Abstract
Prior reports evaluating SARS‐CoV‐2 vaccine efficacy in chronic lymphocytic leukaemia (CLL) used semiquantitative measurements of anti‐S to evaluate immunity; however, neutralization assays were used to assess functional immunity in the trials leading to vaccine approval. Here, we identified decreased rates of seroconversion in vaccinated CLL patients and lower anti‐S levels compared to healthy controls. Notably, we demonstrated similar results with the Roche anti‐S assay and neutralization activity. Durable responses were seen at six months; augmentation with boosters was possible in responding patients. Absence of normal B cells, frequently seen in patients receiving Bruton tyrosine kinase and B‐cell lymphoma 2 inhibitors, was a strong predictor of lack of seroconversion.
Keywords: COVID vaccine, CLL, B cell
A compromised immune response to vaccinations has been a long‐standing concern for patients with chronic lymphocytic leukaemia (CLL) and continues to be in the era of novel agents. 1 Recent publications indicate decreased rates of seroconversion following SARS‐CoV‐2 vaccines in both previously untreated patients with CLL and those undergoing therapy. 2 , 3 , 4 The modality for evaluating immunity in these studies consisted of semiquantitative measurements of antibodies targeting portions of the spike protein receptor binding domain. However, neutralization assays were the key method of assessing functional immune response in trials leading to emergency use authorization of the currently approved vaccines. 5 While the correlation between these assays has been established in healthy subjects, data are lacking in those with inherent and treatment‐related humoral and cellular immunodeficiencies. 6 Additionally, there is limited information regarding the durability of vaccine‐induced responses in this patient population. As risk factors for COVID‐19 overlap with many clinical features of CLL, it is imperative to understand SARS‐CoV‐2 vaccine efficacy in this vulnerable population.
METHODS
This prospective observational study was conducted at the University of Washington/Fred Hutchinson Cancer Research Center and approved by the IRB. Patients were eligible if they had a diagnosis of CLL or small lymphocytic lymphoma (SLL), were ≥18 years‐old, and had no known prior history of SARS‐CoV‐2 infection. Patients were enrolled into three cohorts: (1) previously untreated; (2) currently receiving a Bruton tyrosine kinase (BTK) inhibitor; and (3) currently receiving venetoclax + anti‐CD20 monoclonal antibody (Mab) or completed this regimen within the past year. Baseline testing prior to vaccination consisted of full blood count, quantitative IgG level, lymphocyte subsets, and Roche Elecsys Anti‐SARS‐CoV‐2 (Roche Diagnostics, Indianapolis, IN, USA; anti‐nucleocapsid) serologic testing. Normal B cells were distinguished from malignant B cells by flow cytometry. Normal B cells expressed CD19 and CD20 with polyclonal light‐chain expression (negative for CD5), whereas the malignant cells expressed CD5, CD19, and CD20 (dim) with dim light‐chain restriction. Vaccine response was assessed via the Roche Elecsys Anti‐SARS‐CoV‐2 S, a semiquantitative total antibody assay against the spike protein receptor binding domain (anti‐S), and SARS‐CoV‐2 spike D614G pseudotyped lentivirus neutralization assays. 7 , 8 For the pseudotyped lentivirus assay, serum was heat‐treated at 56°C for 30 min and diluted 1:10 followed by six 1:3 serial dilutions to 1:7290 and run in duplicate to determine ND50 and ND80 values. External quality assurance for pseudotype assay was performed using the EQAPOL SARS‐CoV‐2 antibody assay monitoring programme. Reference interval for a negative result was <0.8 AU/ml on the Roche anti‐S assay and ND50 < 20 for the neutralization assay. Patients underwent serial serologic response assessments prior to their first vaccine dose and approximately one month after (one week after a second mRNA vaccine or four weeks after the single Ad26.COV2.S vaccine). Subsequent serologic testing was performed at 3, 6, and 12 months. Univariate logistic models were created to test for differences between various groups on vaccine status. A McNemar test was used to test concordance between the two assays. All calculations were performed using R (version 4.1.0).
RESULTS
Patient characteristics are in Table 1. Patient enrollment by arm was: previously untreated (n = 14), BTK inhibitor (n = 15), and venetoclax + Mab (n = 8). Anti‐nucleocapsid antibody was non‐reactive in all patients, indicating no evidence of prior SARS‐CoV‐2 infection. The overall seroconversion rate via the Roche anti‐S assay was 41% at the first assessment performed after vaccination. Seroconversion rates for previously untreated, BTK inhibitor, and venetoclax + Mab were 50%, 33% [overall response (OR) 0.5, 95% confidence interval (CI) 0.11–2.24, p‐value 0.37], and 38% (OR 0.6, 95% CI 0.10–3.53), p‐value 0.57). For the patients who seroconverted, the median anti‐S levels were 18.45 AU/ml (range 0.954–1081) overall, 20.4 AU/ml (1.14–1081) previously untreated, 8.7 AU/ml (0.95–45) for BTK inhibitor, and 92.3 AU/ml (3.47–494) for venetoclax + Mab. The neutralization assays are depicted in Figure SS1. The Roche anti‐S assay performed similarly (40% positive, 95% CI 26–56) to the neutralization assay (34% positive, 95% CI 21–51); with non‐significant discordance between the two assays, (p = 0.617; Table S1).
TABLE 1.
Patient characteristics
| Patient characteristics |
Number of patients (n = 37) |
|---|---|
| Age | |
| Median | 65 years |
| Range | 42–81 |
| Sex | |
| Male | 25 |
| Female | 12 |
| Vaccine | |
| BNT162b2 | 29 |
| mRNA‐1273 | 6 |
| Ad26.COV2.S | 2 |
| Time between diagnosis and vaccination (months) | |
| Median | 45 |
| Range | 2–314 |
| Therapy | |
| Previously untreated | 14 |
| BTK inhibitor | 15 |
| Venetoclax + anti‐CD20 antibody | 8 |
| Prior anti‐CD20 monoclonal antibody | |
| <12 months | 8 |
| ≥12 months | 11 |
| IgG (normal 610–1616) | |
| Median | 651 |
| Range | 70–1037 |
| Absolute lymphocyte count | |
| Median | 2.12 × 103/μl |
| Range | 0.52–306 × 103/μl |
| Normal B‐lymphocyte count | Median (range) |
| Previously untreated | 104 cells/μl (8–625 cells/μl) |
| BTK inhibitor | 0 cells/μl (0–121 cells/μl) |
| Venetoclax + anti‐CD20 antibody | 0 cells/μl (0–39 cells/μl) |
The presence of any detectable normal B lymphocytes was associated with a significantly higher ability to mount a serologic response (OR 9.3, 95% CI 1.69–52.13, p = 0.01; Table 1 ) Lack of normal B cells was only identified in patients currently receiving BTK inhibitors or venetoclax + Mab. In the venetoclax + Mab cohort, the only responses were seen in three patients who had recently completed venetoclax (range 3–7 months). All had received anti‐CD20‐Mab more than one year prior and had detectable normal B cells at the time of vaccination: median 11 cells/ml (range 4–39). The remaining five patients who did not seroconvert were still receiving venetoclax at the time of vaccination, had received anti‐CD20‐Mab within the past year, and lacked normal B cells at the time of vaccination. Seroconversion was independent of IgG level (OR 0.85, 95% CI 0.22–3.3, p = 0.82). There was a statistically non‐significantly increased serologic response in previously untreated compared to patients on treatment (OR 1.88, 95% CI 0.48–7.26, p = 0.36). Seroconversion was statistically more likely in patients whose last exposure to anti‐CD20‐Mab was more than one year before (OR 18, 95% CI 1.49–216, p = 0.02). Serologic responses were seen in 10/29 who received BNT162b2 (34%; Pfizer Inc, New York, NY, USA), 4/6 (67%; Moderna Inc, Cambridge, MA, USA) who received mRNA‐1273, and 1/2 who received Ad26.COV2.S (50%; Johnson & Johnson, New Brunswick, NJ, USA).
Of the 15 responding patients, serial assessments were available in 13 at three months and 14 at six months. None had received an additional dose of vaccine prior to three‐month assessments. At three months, 12 of the 13 evaluable had evidence of persistent serologic response. Patients who achieved a serologic response one month after first vaccination were more likely to maintain the response at three months (OR 30, 95% CI 3.2–275, p = 0.0026). The one patient who did not maintain serologic response at three months (<0.4 AU/ml) had only mounted a minimal response of 0.95 AU/ml by one month. At six months, each of the 14 evaluable patients had evidence of ongoing serologic response; median anti‐S level 4109 AU/ml (range 1.71 to >25 000). Seven of the patients had received a booster vaccine dose prior to the six‐month assessment, resulting in increased antibody levels (Figure 1). In the remaining seven who did not receive a booster prior to the six‐month assessment, none experienced a significant decrease in antibody levels compared to the one‐month assessment.
FIGURE 1.
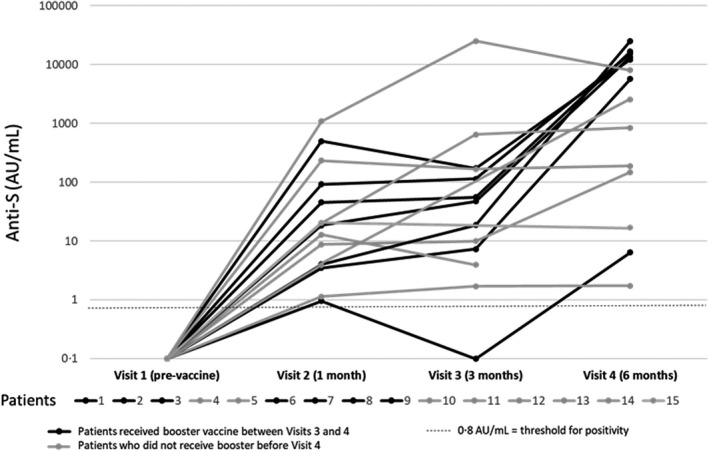
Serologic response overtime in the 15 patients with seroconversion
Of the 37‐patient cohort, one patient developed symptomatic COVID‐19 infection. This patient had completed obinutuzumab three months prior to initial vaccination (BNT162b2 in March/April 2021) but did not seroconvert. They continued to receive venetoclax until July 2021. The patient received a BNT162b2 booster in September 2021 but did not undergo serologic response assessment. Subsequently, the patient developed COVID‐19 in November 2021, received casirivimab/imdevimab, and recovered without significant complications.
DISCUSSION
We identified low rates of seroconversion to the SARS‐CoV‐2 vaccines in CLL, including patients who were previously untreated and those receiving the most utilized therapies, namely BTK and B‐cell lymphoma 2 (BCL2) inhibitors. Vaccine‐induced responses were similar to those in previously published reports of 40%–64%. 2 , 3 , 4 The anti‐S levels produced at one month were relatively low, median 18.45 AU/ml (range 0.954–1081), compared to healthy vaccinated controls. 9 , 10 As a reference, in a prospective observational study conducted amongst healthcare workers utilizing the Roche anti‐S assay, the geometric mean titres in previously uninfected participants were 2881 U/ml for mRNA‐1273 recipients and 1108 U/ml for BNT162b2 recipients.
To confirm the clinical relevance of our findings in patients with humoral deficiency, we demonstrated similar results with the high‐throughput Roche Elecsys anti‐S binding assay and neutralization activity. As such, the Roche anti‐S assay appears to be a reasonable surrogate for neutralization activity in this immunocompromised population. Furthermore, this study is one of the first to demonstrate durability of seroconversion after primary vaccination for at least six months in patients with CLL. As in immunocompetent patients, augmentation of serologic response with a booster dose was possible in responding patients. Whether booster doses should be utilized at earlier time points or repeated is a question for future investigation. It is important to educate patients to continue using precaution as even successfully vaccinated individuals can contract certain variants of SARS‐CoV‐2.
Our study identified that the lack of detectable normal B cells, due to B‐cell‐directed therapy, was a strong predictor of lack of serologic response. While recent publications have shown inferior responses to other types of vaccines in patients receiving BTK inhibitors, our data are the first to identify measurable B cells as a potential predictive biomarker amongst this population. 11 , 12 Data regarding vaccine efficacy in patients receiving venetoclax are limited; however, our findings suggest that relatively rapid B‐cell reconstitution is possible following discontinuation of therapy.
The primary limitation of this analyses was sample size. In addition to small numbers of patients receiving B‐cell‐directed therapy, there was minimal representation of patients receiving the mRNA‐1273 or Ad26.COV2.S vaccines. We have previously reported on the efficacy of Ad26.COV2.S in patients in whom mRNA vaccines were unsuccessful. 13 Additionally, although a thorough analysis of serologic response was performed by both serial anti‐S levels and functional immunity via neutralization assay; analysis of T‐cell immune responses was not performed. While only one patient in our cohort developed COVID‐19, this likely reflects the strict precautions embraced by a vulnerable group of patients. Our larger national trial to further clarify these questions and others regarding the efficacy of the initial vaccination series and subsequent booster vaccines is ongoing (NCT04852822).
AUTHOR CONTRIBUTIONS
Chaitra Ujjani performed research, designed the research study, analysed data, wrote the paper. Mazyar Shadman performed research, designed the research study, analysed data, wrote the paper. Ryan C. Lynch performed research, analysed data. Brian Tu performed research. Philip A. Stevenson analysed data, performed statistical work. Caitlin Grainger performed research. Haiying Zhu performed research. Joshua A. Hill designed the research study, analysed data, wrote the paper. Meei‐Li Huang performed research, analysed data. Leslie Nielsen performed research. Christina Poh performed research. Tyler Sorensen performed research. Ajay K. Gopal performed research, analysed data. Edus H. Warren performed research. Brian G. Till performed research. Sydney Lee performed research. Daria Gausman performed research. Stephen D. Smith performed research. Ted Gooley designed the research study. Alex Greninger performed research, designed the research study, analysed data, wrote the paper.
Supporting information
Table S1 Performance of Roche Anti‐S assay and neutralization assay
Figure S1 Inhibition curve (neutralization activity) of patient serum samples against SARS‐CoV‐2 Spikedelta21‐D614G pseudotyped lentiviral particles with Luciferase‐IReS‐ZsGreen backbone (gifts from Dr. Jesse Bloom).
ACKNOWLEDGEMENTS
Bloom Lab at Fred Hutchinson Cancer Research Center, Dr. Jesse Bloom. Richard Hotes, his foundation helped fund the project.
Ujjani CP, Shadman M, Lynch RC, Tu B, Stevenson PA, Grainger C, et al. The impact of B‐cell‐directed therapy on SARS‐CoV‐2 vaccine efficacy in chronic lymphocytic leukaemia. Br J Haematol. 2022;197:306–309. 10.1111/bjh.18088
REFERENCES
- 1. Shadman M, Ujjani C. Vaccinations in CLL: implications for COVID‐19. Blood. 2021;137(2):144–6. 10.1182/blood.2020009966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. 10.1182/blood.2021011568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roeker LE, Knorr DA, Pessin MS, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047–9. 10.1038/s41375-020-01030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS‐CoV‐2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–3. 10.1016/j.ccell.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Development and Licensure of Vaccines to Prevent COVID‐19: Guidance for Industry . June 2020. https://www.fda.gov/media/139638/download
- 6. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan CW, Yi X, Lenza M, et al. Analytical and clinical evaluation of the semiquantitative elecsys anti‐SARS‐CoV‐2 spike protein receptor binding domain antibody assay on the roche cobas e602 analyzer. Am J Clin Pathol. 2022;157(1):109–18. 10.1093/ajcp/aqab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crawford KHD, Eguia R, Dingens AS, et al. Protocol and reagents for Pseudotyping lentiviral particles with SARS‐CoV‐2 spike protein for neutralization assays. Viruses. 2020;12(5):513. 10.3390/v12050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley BT, Bryan A, Fink SL, et al. Anti‐SARS‐CoV‐2 antibody levels measured by the AdviseDx SARS‐CoV‐2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J Clin Microbiol. 2021;59(9):e0098921. 10.1128/JCM.00989-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steensels D, Pierlet N, Penders J, et al. Comparison of SARS‐CoV‐2 antibody response following vaccination with BNT162b2 and mRNA‐1273. JAMA. 2021;326(15):1533–5. 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun C, Gao J, Couzens L, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656–7. 10.1001/jamaoncol.2016.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185–9. 10.1182/blood.2020008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ujjani C, Greninger AL, Shadman M, et al. Heterologous SARS‐CoV‐2 vaccinations in patients with B‐cell lymphoid malignancies. Am J Hematol. 2022;97(2):E67–9. 10.1002/ajh.26418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Performance of Roche Anti‐S assay and neutralization assay
Figure S1 Inhibition curve (neutralization activity) of patient serum samples against SARS‐CoV‐2 Spikedelta21‐D614G pseudotyped lentiviral particles with Luciferase‐IReS‐ZsGreen backbone (gifts from Dr. Jesse Bloom).


