Abstract
During the 2020 coronavirus (SARS‐CoV‐2) pandemic, several cutaneous lesions were identified, including pseudo‐chilblain, vesicular, urticarial, maculopapular, and livedo/necrosis. A 59‐year‐old obese man with probable COVID‐19 developed painful cyanosis with histopathologic capillary thrombosis of toes, and the cyanosis persisted for nearly 22 months. Shortly after initial exposure to family members with documented SARS‐CoV‐2, he developed upper respiratory symptoms, yet his anti‐SARS‐CoV‐2 antibody and nasal swab RT‐PCR tests were repeatedly negative. Two family members were hospitalized and one of them succumbed with documented SARS‐CoV‐2 pneumonia within 10 days of exposure. Biopsy specimen of the distal toe 16 weeks after initial exposure showed papillary dermal capillary thrombosis with endothelial swelling, telangiectasia, and peri‐eccrine lymphocytic infiltrates resembling pernio. Overall, this is the first case of biopsy specimen of “long COVID toe” following presumed SARS‐CoV‐2 exposure, with a demonstration of thrombotic vasculopathy, toe cyanosis, and pernio‐like pathology.
Keywords: capillary occlusion, COVID toe, COVID‐19, fibrin thrombi, pernio, SARS‐CoV‐2
1. INTRODUCTION
Chilblain‐like acral lesions account for nearly one in five cutaneous COVID‐19 presentations. 1 These chilblain‐like or pseudo‐chilblain lesions around the distal extremities have been termed COVID purpura, or “COVID toe” and may present in the absence of respiratory and gastrointestinal symptoms. 2 , 3 Skin lesions associated with COVID‐19 have been documented in pediatric, adult, and geriatric populations. 4 We describe biopsy findings of a patient who presented with “COVID toe” 16 weeks post‐exposure, with both pernio‐like lymphocyte infiltrate and capillary occlusion by fibrin thrombi (Figure 2B–F).
FIGURE 2.
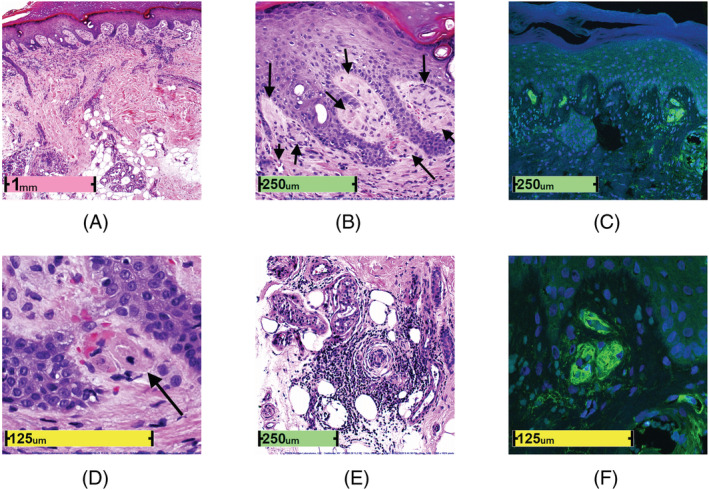
Long COVID toe histopathology. (A) Low‐power photomicrograph (H&E, ×26). (B) Fibrin thrombi occluding capillaries in dermal papillae (H&E, ×130). (C) Fibrin thrombi occluding capillaries in dermal papillae (anti‐fibrin antibody; confocal fluorescence microscopy, ×200). (D) Fibrin thrombi occluding capillaries in dermal papillae (H&E, ×400). (E) Pernio‐like lymphocytic infiltrate in eccrine glands (H&E, ×110). (F) Green fibrin thrombi occluding capillaries in dermal papillae with blue DAPI+ nuclei (fluorescein labeled anti‐fibrin antibody; confocal fluorescence microscopy, ×630)
2. CASE REPORT
A 59‐year‐old obese white male presented to his podiatrist clinic with painful, swollen cyanotic toes of the left foot (Figure 1A–I). This patient reported that 10 weeks earlier, he had been in close contact with two individuals at a family dinner who were diagnosed with COVID‐19 2 days later, and one of whom succumbed to the illness. Following exposure, our patient reported fever and upper respiratory symptoms that resolved within a week. Persistent burning acral pain developed shortly thereafter.
FIGURE 1.
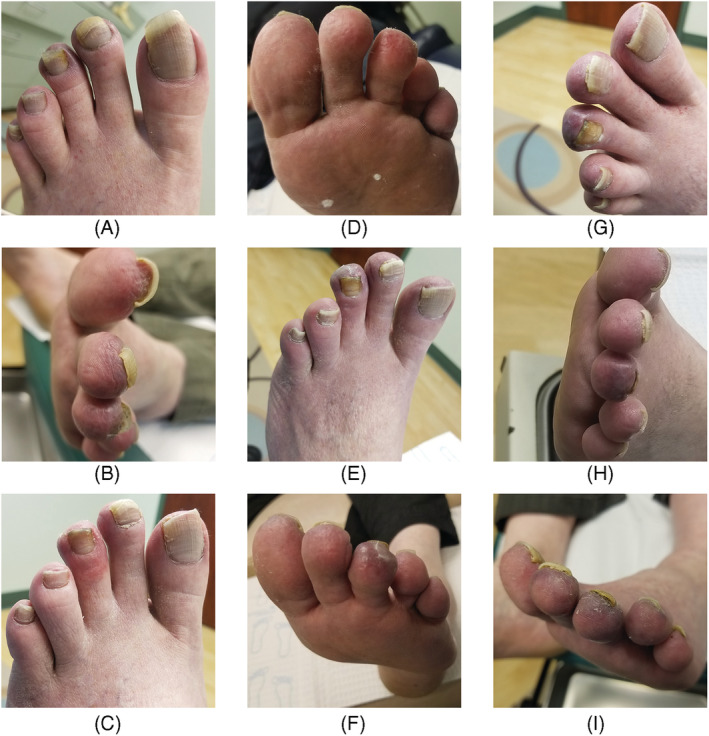
Distal rugosity and cyanosis with proximal erythema persisting for nearly 22 months post‐COVID‐19 exposure. (A, B) May 26, 2020 (12 weeks post‐exposure); (C, D) September 18, 2020 (28 weeks post‐exposure); (E, F) December 2, 2020 (39 weeks post‐exposure); (G, H) January 4, 2021 (44 weeks post‐exposure); (I) May 4, 2021 (first dose: March 23, 2021, second dose: April 29, 2021; 61 weeks post‐exposure)
Physical examination showed toe cyanosis and edema. The toes were tender to palpation with normal capillary refill and normal pedal pulses. The temperature, sensation, range of motion, and muscle strength of the toes were also found to be normal. Radiographs were negative. Past medical history included hypertension; current medications included a daily 50‐mg oral dose of losartan potassium (angiotensin receptor antagonist), a daily 81‐mg oral acetylsalicylic acid, and multivitamins.
Initially, the patient was prescribed celecoxib, a non‐steroidal anti‐inflammatory drug, and advised to keep his toes warm and avoid exposure to cold water or cold temperatures. However, his toes failed to improve and upon return, the third toe appeared more cyanotic than the others, and a 3‐mm punch biopsy specimen was obtained 16 weeks post‐exposure. SARS‐CoV‐2 PCR was repeatedly negative. Anti‐SARS‐CoV‐2 antibody, Epstein–Barr virus PCR, parvovirus B19, C‐reactive protein, ferritin, sedimentation rate, d‐dimer, and prothrombin time were also negative.
Histopathologically, fibrin thrombi occluded many papillary dermal capillaries having endothelial cell swelling and pauci‐cellular inflammatory cell response, mainly deep dermal infiltrate of small lymphocytes in and around eccrine coils (Figure 2E). Fibrin thrombi stained weakly PAS+ and stained intensely with rabbit polyclonal anti‐fibrin antibody by direct immunofluorescence technique (Figure 2C,F; cat. no. RB‐1924‐R2; NeoMarkers; batch #1924R2004A) using epiluminscence microscopy (Olympus BX61 Olympus USA) and confocal fluorescence microscopy (Leica SP8X). Immunohistochemistry for 1A9 monoclonal antibody against SARS‐CoV/SARS‐COV‐2 (COVID‐19) spike protein (dilution 1:200, cat. no. GTX632604; GeneTex, Inc.; batch 43 937) was negative, except for focal weak staining of single epidermal basal keratinocytes and no endothelial staining was observed (not shown). 5 A human lung biopsy specimen with confirmed COVID‐19 positivity was used as positive stain control. The immunostain to spike protein was interpreted as negative because of the low‐intensity staining of a few keratinocytes in the basal layer. The pain diminished with 2% nitroglycerin applied to each toe daily. However, the toes became cyanotic and erythematous and remained so for nearly 22 months, worsening after the second dose of COVID‐19 vaccine (Pfizer‐BioNTech BNT162b2; Figure 1I).
3. DISCUSSION
Generally, there have been reports of epidermal changes, interface dermatitis, papillary dermal changes, lymphocytic vasculitis, lymphocyte infiltrate, along with interstitial and peri‐eccrine mucin deposition associated with COVID toe. 6 The lymphocytic infiltrate may be deep and/or superficial, and perivascular, interstitial, and/or peri‐eccrine. 6 The lesions may also be accompanied by fibrin; however, this is not indicative of systemic coagulopathy. 7 One group examined pernio duration in COVID‐19 patients and found the mean duration was 15 and 12 days for suspected and laboratory‐confirmed COVID‐19, respectively. 7 Of the cases examined in that study, 6.8% had “long‐haul COVID,” with a duration of pernio that persisted over 60 days; two patients sustained pernio lesions for over 133 and 150 days, respectively. 7 Regarding “long haul” COVID‐19, André et al 8 report four patients with acral lesions persisting between 3 and 10 months. Herein, we utilize confocal microscopy (Figure 2C,F) to examine fibrin deposition in a pernio‐like pathology that has been sustained with cyanosis for almost 22 months, or nearly 670 days. This is the longest documented case of long COVID toe, first use of the term long COVID toe, accompanied with histopathology and a clinical timeline with asymptomatic resolution after nearly 22 months.
Given the COVID‐19 exposure, febrile respiratory illness and pseudo‐chilblains, this patient had COVID‐19. Yet neither the virus nor antibodies to the virus could be confirmed for this patient by serology, by RT‐PCR of nasal swab, nor by skin biopsy immunohistochemistry using antibodies to COVID‐19 spike protein. We suspect this represents false‐negative testing because reported false‐negative rates are highly variable and dependent on the timeline between testing and days since exposure; the median false‐negative rate on the day of symptom onset is reported to be 38%. 9 However, this answer is not wholly satisfactory because this patient had no detectable antibody to SARS COV‐2 either. It has also been reported that chilblain‐like lesions can be associated with asymptomatic SARS‐CoV‐2 and the RT‐PCR for the infection may be negative at the time of the chilblain‐like presentation. 7
Our findings, however, follow other observations of cutaneous manifestations of COVID‐19 occurring with negative PCR and serologic results, 1 , 9 , 10 , 11 and given the timing, exposure to and death of a related close‐contact, and histopathological findings, we interpret this patient's dermatological manifestations as because of COVID‐19. There remains the possibility that these lesions may not be caused by COVID‐19; as Daneshjou et al 11 describe, the negative PCR and serology may be because of a dampened immune response, a limitation of the available IHC antibodies, or that the lesions are not associated with COVID‐19.
The patient presented with painful toe cyanosis 10 weeks after close‐contact exposure to COVID‐19 and respiratory illness. Upon histopathological examination, the biopsy specimen (Figure 2A–F) revealed a thrombotic vasculopathy in addition to pernio; the lesions were interpreted as consistent with an exanthem of viral origin. This is the first case of biopsy specimen of long COVID toe with the histopathology showing (a) superficial fibrin thrombi occluding capillaries in dermal papillae, accompanied by (b) endothelial swelling without vasculitis, and (c) pernio‐like lymphocytic infiltrate in eccrine coils. Furthermore, the patient endured symptomatic COVID toe for nearly 22 months.
Nirenberg MS, Requena L, Santonja C, Smith GT, McClain SA. Histopathology of persistent long COVID toe: A case report. J Cutan Pathol. 2022;1‐4. doi: 10.1111/cup.14240
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Galvan Casas C, Catala A, Carretero Hernandez G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. doi: 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;33(6):e13986. doi: 10.1111/dth.13986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen TG, Shrit MA, Feeser TA, Wargo JJ. COVID purpura (toes) case series: a chilblains‐like vasculopathy. Am J Dermatopathol. 2021;43(4):e47‐e50. doi: 10.1097/DAD.0000000000001829 [DOI] [PubMed] [Google Scholar]
- 4. Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID‐19: review of the literature. Dermatopathology. 2020;7(1):3‐16. doi: 10.3390/dermatopathology7010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko CJ, Harigopal M, Gehlhausen JR, Bosenberg M, McNiff JM, Damsky W. Discordant anti‐SARS‐CoV‐2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2021;48(1):47‐52. doi: 10.1111/cup.13866 [DOI] [PubMed] [Google Scholar]
- 6. Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID‐19‐induced chilblains (COVID‐toes) correlated with a published literature review. J Cutan Pathol. 2022;49(1):17‐28. doi: 10.1111/cup.14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMahon DE, Gallman AE, Hruza GJ, et al. Long COVID in the skin: a registry analysis of COVID‐19 dermatological duration. Lancet Infect Dis. 2021;21(3):313‐314. doi: 10.1016/S1473-3099(20)30986-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. André R, Hsieh A, Trellu LT. Chronic acral lesions (“COVID toes”): to add to long post‐COVID‐19 syndrome? Angiology. 2022. doi: 10.1177/00033197211068938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction‐based SARS‐CoV‐2 tests by time since exposure. Ann Intern Med. 2020;173(4):262‐267. doi: 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hubiche T, Le Duff F, Chiaverini C, Giordanengo V, Passeron T. Negative SARS‐CoV‐2 PCR in patients with chilblain‐like lesions. Lancet Infect Dis. 2021;21(3):315‐316. doi: 10.1016/S1473-3099(20)30518-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daneshjou R, Rana J, Dickman M, Yost JM, Chiou A, Ko J. Pernio‐like eruption associated with COVID‐19 in skin of color. JAAD Case Rep. 2020;6(9):892‐897. doi: 10.1016/j.jdcr.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


