—original draft (equal); writing—review and editing (equal). Michelangelo Dini: Data curation (equal); formal analysis (equal); investigation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal). Chiara Rosci: Investigation (equal); methodology (equal); resources (equal); writing—original draft (equal). Antonella Capozza: Investigation (equal); resources (equal); writing—original draft (equal). Elisabetta Groppo: Methodology (equal); resources (equal); writing—original draft (equal). Maria Rita Reitano: Investigation (equal); resources (equal); writing—original draft (equal). Elisa Allocco: Investigation (equal); writing—original draft (equal). B. Poletti: Validation (equal); writing—review and editing (equal). Agostino Brugnera: Formal analysis (equal); methodology (equal); writing—original draft (equal). Francesca Bai: Resources (equal); writing—review and editing (equal). Alessia Monti: Methodology (equal); writing—review and editing (equal). Nicola Ticozzi: Supervision (equal); writing—review and editing (equal). Vincenzo Silani: Resources (equal); writing—original draft (equal); writing—review and editing (equal). Stefano Centanni: Resources (equal); writing—review and editing (equal). Antonella D'Arminio Monforte: Resources (equal); supervision (equal); writing—review and editing (equal). Luca Tagliabue: Resources (equal); writing—original draft (equal); writing—review and editing (equal). Alberto Priori: Conceptualization (equal); resources (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal).
Supporting information
ACKNOWLEDGEMENTS
This study was partially supported by the Romeo and Enrica Invernizzi Foundation. Michelangelo Dini and Roberta Ferrucci were supported from the Research Center (CRC) Aldo Ravelli Center for Neurotechnology and Brain Therapeutics. Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement.
Ferrucci R, Dini M, Rosci C, et al. One‐year cognitive follow‐up of COVID‐19 hospitalized patients. Eur J Neurol. 2022;29:2006–2014. doi: 10.1111/ene.15324
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy‐associated morbidity in COVID‐19 patients. Ann Clin Transl Neurol. 2020;7(11):2221‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moro E, Priori A, Beghi E, et al. The international European Academy of Neurology survey on neurological symptoms in patients with COVID‐19 infection. Eur J Neurol. 2020;27(9):1727‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priori A, ed. Neurology of COVID‐19. Milano University Press; 2021. [PubMed] [Google Scholar]
- 5. Ferrucci R, Dini M, Groppo E, et al. Long‐lasting cognitive abnormalities after COVID‐19. Brain Sci. 2021;11(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain. 2021;144(4):1263‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blazhenets G, Schröter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID‐19 patients. J Nucl Med. 2021;62(7):910‐915. 10.2967/jnumed.121.262128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, De Palma G. Neurological and cognitive sequelae of COVID‐19: a four month follow‐up. J Neurol. 2021;268(12):4422‐4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cristillo V, Pilotto A, Cotti Piccinelli S, et al. Age and subtle cognitive impairment are associated with long‐term olfactory dysfunction after COVID‐19 infection. J Am Geriatr Soc. 2021;69(10):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miskowiak KW, Johnsen S, Sattler SM, et al. Cognitive impairments four months after COVID‐19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Méndez R, Balanzá‐Martínez V, Luperdi SC, et al. Short‐term neuropsychiatric outcomes and quality of life in COVID‐19 survivors. J Intern Med. 2021;290(3):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou H, Lu S, Chen J, et al. The landscape of cognitive function in recovered COVID‐19 patients. J Psychiatr Res. 2020;129:98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID‐19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Heal. 2020;9:100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker HA, Safavynia SA, Evered LA. The ‘third wave’: impending cognitive and functional decline in COVID‐19 survivors. Br J Anaesth. 2021;126(1):44‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS‐CoV‐2 in human and mouse brain. J Exp Med. 2021;218(3):e20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasannejad C, Ely EW, Lahiri S. Long‐term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson‐lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 19. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacLullich AMJ, Ferguson KJ, Miller T, de Rooij SEJA, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santangelo G, Siciliano M, Pedone R, et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci. 2015;36(4):585‐591. [DOI] [PubMed] [Google Scholar]
- 23. Amato MP, Portaccio E, Goretti B, et al. The Rao’s Brief Repeatable Battery and Stroop test: normative values with age, education and gender corrections in an Italian population. Mult Scler J. 2006;12(6):787‐793. [DOI] [PubMed] [Google Scholar]
- 24. Beck AT, Steer RA, Brown GK. Beck Depression Inventory®–II (BDI®–II). Pearson; 1996. [Google Scholar]
- 25. Ghisi M, Flebus GB, Montano A, Sanavio E, Sica C. Beck Depression inventory—II. 1st ed. Giunti Psychometrics; 2006. [Google Scholar]
- 26. Kim H‐Y. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502‐508. [DOI] [PubMed] [Google Scholar]
- 28. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. J Am Med Assoc. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 29. Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post‐COVID‐19 Syndrome: a systematic review and meta‐analysis. Brain Behav Immun. 2021;2022(101):93‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Begum A, Morgan C, Chiu CC, Tylee A, Stewart R. Subjective memory impairment in older adults: aetiology, salience and help seeking. Int J Geriatr Psychiatry. 2012;27(6):612‐620. [DOI] [PubMed] [Google Scholar]
- 31. Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long‐term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20(3):263‐271. [DOI] [PubMed] [Google Scholar]
- 33. Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intens Care Med. 2016;42(5):725‐738. [DOI] [PubMed] [Google Scholar]
- 34. Wilcox EM, Brummel NE, Archer K, Wesley Ely E, Jackson JC, Hopkins RO. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41(9 SUPPL.1):81‐98. [DOI] [PubMed] [Google Scholar]
- 35. Beaud V, Crottaz‐Herbette S, Dunet V, et al. Pattern of cognitive deficits in severe COVID‐19. J Neurol Neurosurg Psychiatry. 2021;92(5):567‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cervós‐Navarro J, Sampaolo S, Hamdorf G. Brain changes in experimental chronic hypoxia. Exp Pathol. 1991;42(4):205‐212. [DOI] [PubMed] [Google Scholar]
- 38. Squire L, Zola‐Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380‐1386. [DOI] [PubMed] [Google Scholar]
- 39. Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996;93(24):13438‐13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 41. Insausti R. Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus. 1993;3(S1):19‐26. [PubMed] [Google Scholar]
- 42. Cooper KW, Brann DH, Farruggia MC, et al. COVID‐19 and the chemical senses: supporting players take center stage. Neuron. 2020;107(2):219‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bulfamante G, Chiumello D, Canevini MP, et al. First ultrastructural autoptic findings of SARS‐Cov‐2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678‐679. [DOI] [PubMed] [Google Scholar]
- 44. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol. 2020;19(11):919‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


